Peptide-Based Subunit Vaccine Design of T- and B-Cells Multi-Epitopes against Zika Virus Using Immunoinformatics Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Zika Polyprotein Sequences
2.2. Preparation and Visualization of Three-Dimensional Protein Structures
2.3. CD8+ T-cell Epitope Prediction
2.4. CD4+ T-cell Epitope Prediction
2.5. T-cell Epitope Shortlisting
2.6. Linear and Conformational B-cell Epitope Prediction
2.7. B-cell Epitope Shortlisting
2.8. Modeling of Protein Structures
2.9. Molecular Docking Simulation
2.10. Molecular Dynamics Simulation
3. Results
3.1. Preparation of Zika Polyprotein Sequence for Epitope Screening
3.2. Selection of HLA Alleles in T-cell Epitope Screening
3.3. Prediction of T-cell Epitopes Recognized by MHC-I and MHC-II
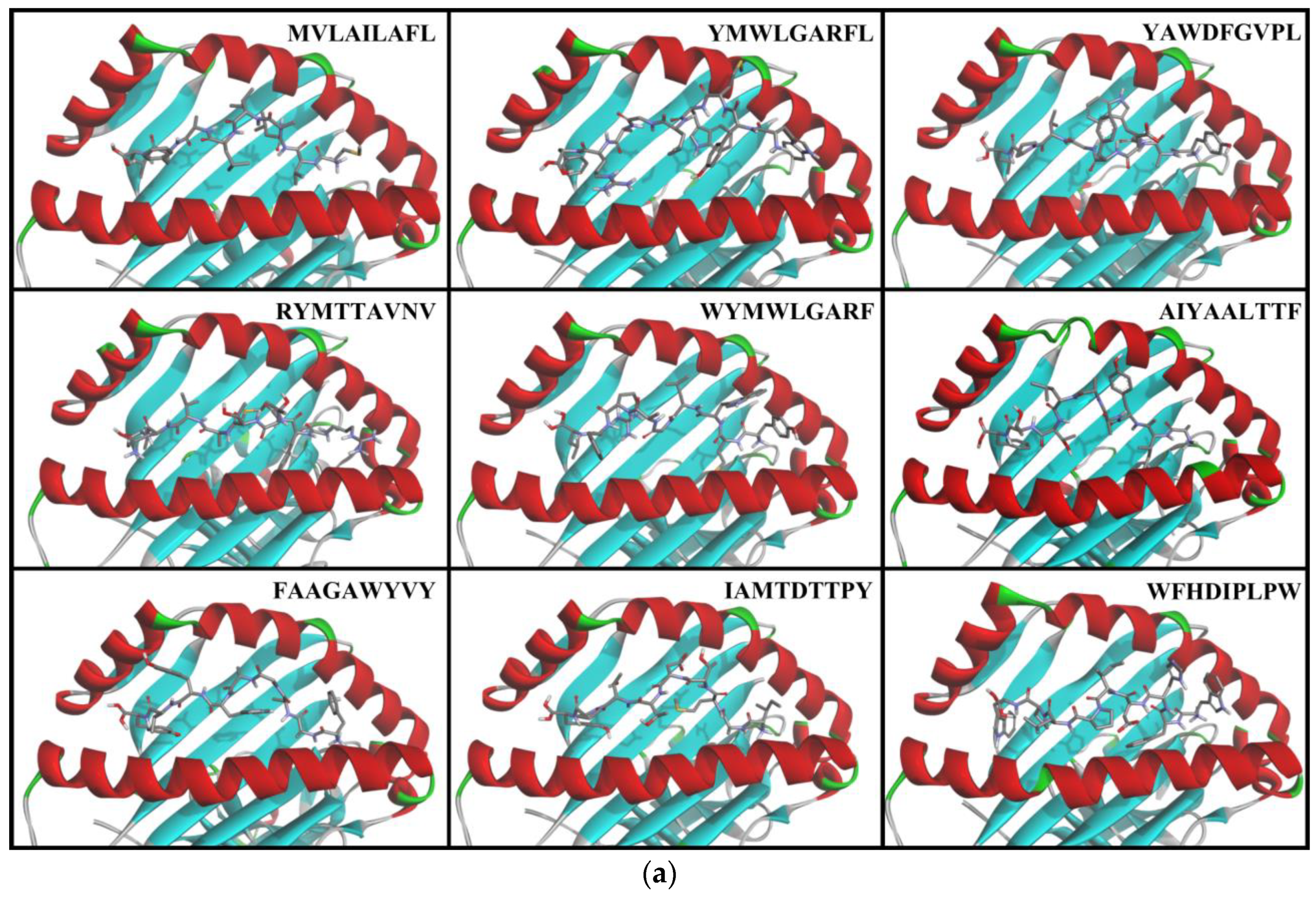
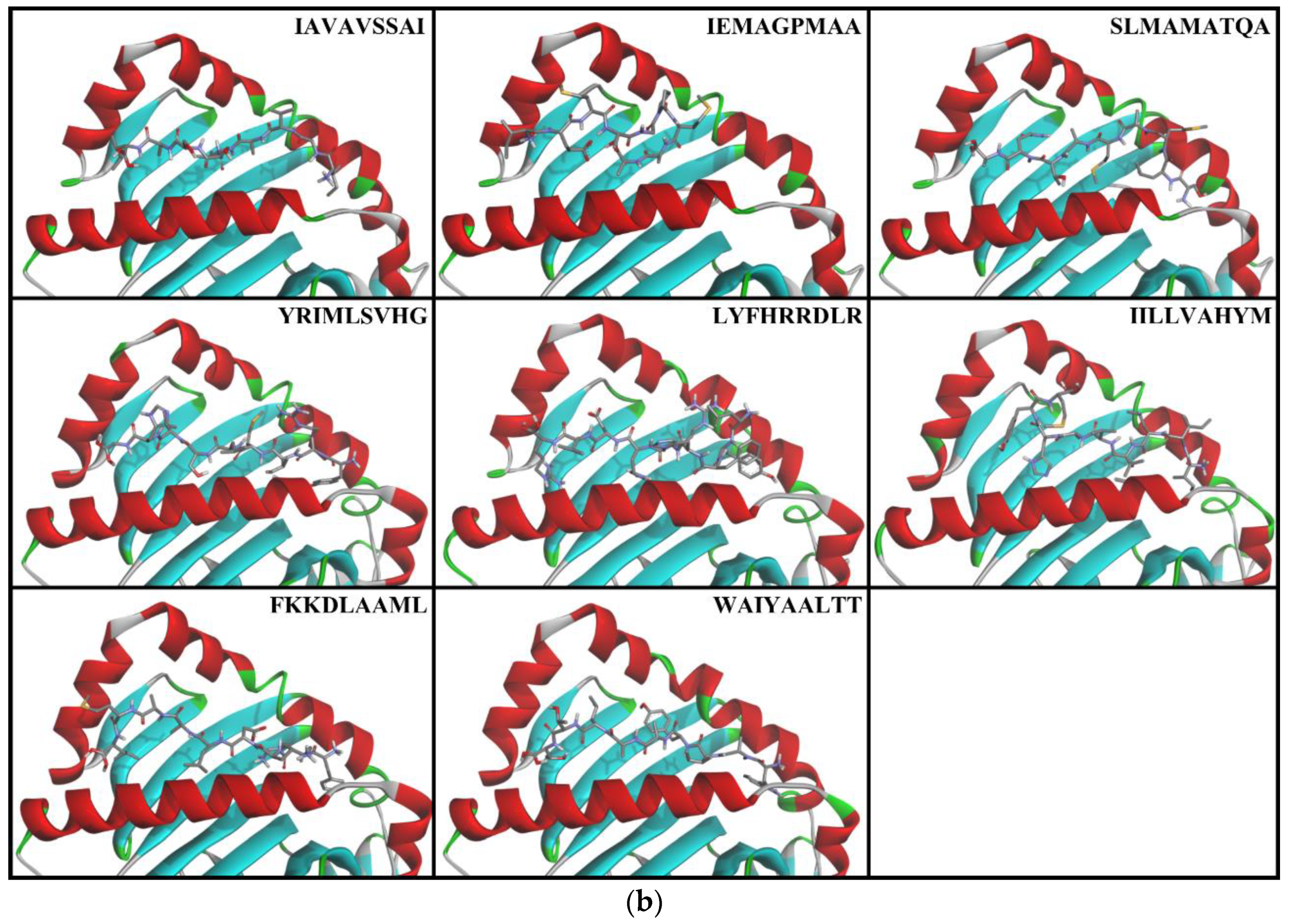
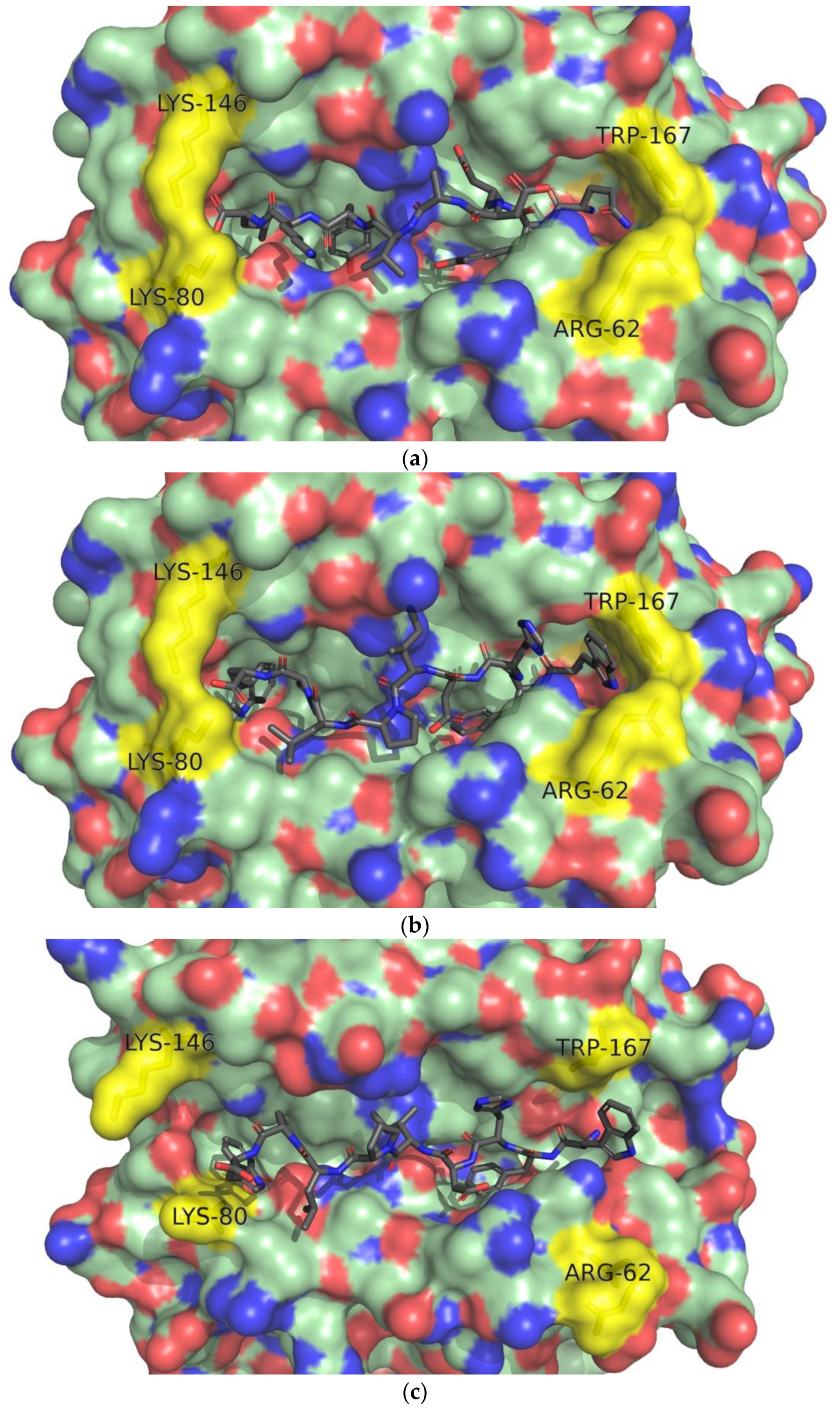
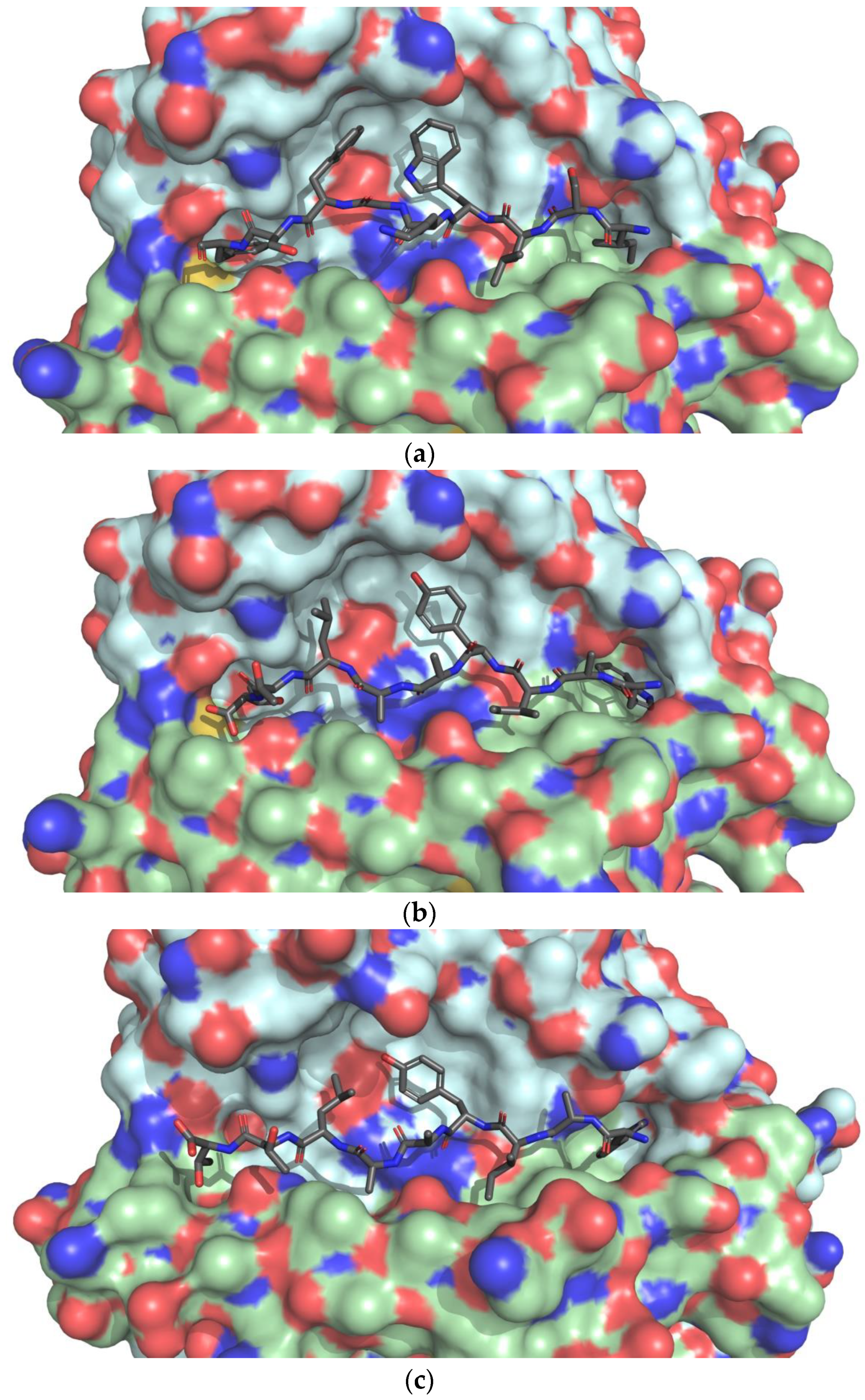
3.4. Docking of T-cell Epitopes to MHC Molecules
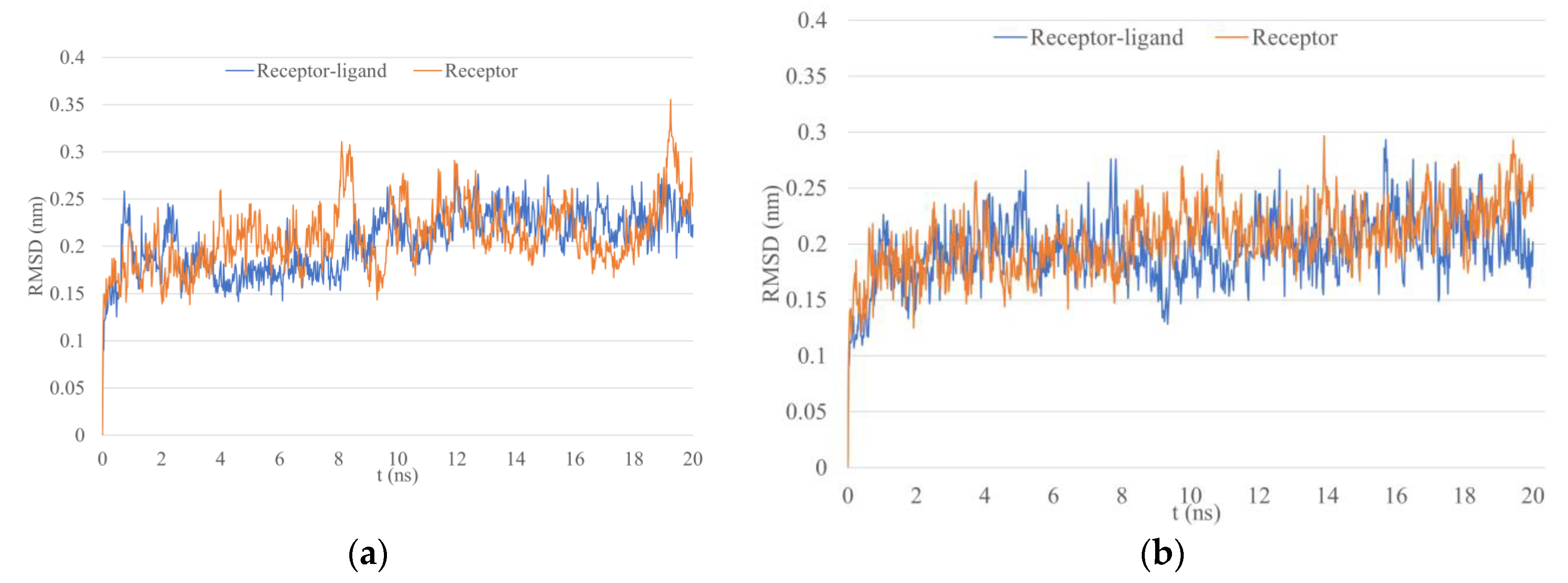
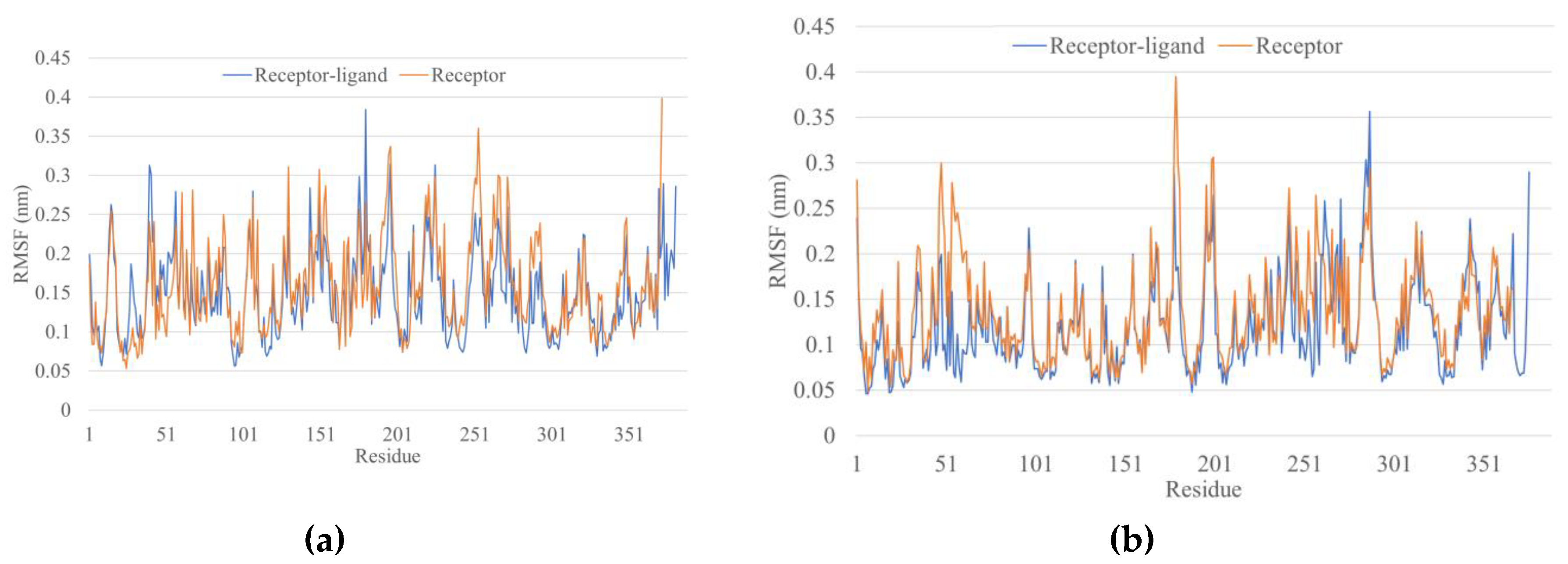
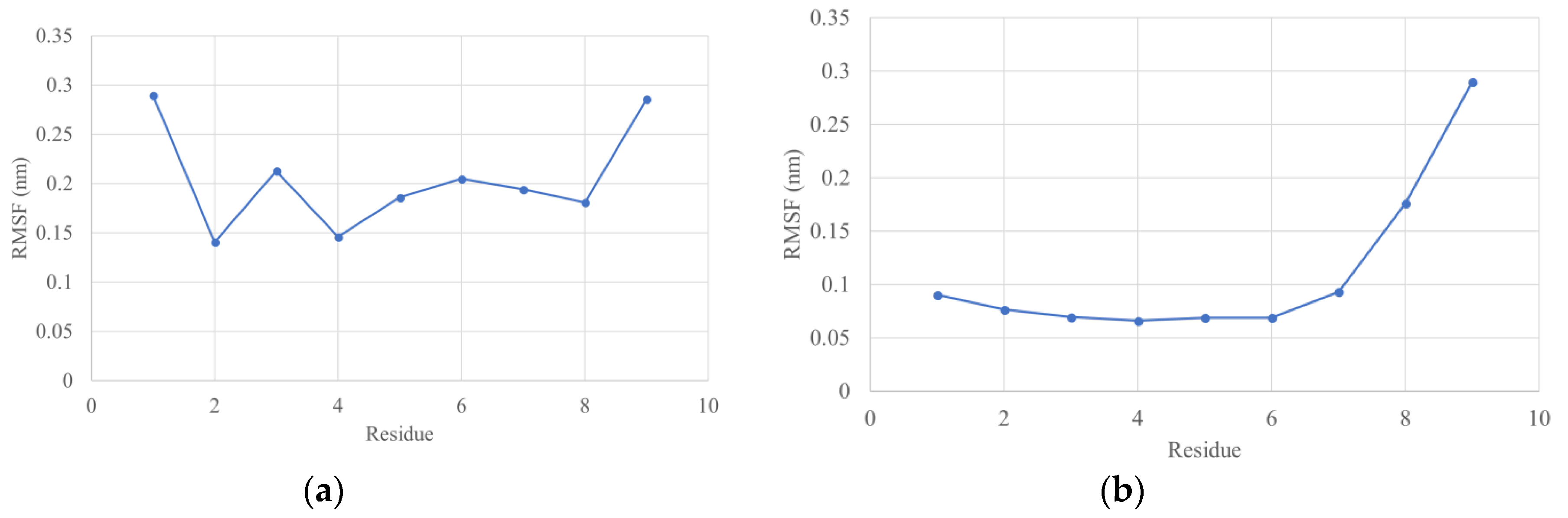
3.5. Molecular Dynamics Simulations
3.6. Prediction of B-cell Linear Epitopes
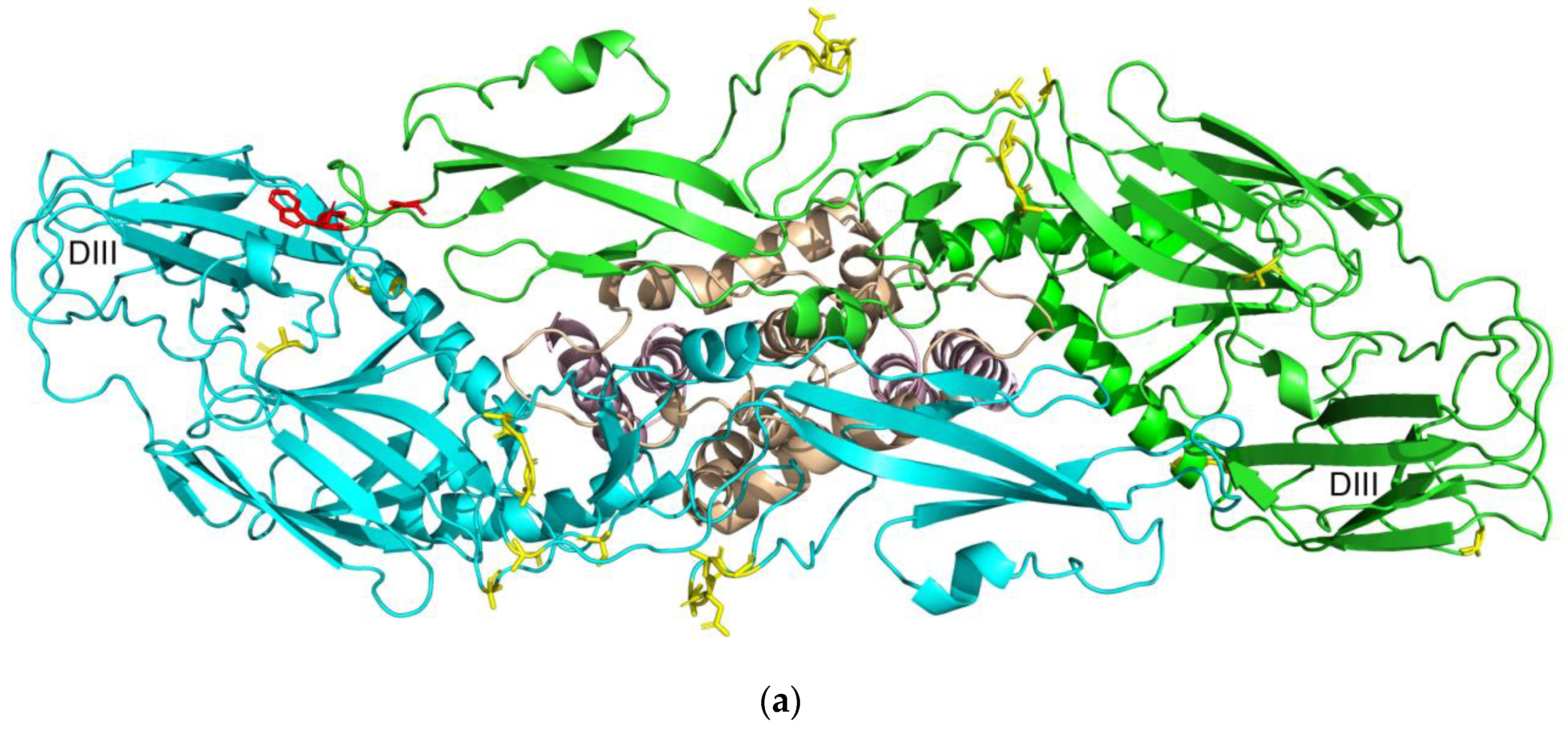
3.7. Prediction of B-cell Conformational Epitopes
4. Discussion
4.1. Prediction of T-cell Epitopes Recognized by Class I and II MHC
4.2. Molecular Docking of T-cell Epitopes to MHC Molecules
4.3. Molecular Dynamics Simulations
4.4. Prediction of B-cell Linear Epitopes
4.5. Prediction of B-cell Conformational Epitopes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gong, Z.; Gao, Y.; Han, G.-Z. Zika Virus: Two or Three Lineages? Trends Microbiol. 2016, 24, 521–522. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Shi, J.; Wang, J.; Tang, S.; Wang, H.; Hu, Z.; Deng, F. Phylogenetic analysis revealed the central roles of two African countries in the evolution and worldwide spread of Zika virus. Virol. Sin. 2016, 31, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Plourde, A.R.; Bloch, E.M. A Literature Review of Zika Virus. Emerg. Infect. Dis. 2016, 22, 1185–1192. [Google Scholar] [CrossRef]
- Slenczka, W. Zika Virus Disease. Microbiol. Spectr. 2016, 4, EI10-0019–2016. [Google Scholar] [CrossRef] [PubMed]
- Schuler-Faccini, L. Possible Association Between Zika Virus Infection and Microcephaly—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Zika Situation Report 5 February 2016: Neurological Syndrome and Congenital Anomalies. Available online: http://www.who.int/emergencies/zika-virus/situation-report/5-february-2016/en/ (accessed on 20 May 2018).
- Yuan, L.; Huang, X.-Y.; Liu, Z.-Y.; Zhang, F.; Zhu, X.-L.; Yu, J.-Y.; Ji, X.; Xu, Y.-P.; Li, G.; Li, C.; et al. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 2017, 358, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Metsky, H.C.; Matranga, C.B.; Wohl, S.; Schaffner, S.F.; Freije, C.A.; Winnicki, S.M.; West, K.; Qu, J.; Baniecki, M.L.; Gladden-Young, A.; et al. Zika virus evolution and spread in the Americas. Nature 2017, 546, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Zanluca, C.; Melo, V.C.A.D.; Mosimann, A.L.P.; Santos, G.I.V.D.; Santos, C.N.D.D.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef]
- De Oliveira, W.K.; de França, G.V.A.; Carmo, E.H.; Duncan, B.B.; de Souza Kuchenbecker, R.; Schmidt, M.I. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: A surveillance-based analysis. Lancet 2017, 390, 861–870. [Google Scholar] [CrossRef]
- Secretaria de Vigilância em Saúde—Ministério da Saúde Monitoramento dos casos de dengue, febre de chikungunya e febre pelo vírus Zika até a Semana Epidemiológica 25, 2017. Bol. Epidemiol. 2017, 48, 1–10.
- Lagunas-Rangel, F.A.; Viveros-Sandoval, M.E.; Reyes-Sandoval, A. Current trends in Zika vaccine development. J. Virus Erad. 2017, 3, 124–127. [Google Scholar] [PubMed]
- Ortiz, J.F.; MacDonald, M.L.; Masterson, P.; Uversky, V.N.; Siltberg-Liberles, J. Rapid evolutionary dynamics of structural disorder as a potential driving force for biological divergence in flaviviruses. Genome Biol. Evol. 2013, 5, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Tirado, S.M.C.; Yoon, K.-J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003, 16, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Vazquez, S. The Complexity of Antibody-Dependent Enhancement of Dengue Virus Infection. Viruses 2010, 2, 2649–2662. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Neto, E.; Rosa, D.S.; Harris, P.E.; Olson, T.; Morrow, A.; Ciotlos, S.; Herst, C.V.; Rubsamen, R.M. An Approach for a Synthetic CTL Vaccine Design against Zika Flavivirus Using Class I and Class II Epitopes Identified by Computer Modeling. Front. Immunol. 2017, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, R.M.; Eddy, W.E.; Tang, W.W.; Miller, R.; Shresta, S. CD8+ T-cells prevent antigen-induced antibody-dependent enhancement of dengue disease in mice. J. Immunol. 2014, 193, 4117–4124. [Google Scholar] [CrossRef] [PubMed]
- Paquin-Proulx, D.; Leal, F.E.; Silveira, C.G.T.; Maestri, A.; Brockmeyer, C.; Kitchen, S.M.; Cabido, V.D.; Kallas, E.G.; Nixon, D.F. T-cell responses in individuals infected with Zika virus and in those vaccinated against Dengue virus. Pathog. Immun. 2017, 2, 274. [Google Scholar] [CrossRef]
- Alam, A.; Ali, S.; Ahamad, S.; Malik, M.Z.; Ishrat, R. From ZikV genome to vaccine: In silico approach for the epitope-based peptide vaccine against Zika virus envelope glycoprotein. Immunology 2016, 149, 386–399. [Google Scholar] [CrossRef]
- Dar, H.; Zaheer, T.; Rehman, M.T.; Ali, A.; Javed, A.; Khan, G.A.; Babar, M.M.; Waheed, Y. Prediction of promiscuous T-cell epitopes in the Zika virus polyprotein: An in silico approach. Asian Pac. J. Trop. Med. 2016, 9, 844–850. [Google Scholar] [CrossRef]
- Dikhit, M.R.; Ansari, M.Y.; Vijaymahantesh; Kalyani; Mansuri, R.; Sahoo, B.R.; Dehury, B.; Amit, A.; Topno, R.K.; Sahoo, G.C.; et al. Computational prediction and analysis of potential antigenic CTL epitopes in Zika virus: A first step towards vaccine development. Infect. Genet. Evol. 2016, 45, 187–197. [Google Scholar] [CrossRef]
- Mirza, M.U.; Rafique, S.; Ali, A.; Munir, M.; Ikram, N.; Manan, A.; Salo-Ahen, O.M.H.; Idrees, M. Towards peptide vaccines against Zika virus: Immunoinformatics combined with molecular dynamics simulations to predict antigenic epitopes of Zika viral proteins. Sci. Rep. 2016, 6, 37313. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Yadav, M.; Verma, R.; Khan, N.S.; Jena, L.; Jain, A.K. Discovery of T-cell Driven Subunit Vaccines from Zika Virus Genome: An Immunoinformatics Approach. Interdiscip. Sci. Comput. Life Sci. 2017, 9, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Rao, R.; Raj, U.; Varadwaj, P. Computational modeling and analysis of prominent T-cell epitopes for assisting in designing vaccine of ZIKA virus. J. Appl. Pharm. Sci. 2017, 7, 116–122. [Google Scholar]
- Soria-Guerra, R.E.; Nieto-Gomez, R.; Govea-Alonso, D.O.; Rosales-Mendoza, S. An overview of bioinformatics tools for epitope prediction: Implications on vaccine development. J. Biomed. Inform. 2015, 53, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Systèmes, D. BIOVIA Discovery Studio Modeling Environment; Dassault Systèmes Biovia: San Diego, CA, USA, 2016. [Google Scholar]
- Schrödinger, L.L.C. The PyMOL Molecular Graphics System, version 2.1; Schrödinger, L.L.C.: New York, NY, USA, 2018.
- Stranzl, T.; Larsen, M.V.; Lundegaard, C.; Nielsen, M. NetCTLpan: Pan-specific MHC class I pathway epitope predictions. Immunogenetics 2010, 62, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Hoof, I.; Peters, B.; Sidney, J.; Pedersen, L.E.; Sette, A.; Lund, O.; Buus, S.; Nielsen, M. NetMHCpan, a method for MHC class I binding prediction beyond humans. Immunogenetics 2009, 61, 1–13. [Google Scholar] [CrossRef]
- Nielsen, M.; Lundegaard, C.; Lund, O.; Keşmir, C. The role of the proteasome in generating cytotoxic T-cell epitopes: Insights obtained from improved predictions of proteasomal cleavage. Immunogenetics 2005, 57, 33–41. [Google Scholar] [CrossRef]
- Peters, B.; Bulik, S.; Tampe, R.; Van Endert, P.M.; Holzhütter, H.-G. Identifying MHC class I epitopes by predicting the TAP transport efficiency of epitope precursors. J. Immunol. 2003, 171, 1741–1749. [Google Scholar] [CrossRef]
- Moutaftsi, M.; Peters, B.; Pasquetto, V.; Tscharke, D.C.; Sidney, J.; Bui, H.-H.; Grey, H.; Sette, A. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat. Biotechnol. 2006, 24, 817–819. [Google Scholar] [CrossRef]
- Nielsen, M.; Andreatta, M. NetMHCpan-3.0; improved prediction of binding to MHC class I molecules integrating information from multiple receptor and peptide length datasets. Genome Med. 2016, 8, 33. [Google Scholar] [CrossRef]
- Andreatta, M.; Nielsen, M. Gapped sequence alignment using artificial neural networks: Application to the MHC class I system. Bioinform. Oxf. Engl. 2016, 32, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Lundegaard, C.; Worning, P.; Lauemøller, S.L.; Lamberth, K.; Buus, S.; Brunak, S.; Lund, O. Reliable prediction of T-cell epitopes using neural networks with novel sequence representations. Protein Sci. Publ. Protein Soc. 2003, 12, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.; Sette, A. Generating quantitative models describing the sequence specificity of biological processes with the stabilized matrix method. BMC Bioinform. 2005, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Sidney, J.; Assarsson, E.; Moore, C.; Ngo, S.; Pinilla, C.; Sette, A.; Peters, B. Quantitative peptide binding motifs for 19 human and mouse MHC class I molecules derived using positional scanning combinatorial peptide libraries. Immunome Res. 2008, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sidney, J.; Dow, C.; Mothé, B.; Sette, A.; Peters, B. A Systematic Assessment of MHC Class II Peptide Binding Predictions and Evaluation of a Consensus Approach. PLoS Comput. Biol. 2008, 4, e1000048. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Lundegaard, C.; Lund, O. Prediction of MHC class II binding affinity using SMM-align, a novel stabilization matrix alignment method. BMC Bioinform. 2007, 8, 238. [Google Scholar] [CrossRef]
- Nielsen, M.; Lund, O. NN-align. An artificial neural network-based alignment algorithm for MHC class II peptide binding prediction. BMC Bioinform. 2009, 10, 296. [Google Scholar] [CrossRef]
- Sturniolo, T.; Bono, E.; Ding, J.; Raddrizzani, L.; Tuereci, O.; Sahin, U.; Braxenthaler, M.; Gallazzi, F.; Protti, M.P.; Sinigaglia, F.; et al. Generation of tissue-specific and promiscuous HLA ligand databases using DNA microarrays and virtual HLA class II matrices. Nat. Biotechnol. 1999, 17, 555–561. [Google Scholar] [CrossRef]
- Andreatta, M.; Karosiene, E.; Rasmussen, M.; Stryhn, A.; Buus, S.; Nielsen, M. Accurate pan-specific prediction of peptide-MHC class II binding affinity with improved binding core identification. Immunogenetics 2015, 67, 641–650. [Google Scholar] [CrossRef]
- Oyarzún, P.; Ellis, J.J.; Bodén, M.; Kobe, B. PREDIVAC: CD4+ T-cell epitope prediction for vaccine design that covers 95% of HLA class II DR protein diversity. BMC Bioinform. 2013, 14, 52. [Google Scholar] [CrossRef]
- Jensen, K.K.; Andreatta, M.; Marcatili, P.; Buus, S.; Greenbaum, J.A.; Yan, Z.; Sette, A.; Peters, B.; Nielsen, M. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology 2018, 154, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Calis, J.J.A.; Maybeno, M.; Greenbaum, J.A.; Weiskopf, D.; De Silva, A.D.; Sette, A.; Keşmir, C.; Peters, B. Properties of MHC class I presented peptides that enhance immunogenicity. PLoS Comput. Biol. 2013, 9, e1003266. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Consortium, O.S.D.D.; Raghava, G.P.S. In Silico Approach for Predicting Toxicity of Peptides and Proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.-H.; Sidney, J.; Dinh, K.; Southwood, S.; Newman, M.J.; Sette, A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinform. 2006, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. TIG 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, M.C.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-2.0: Improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 2017, 45, W24–W29. [Google Scholar] [CrossRef]
- Singh, H.; Ansari, H.R.; Raghava, G.P.S. Improved method for linear B-cell epitope prediction using antigen’s primary sequence. PLoS ONE 2013, 8, e62216. [Google Scholar] [CrossRef]
- Vita, R.; Overton, J.A.; Greenbaum, J.A.; Ponomarenko, J.; Clark, J.D.; Cantrell, J.R.; Wheeler, D.K.; Gabbard, J.L.; Hix, D.; Sette, A.; et al. The immune epitope database (IEDB) 3.0. Nucleic Acids Res. 2015, 43, D405–D412. [Google Scholar] [CrossRef]
- Kringelum, J.V.; Lundegaard, C.; Lund, O.; Nielsen, M. Reliable B-cell Epitope Predictions: Impacts of Method Development and Improved Benchmarking. PLoS Comput. Biol. 2012, 8, e1002829. [Google Scholar] [CrossRef]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. Publ. Protein Soc. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Wallner, B.; Elofsson, A. Can correct protein models be identified? Protein Sci. Publ. Protein Soc. 2003, 12, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Lüthy, R.; Bowie, J.U. VERIFY3D: Assessment of protein models with three-dimensional profiles. Methods Enzymol. 1997, 277, 396–404. [Google Scholar] [PubMed]
- Chen, V.B.; Arendall, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Lamiable, A.; Thévenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tufféry, P. PEP-FOLD3: Faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Verlet, L. Computer “Experiments” on Classical Fluids. I. Thermodynamical Properties of Lennard-Jones Molecules. Phys. Rev. 1967, 159, 98–103. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.; Fraaije, J.G.E.M. LINCS: A Linear Constraint Solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Nosé, S.; Klein, M.L. Constant pressure molecular dynamics for molecular systems. Mol. Phys. 1983, 50, 1055–1076. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology, 5th ed.; Garland Science: New York, NY, USA, 2001; ISBN 978-0-8153-3642-6. [Google Scholar]
- Yuliwulandari, R.; Kashiwase, K.; Nakajima, H.; Uddin, J.; Susmiarsih, T.P.; Sofro, A.S.M.; Tokunaga, K. Polymorphisms of HLA genes in Western Javanese (Indonesia): Close affinities to Southeast Asian populations. Tissue Antigens 2009, 73, 46–53. [Google Scholar] [CrossRef]
- Appanna, R.; Ponnampalavanar, S.; Lum Chai See, L.; Sekaran, S.D. Susceptible and protective HLA class 1 alleles against dengue fever and dengue hemorrhagic fever patients in a Malaysian population. PLoS ONE 2010, 5, e13029. [Google Scholar] [CrossRef]
- Mercado, E.S.; Espino, F.E.; Perez, M.L.M.; Bilar, J.M.; Bajaro, J.D.P.; Huy, N.T.; Baello, B.Q.; Kikuchi, M.; Hirayama, K. HLA-A*33:01 as Protective Allele for Severe Dengue in a Population of Filipino Children. PLoS ONE 2015, 10, e0115619. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Angelo, M.A.; de Azeredo, E.L.; Sidney, J.; Greenbaum, J.A.; Fernando, A.N.; Broadwater, A.; Kolla, R.V.; De Silva, A.D.; de Silva, A.M.; et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T-cells. Proc. Natl. Acad. Sci. USA 2013, 110, E2046–E2053. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, C.; Granados, J.; Vargas-Alarcon, G.; Ruíz-Morales, J.; Villarreal-Garza, C.; Higuera, L.; Hernández-Pacheco, G.; Cutiño-Moguel, T.; Rangel, H.; Figueroa, R.; et al. HLA-DR antigen frequencies in Mexican patients with dengue virus infection: HLA-DR4 as a possible genetic resistance factor for dengue hemorrhagic fever. Hum. Immunol. 2002, 63, 1039–1044. [Google Scholar] [CrossRef]
- Sierra, B.; Alegre, R.; Pérez, A.B.; García, G.; Sturn-Ramirez, K.; Obasanjo, O.; Aguirre, E.; Alvarez, M.; Rodriguez-Roche, R.; Valdés, L.; et al. HLA-A, -B, -C, and -DRB1 allele frequencies in Cuban individuals with antecedents of dengue 2 disease: Advantages of the Cuban population for HLA studies of dengue virus infection. Hum. Immunol. 2007, 68, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zimmet, P.; Serjeantson, S.W. HLA-DR,DQ sequence polymorphisms in Polynesians, Micronesians, and Javanese. Hum. Immunol. 1992, 34, 153–161. [Google Scholar] [CrossRef]
- Lan, N.T.P.; Kikuchi, M.; Huong, V.T.Q.; Ha, D.Q.; Thuy, T.T.; Tham, V.D.; Tuan, H.M.; Tuong, V.V.; Nga, C.T.P.; Van Dat, T.; et al. Protective and Enhancing HLA Alleles, HLA-DRB1*0901 and HLA-A*24, for Severe Forms of Dengue Virus Infection, Dengue Hemorrhagic Fever and Dengue Shock Syndrome. PLoS Negl. Trop. Dis. 2008, 2, e304. [Google Scholar] [CrossRef] [PubMed]
- Mack, S.J.; Bugawan, T.L.; Moonsamy, P.V.; Erlich, J.A.; Trachtenberg, E.A.; Paik, Y.K.; Begovich, A.B.; Saha, N.; Beck, H.P.; Stoneking, M.; et al. Evolution of Pacific/Asian populations inferred from HLA class II allele frequency distributions. Tissue Antigens 2000, 55, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Förster, C. In silico predictive model to determine vector-mediated transport properties for the blood–brain barrier choline transporter. Adv. Appl. Bioinform. Chem. AABC 2014, 7, 23–36. [Google Scholar] [CrossRef]
- Lobanov, M.Y.; Bogatyreva, N.S.; Galzitskaya, O.V. Radius of gyration as an indicator of protein structure compactness. Mol. Biol. 2008, 42, 623–628. [Google Scholar] [CrossRef]
- Fan, Q.R.; Wiley, D.C. Structure of Human Histocompatibility Leukocyte Antigen (Hla)-Cw4, a Ligand for the Kir2d Natural Killer Cell Inhibitory Receptor. J. Exp. Med. 1999, 190, 113–124. [Google Scholar] [CrossRef]
- Fleischmann, G.; Fisette, O.; Thomas, C.; Wieneke, R.; Tumulka, F.; Schneeweiss, C.; Springer, S.; Schäfer, L.V.; Tampé, R. Mechanistic Basis for Epitope Proofreading in the Peptide-Loading Complex. J. Immunol. 2015, 195, 4503–4513. [Google Scholar] [CrossRef] [PubMed]
- Ho, Z.J.M.; Hapuarachchi, H.C.; Barkham, T.; Chow, A.; Ng, L.C.; Lee, J.M.V.; Leo, Y.S.; Prem, K.; Lim, Y.H.G.; de Sessions, P.F.; et al. Outbreak of Zika virus infection in Singapore: an epidemiological, entomological, virological, and clinical analysis. Lancet Infect. Dis. 2017, 17, 813–821. [Google Scholar] [CrossRef]
- Sant’Angelo, D.B.; Robinson, E.; Janeway, C.A., Jr.; Denzin, L.K. Recognition of core and flanking amino acids of MHC class II-bound peptides by the T-cell receptor. Eur. J. Immunol. 2002, 32, 2510–2520. [Google Scholar] [CrossRef]
- Quintero-Hernández, V.; Jiménez-Vargas, J.M.; Gurrola, G.B.; Valdivia, H.H.F.; Possani, L.D. Scorpion venom components that affect ion-channels function. Toxicon Off. J. Int. Soc. Toxinol. 2013, 76, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huang, Q.; Huang, R.; Deng, F.; Yue, X.; Yin, J.; Zhao, W.; Chen, Y.; Wen, L.; Zhou, J.; et al. B-cells Are Indispensable for a Novel Mouse Model of Primary Sjögren’s Syndrome. Front. Immunol. 2017, 8, 1384. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, G.F. Major Histocompatibility Complex: Interaction with Peptides. In eLS; John Wiley & Sons, Ltd: Chichester, UK, 2011; ISBN 978-0-470-01617-6. [Google Scholar]
- Hauser, A.S.; Windshügel, B. LEADS-PEP: A Benchmark Data Set for Assessment of Peptide Docking Performance. J. Chem. Inf. Model. 2016, 56, 188–200. [Google Scholar] [CrossRef]
- Rentzsch, R.; Renard, B.Y. Docking small peptides remains a great challenge: An assessment using AutoDock Vina. Brief. Bioinform. 2015, 16, 1045–1056. [Google Scholar] [CrossRef]
- Lin, H.H.; Zhang, G.L.; Tongchusak, S.; Reinherz, E.L.; Brusic, V. Evaluation of MHC-II peptide binding prediction servers: Applications for vaccine research. BMC Bioinform. 2008, 9, S22. [Google Scholar] [CrossRef]
- Lin, H.H.; Ray, S.; Tongchusak, S.; Reinherz, E.L.; Brusic, V. Evaluation of MHC class I peptide binding prediction servers: Applications for vaccine research. BMC Immunol. 2008, 9, 8. [Google Scholar] [CrossRef]
- Westermarck, J.; Ivaska, J.; Corthals, G.L. Identification of Protein Interactions Involved in Cellular Signaling. Mol. Cell. Proteomics MCP 2013, 12, 1752–1763. [Google Scholar] [CrossRef]
- Lodish, H.; Berk, A.; Zipursky, S.L.; Matsudaira, P.; Baltimore, D.; Darnell, J. Molecular Cell Biology, 4th ed.; W.H. Freeman: New York, NY, USA, 2000; ISBN 978-0-7167-3136-8. [Google Scholar]
- Rognan, D.; Scapozza, L.; Folkers, G.; Daser, A. Molecular Dynamics Simulation of MHC-Peptide Complexes as a Tool for Predicting Potential T-cell Epitopes. Biochemistry 1994, 33, 11476–11485. [Google Scholar] [CrossRef] [PubMed]
- Ooi, J.D.; Petersen, J.; Tan, Y.H.; Huynh, M.; Willett, Z.J.; Ramarathinam, S.H.; Eggenhuizen, P.J.; Loh, K.L.; Watson, K.A.; Gan, P.Y.; et al. Dominant protection from HLA-linked autoimmunity by antigen-specific regulatory T-cells. Nature 2017, 545, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Saisawang, C.; Kuadkitkan, A.; Auewarakul, P.; Smith, D.R.; Ketterman, A.J. Glutathionylation of dengue and Zika NS5 proteins affects guanylyltransferase and RNA dependent RNA polymerase activities. PLoS ONE 2018, 13, e0193133. [Google Scholar] [CrossRef] [PubMed]
- French, S.; Robson, B. What is a conservative substitution? J. Mol. Evol. 1983, 19, 171–175. [Google Scholar] [CrossRef]
- Barlow, D.J.; Edwards, M.S.; Thornton, J.M. Continuous and discontinuous protein antigenic determinants. Nature 1986, 322, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.-M.; Zhang, W.; Holdaway, H.A.; Li, L.; Kostyuchenko, V.A.; Chipman, P.R.; Kuhn, R.J.; Rossmann, M.G.; Chen, J. Structure of the Immature Dengue Virus at Low pH Primes Proteolytic Maturation. Science 2008, 319, 1834–1837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Corver, J.; Chipman, P.R.; Zhang, W.; Pletnev, S.V.; Sedlak, D.; Baker, T.S.; Strauss, J.H.; Kuhn, R.J.; Rossmann, M.G. Structures of immature flavivirus particles. EMBO J. 2003, 22, 2604–2613. [Google Scholar] [CrossRef]
- Sirohi, D.; Chen, Z.; Sun, L.; Klose, T.; Pierson, T.C.; Rossmann, M.G.; Kuhn, R.J. The 3.8 Å resolution cryo-EM structure of Zika virus. Science 2016, 352, 467–470. [Google Scholar] [CrossRef]
- Xu, X.; Song, H.; Qi, J.; Liu, Y.; Wang, H.; Su, C.; Shi, Y.; Gao, G.F. Contribution of intertwined loop to membrane association revealed by Zika virus full-length NS1 structure. EMBO J. 2016, 35, 2170–2178. [Google Scholar] [CrossRef]
- Jones, C.T.; Ma, L.; Burgner, J.W.; Groesch, T.D.; Post, C.B.; Kuhn, R.J. Flavivirus Capsid Is a Dimeric Alpha-Helical Protein. J. Virol. 2003, 77, 7143–7149. [Google Scholar] [CrossRef]
- Wen, D.; Li, S.; Dong, F.; Zhang, Y.; Lin, Y.; Wang, J.; Zou, Z.; Zheng, A. N-glycosylation of Viral E Protein Is the Determinant for Vector Midgut Invasion by Flaviviruses. mBio 2018, 9, e00046-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yan, J.; Gao, G.F. Monoclonal Antibodies against Zika Virus: Therapeutics and Their Implications for Vaccine Design. J. Virol. 2017, 91, e01049-17. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Song, J.; Lu, X.; Deng, Y.-Q.; Musyoki, A.M.; Cheng, H.; Zhang, Y.; Yuan, Y.; Song, H.; Haywood, J.; et al. Structures of the Zika Virus Envelope Protein and Its Complex with a Flavivirus Broadly Protective Antibody. Cell Host Microbe 2016, 19, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Miller, A.; Sapparapu, G.; Fernandez, E.; Klose, T.; Long, F.; Fokine, A.; Porta, J.C.; Jiang, W.; Diamond, M.S.; et al. A human antibody against Zika virus crosslinks the E protein to prevent infection. Nat. Commun. 2017, 8, 14722. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.; Liu, X.; Dai, L.; Ma, T.; Qi, J.; Wong, G.; Peng, R.; Liu, S.; Li, J.; et al. Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus. Sci. Transl. Med. 2016, 8, 369ra179. [Google Scholar] [CrossRef] [PubMed]
- Robbiani, D.F.; Bozzacco, L.; Keeffe, J.R.; Khouri, R.; Olsen, P.C.; Gazumyan, A.; Schaefer-Babajew, D.; Avila-Rios, S.; Nogueira, L.; Patel, R.; et al. Recurrent Potent Human Neutralizing Antibodies to Zika Virus in Brazil and Mexico. Cell 2017, 169, 597–609. [Google Scholar] [CrossRef]
- Zhao, H.; Fernandez, E.; Dowd, K.A.; Speer, S.D.; Platt, D.J.; Gorman, M.J.; Govero, J.; Nelson, C.A.; Pierson, T.C.; Diamond, M.S.; et al. Structural Basis of Zika Virus-Specific Antibody Protection. Cell 2016, 166, 1016–1027. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Phoo, W.W.; Loh, Y.R.; Li, R.; Yang, H.Y.; Jansson, A.E.; Hill, J.; Keller, T.H.; Nacro, K.; et al. Structural Insights into the Inhibition of Zika Virus NS2B-NS3 Protease by a Small-Molecule Inhibitor. Structure 2018, 26, 555–564. [Google Scholar] [CrossRef]
- Badawi, M.M.; Osman, M.M.; Alla, A.F.; Ahmedani, A.M.; Abdalla, M.H.; Gasemelseed, M.M.; Elsayed, A.A.; Salih, M.A. Highly conserved epitopes of Zika envelope glycoprotein may act as a novel peptide vaccine with high coverage: Immunoinformatics approach. Am. J. Biomed. Res. 2016, 4, 46–60. [Google Scholar]
- Roider, J.; Meissner, T.; Kraut, F.; Vollbrecht, T.; Stirner, R.; Bogner, J.R.; Draenert, R. Comparison of experimental fine-mapping to in silico prediction results of HIV-1 epitopes reveals ongoing need for mapping experiments. Immunology 2014, 143, 193–201. [Google Scholar] [CrossRef]
- Cravo, P.; Machado, R.B.; Leite, J.A.; Leda, T.; Suwanarusk, R.; Bittencourt, N.; Albrecht, L.; Judice, C.; Lopes, S.C.; Lacerda, M.V.; et al. In silico epitope mapping and experimental evaluation of the Merozoite Adhesive Erythrocytic Binding Protein (MAEBL) as a malaria vaccine candidate. Malar. J. 2018, 17, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Agallou, M.; Athanasiou, E.; Koutsoni, O.; Dotsika, E.; Karagouni, E. Experimental validation of multi-epitope peptides including promising MHC class I-and II-restricted epitopes of four known Leishmania infantum proteins. Front. Immunol. 2014, 5, 268. [Google Scholar] [CrossRef] [PubMed]
- Liljeroos, L.; Malito, E.; Ferlenghi, I.; Bottomley, M.J. Structural and computational biology in the design of immunogenic vaccine antigens. J. Immunol. Res. 2015, 2015, 17. [Google Scholar] [CrossRef] [PubMed]

| HLA Class | HLA Allele | Common | Protective | References |
|---|---|---|---|---|
| I | HLA-A*02:01 | ✓ | [73] | |
| HLA-A*03:01 | ✓ | [74] | ||
| HLA-A*11:01 | ✓ | [73] | ||
| HLA-A*24:02 | ✓ | [73] | ||
| HLA-A*24:07 | ✓ | [73] | ||
| HLA-A*33:01 | ✓ | [75] | ||
| HLA-A*33:03 | ✓ | [73] | ||
| HLA-B*15:02 | ✓ | [73] | ||
| HLA-B*15:13 | ✓ | [73] | ||
| HLA-B*18:01 | ✓ | [74] | ||
| HLA-B*35:01 | ✓ | [74,76] | ||
| HLA-B*35:05 | ✓ | [73] | ||
| HLA-B*44:03 | ✓ | [73] | ||
| II | HLA-DRB1*03:01 | ✓ | [73] | |
| HLA-DRB1*04:01 | ✓ | [77,78] | ||
| HLA-DRB1*07:01 | ✓ | ✓ | [73,78,79] | |
| HLA-DRB1*09:01 | ✓ | [80] | ||
| HLA-DRB1*12:02 | ✓ | [73,79] | ||
| HLA-DRB1*15:01 | ✓ | [73] | ||
| HLA-DRB1*15:02 | ✓ | [73,79,81] | ||
| HLA-DQA1*01:01 | ✓ | [79] | ||
| HLA-DQA1*01:02 | ✓ | [79] | ||
| HLA-DQA1*06:01 | ✓ | [79] | ||
| HLA-DQB1*03:01 | ✓ | [79,81] | ||
| HLA-DQB1*05:01 | ✓ | [79,81] |
| Pos | Protein | Epitope | NA | SA | SAS | SEA | Oce | WI | WAf | CAf | CA | Avg 1 | Auto-Immunity 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2310 | NS4B | AIYAALTTF | 81.62 | 63.67 | 75.25 | 81.47 | 78.14 | 66.07 | 75.72 | 85.91 | 2.97 | 75.98 | ○ |
| 1488 | NS2B | FAAGAWYVY | 81.84 | 73.23 | 76.47 | 74.99 | 56.09 | 75.05 | 78.11 | 80.77 | 7.02 | 74.57 | ○ |
| 2862 | NS5 | IAMTDTTPY | 72.99 | 50.65 | 69.71 | 75.12 | 62.86 | 71.10 | 64.16 | 74.85 | 6.44 | 67.68 | ○ |
| 664 | Env | MMLELDPPF | 92.11 | 89.51 | 77.07 | 91.02 | 83.26 | 85.29 | 84.11 | 89.86 | 5.70 | 86.53 | ○ |
| 46 | Capsid | MVLAILAFL | 89.18 | 81.56 | 74.70 | 63.25 | 59.63 | 81.05 | 87.53 | 93.37 | 7.95 | 78.78 | ○ |
| 1744 | NS3 | RYMTTAVNV | 80.88 | 70.87 | 76.55 | 78.60 | 81.71 | 41.77 | 79.54 | 81.32 | 2.19 | 73.91 | ○ |
| 507 | Env | WFHDIPLPW | 74.92 | 68.36 | 60.35 | 80.50 | 79.53 | 53.02 | 69.01 | 70.19 | 3.75 | 69.49 | ○ |
| 2996 | NS5 | WYMWLGARF | 74.49 | 67.56 | 57.78 | 77.88 | 75.46 | 59.09 | 70.34 | 70.83 | 1.39 | 69.18 | ○ |
| 2356 | NS4B | YAWDFGVPL | 97.13 | 92.59 | 90.73 | 92.52 | 84.57 | 81.90 | 94.71 | 94.86 | 2.98 | 91.13 | ○ |
| 2997 | NS5 | YMWLGARFL | 89.75 | 86.09 | 77.98 | 78.90 | 69.37 | 54.85 | 90.41 | 84.46 | 0.00 | 78.98 | ○ |
| Pos | Protein | Epitope | NA | CA | SA | SAS | SEA | Oce | WI | WAf | CAf | Avg | SB | WB | SB + (WB/2) | Auto-Immunity 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 84 | Capsid | FKKDLAAML | 100.00 | 99.98 | 99.78 | 99.82 | 89.63 | 98.77 | 60.73 | 99.73 | 99.83 | 94.25 | 10 | 9 | 14.5 | ○ |
| 2445 | NS4B | IAVAVSSAI | 99.46 | 100.00 | 99.79 | 99.29 | 96.87 | 99.21 | 99.13 | 99.82 | 97.47 | 99.00 | 11 | 9 | 15.5 | ○ |
| 1398 | NS2B | IEMAGPMAA | 100.00 | 100.00 | 100.00 | 99.72 | 99.44 | 99.93 | 99.70 | 99.94 | 99.71 | 99.83 | 7 | 7 | 10.5 | ○ |
| 2382 | NS4B | IILLVAHYM | 99.95 | 99.19 | 98.50 | 97.04 | 74.51 | 94.90 | 71.01 | 87.61 | 92.44 | 90.57 | 5 | 11 | 10.5 | ○ |
| 2396 | NS4B | LQAAAARAA | 100.00 | 99.99 | 99.91 | 99.35 | 98.89 | 99.91 | 97.86 | 99.42 | 99.29 | 99.40 | 11 | 2 | 12 | × |
| 3287 | NS5 | LYFHRRDLR | 99.94 | 99.05 | 96.53 | 96.46 | 62.07 | 90.11 | 69.54 | 89.41 | 93.30 | 88.49 | 9 | 3 | 10.5 | ○ |
| 2335 | NS4B | SLMAMATQA | 99.00 | 99.93 | 99.64 | 98.43 | 98.56 | 99.37 | 99.94 | 99.95 | 99.13 | 99.33 | 11 | 7 | 14.5 | ○ |
| 557 | Env | TALAGALEA | 99.74 | 100.00 | 100.00 | 98.00 | 99.19 | 99.80 | 99.32 | 99.51 | 98.91 | 99.39 | 10 | 2 | 11 | × |
| 2309 | NS4B | WAIYAALTT | 99.81 | 100.00 | 99.89 | 98.86 | 99.79 | 99.83 | 98.60 | 99.61 | 98.61 | 99.44 | 6 | 11 | 11.5 | ○ |
| 427 | Env | YRIMLSVHG | 99.95 | 92.92 | 96.84 | 97.70 | 86.77 | 97.30 | 88.96 | 98.29 | 95.73 | 94.94 | 11 | 5 | 13.5 | ○ |
| Protein | Peptide | HLA | Receptor | Binding Energy (kcal/mol) | Ki | #H Bond |
|---|---|---|---|---|---|---|
| Capsid | MVLAILAFL | A*02:01 | 2GIT | −9 | 4.52 × 10−7 | 9 |
| NS5 | YMWLGARFL | A*02:01 | 2GIT | −9.3 | 2.78 × 10−7 | 8 |
| NS4B | YAWDFGVPL | A*02:01 | 2GIT | −10.3 | 5.48 × 10−8 | 7 |
| NS3 | RYMTTAVNV | A*24:02 | 2X4O | −8.9 | 5.32 × 10−7 | 9 |
| NS5 | WYMWLGARF | A*24:02 | 2X4O | −7.5 | 5.16 × 10−6 | 7 |
| NS4B | AIYAALTTF | B*15:01 | 5TXS | −9.9 | 1.05 × 10−7 | 12 |
| NS2B | FAAGAWYVY | B*35:01 | 3LKN | −10.1 | 7.58 × 10−8 | 9 |
| NS5 | IAMTDTTPY | B*35:01 | 3LKN | −9.8 | 1.23 × 10−7 | 8 |
| Env | WFHDIPLPW | C*04:01 | 1QQD | −11 | 1.76 × 10−8 | 8 |
| Protein | Peptide | HLA | Receptor 1 | Binding Energy (kcal/mol) | Ki | #H Bond |
|---|---|---|---|---|---|---|
| NS4B | IAVAVSSAI | DQA1*05:01/DQB1*03:03 | 1UVQ* | −6.8 | 1.61 × 10−5 | 4 |
| NS2B | IEMAGPMAA | DQA1*05:01/DQB1*03:03 | 1UVQ* | −6.3 | 3.62 × 10−5 | 4 |
| NS4B | SLMAMATQA | DQA1*02:01/DQB1*03:03 | 1UVQ* | −7.3 | 7.14 × 10−6 | 6 |
| Env | YRIMLSVHG | DRB1*11:01 | 4MD5* | −7.3 | 7.14 × 10−6 | 9 |
| NS5 | LYFHRRDLR | DRB1*03:01 | 1A6A | −8.5 | 1.02 × 10−6 | 12 |
| NS4B | IILLVAHYM | DRB1*15:01 | 1BX2 | −6.5 | 2.62 × 10−5 | 6 |
| Capsid | FKKDLAAML | DRB1*09:01 | 5V4M* | −8 | 2.29 × 10−6 | 5 |
| NS4B | WAIYAALTT | DRB1*15:02 | 5V4M* | −10.4 | 4.66 × 10−8 | 7 |
| Protein | Position | Length | Peptide 1 |
|---|---|---|---|
| prM | 56 | 10 | LDEGVEPDDV |
| 84 | 20 | KKGEARRSRRAVTLPSHSTR | |
| M | 25 | 7 | YTKHLIR |
| E | 89 | 15 | QYVCKRTLVDRGWGN |
| 146 | 8 | SQHSGMIV | |
| 216 | 7 | EWFHDIP | |
| 428 | 9 | AWDFGSVGG | |
| NS1 | 28 | 13 | WRDRYKYHPDSPR |
| 141 | 8 | KECPLKHR | |
| 338 | 11 | RKEPESNLVRS | |
| NS3 | 6 | 7 | DVPAPKE |
| 27 | 7 | TRRLLGS | |
| 65 | 11 | LDPYWGDVKQD | |
| 82 | 9 | PWKLDAAWD | |
| 594 | 11 | WMDARVCSDHA | |
| NS5 | 34 | 15 | EVCREEARRALKDGV |
| 153 | 9 | SPEVEEART | |
| 247 | 17 | PRRPVKYEEDVNLGSGT | |
| 353 | 9 | QRVFKEKVD | |
| 363 | 7 | RVPDPQE | |
| 414 | 24 | FEEEKEWKTAVEAVNDPRFWALVD | |
| 598 | 9 | QDQRGSGQV |
| Protein | PDB ID |
|---|---|
| C | 5YGH |
| E | 5IRE |
| prM | 4B03 via homology modeling |
| M | 5IRE |
| NS1 | 5GS6 via point mutation: H1R |
| NS2B | 5YOF |
| NS3 (protease domain) | 5YOF via homology modeling |
| NS3 (helicase domain) | 5VI7 via homology modeling |
| NS5 | 5U0B via homology modeling |
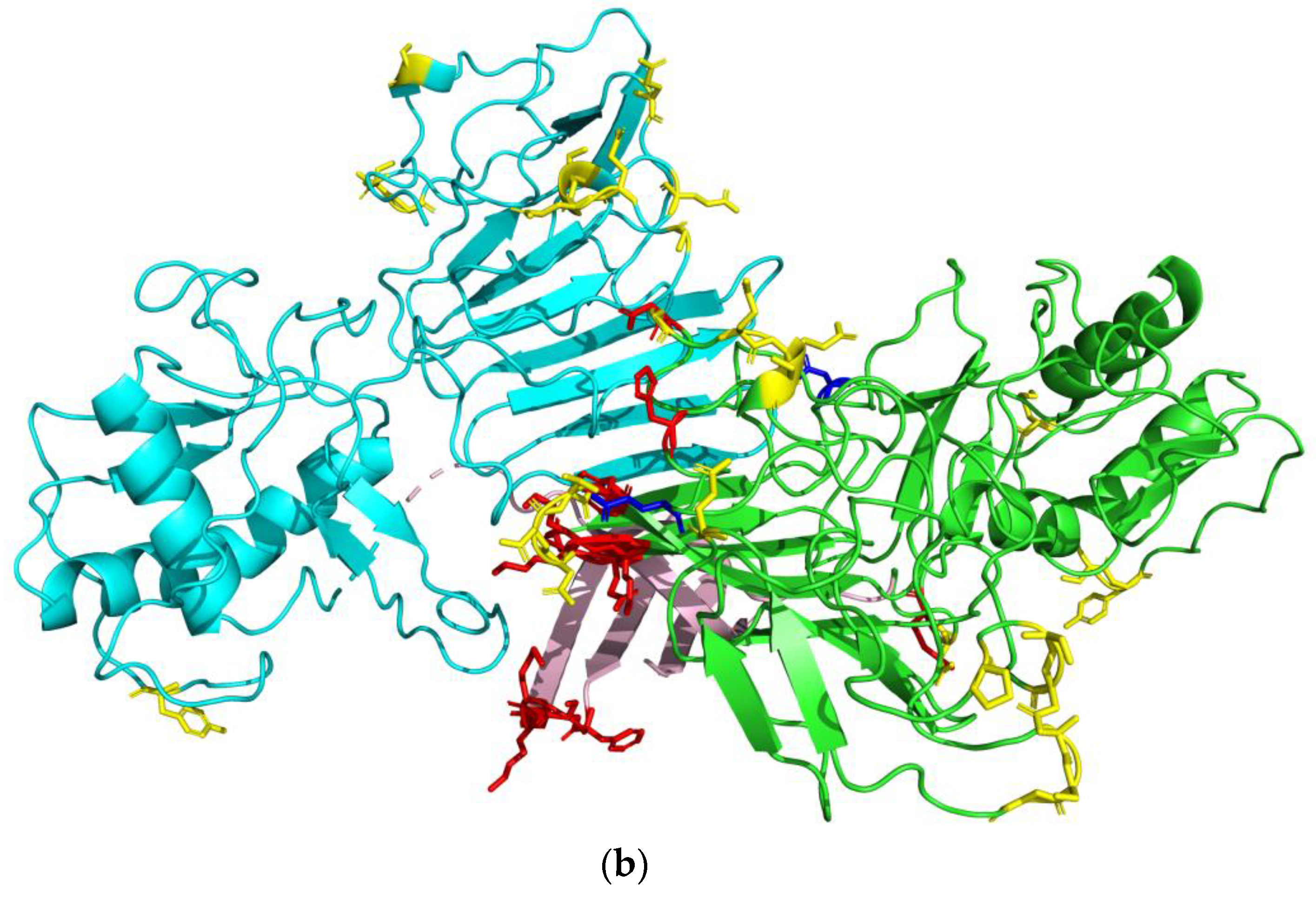
| Protein | Epitope Residues 1 |
|---|---|
| C | Chain A*: PRO26, PHE27, LYS74, LYS75, ASN96, ALA97, ARG98 |
| E | Chain C: ASN52, SER129, GLU133, THR156, ALA229, ASP230, THR231, GLY232, ASP278, GLY279, ALA280, THR405, ILE407 Chain E*: ASN52, TRP101, GLY102, GLY109, GLU133, THR156, ALA229, ASP230, THR231, GLY232, ASP278, GLY279, ALA280, GLN350, THR406, ILE407 |
| NS1 | Chain A*: PHE8, SER9, LYS10, LYS11, ASP30, ARG31, LYS116, SER121, TYR122, ASP157, GLY190, LYS191, GLU192, GLU205, LYS206, ASN207, ASP208, THR209, TRP210, ASP234, GLY235, GLU237, GLU238, SER239, HIS253, GLU258, ALA303, SER304, GLY305, GLU315, PRO341, SER343, ASN344 Chain B: TYR122, GLY235, GLU237, GLU238, SER239, GLU258, ALA303, SER304, GLY305, GLU315, SER343 |
| prM | HIS82, HIS83, LYS84, LYS85, GLY86, GLU87, ALA88, ARG89, ARG90, SER91, ARG92, ARG93, ALA94, VAL95, THR96, LEU97, PRO98, SER99, HIS100, SER101, THR102, ARG103, LYS104, LEU105, GLN106, THR107, ARG108, SER109, GLN110, THR111, TRP112, LEU113, GLU114, SER115, ARG116, GLU117, TYR118, THR119, LYS120, HIS121 |
| M | ALA1, THR 3, LEU4, PRO5, SER6, HIS7, SER8, THR9, ARG10, LYS11, LEU12, GLN13, THR14, ARG15, SER16, GLN17, THR18, TRP19, LEU20, GLU21, SER22, ARG23, GLU24, TYR25, THR26, LYS27 |
| NS2B | ASP50, MET51, TYR52, ILE53, GLU54, ARG55, ALA56, GLY57, ASP58, ILE59, THR60, TRP61, GLU62, LYS63, ASP64, ALA65, GLU66, VAL67, THR68, GLY69, ASN70, SER71, PRO72, ARG73, GLU80, GLY82 |
| NS3-P | ARG13, ARG14, LEU15, LEU16, GLY17, GLY46, GLU47, ASP51, LYS104, ASP105, GLU156 |
| NS3-H | ARG147, ASP148, ASP152, SER153, ASN154, SER155, PRO156, ILE157, MET158, ASP159, THR160, SER180, GLY181, LYS182, LYS223, HIS224, GLN225, GLU226, LYS243, ASP245, GLY285, ARG286, ASN287, PRO288, ASN289, LYS290, PRO291, GLY292, ASP293, TRP405, HIS408, GLY409, GLU410, LYS411 |
| NS5 | ALA10, ASN13, GLN14, MET15, SER16, ALA17, LEU18, GLU19, PHE20, TYR21, SER22, TYR23, LYS24, LYS25, SER26, GLY27, GLY103, PRO104, GLY105, GLN234, LEU237, GLY238, MET240, ASP241, GLY242, PRO243, ARG244, ARG245, PRO246, VAL247, LYS248, TYR249, SER264, CYS265, ALA266, GLU267, ALA268, ASN270, MET271, LYS272, GLU279, ARG282, ALA286, GLU287, THR288, TRP289, PHE290, PHE291, GLU293, TYR297, ARG298, THR299, TRP300, ALA301, TYR302, GLY304, TYR306, GLU307, ALA308, PRO309, THR310, SER313, ALA314, ASP342, THR343, THR344, GLN348, VAL351, PHE352, LYS353, GLU354, LYS355, VAL356, ASP357, THR358, ARG359, VAL360, PRO361, ASP362, LYS384, HIS385, MET452, GLY453, GLN459, LYS466, GLY467, SER468, ARG469, ARG521, ILE522, PRO523, GLY525, ARG526, ALA533, GLY534, LEU578, LYS583, GLY584, LYS585, THR586, GLU625, GLU628, MET629, GLN630, TRP633, LEU634, ARG636, ARG637, GLU639, ASN643, GLN646, SER647, ASP669, ASP670, ARG671, HIS674, LYS687, ASP688, THR689, GLN690, GLU691, TRP692, LYS693, PRO694, THR696, ASP699, ASN700, HIS715, LEU716, LYS717, ASP718, GLY719, ARG720, SER721, ILE816, GLU817, GLU818, ASP820, MET822, GLU823, ASP824, LYS825, THR826, PRO827, THR829, GLY838, LYS839, ARG840, GLY850, ARG852 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasasty, V.D.; Grazzolie, K.; Rosmalena, R.; Yazid, F.; Ivan, F.X.; Sinaga, E. Peptide-Based Subunit Vaccine Design of T- and B-Cells Multi-Epitopes against Zika Virus Using Immunoinformatics Approaches. Microorganisms 2019, 7, 226. https://doi.org/10.3390/microorganisms7080226
Prasasty VD, Grazzolie K, Rosmalena R, Yazid F, Ivan FX, Sinaga E. Peptide-Based Subunit Vaccine Design of T- and B-Cells Multi-Epitopes against Zika Virus Using Immunoinformatics Approaches. Microorganisms. 2019; 7(8):226. https://doi.org/10.3390/microorganisms7080226
Chicago/Turabian StylePrasasty, Vivitri Dewi, Karel Grazzolie, Rosmalena Rosmalena, Fatmawaty Yazid, Fransiskus Xaverius Ivan, and Ernawati Sinaga. 2019. "Peptide-Based Subunit Vaccine Design of T- and B-Cells Multi-Epitopes against Zika Virus Using Immunoinformatics Approaches" Microorganisms 7, no. 8: 226. https://doi.org/10.3390/microorganisms7080226
APA StylePrasasty, V. D., Grazzolie, K., Rosmalena, R., Yazid, F., Ivan, F. X., & Sinaga, E. (2019). Peptide-Based Subunit Vaccine Design of T- and B-Cells Multi-Epitopes against Zika Virus Using Immunoinformatics Approaches. Microorganisms, 7(8), 226. https://doi.org/10.3390/microorganisms7080226






