Gut Dysbiosis and the Intestinal Microbiome: Streptococcus thermophilus a Key Probiotic for Reducing Uremia
Abstract
1. Introduction
What this Narrative Review Proposes to Add
2. Materials and Methods
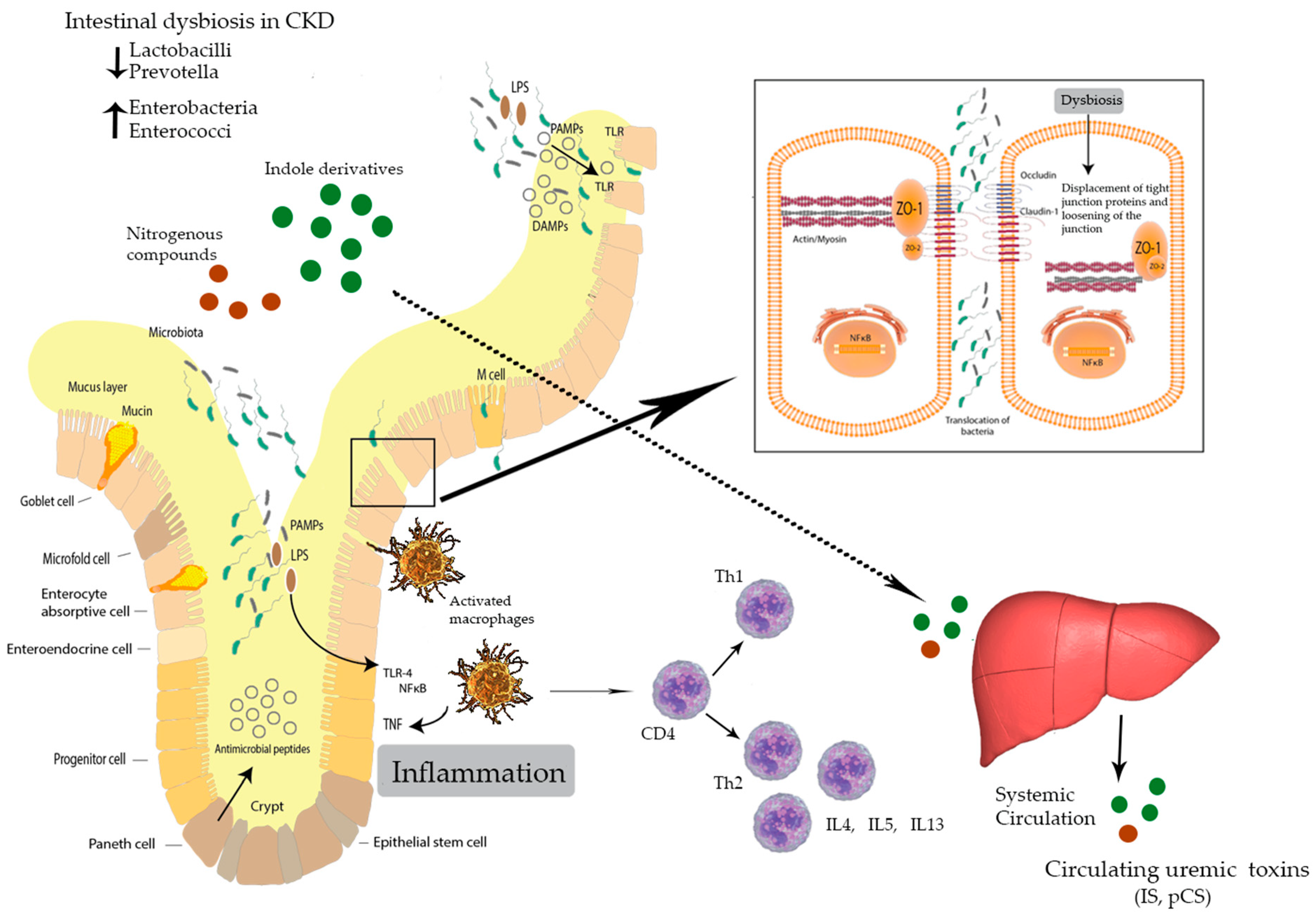
3. Intestinal Dysbiosis
4. Uremia and the Intestinal Microbiome
5. Inflammation, Immunity, and CKD
6. Probiotics, Prebiotics, Synbiotics, and CKD
7. Discussion
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karl, J.P.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front. Microbiol. 2018, 9, 2013. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Chen, J.; Clarke, S. The vermiform appendix: An immunological organ sustaining a microbiome inoculum. Clin. Sci. 2019, 133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Amdur, R.L.; Feldman, H.I.; Gupta, J.; Yang, W.; Kanetsky, P.; Shlipak, M.; Rahman, M.; Lash, J.P.; Townsend, R.R.; Ojo, A.; et al. Inflammation and Progression of CKD: The CRIC Study. Clin. J. Am. Soc. Nephrol. 2016, 11, 1546–1556. [Google Scholar] [CrossRef]
- Vitetta, L.; Gobe, G. Uremia and chronic kidney disease: The role of the gut microflora and therapies with pro- and prebiotics. Mol. Nutr. Food Res. 2013, 57, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Kesper, M.S.; Marschner, J.A.; Konrad, L.; Ryu, M.; Kumar Vr, S.; Kulkarni, O.P.; Mulay, S.R.; Romoli, S.; Demleitner, J.; et al. Intestinal Dysbiosis, Barrier Dysfunction, and Bacterial Translocation Account for CKD-Related Systemic Inflammation. J. Am. Soc. Nephrol. 2017, 28, 76–83. [Google Scholar] [CrossRef]
- Jansen, J.; Jankowski, J.; Gajjala, P.R.; Wetzels, J.F.; Masereeuw, R. Disposition and clinical implications of protein-bound uremic toxins. Clin. Sci. 2017, 131, 1631–1647. [Google Scholar] [CrossRef]
- Nallu, A.; Sharma, S.; Ramezani, A.; Muralidharan, J.; Raj, D. Gut microbiome in chronic kidney disease: Challenges and opportunities. Transl. Res. 2017, 179, 24–37. [Google Scholar] [CrossRef]
- Yang, J.; Lim, S.Y.; Ko, Y.S.; Lee, H.Y.; Oh, S.W.; Kim, M.G.; Cho, W.Y.; Jo, S.K. Intestinal barrier disruption and dysregulated mucosal immunity contribute to kidney fibrosis in chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 419–428. [Google Scholar] [CrossRef]
- Vitetta, L.; Vitetta, G.; Hall, S. Immunological Tolerance and Function: Associations Between Intestinal Bacteria, Probiotics, Prebiotics, and Phages. Front. Immunol. 2018, 9, 2240. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, E.T.; Palacios, T.; Thomsen, M.; Vitetta, L. Intestinal Microbiome Shifts, Dysbiosis, Inflammation, and Non-alcoholic Fatty Liver Disease. Front. Microbiol. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R. Introduction to the Toxins Special Issue on “Novel Issues in Uremic Toxicity”. Toxins 2018, 10, 388. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Glassock, R.J.; De Broe, M.E. Epidemiology of chronic kidney disease: Think (at least) twice! Clin. Kidney J. 2017, 10, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Tarng, D.-C. Diet, gut microbiome and indoxyl sulphate in chronic kidney disease patients. Nephrology 2018, 23 (Suppl. 4), 16–20. [Google Scholar] [CrossRef]
- Al Khodor, S.; Shatat, I.F. Gut microbiome and kidney disease: A bidirectional relationship. Pediatr. Nephrol. 2017, 32, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Chen, D.-Q.; Chen, L.; Liu, J.-R.; Vaziri, N.D.; Guo, Y.; Zhao, Y.-Y. Microbiome–metabolome reveals the contribution of gut–kidney axis on kidney disease. J. Transl. Med. 2019, 17, 5. [Google Scholar] [CrossRef]
- Hida, M.; Aiba, Y.; Sawamura, S.; Suzuki, N.; Satoh, T.; Koga, Y. Inhibition of the Accumulation of Uremic Toxins in the Blood and Their Precursors in the Feces after Oral Administration of Lebenin, a Lactic Acid Bacteria Preparation, to Uremic Patients Undergoing Hemodialysis. Nephron 1996, 74, 349–355. [Google Scholar] [CrossRef]
- Klammt, S.; Wojak, H.J.; Mitzner, A.; Koball, S.; Rychly, J.; Reisinger, E.C.; Mitzner, S. Albumin-binding capacity (ABiC) is reduced in patients with chronic kidney disease along with an accumulation of protein-bound uraemic toxins. Nephrol. Dial. Transplant. 2012, 27, 2377–2383. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Vaziri, N.D. The Leaky Gut and Altered Microbiome in Chronic Kidney Disease. J. Ren. Nutr. 2017, 27, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Yuan, J.; Rahimi, A.; Ni, Z.; Said, H.; Subramanian, V.S. Disintegration of colonic epithelial tight junction in uremia: A likely cause of CKD-associated inflammation. Nephrol. Dial. Transplant. 2012, 27, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, D.A.; Piccolo, B.D.; Vaziri, N.D.; Liu, S.; Lau, W.L.; Khazaeli, M.; Nazertehrani, S.; Moore, M.E.; Marco, M.L.; Martin, R.J.; et al. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am. J. Physiol. Physiol. 2016, 310, F857–F871. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Fouque, D.; Soulage, C.O. The Role of Gut Microbiota and Diet on Uremic Retention Solutes Production in the Context of Chronic Kidney Disease. Toxins 2018, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Glorieux, G. Gut-derived metabolites and chronic kidney disease: The Forest (F)or the trees? Clin. J. Am. Soc. Nephrol. 2018, 13, 1311–1313. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.-J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013, 83, 1010–1016. [Google Scholar] [CrossRef]
- Joossens, M.; Faust, K.; Gryp, T.; Nguyen, A.T.L.; Wang, J.; Eloot, S.; Schepers, E.; Dhondt, A.; Pletinck, A.; Vieira-Silva, S.; et al. Gut microbiota dynamics and uraemic toxins: One size does not fit all. Gut 2018. [Google Scholar] [CrossRef]
- Gupta, J.; Mitra, N.; Kanetsky, P.A.; Devaney, J.; Wing, M.R.; Reilly, M.; Shah, V.O.; Balakrishnan, V.S.; Guzman, N.J.; Girndt, M.; et al. Association between Albuminuria, Kidney Function, and Inflammatory Biomarker Profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012, 7, 1938–1946. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P. Inflammation in end-stage renal disease—What have we learned in 10 years? Semin. Dial. 2010, 23, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Axelsson, J.; Machowska, A.; Heimbürger, O.; Bárány, P.; Lindholm, B.; Lindström, K.; Stenvinkel, P.; Qureshi, A.R. Biomarkers of Cardiovascular Disease and Mortality Risk in Patients with Advanced CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Voltan, R.; Rimondi, E.; Melloni, E.; Milani, D.; Cervellati, C.; Gemmati, D.; Celeghini, C.; Secchiero, P.; Zauli, G.; et al. TRAIL, OPG, and TWEAK in kidney disease: Biomarkers or therapeutic targets? Clin. Sci. 2019, 133, 1145–1166. [Google Scholar] [CrossRef] [PubMed]
- Feigerlová, E.; Battaglia-Hsu, S.-F. IL-6 signaling in diabetic nephropathy: From pathophysiology to therapeutic perspectives. Cytokine Growth Factor Rev. 2017, 37, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Glorieux, G.; Vanholder, R. The Gut: The Forgotten Organ in Uremia? Blood Purif. 2010, 29, 130–136. [Google Scholar] [CrossRef]
- Vitetta, L. Probiotics Can Break the Toxic Relationship Between the Intestinal Microbiome and the Kidney. Dig. Dis. Sci. 2019, 64, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Harrison, O.J. Homeostatic immunity and the microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, Y.; Wang, X.M.; Lu, J.; Lee, V.W.; Ye, Q.; Nguyen, H.; Zheng, G.; Zhao, Y.; Alexander, S.I.; et al. Renal F4/80+ CD11c+ mononuclear phagocytes display phenotypic and functional characteristics of macrophages in health and in adriamycin nephropathy. J. Am. Soc. Nephrol. 2015, 26, 349–363. [Google Scholar] [CrossRef]
- Sansonetti, P. Host-pathogen interactions: The seduction of molecular cross talk. Gut 2002, 50 (Suppl. 3), III2–III8. [Google Scholar] [CrossRef]
- Nelson, P.J.; Rees, A.J.; Griffin, M.D.; Hughes, J.; Kurts, C.; Duffield, J. The renal mononuclear phagocytic system. J. Am. Soc. Nephrol. 2012, 23, 194–203. [Google Scholar] [CrossRef]
- Koppe, L.; Mafra, D.; Fouque, D. Probiotics and chronic kidney disease. Kidney Int. 2015, 88, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Pisano, A.; D’Arrigo, G.; Coppolino, G.; Bolignano, D. Biotic Supplements for Renal Patients: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1224. [Google Scholar] [CrossRef] [PubMed]
- Pavan, M. Influence of prebiotic and probiotic supplementation on the progression of chronic kidney disease. Minerva Urol. Nefrol. 2016, 68, 222–226. [Google Scholar] [PubMed]
- Ranganathan, N.; Friedman, E.A.; Tam, P.; Rao, V.; Ranganathan, P.; Dheer, R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: A 6-month pilot scale trial in Canada. Curr. Med. Res. Opin. 2009, 25, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, N.; Ranganathan, P.; Friedman, E.A.; Joseph, A.; Delano, B.; Goldfarb, D.S.; Tam, P.; Rao, A.V.; Anteyi, E.; Musso, C.G. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv. Ther. 2010, 27, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Guida, B.; Germano, R.; Trio, R.; Russo, D.; Memoli, B.; Grumetto, L.; Barbato, F.; Cataldi, M. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: A randomized clinical trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Pechenyak, B.; Vyas, U.; Ranganathan, P.; Weinberg, A.; Liang, P.; Mallappallil, M.C.; Norin, A.J.; Friedman, E.A.; Saggi, S.J. Randomized Controlled Trial of Strain-Specific Probiotic Formulation (Renadyl) in Dialysis Patients. BioMed Res. Int. 2014, 2014, 568571. [Google Scholar] [CrossRef] [PubMed]
- Saggi, S.J.; Mercier, K.; Gooding, J.R.; Friedman, E.; Vyas, U.; Ranganathan, N.; Ranganathan, P.; McRitchie, S.; Sumner, S. Metabolic profiling of a chronic kidney disease cohort reveals metabolic phenotype more likely to benefit from a probiotic. Int. J. Probiotics Prebiotics 2017, 12, 43–54. [Google Scholar] [PubMed]
- Rossi, M.; Johnson, D.W.; Morrison, M.; Pascoe, E.M.; Coombes, J.S.; Forbes, J.M.; Szeto, C.-C.; McWhinney, B.C.; Ungerer, J.P.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, H.; Heidari, F.; Mozaffari-Khosravi, H.; Nouri-Majelan, N.; Dehghani, A. Synbiotic Supplementations for Azotemia in Patients With Chronic Kidney Disease: A Randomized Controlled Trial. Iran. J. Kidney Dis. 2016, 10, 351–357. [Google Scholar] [PubMed]
- Borges, N.A.; Carmo, F.L.; Stockler-Pinto, M.B.; de Brito, J.S.; Dolenga, C.J.; Ferreira, D.C.; Nakao, L.S.; Rosado, A.; Fouque, D.; Mafra, D. Probiotic Supplementation in Chronic Kidney Disease: A Double-blind, Randomized, Placebo-controlled Trial. J. Ren. Nutr. 2018, 28, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.S.; Paik, K.H.; Cho, H.Y. p-Cresyl sulphate and indoxyl sulphate in pediatric patients on chronic dialysis. Korean J. Pediatr. 2013, 56, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-Y.; Lu, J.-R.; Chen, B.-J.; Wu, C.; Chen, Y.-P.; Chen, M.-J. Selection of uremic toxin-reducing probiotics in vitro and in vivo. J. Funct. Foods 2014, 7, 407–415. [Google Scholar] [CrossRef]
- Patra, A.; Mandal, A.; Samanta, A.; Mahapatra, T.D.; Mandal, S.; Roy, S.; Pradhan, S.; Nandi, D.K.; Mondal, K. Therapeutic effect of Streptococcus thermophilus (MTCC 1938) on acetaminophen induced uremia in experimental rats. Indian J. Biotechnol. 2014, 13, 318–323. [Google Scholar]
- Mandal, A.; Patra, A.; Mandal, S.; Roy, S.; Mahapatra, S.D.; Mahapatra, T.D.; Paul, T.; Das, K.; Mondal, K.C.; Nandi, D.K. Therapeutic potential of different commercially available synbiotic on acetaminophen-induced uremic rats. Clin. Exp. Nephrol. 2015, 19, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Wing, M.R.; Patel, S.S.; Ramezani, A.; Raj, D.S. Gut microbiome in chronic kidney disease. Exp. Physiol. 2016, 101, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Deveau, H.; Barrangou, R.; Garneau, J.E.; Labonté, J.; Fremaux, C.; Boyaval, P.; Romero, D.A.; Horvath, P.; Moineau, S. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J. Bacteriol. 2008, 190, 1390–1400. [Google Scholar] [CrossRef]
- Carding, S.R.; Davis, N.; Hoyles, L. Review article: The human intestinal virome in health and disease. Aliment. Pharmacol. Ther. 2017, 46, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Gindin, M.; Febvre, H.P.; Rao, S.; Wallace, T.C.; Weir, T.L. Bacteriophage for Gastrointestinal Health (PHAGE) Study: Evaluating the Safety and Tolerability of Supplemental Bacteriophage Consumption. J. Am. Coll. Nutr. 2019, 38, 68–75. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Coresh, J.; Grams, M.E.; Steffen, L.M.; Anderson, C.A.; Appel, L.J.; Crews, D.C. Dietary Acid Load and Incident Chronic Kidney Disease: Results from the ARIC Study. Am. J. Nephrol. 2015, 42, 427–435. [Google Scholar] [CrossRef] [PubMed]
| CKD Stages | eGFR 1 (mL/min/1.73 m2) | Report | Mean (SD) % Protein-Bound Uremic Toxins 1 | Mean (SD) Serum Levels Indoxyl Sulphate 1 (µmol/L) |
|---|---|---|---|---|
| Stage 1 | 90 mL min−1 | Normal renal function with abnormal urine report or structural abnormalities or a genetic trait indicating kidney disease. | 118 (12) | 3.9 (1.1) |
| Stage 2 | 60–89 mL min−1 | Mildly ↓ renal function and other reports (as for Stage 1) indicating kidney disease. | ||
| Stage 3 stage (a) | 45–59 mL min−1 | Moderately ↓ kidney function | 111 (11) | 6.2 (3.2) |
| Stage 3 stage (b) | 30–44 mL min−1 | |||
| Stage 4 | 15–29 mL min−1 | Severely ↓ kidney function | 99 (8) | 16.2 (14.9) |
| Stage 5 | <15 mL min−1 or patient on dialysis | Very severe or end stage kidney disease (often referred to as established kidney failure) | 79 (9) | 56.1 (28.6) |
| Human Studies | |||
|---|---|---|---|
| Probiotics Administered | Intervention Details | Results | PubMed ID [Reference] |
| Lactobacillus acidophilus KB31 Streptococcus thermophilus KB27 Bifidobacterium longum KB35 15 × 109 CFU/day | Single-center, prospective, DBRCT cross-over|n = 13|CKD stage 3–4| 6 months | ↓ BUN ↓ Uric acid concentration ↑ QoL | PMID 19558344 [44] |
| L. acidophilus KB31 S. thermophilus KB27 B. longum KB35 15 × 109 CFU/day | Multicenter, prospective, DBRCT cross-over|n = 46|CKD stage 3–4| 6 months | ↓ BUN ↑ QoL | PMID 20721651 [45] |
| Synbiotic: L. plantarum, L. casei subsp rhamnosus, L. gasseri, L acidophilus, L. salivarius, L. sporogenes, B. infantis, B. longum, S. thermophilus and previotic inulin (VB Beneo Synergy 1), and resistant tapioca starch | Single-center|DBRCT cross-over|n = 30|CKD non dialyzed stage 3–4|4 weeks | ↓ Plasma pCS | PMID 24929795 [46] |
| S. thermophilus KB 19, L. acidophilus KB 27 B. longum KB 31 | Single-center|DBRCT cross-over|n = 22|KD| 8 weeks | ↑ QoL trend toward ↓ serum IS glucuronide ↓ C-reactive protein | PMID 25147806 [47] |
| S. thermophilus KB 19, L. acidophilus KB 27, B. longum KB 31 270 × 109 CFU/day | Single-center|n = 27| CKD Stage III–IV Dose escalation study from 30 × 109/day to 90 × 109/t.i.d. 4 months | ↓ BUN in a subset of plasma samples from 16 subjects | PMID 30774576 [48] |
| Synbiotic Lactobacillus, Bifidobacteria, Streptococcus genera + prebiotics (inulin fructooligosaccarides galacto-oligosaccarides) | DBRCT|n = 37|CKD stage 4–5|6 weeks [4-week] washout|crossover|Dietary advice (protein 0.8 g/kg BW/d) | 1° outcomes: level of IS 2° outcomes: levels of pCS; LPS, TMAO, inflammation, and OS markers; RF; QoL ↓Serum pCS only | PMID 26772193 [49] |
| Synbiotic: prebiotic + probiotic | Prospective observation PCRT|n = 24|CKD stage 3–4|12 mo|Dietary advice (protein 0.8 g/kg BW/d) | Slowing progression of CKD | PMID 24990390 [43] |
| L. casei, L. acidophilus, L. bugaricus, L. rhamnosus, B. breve, B. longum, S. thermophilus, and fructo-oligosacharide prebiotic 500 mg b.i.d. capsules | RCT|n = 66 | CKD stage 3–4|6 weeks | ↓ serum urea ↓ serum nitrogen | PMID 27903994 [50] |
| L. acidophilus S. thermophilus B. longum 90 × 109 CFU/day | Single center|DBRCT| N = 46|HD|3 months | No reduction in… → uremic toxins → inflammatory markers | PMID 28888762 [51] |
| VSL#3 S. thermophilus B. breve, B. longum B. infantis, L. acidophilus L. plantarum, L. paracasei L. delbrueckii spp bulgaricus 450 × 109 CFU/sachet/day | Single center|n = 16 pediatric patients|HD 3 months | No reduction in… → uremic toxins IS and pCS | PMID 23646054 [52] |
| Animal Studies | |||
| L. plantarum subsp. plantarum BCRC12251 L. paracasei spp. paracasei BCRC12188 S. salivarius spp. thermophilus BCRC13869 at 3 × 109 CFU/kg BW | Rats with cisplatin-induced kidney injury were administered probiotic mix for 5 days. | ↓ Phenol ↓ pCS ↓ IS | No PMID [53] |
| S. thermophilus MTCC1938 At 1 × 109 CFU/mL/100 g BW | Acetaminophen-induced uremic rats were given probiotic for 15 days. | ↓ plasma urea ↓ plasma creatinine ↓ urinary protein ↓ urinary glucose ↑ glutathione | No PMID [54] |
| Commercially available combination formulations at a dose of ≥109 CFU/day Ecobion: L. acidophilus, L rhamnosus B. longum, B. bifidum S. boulardii, S. thermophilus Fructo- oligo-saccharide VSL#3: S. thermophilus B. breve, B. longum B. infantis, L. acidophilus L. plantarum, L. paracasei L. delbrueckii spp. bulgaricus | Acetaminophen-induced uremic rats given one of seven symbiotic combinations for 3 weeks. | VSL#3: ↓ plasma urea |creatinine ↓ glomerular necrosis ↑ CAT, SOD, glutathione | PMID 24740592 [55] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitetta, L.; Llewellyn, H.; Oldfield, D. Gut Dysbiosis and the Intestinal Microbiome: Streptococcus thermophilus a Key Probiotic for Reducing Uremia. Microorganisms 2019, 7, 228. https://doi.org/10.3390/microorganisms7080228
Vitetta L, Llewellyn H, Oldfield D. Gut Dysbiosis and the Intestinal Microbiome: Streptococcus thermophilus a Key Probiotic for Reducing Uremia. Microorganisms. 2019; 7(8):228. https://doi.org/10.3390/microorganisms7080228
Chicago/Turabian StyleVitetta, Luis, Hannah Llewellyn, and Debbie Oldfield. 2019. "Gut Dysbiosis and the Intestinal Microbiome: Streptococcus thermophilus a Key Probiotic for Reducing Uremia" Microorganisms 7, no. 8: 228. https://doi.org/10.3390/microorganisms7080228
APA StyleVitetta, L., Llewellyn, H., & Oldfield, D. (2019). Gut Dysbiosis and the Intestinal Microbiome: Streptococcus thermophilus a Key Probiotic for Reducing Uremia. Microorganisms, 7(8), 228. https://doi.org/10.3390/microorganisms7080228





