Metabolite Profiling: A Tool for the Biochemical Characterisation of Mycobacterium sp.
Abstract
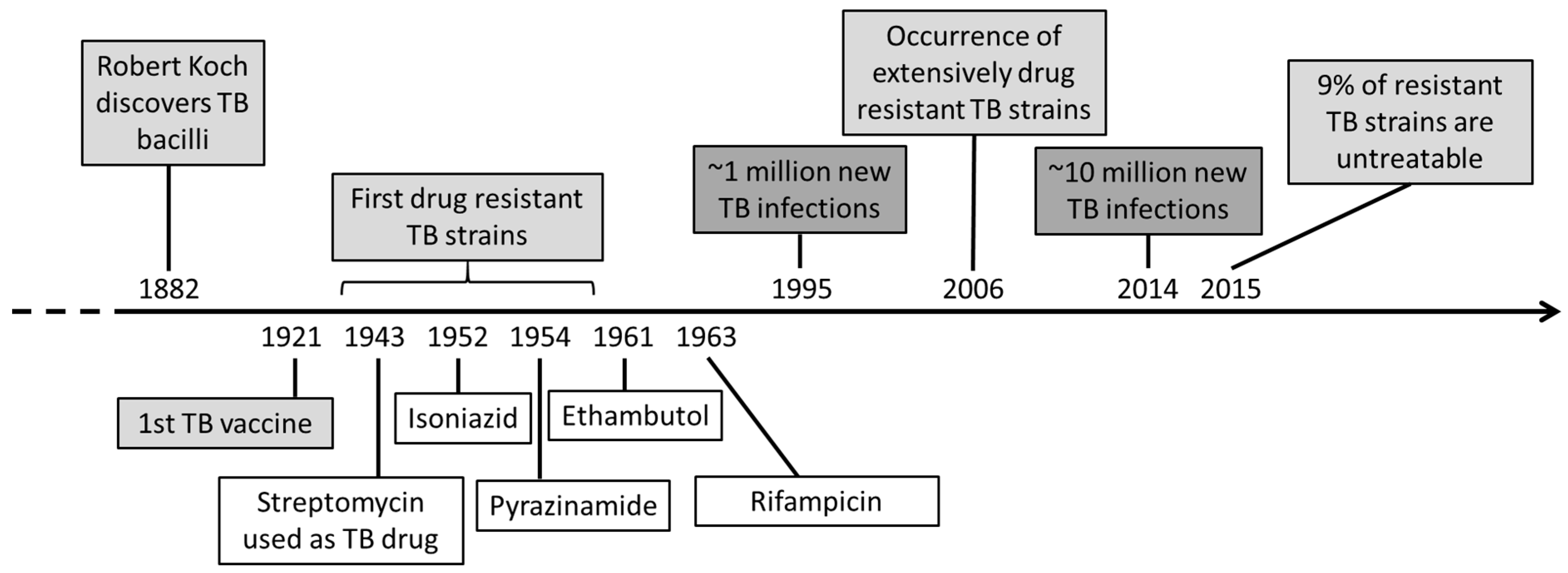
1. Introduction
2. Metabolite Profiling a “New” Approach for Drug Discovery
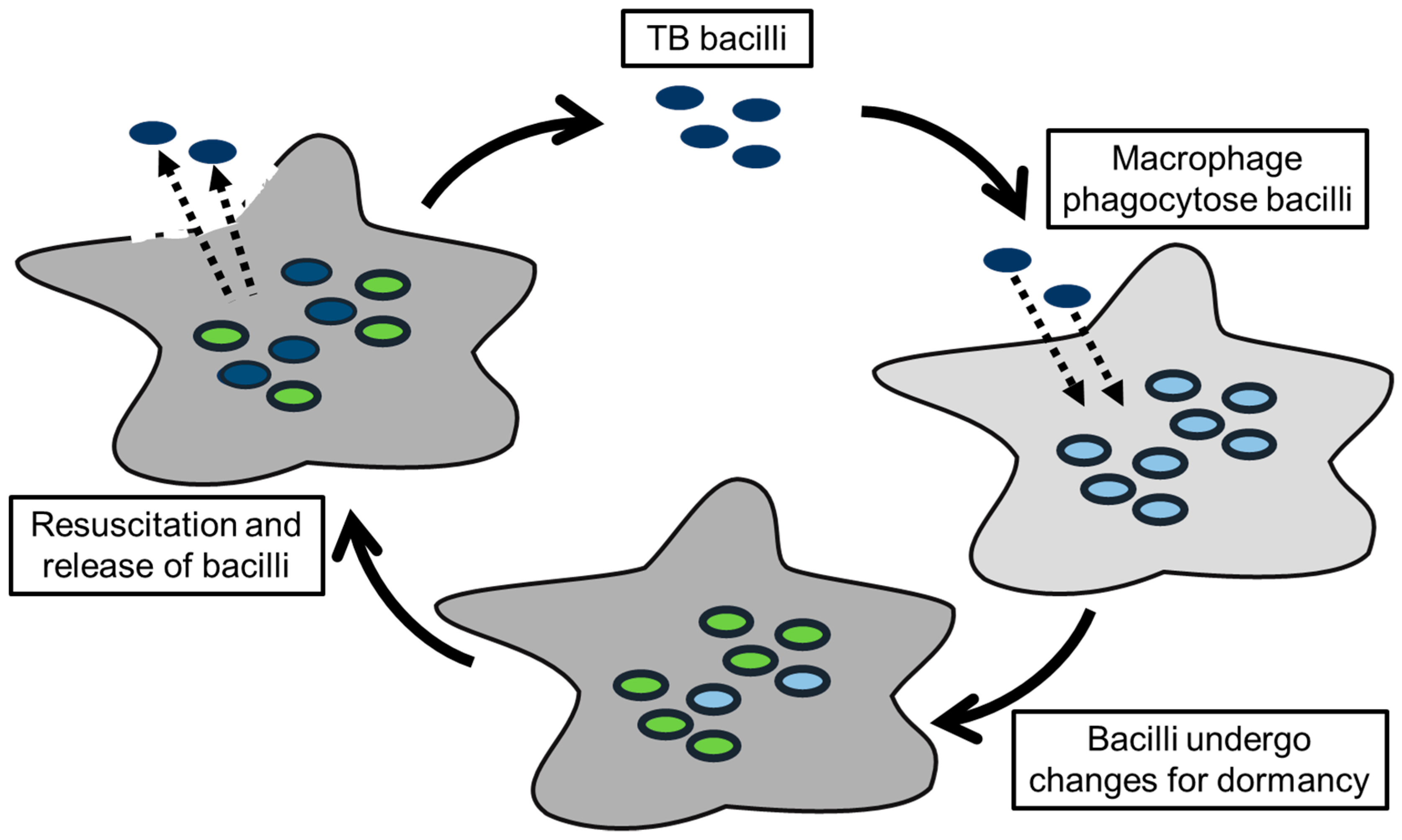
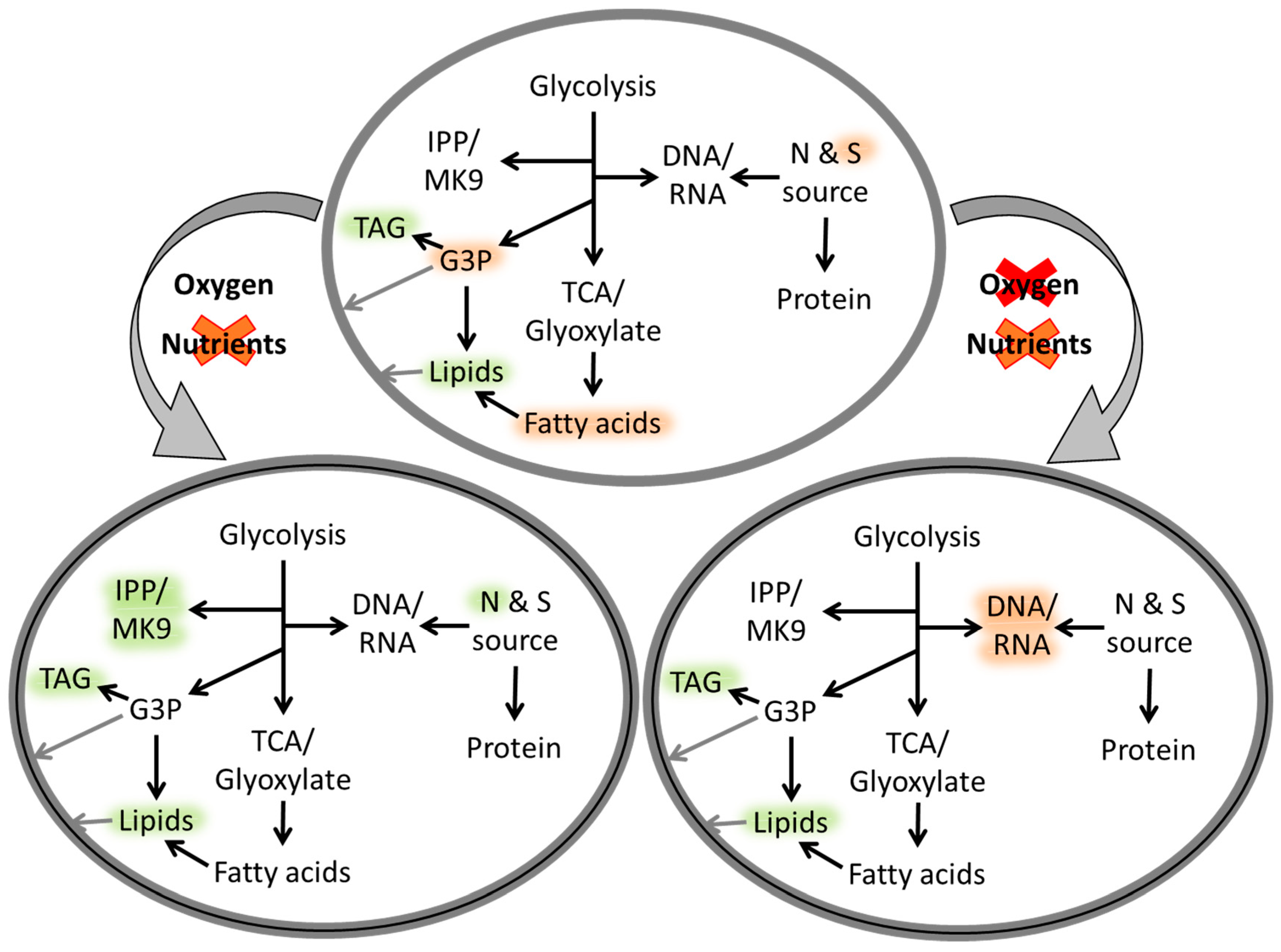
2.1. Understanding Mtb Properties through Metabolite Studies
2.2. New Drug Targets and Understanding Drug Resistance Based on Metabolic Studies
3. Advantages and Disadvantages of Metabolite Profiling Studies
3.1. Model Organisms for Mtb
3.2. Heterogeneous Cultures
3.3. In Vitro Versus in Vivo Studies
3.4. Limitation to Metabolite Profiling
4. Conclusions
Funding
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2018; WHO Press: Geneva, Switzerland, 2018; p. 1. [Google Scholar]
- Luca, S.; Mihaescu, T. History of BCG Vaccine. Mædica 2013, 8, 53–55. [Google Scholar]
- Lugosi, L. Theoretical and methodological aspects of BCG vaccine from the discovery of Calmette and Guérin to molecular biology. A review. Tuber. Lung Dis. 1992, 73, 252–261. [Google Scholar] [CrossRef]
- Brosch, R.; Pym, A.S.; Gordon, S.V.; Cole, S.T. The evolution of mycobacterial pathogenicity: Clues from comparative genomics. Trends Microbiol. 2001, 9, 452–458. [Google Scholar] [CrossRef]
- Russell, D.G.; Cardona, P.J.; Kim, M.J.; Allain, S.; Altare, F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat. Immunol. 2009, 10, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Anuchin, A.M.; Mulyukin, A.L.; Suzina, N.E.; Duda, V.I.; El-Registan, G.I.; Kaprelyants, A.S. Dormant forms of Mycobacterium smegmatis with distinct morphology. Microbiology 2009, 155, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.C.; Rubin, E.J. Letting sleeping dos lie: Does dormancy play a role in tuberculosis? Annu. Rev. Microbiol. 2010, 64, 293–311. [Google Scholar] [CrossRef]
- Gago, G.; Diacovich, L.; Arabolaza, A.; Tsai, S.C.; Gramajo, H. Fatty acid biosynthesis in actinomycetes. FEMS Microbiol. Rev. 2011, 35, 475–497. [Google Scholar] [CrossRef]
- Karakousis, P.C.; Williams, E.P.; Bishai, W.R. Altered expression of isoniazid-regulated genes in drug-treated dormant Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2008, 61, 323–331. [Google Scholar] [CrossRef]
- Niederweis, M. Nutrient acquisition by mycobacteria. Microbiology 2008, 154, 679–692. [Google Scholar] [CrossRef]
- WHO. Treatment of Tuberculosis Guidelines, 4th ed.; WHO Press: Geneva, Switzerland, 2010; p. 83. [Google Scholar]
- Da Silva, P.E.A.; Palomino, J.C. Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: Classical and new drugs. J. Antimicrob. Chemother. 2011, 66, 1417–1430. [Google Scholar] [CrossRef]
- Jamshidi, N.; Palsson, B. Investigating the metabolic capabilities of Mycobacterium tuberculosis H37Rv using the in silico strain iNJ661 and proposing alternative drug targets. BMC Syst. Biol. 2007, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Beste, D.J.; Bonde, B.; Hawkins, N.; Ward, J.L.; Beale, M.H.; Noack, S.; Nöh, K.; Kruger, N.J.; Ratcliffe, R.G.; McFadden, J. 13C metabolic flux analysis identifies an unusual route for pyruvate dissimilation in mycobacteria which requires isocitrate lyase and carbon dioxide fixation. PLoS Pathog. 2011, 7, e1002091. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, L.P.S.; Fischer, S.M.; Marrero, J.; Nathan, C.; Ehrt, S.; Rhee, K.Y. Metabolomics of Mycobacterium tuberculosis Reveals Compartmentalized Co-Catabolism of Carbon Substrates. Chem. Biol. 2010, 17, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Boshoff, H.I.; Lun, D.S. Systems biology approaches to understanding mycobacterial survival mechanisms. Drug Discov. Today Dis. Mech. 2010, 7, e75–e82. [Google Scholar] [CrossRef][Green Version]
- Wheeler, P.R.; Coldham, N.G.; Keating, L.; Gordon, S.V.; Wooff, E.E.; Parish, T.; Hewinson, R.G. Functional demonstration of reverse transsulfuration in the Mycobacterium tuberculosis complex reveals that methionine is the preferred sulfur source for pathogenic Mycobacteria. J. Biol. Chem. 2005, 280, 8069–8078. [Google Scholar] [CrossRef]
- Sareen, D.; Newton, G.L.; Fahey, R.C.; Buchmeier, N.A. Mycothiol is essential for growth of Mycobacterium tuberculosis Erdman. J. Bacteriol. 2003, 185, 6736–6740. [Google Scholar] [CrossRef] [PubMed]
- Beste, D.J.V.; Hooper, T.; Stewart, G.; Bonde, B.; Avignone-Rossa, C.; Bushell, M.; Wheeler, P.; Klamt, S.; Kierzek, A.M.; McFadden, J. GSMN-TB: A web-based genome scale network model of Mycobacterium tuberculosis metabolism. Genome Biol. 2007, 8, R89. [Google Scholar] [CrossRef]
- Harrigan, G.G.; Goodacre, R. Introduction. In Metabolic Profiling—Its Role in Biomarker Discovery and Gene Function Analysis; Harrigan, G.G., Goodacre, R., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2003; pp. 1–9. [Google Scholar]
- Halket, J.M.; Waterman, D.; Przyborowska, A.M.; Patel, R.K.; Fraser, P.D.; Bramley, P.M. Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. J. Exp. Bot. 2005, 56, 219–243. [Google Scholar] [CrossRef]
- Layre, E.; Sweet, L.; Hong, S.; Madigan, C.A.; Desjardins, D.; Young, D.C.; Cheng, T.Y.; Annand, J.W.; Kim, K.; Shamputa, I.C.; et al. A Comparative Lipidomics Platform for Chemotaxonomic Analysis of Mycobacterium tuberculosis. Chem. Biol. 2011, 18, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Sartain, M.J.; Dick, D.L.; Rithner, C.D.; Crick, D.C.; Belisle, J.T. Lipidomic analyses of Mycobacterium tuberculosis based on accurate mass measurements and the novel “Mtb LipidDB”. J. Lipid Res. 2011, 52, 861–872. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Lee, K.-C.; Curreem, S.O.T.; Chow, W.-N.; To, K.K.W.; Hung, I.F.N.; Ho, D.T.Y.; Sridhar, S.; Li, I.W.S.; Ding, V.S.Y.; et al. Metabolomic Profiling of Plasma from Patients with Tuberculosis by Use of Untargeted Mass Spectrometry Reveals Novel Biomarkers for Diagnosis. J. Clin. Microbiol. 2015, 53, 3750. [Google Scholar] [CrossRef] [PubMed]
- Olivier, I.; Loots, D.T. A metabolomics approach to characterise and identify various Mycobacterium species. J. Microbiol. Methods 2012, 88, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.J.; Shui, W.; Myers, S.; Feng, X.; Bertozzi, C.; Keasling, J.D. Central metabolism in Mycobacterium smegmatis during the transition from O(2)-rich to O(2)-poor conditions as studied by isotopomer-assisted metabolite analysis. Biotechnol. Lett. 2009, 31, 1233–1240. [Google Scholar]
- Cunningham, A.F.; Spreadbury, C.L. Mycobacterial stationary phase induced by low oxygen tension: Cell wall thickening and localization of the 16-kilodalton alpha-crystallin homolog. J. Bacteriol. 1998, 180, 801–808. [Google Scholar]
- Sassetti, C.M.; Rubin, E.J. Genetic requirements for mycobacterial survival during infection. Proc. Natl. Acad. Sci. USA 2003, 100, 12989–12994. [Google Scholar]
- Esmail, H.; Barry, C.E., 3rd; Wilkinson, R.J. Understanding latent tuberculosis: The key to improved diagnostic and novel treatment strategies. Drug Discov. Today 2012, 17, 514–521. [Google Scholar]
- Crick, D.C.; Schulbach, M.C.; Zink, E.E.; Macchia, M.; Barontini, S.; Besra, G.S.; Brennan, P.J. Polyprenyl phosphate biosynthesis in Mycobacterium tuberculosis and Mycobacterium smegmatis. J. Bacteriol. 2000, 182, 5771–5778. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Kruthiventi, A.K.; Prasad, J.V.; Kumar, S.P.; Srinu, G.; Chatterji, D. Inhibition of mycobacterial growth by plumbagin derivatives. Chem. Biol. Drug Des. 2010, 76, 34–42. [Google Scholar] [CrossRef]
- Provvedi, R.; Kocíncová, D.; Donà, V.; Euphrasie, D.; Daffé, M.; Etienne, G.; Manganelli, R.; Reyrat, J.M. SigF controls carotenoid pigment production and affects transformation efficiency and hydrogen peroxide sensitivity in Mycobacterium smegmatis. J. Bacteriol. 2008, 190, 7859–7863. [Google Scholar]
- Besra, G.S.; Sievert, T.; Lee, R.E.; Slayden, R.A.; Brennan, P.J.; Takayama, K. Identification of the apparent carrier in mycolic acid synthesis. Proc. Natl. Acad. Sci. USA 1994, 91, 12735. [Google Scholar]
- Prach, L.; Kirby, J.; Keasling, J.D.; Alber, T. Diterpene production in Mycobacterium tuberculosis. FEBS J. 2010, 277, 3588–3595. [Google Scholar] [PubMed]
- Mann, F.M.; Xu, M.; Davenport, E.K.; Peters, R.J. Functional characterization and evolution of the isotuberculosinol operon in Mycobacterium tuberculosis and related Mycobacteria. Front. Microbiol. 2012, 3, 368. [Google Scholar] [PubMed]
- Dhiman, R.K.; Mahapatra, S.; Slayden, R.A.; Boyne, M.E.; Lenaerts, A.; Hinshaw, J.C.; Angala, S.K.; Chatterjee, D.; Biswas, K.; Narayanasamy, P.; et al. Menaquinone synthesis is critical for maintaining mycobacterial viability during exponential growth and recovery from non-replicating persistence. Mol. Microbiol. 2009, 72, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Viveiros, M.; Krubasik, P.; Sandmann, G.; Houssaini-Iraqui, M. Structural and functional analysis of the gene cluster encoding carotenoid biosynthesis in Mycobacterium aurum A+. FEMS Microbiol. Lett. 2000, 187, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chang, W.-C.; Xiao, Y.; Liu, H.-W.; Liu, P. Methylerythritol Phosphate Pathway of Isoprenoid Biosynthesis. Annu. Rev. Biochem. 2013, 82, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Wayne, L.G.; Sohaskey, C.D. Nonreplicating persistence of mycobacterium tuberculosis. Annu. Rev. Microbiol. 2001, 55, 139–163. [Google Scholar] [PubMed]
- Hampshire, T.; Soneji, S.; Bacon, J.; James, B.W.; Hinds, J.; Laing, K.; Stabler, R.A.; Marsh, P.D.; Butcher, P.D. Stationary phase gene expression of Mycobacterium tuberculosis following a progressive nutrient depletion: A model for persistent organisms? Tuberculosis 2004, 84, 228–238. [Google Scholar] [CrossRef]
- Sirakova, T.D.; Dubey, V.S.; Deb, C.; Daniel, J.; Korotkova, T.A.; Abomoelak, B.; Kolattukudy, P.E. Identification of a diacylglycerol acyltransferase gene involved in accumulation of triacylglycerol in Mycobacterium tuberculosis under stress. Microbiology 2006, 152, 2717–2725. [Google Scholar] [CrossRef]
- Rhee, K.Y.; de Carvalho, L.P.S.; Bryk, R.; Ehrt, S.; Marrero, J.; Park, S.W.; Schnappinger, D.; Venugopal, A.; Nathan, C. Central carbon metabolism in Mycobacterium tuberculosis: An unexpected frontier. Trends Microbiol. 2011, 19, 307–314. [Google Scholar]
- Eoh, H.; Rhee, K.Y. Methylcitrate cycle defines the bactericidal essentiality of isocitrate lyase for survival of Mycobacterium tuberculosis on fatty acids. Proc. Natl. Acad. Sci. USA 2014, 111, 4976–4981. [Google Scholar] [CrossRef]
- Wayne, L.G.; Hayes, L.G. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 1996, 64, 2062–2069. [Google Scholar]
- Drapal, M.; Wheeler, P.R.; Fraser, P.D. Metabolite analysis of Mycobacterium species under aerobic and hypoxic conditions reveals common metabolic traits. Microbiology 2016, 162, 1456–1467. [Google Scholar] [CrossRef]
- Eoh, H.; Rhee, K.Y. Multifunctional essentiality of succinate metabolism in adaptation to hypoxia in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2013, 110, 6554–6559. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Chandra, H.; Bhatnagar, R. Poly-L-glutamate/glutamine synthesis in the cell wall of Mycobacterium bovis is regulated in response to nitrogen availability. BMC Microbiol. 2013, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Halouska, S.; Chacon, O.; Fenton, R.J.; Zinniel, D.K.; Barletta, R.G.; Powers, R. Use of NMR metabolomics to analyze the targets of D-cycloserine in mycobacteria: Role of D-alanine racemase. J. Proteome Res. 2007, 6, 4608–4614. [Google Scholar] [CrossRef]
- Wayne, L.G.; Sramek, H.A. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 1994, 38, 2054–2058. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Arora, G.; Sajid, A.; Maji, A.; Bhat, A.; Virmani, R.; Upadhyay, S.; Nandicoori, V.K.; Sengupta, S.; Singh, Y. Regulation of homocysteine metabolism by Mycobacterium tuberculosis S-adenosylhomocysteine hydrolase. Sci. Rep. 2013, 3, 2264. [Google Scholar] [CrossRef]
- Banks, E.C.; Doughty, S.W.; Toms, S.M.; Wheelhouse, R.T.; Nicolaou, A. Inhibition of cobalamin-dependent methionine synthase by substituted benzo-fused heterocycles. FEBS J. 2007, 274, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R.K.; Schaeffer, M.L.; Bailey, A.M.; Testa, C.A.; Scherman, H.; Crick, D.C. 1-Deoxy-D-xylulose 5-phosphate reductoisomerase (IspC) from Mycobacterium tuberculosis: Towards understanding mycobacterial resistance to fosmidomycin. J. Bacteriol. 2005, 187, 8395–8402. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, P.J.; Carlson, B.L.; Siridechadilok, B.; Pratt, M.R.; Senaratne, R.H.; Mougous, J.D.; Riley, L.W.; Williams, S.J.; Bertozzi, C.R. Trehalose is required for growth of Mycobacterium smegmatis. J. Biol. Chem. 2004, 279, 28835–28843. [Google Scholar] [CrossRef]
- Balganesh, M.; Dinesh, N.; Sharma, S.; Kuruppath, S.; Nair, A.V.; Sharma, U. Efflux pumps of Mycobacterium tuberculosis play a significant role in antituberculosis activity of potential drug candidates. Antimicrob. Agents Chemother. 2012, 56, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Drapal, M.; Wheeler, P.R.; Fraser, P.D. The assessment of changes to the nontuberculous mycobacterial metabolome in response to anti-TB drugs. FEMS Microbiol. Lett. 2018, 365, fny153. [Google Scholar] [CrossRef]
- Cloete, R.; Oppon, E.; Murungi, E.; Schubert, W.-D.; Christoffels, A. Resistance related metabolic pathways for drug target identification in Mycobacterium tuberculosis. BMC Bioinform. 2016, 17, 75. [Google Scholar] [CrossRef]
- Derewacz, D.K.; Goodwin, C.R.; McNees, C.R.; McLean, J.A.; Bachmann, B.O. Antimicrobial drug resistance affects broad changes in metabolomic phenotype in addition to secondary metabolism. Proc. Natl. Acad. Sci. USA 2013, 110, 2336. [Google Scholar] [CrossRef]
- Nguyen, L. Antibiotic resistance mechanisms in M. tuberculosis: An update. Arch. Toxicol. 2016, 90, 1585–1604. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, J.S.; Sharma, D. Mycobacterium smegmatis and tuberculosis. Trends Microbiol. 2002, 10, 68–70. [Google Scholar] [CrossRef]
- Barry, I.I.I.C.E. Mycobacterium smegmatis: An absurd model for tuberculosis? Trends Microbiol. 2001, 9, 473–474. [Google Scholar] [CrossRef]
- Reyrat, J.M.; Kahn, D. Mycobacterium smegmatis: An absurd model for tuberculosis? Trends Microbiol. 2001, 9, 472–474. [Google Scholar] [CrossRef]
- Gadagkar, R.; Gopinathan, K. Growth of mycobacterium smegmatis in minimal and complete media. J. Biosci. 1980, 2, 337–348. [Google Scholar] [CrossRef]
- Scherzinger, D.; Scheffer, E.; Bär, C.; Ernst, H.; Al-Babili, S. The Mycobacterium tuberculosis ORF Rv0654 encodes a carotenoid oxygenase mediating central and excentric cleavage of conventional and aromatic carotenoids. FEBS J. 2010, 277, 4662–4673. [Google Scholar] [CrossRef]
- Smeulders, M.J.; Keer, J.; Speight, R.A.; Williams, H.D. Adaptation of Mycobacterium smegmatis to stationary phase. J. Bacteriol. 1999, 181, 270–283. [Google Scholar]
- Beste, D.J.; Peters, J.; Hooper, T.; Avignone-Rossa, C.; Bushell, M.E.; McFadden, J. Compiling a molecular inventory for Mycobacterium bovis BCG at two growth rates: Evidence for growth rate-mediated regulation of ribosome biosynthesis and lipid metabolism. J. Bacteriol. 2005, 187, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- McKinney, J.D.; Honer zu Bentrup, K.; Munoz-Elias, E.J.; Miczak, A.; Chen, B.; Chan, W.T.; Swenson, D.; Sacchettini, J.C.; Jacobs, W.R., Jr.; Russell, D.G. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 2000, 406, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.G.; VanderVen, B.C.; Lee, W.; Abramovitch, R.B.; Kim, M.J.; Homolka, S.; Niemann, S.; Rohde, K.H. Mycobacterium tuberculosis wears what it eats. Cell Host Microbe 2010, 8, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, J.C.; du Preez, I.; Loots du, T. A comparison of four sputum pre-extraction preparation methods for identifying and characterising Mycobacterium tuberculosis using GCxGC-TOFMS metabolomics. J. Microbiol. Methods 2012, 91, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Lorian, V. Differences between in vitro and in vivo studies. Antimicrob. Agents Chemother. 1988, 32, 1600–1601. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Schito, M. Tuberculosis Diagnostics in 2015: Landscape, Priorities, Needs, and Prospects. J. Infect. Dis. 2015, 211 (suppl_2), S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, W.; Lang, Y.; Qu, Y.; Chu, F.; Chen, J.; Cui, L. Mass spectrometry-based metabolomics for tuberculosis meningitis. Clin. Chim. Acta 2018, 483, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Banday, K.M.; Pasikanti, K.K.; Chan, E.C.Y.; Singla, R.; Rao, K.V.S.; Chauhan, V.S.; Nanda, R.K. Use of Urine Volatile Organic Compounds To Discriminate Tuberculosis Patients from Healthy Subjects. Anal. Chem. 2011, 83, 5526–5534. [Google Scholar] [CrossRef]
- Weiner, J.; Maertzdorf, J.; Sutherland, J.S.; Duffy, F.J.; Thompson, E.; Suliman, S.; McEwen, G.; Thiel, B.; Parida, S.K.; Zyla, J.; et al. Metabolite changes in blood predict the onset of tuberculosis. Nat. Commun. 2018, 9, 5208. [Google Scholar] [CrossRef]
- Collins, J.M.; Walker, D.I.; Jones, D.P.; Tukvadze, N.; Liu, K.H.; Tran, V.T.; Uppal, K.; Frediani, J.K.; Easley, K.A.; Shenvi, N.; et al. High-resolution plasma metabolomics analysis to detect Mycobacterium tuberculosis-associated metabolites that distinguish active pulmonary tuberculosis in humans. PLoS ONE 2018, 13, e0205398. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, N.; Sauer, U. Novel biological insights through metabolomics and 13C-flux analysis. Curr. Opin. Microbiol. 2009, 12, 553–558. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drapal, M.; Fraser, P.D. Metabolite Profiling: A Tool for the Biochemical Characterisation of Mycobacterium sp. Microorganisms 2019, 7, 148. https://doi.org/10.3390/microorganisms7050148
Drapal M, Fraser PD. Metabolite Profiling: A Tool for the Biochemical Characterisation of Mycobacterium sp. Microorganisms. 2019; 7(5):148. https://doi.org/10.3390/microorganisms7050148
Chicago/Turabian StyleDrapal, Margit, and Paul D. Fraser. 2019. "Metabolite Profiling: A Tool for the Biochemical Characterisation of Mycobacterium sp." Microorganisms 7, no. 5: 148. https://doi.org/10.3390/microorganisms7050148
APA StyleDrapal, M., & Fraser, P. D. (2019). Metabolite Profiling: A Tool for the Biochemical Characterisation of Mycobacterium sp. Microorganisms, 7(5), 148. https://doi.org/10.3390/microorganisms7050148




