ITS rDNA Gene Analysis Versus MALDI-TOF MS For Identification of Neoscytalidium dimidiatum Isolated from Onychomycosis and Dermatomycosis Cases in Medellin (Colombia)
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Isolates
2.2. Molecular Identification
2.2.1. Preparation of Mycelium for the Extraction of DNA
2.2.2. DNA Extraction
2.2.3. PCR amplification
2.2.4. DNA Sequence Processing and Molecular Taxonomic Analysis
2.3. MALDI-TOF MS Identification
2.3.1. Protein Extraction
2.3.2. Constructing the Reference Mass Spectra
2.3.3. Identification of Clinical Isolates
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slippers, B.; Boissin, E.; Phillips, A.J.; Groenewald, J.Z.; Lombard, L.; Wingfield, M.J.; Postma, A.; Burgess, T.; Crous, P.W. Phylogenetic lineages in the Botryosphaeriales: A systematic and evolutionary framework. Stud. Mycol. 2013, 76, 31–49. [Google Scholar] [CrossRef]
- Huang, S.K.; Tangthirasunun, N.; Phillips, A.J.; Dai, D.Q.; Wanasinghe, D.N.; Wen, T.C.; Bahkali, A.H.; Hyde, K.D.; Kang, J.C. Morphology and Phylogeny of Neoscytalidium orchidacearum sp. nov. (Botryosphaeriaceae). Mycobiology 2016, 44, 79–84. [Google Scholar] [CrossRef]
- Calvillo-Medina, R.P.; Martinez-Neria, M.; Mena-Portales, J.; Barba-Escoto, L.; Raymundo, T.; Campos-Guillen, J.; Jones, G.H.; Reyes-Grajeda, J.P.; Gonzalez, Y.M.J.A.; Bautista-de Lucio, V.M. Identification and biofilm development by a new fungal keratitis aetiologic agent. Mycoses 2019, 62, 62–72. [Google Scholar] [CrossRef]
- Di Chiacchio, N.; Noriega, L.F.; Gioia Di Chiacchio, N.; Ocampo-Garza, J. Superficial black onychomycosis due to Neoscytalidium dimidiatum. J. Eur. Acad. Derm. Venereol. 2017, 31, e453–e455. [Google Scholar] [CrossRef]
- Morales-Cardona, C.A.; Valbuena-Mesa, M.C.; Alvarado, Z.; Solorzano-Amador, A. Non-dermatophyte mould onychomycosis: A clinical and epidemiological study at a dermatology referral centre in Bogota, Colombia. Mycoses 2014, 57, 284–293. [Google Scholar] [CrossRef]
- Bakhshizadeh, M.; Hashemian, H.R.; Najafzadeh, M.J.; Dolatabadi, S.; Zarrinfar, H. First report of rhinosinusitis caused by Neoscytalidium dimidiatum in Iran. J. Med. Microbiol. 2014, 63, 1017–1019. [Google Scholar] [CrossRef]
- Dionne, B.; Neff, L.; Lee, S.A.; Sutton, D.A.; Wiederhold, N.P.; Lindner, J.; Fan, H.; Jakeman, B. Pulmonary fungal infection caused by Neoscytalidium dimidiatum. J. Clin. Microbiol. 2015, 53, 2381–2384. [Google Scholar] [CrossRef]
- Garinet, S.; Tourret, J.; Barete, S.; Arzouk, N.; Meyer, I.; Frances, C.; Datry, A.; Mazier, D.; Barrou, B.; Fekkar, A. Invasive cutaneous Neoscytalidium infections in renal transplant recipients: A series of five cases. Bmc. Infect. Dis. 2015, 15, 535. [Google Scholar] [CrossRef]
- Elinav, H.; Izhar, U.; Benenson, S.; Admon, D.; Hidalgo-Grass, C.; Polacheck, I.; Korem, M. Invasive Scytalidium dimidiatum infection in an immunocompetent adult. J. Clin. Microbiol. 2009, 47, 1259–1263. [Google Scholar] [CrossRef]
- Yang, S.J.; Ng, C.Y.; Wu, T.S.; Huang, P.Y.; Wu, Y.M.; Sun, P.L. Deep cutaneous Neoscytalidium dimidiatum infection: Successful outcome with amphotericin B therapy. Mycopathologia 2019, 184, 169–176. [Google Scholar] [CrossRef]
- Phillips, A.J.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–67. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Dingle, T.C.; Butler-Wu, S.M. MALDI-TOF mass spectrometry for microorganism identification. Clin. Lab. Med. 2013, 33, 589–609. [Google Scholar] [CrossRef]
- SanitáLima, M.; Coutinho de Lucas, R.; Lima, N.; Polizeli Mde, L.M.; Santos, C. Fungal community ecology using MALDI-TOF MS demands curated mass spectral databases. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Rodrigues, P.; Venâncio, A.; Kozakiewicz, Z.; Lima, N. A polyphasic approach to the identification of aflatoxigenic and non-aflatoxigenic strains of Aspergillus section Flavi isolated from Portuguese almonds. Int. J. Food Microbiol. 2009, 129, 187–193. [Google Scholar] [CrossRef]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Sninsky, J., White, T., Eds.; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Packeu, A.; Hendrickx, M.; Beguin, H.; Martiny, D.; Vandenberg, O.; Detandt, M. Identification of the Trichophyton mentagrophytes complex species using MALDI-TOF mass spectrometry. Med. Mycol. 2013, 51, 580–585. [Google Scholar] [CrossRef]
- Cassagne, C.; Ranque, S.; Normand, A.C.; Fourquet, P.; Thiebault, S.; Planard, C.; Hendrickx, M.; Piarroux, R. Mould routine identification in the clinical laboratory by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. PLoS ONE 2011, 6, e28425. [Google Scholar] [CrossRef]
- Schoch, C.L.; Robbertse, B.; Robert, V.; Vu, D.; Cardinali, G.; Irinyi, L.; Meyer, W.; Nilsson, R.H.; Hughes, K.; Miller, A.N.; et al. Finding needles in haystacks: Linking scientific names, reference specimens and molecular data for Fungi. Database (Oxford) 2014, 2014. [Google Scholar] [CrossRef]
- Farr, D.F.; Elliott, M.; Rossman, A.Y.; Edmonds, R.L. Fusicoccum arbuti sp. nov. causing cankers on pacific madrone in western North America with notes on Fusicoccum dimidiatum, the correct name for Scytalidium dimidiatum and Nattrassia mangiferae. Mycologia 2005, 97, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Nkondjo Minkoumou, S.; Fabrizi, V.; Papini, M. Onychomycosis in Cameroon: A clinical and epidemiological study among dermatological patients. Int. J. Derm. 2012, 51, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Dias, N.; Santos, C.; Lima, N. The use of MALDI-TOF ICMS as an alternative tool for Trichophyton rubrum identification and typing. Enferm. Infecc. Microbiol. Clin. 2014, 32, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, C.; Berger, F.; Gantier, J.C. Epidemiology of superficial fungal diseases in French Guiana: A three-year retrospective analysis. Med. Mycol. 2011, 49, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Lavorato, F.G.; Guimaraes, D.A.; Premazzi, M.G.; Pineiro-Maceira, J.M.; Bernardes-Engemann, A.R.; Orofino-Costa, R. Performance of mycology and histopathology tests for the diagnosis of toenail onychomycosis due to filamentous fungi: Dermatophyte and non-dermatophyte moulds. Mycoses 2017, 60, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Oycka, C.A.; Gugnani, H.C. Keratin degradation by Scytalidium species and Fusarium solani. Mycoses 1998, 41, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.G.; Martinez, C.; Zapata, B.; Sanclemente, G.; Gallego, M.; Mesa, A.C. In vitro activity of fluconazole, itraconazole, voriconazole and terbinafine against fungi causing onychomycosis. Clin. Exp. Derm. 2010, 35, 658–663. [Google Scholar] [CrossRef]
- Zuluaga, A.; Tabares, A.M.; Arango, M. Importancia creciente de los géneros Fusarium y Scytalidium como agentes de onicomicosis. Rev. Asoc. Col. Dermat. 2001, 9, 593–599. [Google Scholar]
- Zuluaga, A.; de Bedout, C.; Tabares, A.; Cano, L.; Restrepo, A.; Arango, M. Comportamiento de los agentes etiológicos de las onicomicosis en un laboratorio de micología de referencia (Medellín 1994–2003). Med. Cutan. Iber. Lat. Am. 2005, 33, 251–256. [Google Scholar]
- Machouart-Dubach, M.; Lacroix, C.; Vaury, C.; Feuillhade de Chauvin, M.; Bellanné, C.; Derouin, F.; Lorenzo, F. Nucleotide structure of the Scytalidium hyalinum and Scytalidium dimidiatum 18S subunit ribosomal RNA gene: Evidence for the insertion of a group IE intron in the rDNA gene of S. dimidiatum. FEMS Microbiol. Lett. 2001, 208, 187–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alshawa, K.; Beretti, J.L.; Lacroix, C.; Feuilhade, M.; Dauphin, B.; Quesne, G.; Hassouni, N.; Nassif, X.; Bougnoux, M.E. Successful identification of clinical dermatophyte and Neoscytalidium species by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2012, 50, 2277–2281. [Google Scholar] [CrossRef] [PubMed]
- Buskirk, A.D.; Hettick, J.M.; Chipinda, I.; Law, B.F.; Siegel, P.D.; Slaven, J.E.; Green, B.J.; Beezhold, D.H. Fungal pigments inhibit the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis of darkly pigmented fungi. Anal. Biochem. 2011, 411, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.L.; Lee, T.F.; Wu, C.J.; Teng, S.H.; Teng, L.J.; Ko, W.C.; Hsueh, P.R. Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry can accurately differentiate Aeromonas dhakensis from A. hydrophila, A. caviae, and A. veronii. J. Clin. Microbiol. 2014, 52, 2625–2628. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Fungal DNA barcoding. Genome 2016, 59, 913–932. [Google Scholar] [CrossRef] [PubMed]
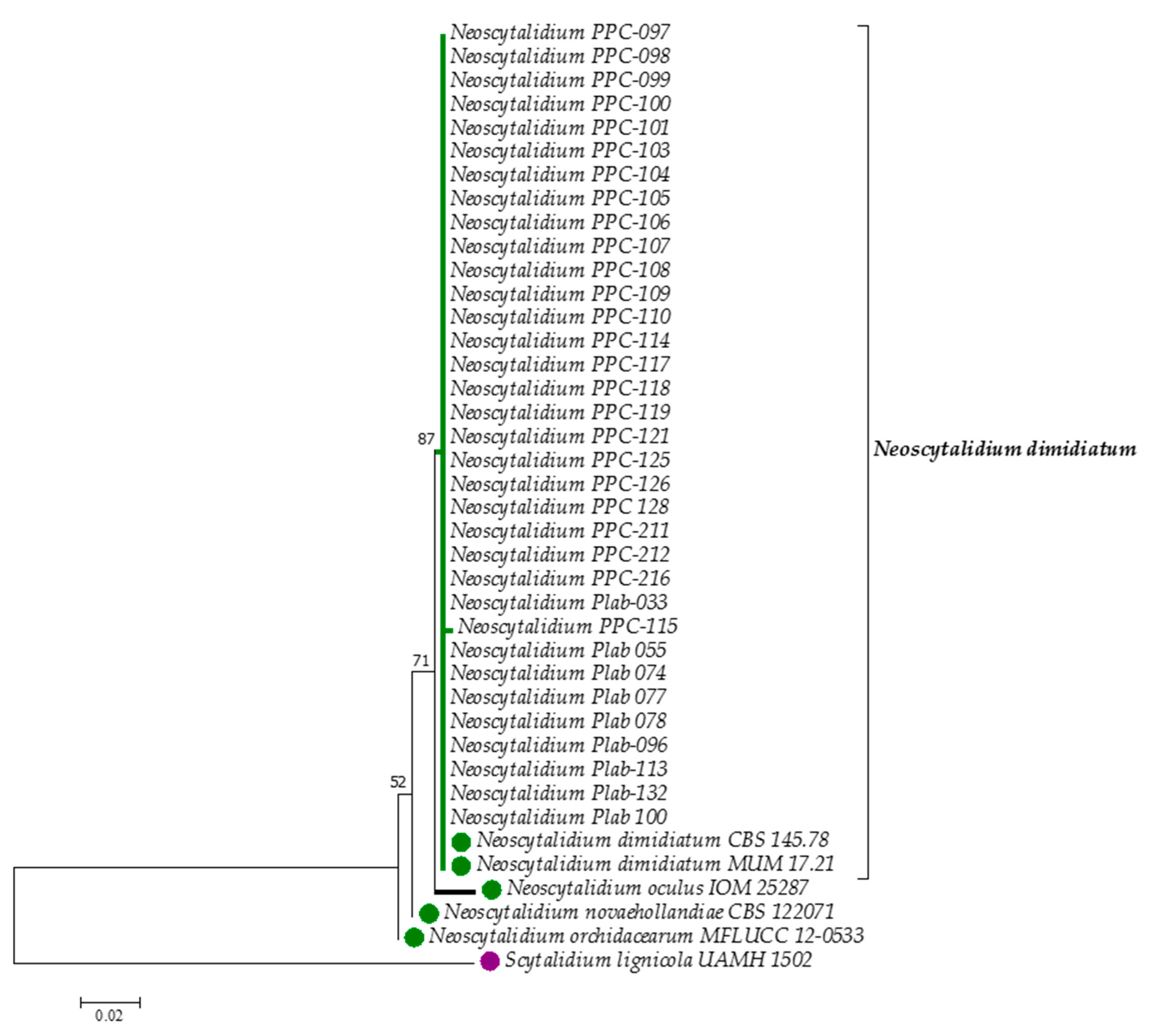
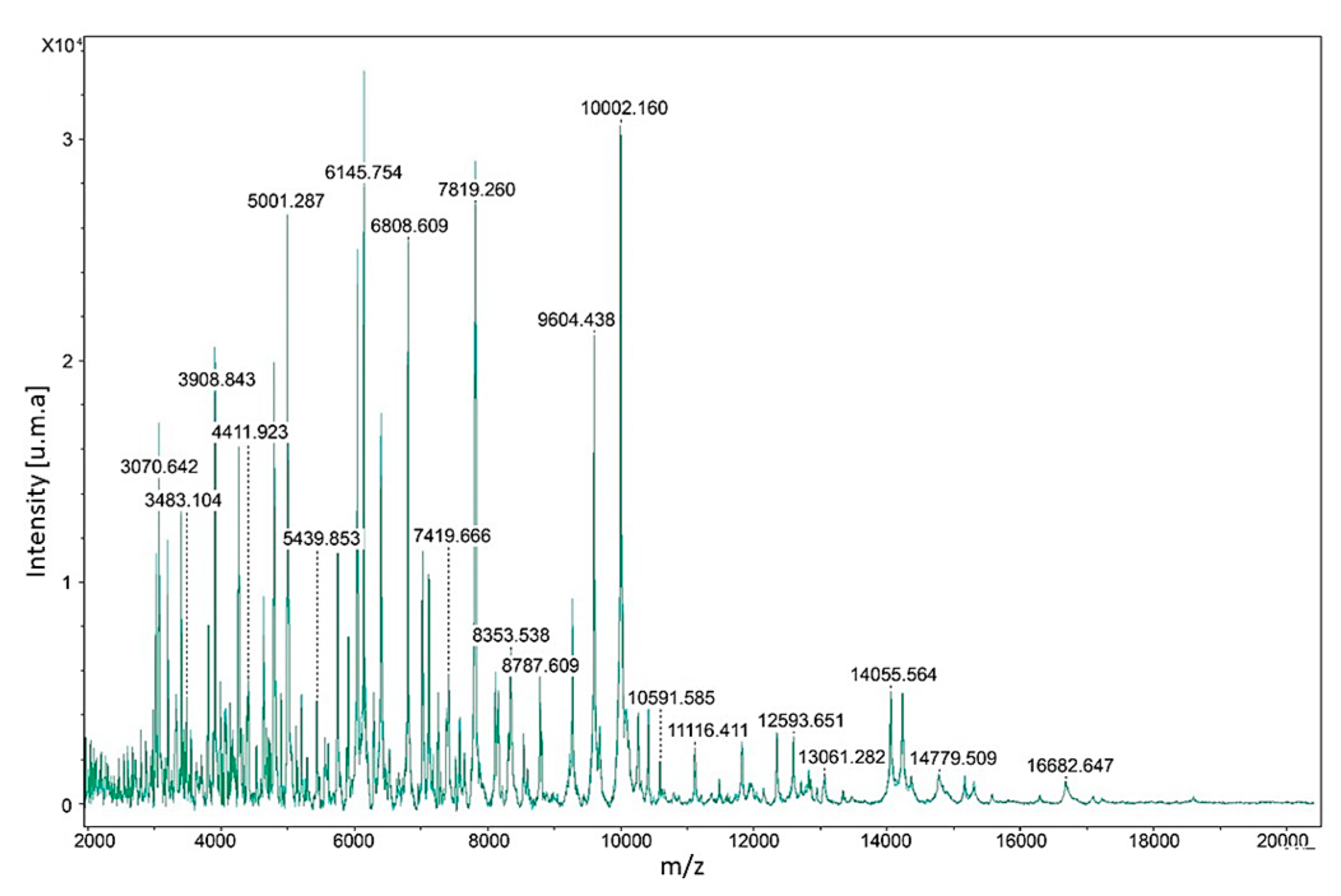
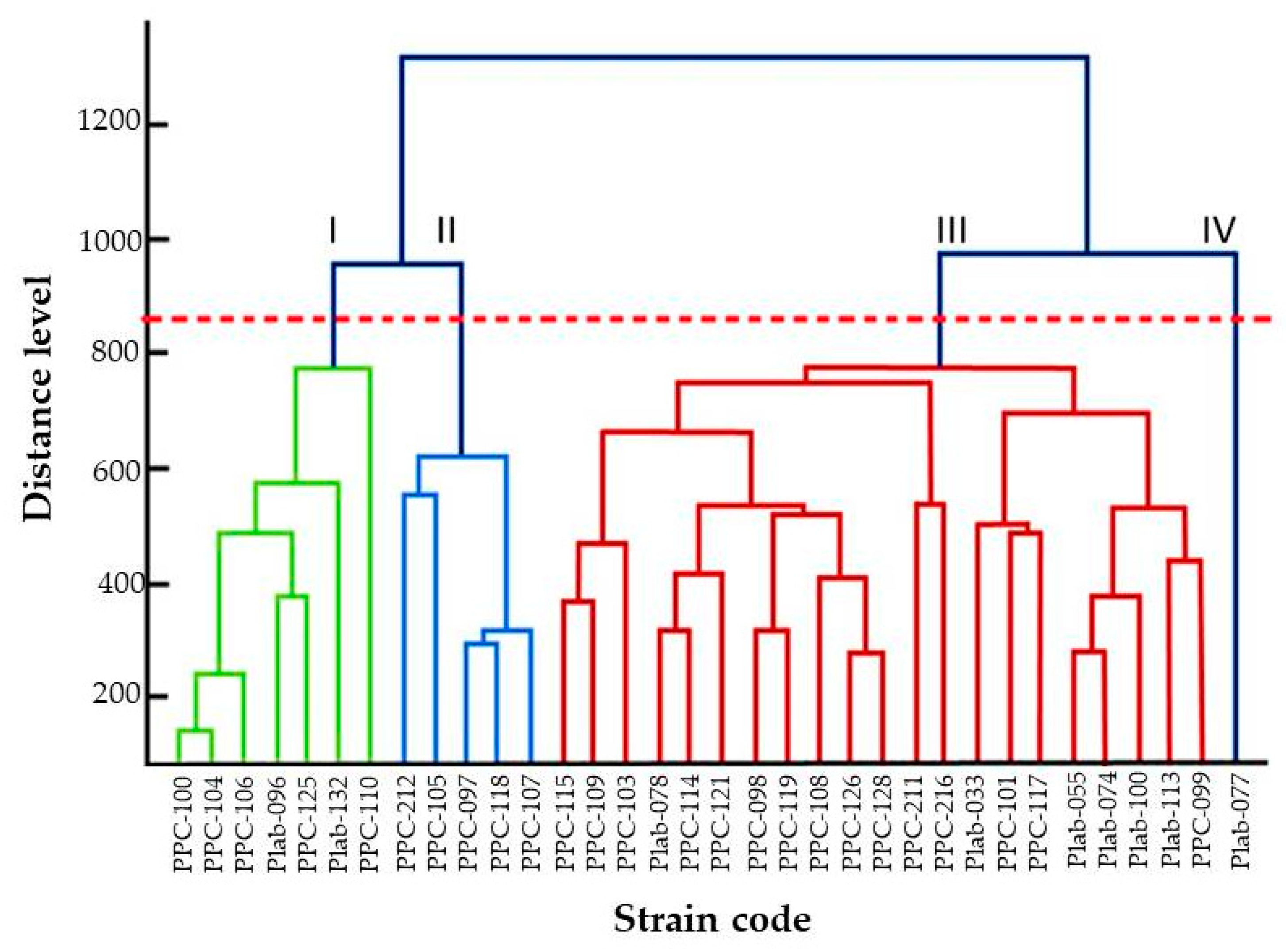
| Strain | Collection Number | Strain Code | Origin | Country | GenBank ac. Number |
|---|---|---|---|---|---|
| N. dimidiatum | N/A | CBS 145.78 T | onychomycosis | UK | KF531816 |
| N. dimidiatum | MUM 17.21 | UdeA 8023 | onychomycosis | Colombia | MN371274 |
| N. dimidiatum | MUM 19.44 | PPC-097 | onychomycosis | Colombia | MN257445 |
| N. dimidiatum | MUM 19.45 | PPC-098 | onychomycosis | Colombia | MN257446 |
| N. dimidiatum | MUM 19.46 | PPC-099 | onychomycosis | Colombia | MN257447 |
| N. dimidiatum | MUM 19.47 | PPC-100 | dermatomycosis | Colombia | MN257448 |
| N. dimidiatum | MUM 19.48 | PPC-101 | onychomycosis | Colombia | MN257449 |
| N. dimidiatum | MUM 19.49 | PPC-103 | onychomycosis | Colombia | MN257450 |
| N. dimidiatum | MUM 19.50 | PPC-104 | onychomycosis | Colombia | MN257451 |
| N. dimidiatum | MUM 19.51 | PPC-105 | onychomycosis | Colombia | MN257452 |
| N. dimidiatum | MUM 19.52 | PPC-106 | onychomycosis | Colombia | MN257453 |
| N. dimidiatum | MUM 19.53 | PPC-107 | onychomycosis | Colombia | MN257454 |
| N. dimidiatum | MUM 19.54 | PPC-108 | onychomycosis | Colombia | MN257455 |
| N. dimidiatum | MUM 19.55 | PPC-109 | onychomycosis | Colombia | MN257456 |
| N. dimidiatum | MUM 19.56 | PPC-110 | onychomycosis | Colombia | MN257457 |
| N. dimidiatum | MUM 19.57 | PPC-114 | onychomycosis | Colombia | MN257458 |
| N. dimidiatum | MUM 19.58 | PPC-115 | onychomycosis | Colombia | MN257459 |
| N. dimidiatum | MUM 19.59 | PPC-117 | onychomycosis | Colombia | MN257460 |
| N. dimidiatum | MUM 19.60 | PPC-118 | onychomycosis | Colombia | MN257461 |
| N. dimidiatum | MUM 19.61 | PPC-119 | onychomycosis | Colombia | MN257462 |
| N. dimidiatum | MUM 19.62 | PPC-121 | onychomycosis | Colombia | MN257463 |
| N. dimidiatum | MUM 19.63 | PPC-125 | onychomycosis | Colombia | MN257464 |
| N. dimidiatum | MUM 19.64 | PPC-126 | dermatomycosis | Colombia | MN257465 |
| N. dimidiatum | MUM 19.65 | PPC-128 | onychomycosis | Colombia | MN257466 |
| N. dimidiatum | MUM 19.66 | PPC-211 | onychomycosis | Colombia | MN257467 |
| N. dimidiatum | MUM 19.67 | PPC-212 | onychomycosis | Colombia | MN257468 |
| N. dimidiatum | MUM 19.68 | PPC-216 | onychomycosis | Colombia | MN257469 |
| N. dimidiatum | MUM 19.69 | Plab-033 | onychomycosis | Colombia | MN257470 |
| N. dimidiatum | MUM 19.70 | Plab-055 | onychomycosis | Colombia | MN257471 |
| N. dimidiatum | MUM 19.71 | Plab-074 | onychomycosis | Colombia | MN257472 |
| N. dimidiatum | MUM 19.72 | Plab-077 | onychomycosis | Colombia | MN257473 |
| N. dimidiatum | MUM 19.73 | Plab-078 | onychomycosis | Colombia | MN257474 |
| N. dimidiatum | MUM 19.74 | Plab-096 | onychomycosis | Colombia | MN257475 |
| N. dimidiatum | MUM 19.75 | Plab-100 | onychomycosis | Colombia | MN257476 |
| N. dimidiatum | MUM 19.76 | Plab-113 | onychomycosis | Colombia | MN257477 |
| N. dimidiatum | MUM 19.77 | Plab-132 | onychomycosis | Colombia | MN257478 |
| N. orchidacearum | N/A | MFLUCC 12-0533 | environmental | Thailand | KU179865 |
| N. novaehollandiae | N/A | CBS 122071 | environmental | Australia | KF766207 |
| N. oculus | N/A | IOM 25287 | clinical | Mexico | MG764431 |
| S. lignicola | N/A | UAMH 1502 | environmental | Italy | NR_121314.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flórez-Muñoz, S.V.; Gómez-Velásquez, J.C.; Loaiza-Díaz, N.; Soares, C.; Santos, C.; Lima, N.; Mesa-Arango, A.C. ITS rDNA Gene Analysis Versus MALDI-TOF MS For Identification of Neoscytalidium dimidiatum Isolated from Onychomycosis and Dermatomycosis Cases in Medellin (Colombia). Microorganisms 2019, 7, 306. https://doi.org/10.3390/microorganisms7090306
Flórez-Muñoz SV, Gómez-Velásquez JC, Loaiza-Díaz N, Soares C, Santos C, Lima N, Mesa-Arango AC. ITS rDNA Gene Analysis Versus MALDI-TOF MS For Identification of Neoscytalidium dimidiatum Isolated from Onychomycosis and Dermatomycosis Cases in Medellin (Colombia). Microorganisms. 2019; 7(9):306. https://doi.org/10.3390/microorganisms7090306
Chicago/Turabian StyleFlórez-Muñoz, Sindy V., Juan C. Gómez-Velásquez, Natalia Loaiza-Díaz, Célia Soares, Carla Santos, Nelson Lima, and Ana C. Mesa-Arango. 2019. "ITS rDNA Gene Analysis Versus MALDI-TOF MS For Identification of Neoscytalidium dimidiatum Isolated from Onychomycosis and Dermatomycosis Cases in Medellin (Colombia)" Microorganisms 7, no. 9: 306. https://doi.org/10.3390/microorganisms7090306
APA StyleFlórez-Muñoz, S. V., Gómez-Velásquez, J. C., Loaiza-Díaz, N., Soares, C., Santos, C., Lima, N., & Mesa-Arango, A. C. (2019). ITS rDNA Gene Analysis Versus MALDI-TOF MS For Identification of Neoscytalidium dimidiatum Isolated from Onychomycosis and Dermatomycosis Cases in Medellin (Colombia). Microorganisms, 7(9), 306. https://doi.org/10.3390/microorganisms7090306





