Should We Not Further Study the Impact of Microbial Activity on Snow and Polar Atmospheric Chemistry?
Abstract
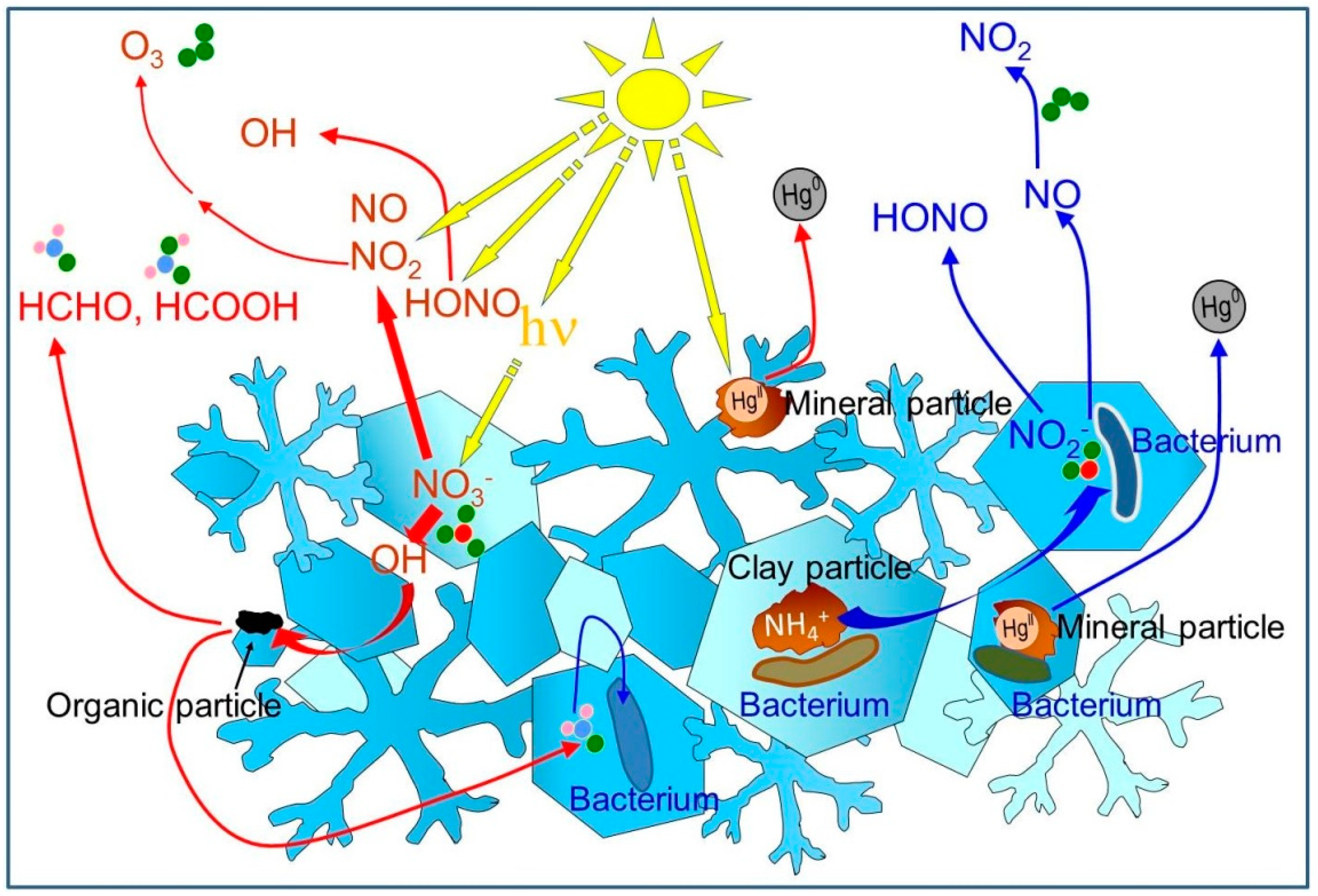
1. Introduction
2. Biologists Detect and Identify Microbes in Snow
3. Chemists Detect Photochemical Reactions in Snow
4. Chemists Detect Impact of Microbial Metabolism in Snow
5. The Need for Collaborations
Funding
Acknowledgments
Conflicts of Interest
References
- Carpenter, E.J.; Lin, S.; Capone, D.G. Bacterial activity in South Pole snow. Appl. Environ. Microbiol. 2000, 66, 4514–4517. [Google Scholar] [CrossRef] [PubMed]
- Tung, H.C.; Bramall, N.E.; Price, P.B. Microbial origin of excess methane in glacial ice and implications for life on Mars. Proc. Natl. Acad. Sci. USA 2005, 102, 18292–18296. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Taş, N. The microbial ecology of permafrost. Nat. Rev. Genet. 2014, 12, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Margesin, R. Psychrophilic lifestyles: Mechanisms of adaptation and biotechnological tools. Appl. Microbiol. Biotechnol. 2019, 103, 2857–2871. [Google Scholar] [CrossRef] [PubMed]
- Hodson, A.; Anesio, A.M.; Tranter, M.; Fountain, A.; Osborn, M.; Priscu, J.; Laybourn-Parry, J.; Sattler, B. Glacial ecosystems. Ecol. Monogr. 2008, 78, 41–67. [Google Scholar] [CrossRef]
- Amoroso, A.; Domine, F.; Esposito, G.; Morin, S.; Savarino, J.; Nardino, M.; Montagnoli, M.; Bonneville, J.-M.; Clement, J.-C.; Ianniello, A.; et al. Microorganisms in dry polar snow are involved in the exchanges of reactive nitrogen species with the atmosphere. Environ. Sci. Technol. 2010, 44, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Domine, F.; Shepson, P.B. Air-snow interactions and atmospheric chemistry. Science 2002, 297, 1506–1510. [Google Scholar] [CrossRef] [PubMed]
- Stibal, M.; Šabacká, M.; Zarsky, J. Biological processes on glacier and ice sheet surfaces. Nat. Geosci. 2012, 5, 771–774. [Google Scholar] [CrossRef]
- Antony, R.; Willoughby, A.S.; Grannas, A.M.; Catanzano, V.; Sleighter, R.L.; Thamban, M.; Hatcher, P.G.; Nair, S. Molecular insights on dissolved organic matter transformation by supraglacial microbial communities. Environ. Sci. Technol. 2017, 51, 4328–4337. [Google Scholar] [CrossRef]
- Maccario, L.; Vogel, T.M.; Larose, C. Potential drivers of microbial community structure and function in Arctic spring snow. Front. Microbiol. 2014, 5, 5. [Google Scholar] [CrossRef]
- Segawa, T.; Miyamoto, K.; Ushida, K.; Agata, K.; Okada, N.; Kohshima, S. Seasonal change in bacterial flora and biomass in mountain snow from the Tateyama Mountains, Japan, analyzed by 16S rRNA gene sequencing and real-time PCR. Appl. Environ. Microbiol. 2005, 71, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Pratt, K.A.; Custard, K.D.; Shepson, P.B.; Douglas, T.A.; Pöhler, D.; General, S.; Zielcke, J.; Simpson, W.R.; Platt, U.; Tanner, D.J.; et al. Photochemical production of molecular bromine in Arctic surface snowpacks. Nat. Geosci. 2013, 6, 351–356. [Google Scholar] [CrossRef]
- Mudryk, L.R.; Derksen, C.; Howell, S.; Laliberté, F.; Thackeray, C.; Sospedra-Alfonso, R.; Vionnet, V.; Kushner, P.J.; Brown, R. Canadian snow and sea ice: historical trends and projections. Cryosphere 2018, 12, 1157–1176. [Google Scholar] [CrossRef]
- Amato, P.; Hennebelle, R.; Magand, O.; Sancelme, M.; Delort, A.-M.; Barbante, C.; Boutron, C.; Ferrari, C.; Hennebelle, R. Bacterial characterization of the snow cover at Spitzberg, Svalbard. FEMS Microbiol. Ecol. 2007, 59, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Steffen, A.; Amyot, M.; Ariya, P.; Aspmo, K.; Bottenheim, J.; Cobbett, F.; Ebinghaus, R.; Goodsite, M.E.; Poulain, A.J.; Scherz, C.; et al. A synthesis of atmospheric mercury depletion event chemistry in the atmosphere and snow. Atmos. Chem. Phys. Discuss. 2008, 8, 1445–1482. [Google Scholar] [CrossRef]
- Møller, A.K.; Barkay, T.; Al-Soud, W.A.; Sørensen, S.J.; Skov, H.; Kroer, N. Diversity and characterization of mercury-resistant bacteria in snow, freshwater and sea-ice brine from the High Arctic. FEMS Microbiol. Ecol. 2011, 75, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.P.; Blum, J.D.; Keeler, G.J.; Douglas, T.A. Investigation of the deposition and emission of mercury in arctic snow during an atmospheric mercury depletion event. J. Geophys. Res. Space Phys. 2008, 113, 17304. [Google Scholar] [CrossRef]
- Ariya, P.A.; Domine, F.; Kos, G.; Amyot, M.; Côté, V.; Vali, H.; Lauzier, T.; Kuhs, W.F.; Techmer, K.; Heinrichs, T.; et al. Snow—A photobiochemical exchange platform for volatile and semi-volatile organic compounds with the atmosphere. Environ. Chem. 2011, 8, 62–73. [Google Scholar] [CrossRef]
- Larose, C.; Dommergue, A.; Vogel, T.M. Microbial nitrogen cycling in Arctic snowpacks. Environ. Res. Lett. 2013, 8, 035004. [Google Scholar] [CrossRef]
- Sowers, T. N2O record spanning the penultimate deglaciation from the Vostok ice core. J. Geophys. Res. Space Phys. 2001, 106, 31903–31914. [Google Scholar] [CrossRef]
- Price, P.B.; Sowers, T. Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc. Natl. Acad. Sci. USA 2004, 101, 4631–4636. [Google Scholar] [CrossRef] [PubMed]
- Schilt, A.; Baumgartner, M.; Blunier, T.; Schwander, J.; Spahni, R.; Fischer, H.; Stocker, T.F. Glacial-interglacial and millennial-scale variations in the atmospheric nitrous oxide concentration during the last 800,000 years. Quat. Sci. Rev. 2010, 29, 182–192. [Google Scholar] [CrossRef]
- Miteva, V.; Sowers, T.; Schupbach, S.; Fischer, H.; Brenchley, J. Geochemical and microbiological studies of nitrous oxide variations within the new neem Greenland ice core during the last glacial period. Geomicrobiol. J. 2016, 33, 647–660. [Google Scholar] [CrossRef]
- Junge, C.E. Sulfur in the atmosphere. J. Geophys. Res. Space Phys. 1960, 65, 227–237. [Google Scholar] [CrossRef]
- Wilson, A.T.; House, D.A. Chemical composition of south polar snow. J. Geophys. Res. Space Phys. 1965, 70, 5515–5518. [Google Scholar] [CrossRef]
- Honrath, R.E.; Peterson, M.C.; Guo, S.; Dibb, J.E.; Shepson, P.B.; Campbell, B. Evidence of NOx production within or upon ice particles in the Greenland snowpack. Geophys. Res. Lett. 1999, 26, 695–698. [Google Scholar] [CrossRef]
- Hahn, J.; Crutzen, P.J. The role of fixed nitrogen in atmospheric photochemistry. Philos. Trans. R. Soc. B Boil. Sci. 1982, 296, 521–541. [Google Scholar] [CrossRef]
- Sumner, A.L.; Shepson, P.B. Snowpack production of formaldehyde and its effect on the Arctic troposphere. Nature 1999, 398, 230–233. [Google Scholar] [CrossRef]
- Swanson, A.L.; Blake, N.J.; Dibb, J.E.; Albert, M.R.; Blake, D.R.; Rowland, F.S. Photochemically induced production of CH3Br, CH3I, C2H5I, ethene, and propene within surface snow at Summit, Greenland. Atmos. Environ. 2002, 36, 2671–2682. [Google Scholar] [CrossRef]
- Chen, G.; Davis, D.; Crawford, J.; Nowak, J.B.; Eisele, F.; Mauldin, R.L., III; Tanner, D.; Buhr, M.; Shetter, R.; Lefer, B.; et al. An investigation of South Pole HOx chemistry: Comparison of model results with ISCAT observations. Geophys. Res. Lett. 2001, 28, 3633–3636. [Google Scholar] [CrossRef]
- Steffen, A.; Schroeder, W.; Bottenheim, J.; Narayan, J.; Fuentes, J.D. Atmospheric mercury concentrations: Measurements and profiles near snow and ice surfaces in the Canadian Arctic during alert 2000. Atmos. Environ. 2002, 36, 2653–2661. [Google Scholar] [CrossRef]
- Sun, J.M.; Ariya, P. Atmospheric organic and bio-aerosols as cloud condensation nuclei (CCN): A review. Atmos. Environ. 2006, 40, 795–820. [Google Scholar] [CrossRef]
- Bock, J.; Savarino, J.; Picard, G. Air–snow exchange of nitrate: A modelling approach to investigate physicochemical processes in surface snow at Dome C, Antarctica. Atmos. Chem. Phys. Discuss. 2016, 16, 12531–12550. [Google Scholar] [CrossRef]
- Barret, M.; Houdier, S.; Domine, F. Thermodynamics of the formaldehyde−water and formaldehyde−ice systems for atmospheric applications. J. Phys. Chem. A 2011, 115, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Rohde, R.A.; Price, P.B. Diffusion-controlled metabolism for long-term survival of single isolated microorganisms trapped within ice crystals. Proc. Natl. Acad. Sci. USA 2007, 104, 21021. [Google Scholar] [CrossRef] [PubMed]
- Amato, P.; Joly, M.; Besaury, L.; Oudart, A.; Taib, N.; Moné, A.I.; Deguillaume, L.; Delort, A.-M.; Debroas, D. Active microorganisms thrive among extremely diverse communities in cloud water. PLoS ONE 2017, 12, e0182869. [Google Scholar] [CrossRef] [PubMed]
- Domine, F.; Bock, J.; Voisin, D.; Donaldson, D.J. Can we model snow photochemistry? Problems with the current approaches. J. Phys. Chem. A 2013, 117, 4733–4749. [Google Scholar] [CrossRef] [PubMed]
- Antony, R.; Grannas, A.M.; Willoughby, A.S.; Sleighter, R.L.; Thamban, M.; Hatcher, P.G. Origin and sources of dissolved organic matter in snow on the East Antarctic ice sheet. Environ. Sci. Technol. 2014, 48, 6151–6159. [Google Scholar] [CrossRef]
- Redeker, K.R.; Chong, J.P.J.; Aguion, A.; Hodson, A.; Pearce, D.A. Microbial metabolism directly affects trace gases in (sub) polar snowpacks. J. R. Soc. Interface 2017, 14, 20170729. [Google Scholar] [CrossRef]
- Legrand, M.R.; Kirchner, S. Origins and variations of nitrate in south polar precipitation. J. Geophys. Res. Space Phys. 1990, 95, 3493–3507. [Google Scholar] [CrossRef]
- Antony, R.; Sanyal, A.; Kapse, N.; Dhakephalkar, P.K.; Thamban, M.; Nair, S. Microbial communities associated with Antarctic snow pack and their biogeochemical implications. Microbiol. Res. 2016, 192, 192–202. [Google Scholar] [CrossRef]
- Zhang, C.L.; Dang, H.; Azam, F.; Benner, R.; Legendre, L.; Passow, U.; Polimene, L.; Robinson, C.; Suttle, C.A.; Jiao, N. Evolving paradigms in biological carbon cycling in the ocean. Nat. Sci. Rev. 2018, 5, 481–499. [Google Scholar] [CrossRef]
- Tang, K.; Lin, Y.; Han, Y.; Jiao, N. Characterization of potential polysaccharide utilization systems in the marine bacteroidetes gramella flava JLT2011 using a multi-omics approach. Front. Microbiol. 2017, 8, 13. [Google Scholar] [CrossRef]
- De Corte, D.; Srivastava, A.; Koski, M.; Garcia, J.A.L.; Takaki, Y.; Yokokawa, T.; Nunoura, T.; Elisabeth, N.H.; Sintes, E.; Herndl, G.J. Metagenomic insights into zooplankton-associated bacterial communities. Environ. Microbiol. 2018, 20, 492–505. [Google Scholar] [CrossRef]
- Cohen, J.; Ye, H.; Jones, J. Trends and variability in rain-on-snow events. Geophys. Res. Lett. 2015, 42, 7115–7122. [Google Scholar] [CrossRef]
- Hood, E.; Fellman, J.; Spencer, R.G.M.; Hernes, P.J.; Edwards, R.; D’Amore, D.; Scott, D. Glaciers as a source of ancient and labile organic matter to the marine environment. Nature 2009, 462, U1044–U1100. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domine, F. Should We Not Further Study the Impact of Microbial Activity on Snow and Polar Atmospheric Chemistry? Microorganisms 2019, 7, 260. https://doi.org/10.3390/microorganisms7080260
Domine F. Should We Not Further Study the Impact of Microbial Activity on Snow and Polar Atmospheric Chemistry? Microorganisms. 2019; 7(8):260. https://doi.org/10.3390/microorganisms7080260
Chicago/Turabian StyleDomine, Florent. 2019. "Should We Not Further Study the Impact of Microbial Activity on Snow and Polar Atmospheric Chemistry?" Microorganisms 7, no. 8: 260. https://doi.org/10.3390/microorganisms7080260
APA StyleDomine, F. (2019). Should We Not Further Study the Impact of Microbial Activity on Snow and Polar Atmospheric Chemistry? Microorganisms, 7(8), 260. https://doi.org/10.3390/microorganisms7080260





