The Architecture of Monospecific Microalgae Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Strains and Planktonic Culture Maintenance
2.2. Biofilms Cultivation: Inoculum, Initial Adhesion, and Growth
2.3. Confocal Laser Scanning Microscopy (CLSM): Cells and Matrix Characterization
2.4. Image Analysis
2.5. ATR-FTIR Spectroscopy
2.6. Statistics
3. Results
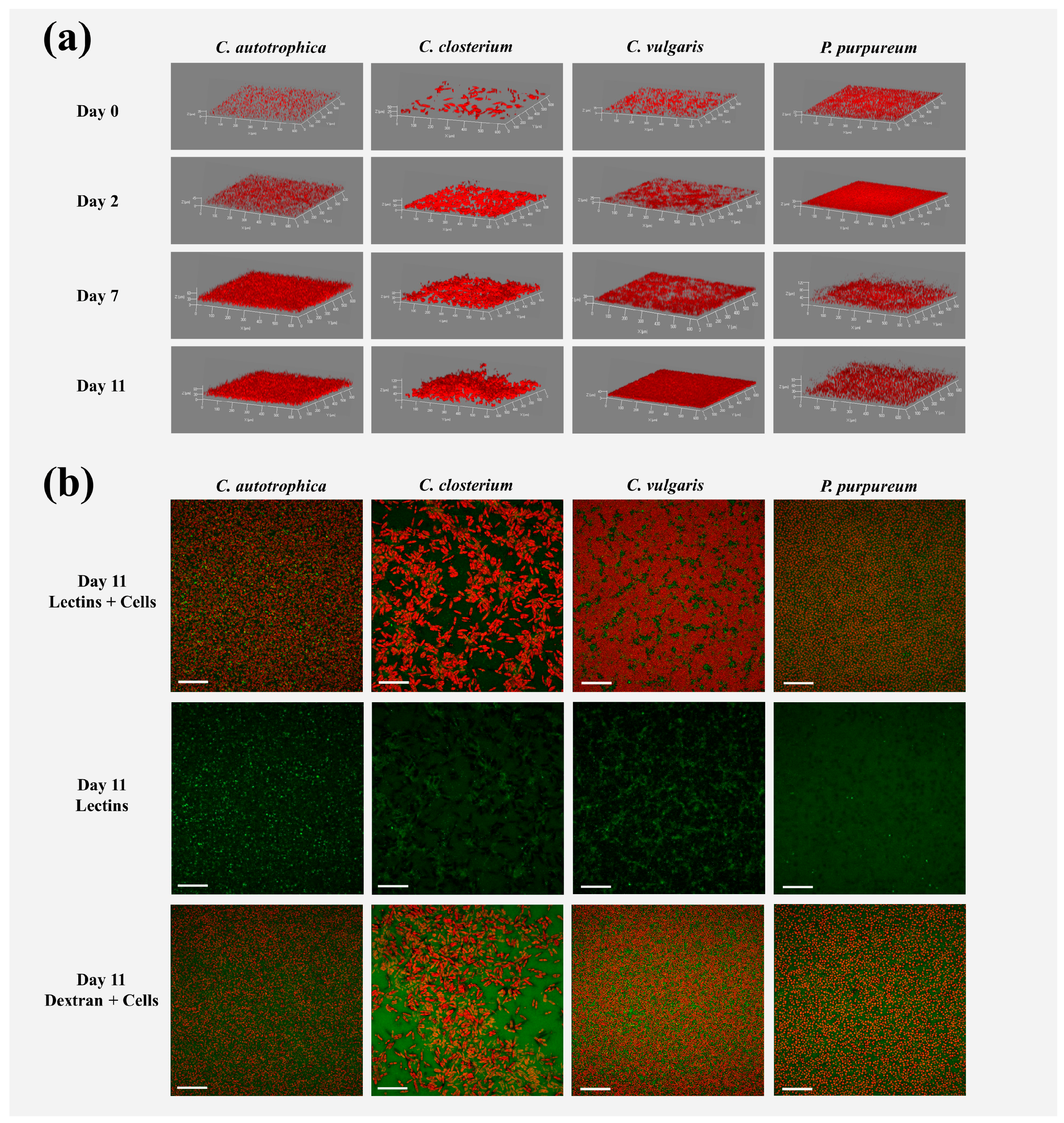
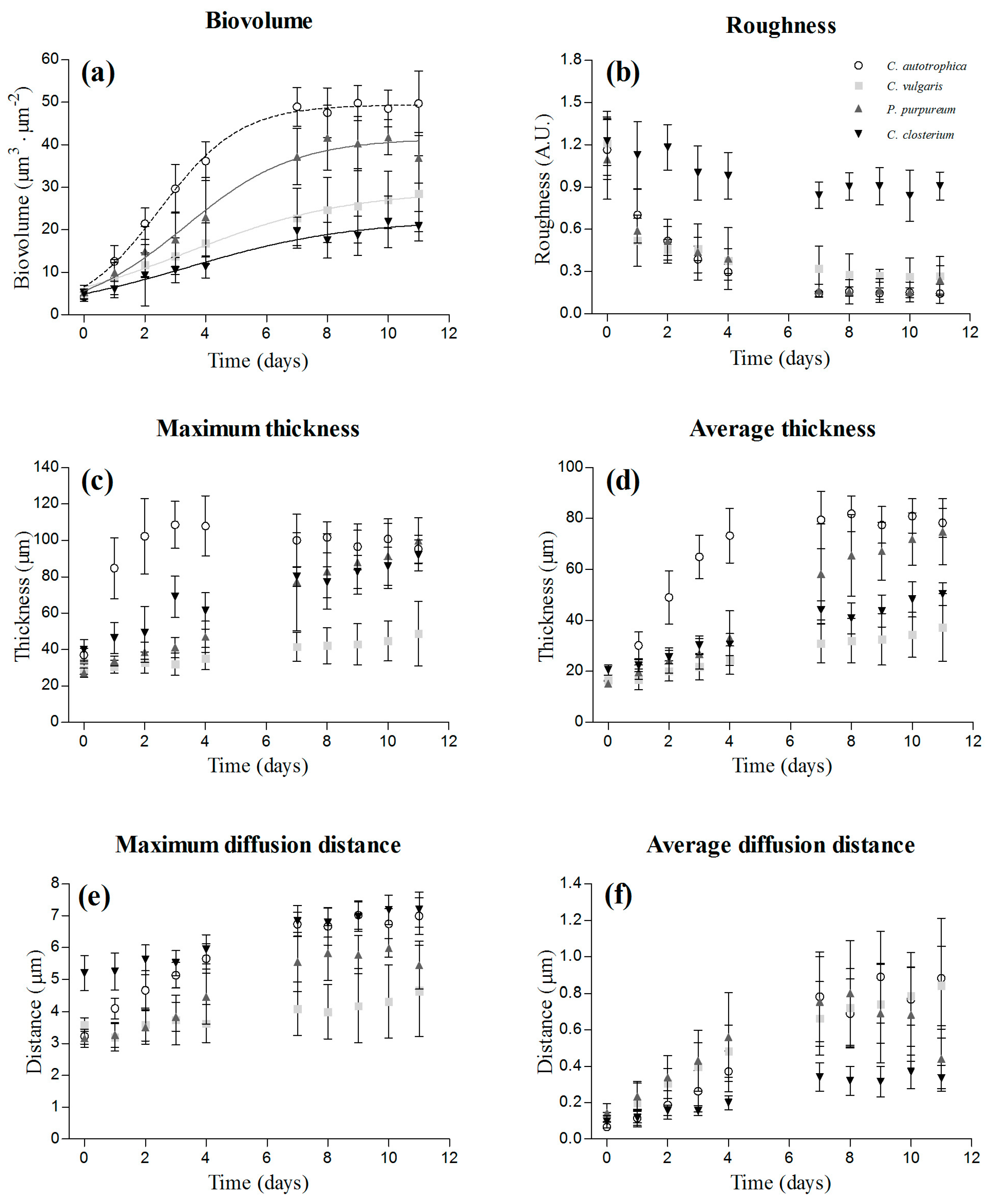
3.1. Biofilm Development over Time: Structural Characteristics
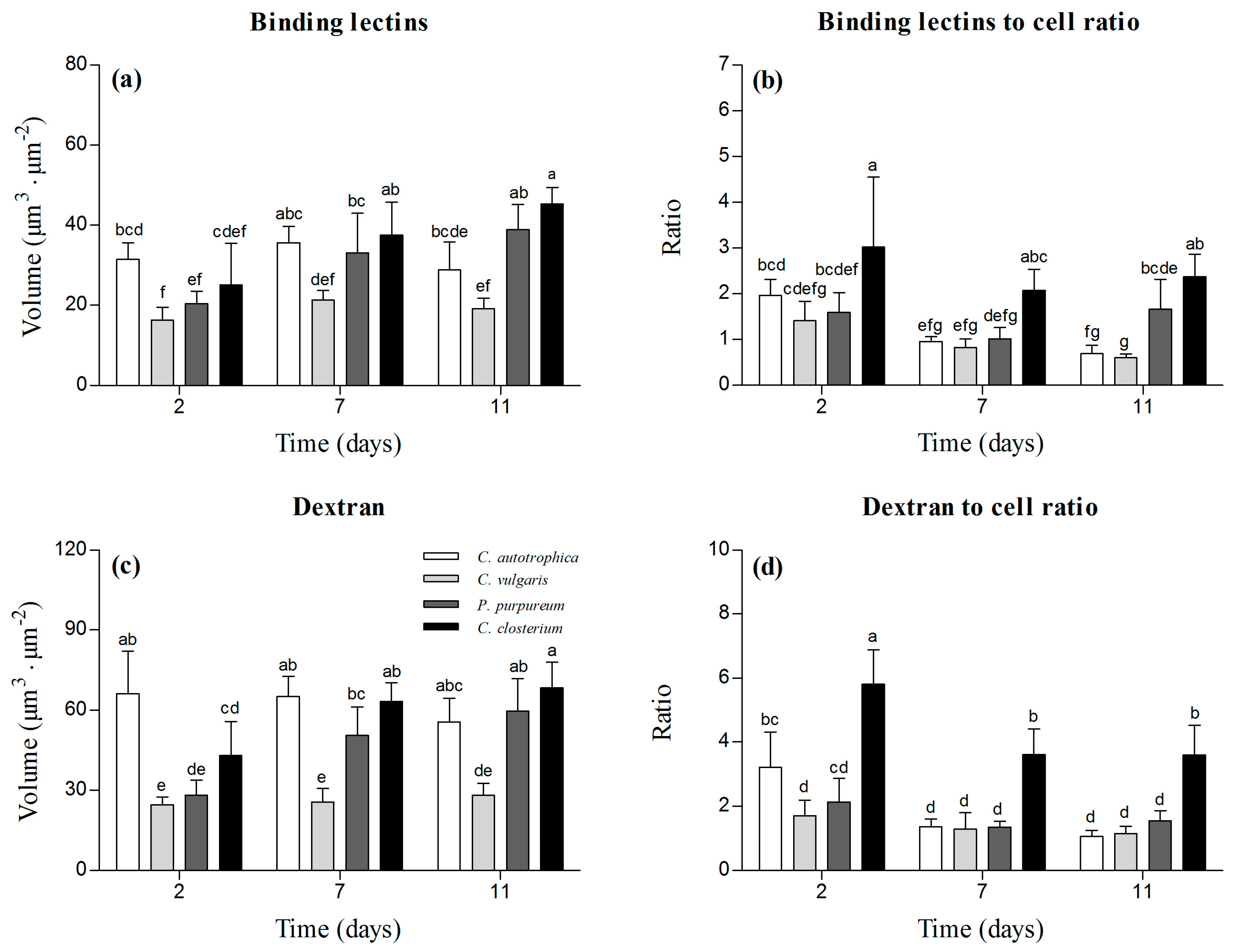
3.2. Matrix Characterization: Lectins and Dextran Volumes
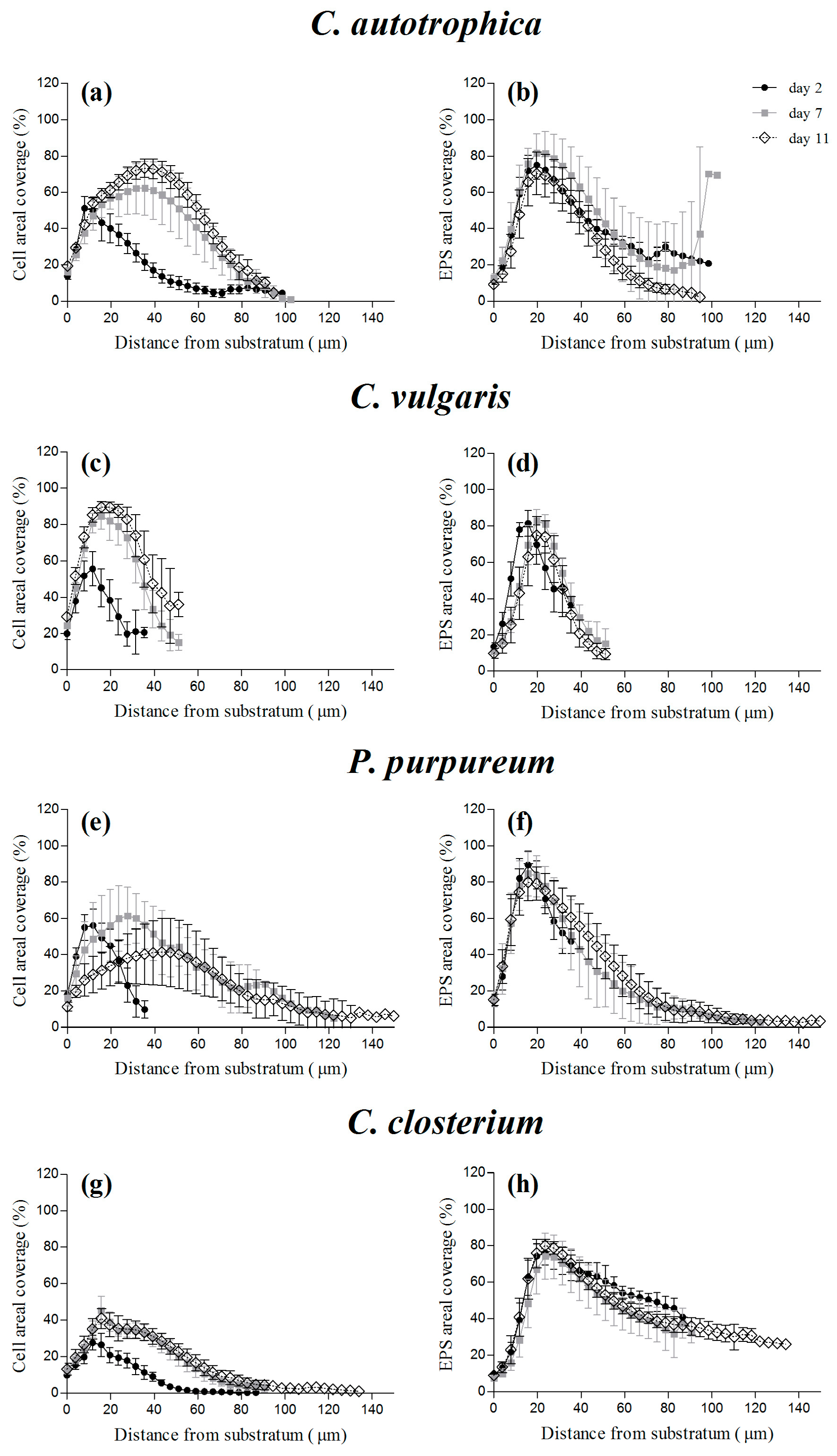
3.3. Areal Coverage over Depth: Cells vs. Matrix Vertical Profiles
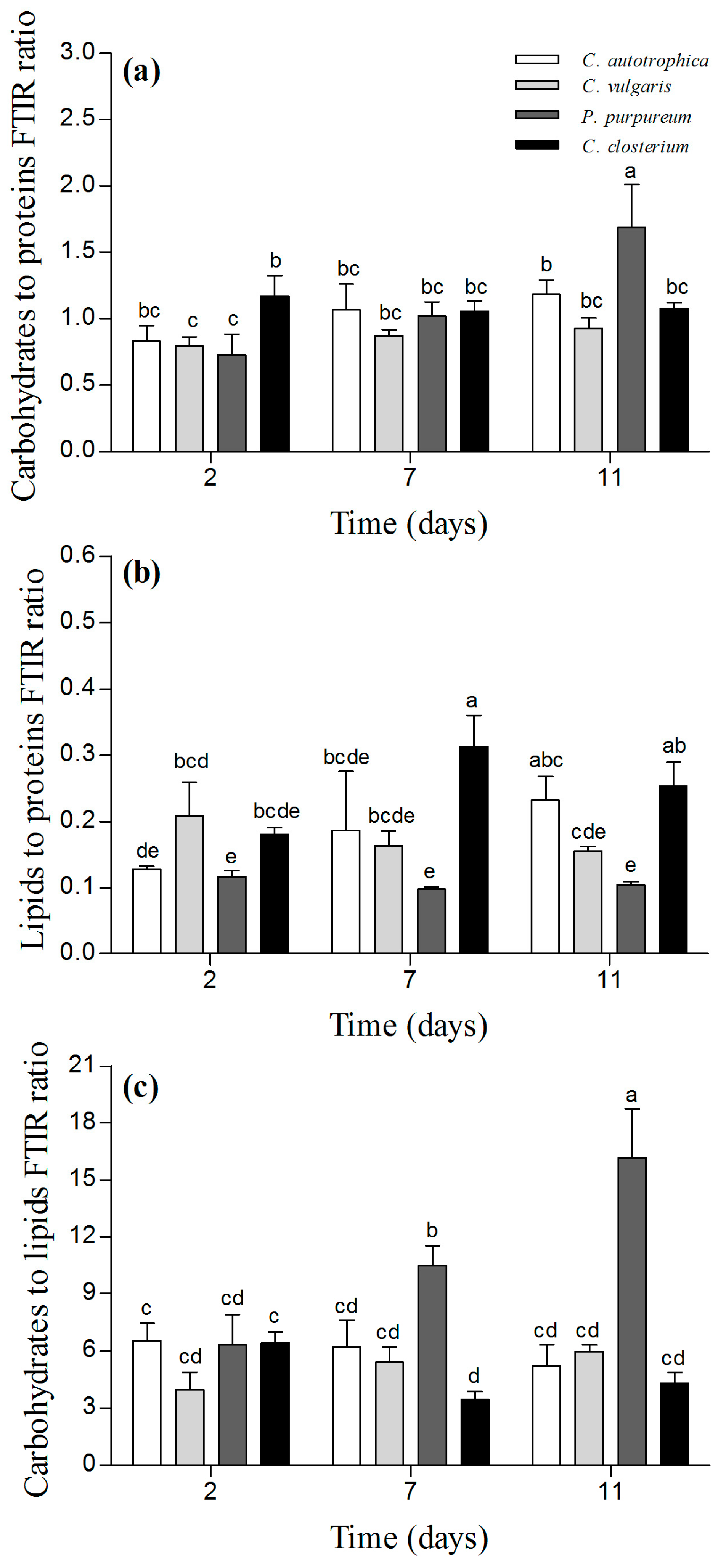
3.4. Biofilm Biochemical Characterization by ATR-FTIR Spectroscopy and Correlation Analysis with Structural Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jorquera, O.; Kiperstok, A.; Sales, E.A.; Embiruçu, M.; Ghirardi, M.L. Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour. Technol. 2010, 101, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, A.; Kinney, K.; Katz, L.; Berberoglu, H. Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor. Bioresour. Technol. 2012, 114, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Berner, F.; Heimann, K.; Sheehan, M. Microalgal biofilms for biomass production. J. Appl. Phycol. 2015, 27, 1793–1804. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenerg. 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Di Pippo, F.; Ellwood, N.T.W.; Gismondi, A.; Bruno, L.; Rossi, F.; Magni, P.; De Philippis, R. Characterization of exopolysaccharides produced by seven biofilm-forming cyanobacterial strains for biotechnological applications. J. Appl. Phycol. 2013, 25, 1697–1708. [Google Scholar] [CrossRef]
- Sutherland, I.W. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- de Beer, D.; Stoodley, P.; Roe, F.; Lewandowski, Z. Effects of biofilm structures on oxygen distribution and mass transport. Biotechnol. Bioeng. 1994, 43, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.N.; de Brouwer, J.F.; Almeida, J.S.; Stal, L.J.; Xavier, J.B. Analysis of a marine phototrophic biofilm by confocal laser scanning microscopy using the new image quantification software PHLIP. BMC Ecol. 2006, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef]
- Bridier, A.; Dubois-Brissonnet, F.; Boubetra, A.; Thomas, V.; Briandet, R. The biofilm architecture of sixty opportunistic pathogens deciphered using a high throughput CLSM method. J. Microbiol. Methods 2010, 82, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Srinandan, C.S.; Jadav, V.; Cecilia, D.; Nerurkar, A.S. Nutrients determine the spatial architecture of Paracoccus sp. biofilm. Biofouling 2010, 26, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Staudt, C.; Horn, H.; Hempel, D.C.; Neu, T.R. Volumetric measurements of bacterial cells and extracellular polymeric substance glycoconjugates in biofilms. Biotechnol. Bioeng. 2004, 88, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Desmond, P.; Best, J.P.; Morgenroth, E.; Derlon, N. Linking composition of extracellular polymeric substances (EPS) to the physical structure and hydraulic resistance of membrane biofilms. Water Res. 2018, 132, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Garny, K.; Neu, T.R.; Horn, H.; Volke, F.; Manz, B. Combined application of 13C NMR spectroscopy and confocal laser scanning microscopy—Investigation on biofilm structure and physico-chemical properties. Chem. Eng. Sci. 2010, 65, 4691–4700. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Decho, A.W. Biochemical characterization of cyanobacterial extracellular polymers (EPS) from modern marine stromatolites (Bahamas). Prep. Biochem. Biotechnol. 2000, 30, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Zhao, F.; Han, Q.; Zhao, A.; Malakar, P.K.; Liu, H.; Pan, Y.; Zhao, Y. High correlation between structure development and chemical variation during biofilm formation by Vibrio parahaemolyticus. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.K.; Hutchison, J.R.; Renslow, R.S.; Kim, Y.-M.; Chrisler, W.B.; Engelmann, H.E.; Dohnalkova, A.C.; Hu, D.; Metz, T.O.; Fredrickson, J.K.; et al. Phototrophic biofilm assembly in microbial-mat-derived unicyanobacterial consortia: model systems for the study of autotroph-heterotroph interactions. Front. Microbiol. 2014, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- David, C.; Bühler, K.; Schmid, A. Stabilization of single species Synechocystis biofilms by cultivation under segmented flow. J. Ind. Microbiol. Biotechnol. 2015, 42, 1083–1089. [Google Scholar] [CrossRef]
- Kernan, C.; Chow, P.P.; Christianson, R.J.; Huang, J. Experimental and computational investigation of biofilm formation by Rhodopseudomonas palustris growth under two metabolic modes. PLoS ONE 2015, 10, e0129354. [Google Scholar] [CrossRef]
- Norcy, T.L.; Niemann, H.; Proksch, P.; Linossier, I.; Vallée-Réhel, K.; Hellio, C.; Faÿ, F. Anti-biofilm effect of biodegradable coatings based on Hemibastadin derivative in marine environment. Int. J. Mol. Sci. 2017, 18, 1520. [Google Scholar] [CrossRef] [PubMed]
- Le Norcy, T.; Faÿ, F.; Obando, C.Z.; Hellio, C.; Réhel, K.; Linossier, I. A new method for evaluation of antifouling activity of molecules against microalgal biofilms using confocal laser scanning microscopy-microfluidic flow-cells. Int. Biodet. Biodegr. 2019, 139, 54–61. [Google Scholar] [CrossRef]
- Barranguet, C.; van Beusekom, S.A.M.; Veuger, B.; Neu, T.R.; Manders, E.M.M.; Sinke, J.J.; Admiraal, W. Studying undisturbed autotrophic biofilms: still a technical challenge. Aquat. Microb. Ecol. 2004, 34, 1–9. [Google Scholar] [CrossRef]
- Neu, T.R.; Swerhone, G.D.W.; Böckelmann, U.; Lawrence, J.R. Effect of CNP on composition and structure of lotic biofilms as detected with lectin-specific glycoconjugates. Aquat. Microb. Ecol. 2005, 38, 283–294. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Bioactivity and applications of polysaccharides from marine microalgae. In Polysaccharides: Bioactivity and Biotechnology; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1683–1727. ISBN 978-3-319-16298-0. [Google Scholar]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.W.; Bold, H.C. Phycological Studies IV. Some Soil Algae from Enchanted Rock and Related Algal Species; University of Texas Publication: Austin, TX, USA, 1963; Volume 6318, p. 95. [Google Scholar]
- Lyman, J.; Fleming, R.H. Composition of sea water. J. Mar. Res. 1940, 134–146. [Google Scholar]
- Walne, P.R. Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria and Mytilus. Fish. Invest. Ser. 1970, 2, 26. [Google Scholar]
- Assaf, D.; Steinberg, D.; Shemesh, M. Lactose triggers biofilm formation by Streptococcus mutans. Int. Dairy J. 2015, 42, 51–57. [Google Scholar] [CrossRef]
- Klein, M.I.; Duarte, S.; Xiao, J.; Mitra, S.; Foster, T.H.; Koo, H. Structural and molecular basis of the role of starch and sucrose in Streptococcus mutans biofilm development. Appl. Environ. Microbiol. 2009, 75, 837–841. [Google Scholar] [CrossRef]
- Koo, H.; Xiao, J.; Klein, M.I.; Jeon, J.G. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J. Bacteriol. 2010, 192, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.R.; Wolfaardt, G.M.; Korber, D.R. Determination of diffusion coefficients in biofilms by Confocal Laser Microscopy. Appl. Environ. Microbiol. 1994, 60, 1166–1173. [Google Scholar] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Otsu, N. A treshold selection method from gray-level histograms. IEEE Trans. Syst. Man and Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org/ (accessed on 29 May 2018).
- Tsai, Y.-P. Impact of flow velocity on the dynamic behaviour of biofilm bacteria. Biofouling. 2005, 21, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Miao, L.; Hou, J.; Wang, P.; Qian, J.; Dai, S. The effect of flow velocity on the distribution and composition of extracellular polymeric substances in biofilms and the detachment mechanism of biofilms. Water Sci. Technol. 2014, 69, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84). 2017. Available online: https://github.com/taiyun/corrplot (accessed on 11 January 2019).
- Kolderman, E.; Bettampadi, D.; Samarian, D.; Dowd, S.E.; Foxman, B.; Jakubovics, N.S.; Rickard, A.H. L-Arginine destabilizes oral multi-species biofilm communities developed in human saliva. PLoS ONE 2015, 10, e0121835. [Google Scholar] [CrossRef]
- Li, L.; Jeon, Y.; Lee, S.-H.; Ryu, H.; Santo Domingo, J.W.; Seo, Y. Dynamics of the physiochemical and community structures of biofilms under the influence of algal organic matter and humic substances. Water Res. 2019, 158, 136–145. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Silva, W.J.; Jin, L.J.; Samaranayake, Y.H.; Samaranayake, L.P. Architectural analysis, viability assessment and growth kinetics of Candida albicans and Candida glabrata biofilms. Arch. Oral Biol. 2009, 54, 1052–1060. [Google Scholar] [CrossRef]
- Schnurr, P.J.; Espie, G.S.; Allen, D.G. The effect of light direction and suspended cell concentrations on algal biofilm growth rates. Appl. Microbiol. Biotechnol. 2014, 98, 8553–8562. [Google Scholar] [CrossRef]
- Rincon, S.M.; Urrego, N.F.; Avila, K.J.; Romero, H.M.; Beyenal, H. Photosynthetic activity assessment in mixotrophically cultured Chlorella vulgaris biofilms at various developmental stages. Algal Res. 2019, 38, 101408. [Google Scholar] [CrossRef]
- Battin, T.J.; Kaplan, L.A.; Newbold, J.D.; Cheng, X.; Hansen, C. Effects of current velocity on the nascent architecture of stream microbial biofilms. Appl. Environ. Microbiol. 2003, 69, 5443–5452. [Google Scholar] [CrossRef] [PubMed]
- Picioreanu, C.; Loosdrecht, M.C.M.V.; Heijnen, J.J. A theoretical study on the effect of surface roughness on mass transport and transformation in biofilms. Biotechnol. Bioeng. 2000, 68, 355–369. [Google Scholar] [CrossRef]
- Picioreanu, C.; Loosdrecht, M.C.M.V.; Heijnen, J.J. Effect of diffusive and convective substrate transport on biofilm structure formation: A two-dimensional modeling study. Biotechnol. Bioeng. 2000, 69, 504–515. [Google Scholar] [CrossRef]
- Alpkvist, E.; Picioreanu, C.; Loosdrecht, M.C.M.V.; Heyden, A. Three-dimensional biofilm model with individual cells and continuum EPS matrix. Biotechnol. Bioeng. 2006, 94, 961–979. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.A.; Bueno-Silva, B.; Galvão, L.C.D.C.; Duarte, M.C.T.; Sartoratto, A.; Figueira, G.M.; Alencar, S.M.D.; Rosalen, P.L. The Effect of Essential Oils and Bioactive Fractions on Streptococcus mutans and Candida albicans biofilms: A Confocal Analysis. Available online: https://www.hindawi.com/journals/ecam/2015/871316/ (accessed on 11 February 2019).
- Rodriguez, D.; Einarsson, B.; Carpio, A. Biofilm growth on rugose surfaces. Phys. Rev. E 2012, 86, 061914. [Google Scholar] [CrossRef]
- Wagner, M.; Ivleva, N.P.; Haisch, C.; Niessner, R.; Horn, H. Combined use of confocal laser scanning microscopy (CLSM) and Raman microscopy (RM): Investigations on EPS–Matrix. Water Res. 2009, 43, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Lu, W.-T.; Shan, Q.; Cao, J.-S. Characteristics of extracellular polymeric substances of phototrophic biofilms at different aquatic habitats. Carbohydr. Polym. 2014, 106, 1–6. [Google Scholar] [CrossRef]
- Polizzi, B.; Bernard, O.; Ribot, M. A time-space model for the growth of microalgae biofilms for biofuel production. J. Theor. Biol. 2017, 432, 55–79. [Google Scholar] [CrossRef]
| Microalgae Species | μ (d−1) | Maximal Biovolume (μm3·μm−2) |
|---|---|---|
| C. autotrophica | 0.72 a (0.24) | 50.47 a (4.09) |
| C. vulgaris | 0.45 b (0.14) | 31.22 c (8.49) |
| P. purpureum | 0.65 ab (0.20) | 43.02 b (4.92) |
| C. closterium | 0.43 b (0.13) | 22.42 d (2.59) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanesi, A.; Paule, A.; Bernard, O.; Briandet, R.; Lopes, F. The Architecture of Monospecific Microalgae Biofilms. Microorganisms 2019, 7, 352. https://doi.org/10.3390/microorganisms7090352
Fanesi A, Paule A, Bernard O, Briandet R, Lopes F. The Architecture of Monospecific Microalgae Biofilms. Microorganisms. 2019; 7(9):352. https://doi.org/10.3390/microorganisms7090352
Chicago/Turabian StyleFanesi, Andrea, Armelle Paule, Olivier Bernard, Romain Briandet, and Filipa Lopes. 2019. "The Architecture of Monospecific Microalgae Biofilms" Microorganisms 7, no. 9: 352. https://doi.org/10.3390/microorganisms7090352
APA StyleFanesi, A., Paule, A., Bernard, O., Briandet, R., & Lopes, F. (2019). The Architecture of Monospecific Microalgae Biofilms. Microorganisms, 7(9), 352. https://doi.org/10.3390/microorganisms7090352





