Inhibitory Effect of 7-Demethoxytylophorine on Penicillium italicum and its Possible Mechanism
Abstract
:1. Introduction
2. Materials and Methods
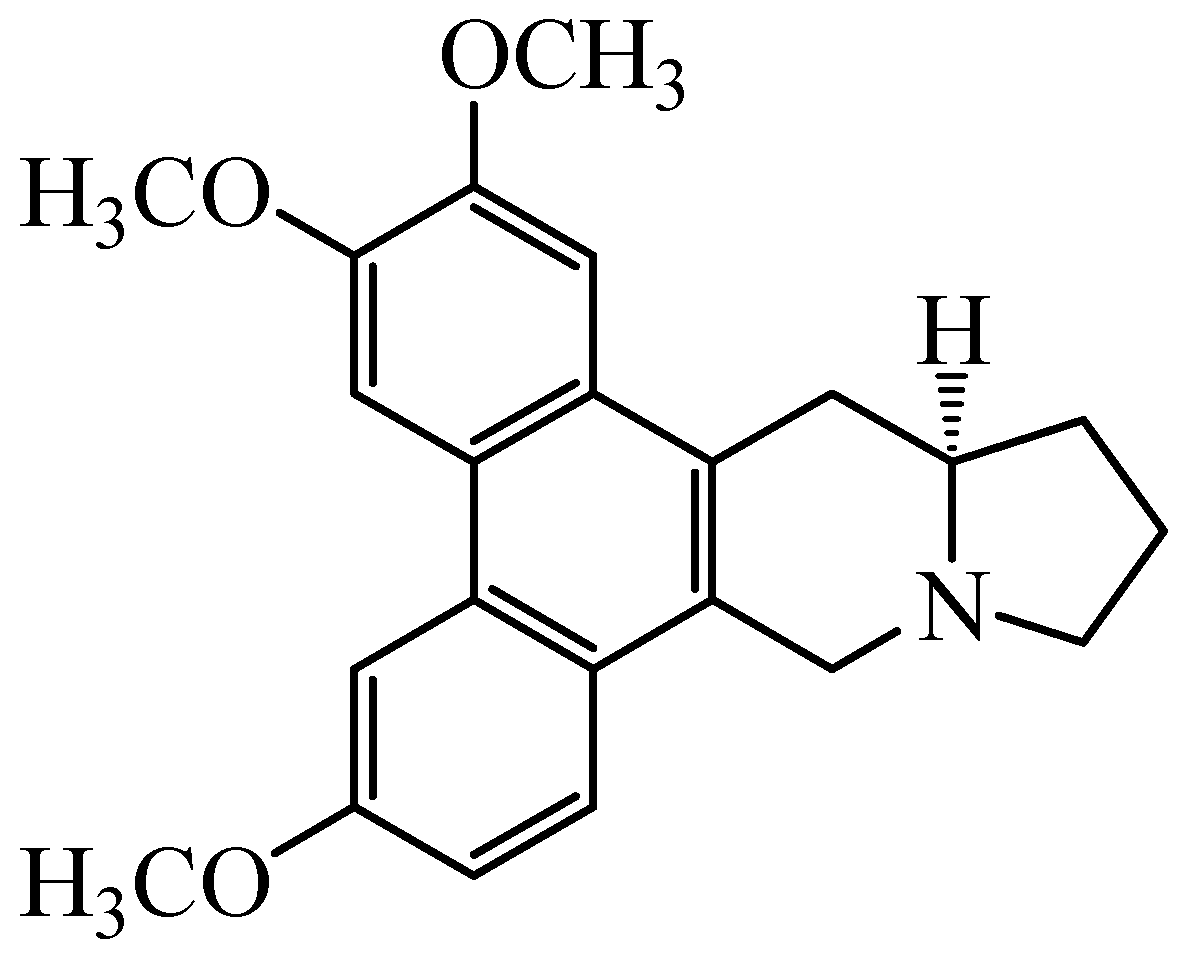
2.1. Chemicals
2.2. Fungal Pathogen and Medium
2.3. Antifungal Activity Assays
2.4. Determination of Membrane Permeability
2.5. Assay for Reducing Sugars and Soluble Proteins Content
2.6. Assay for the Release of Reducing Sugars and Soluble Proteins
2.7. Assay for Activities of MDH and SDH
2.8. Statistical Analysis
3. Results
3.1. Mycelial Growth and Spore Germination
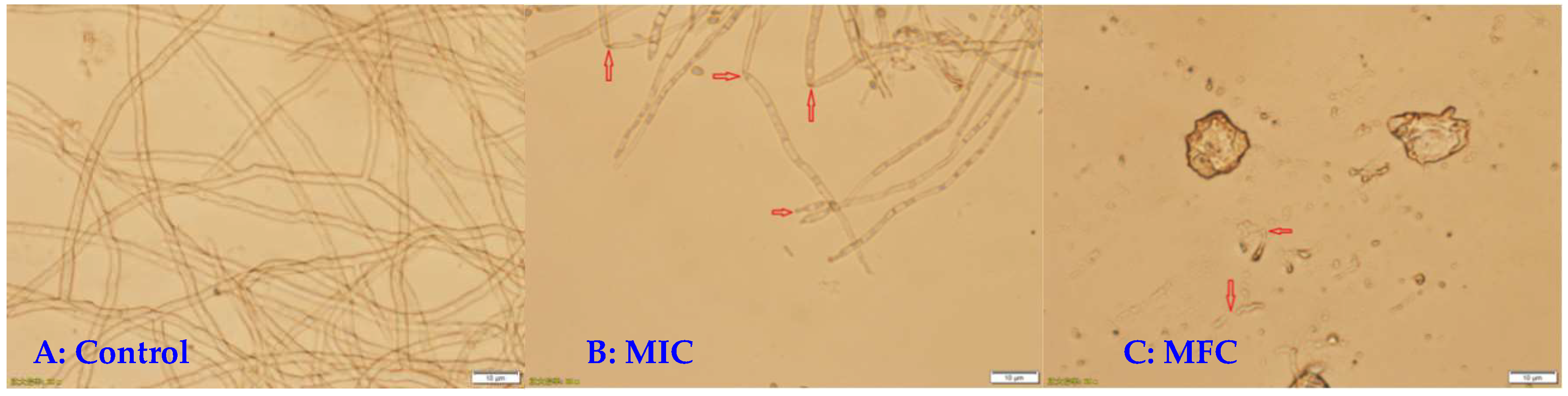
3.2. Light Microscopy
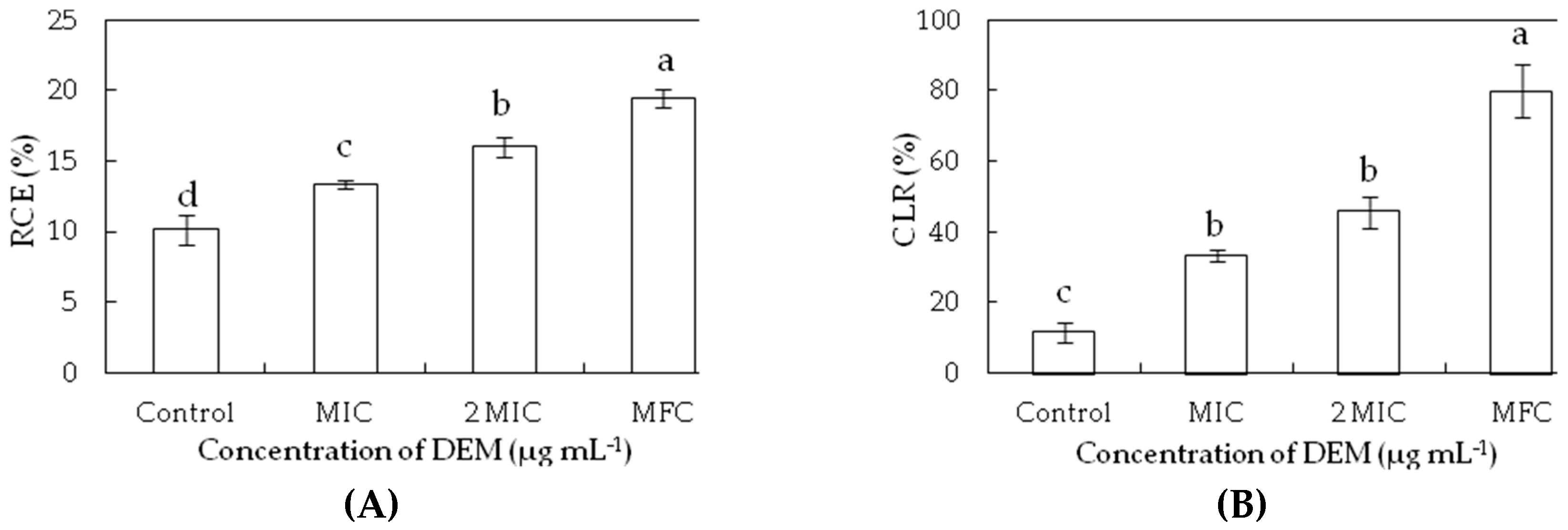
3.3. Membrane Permeability
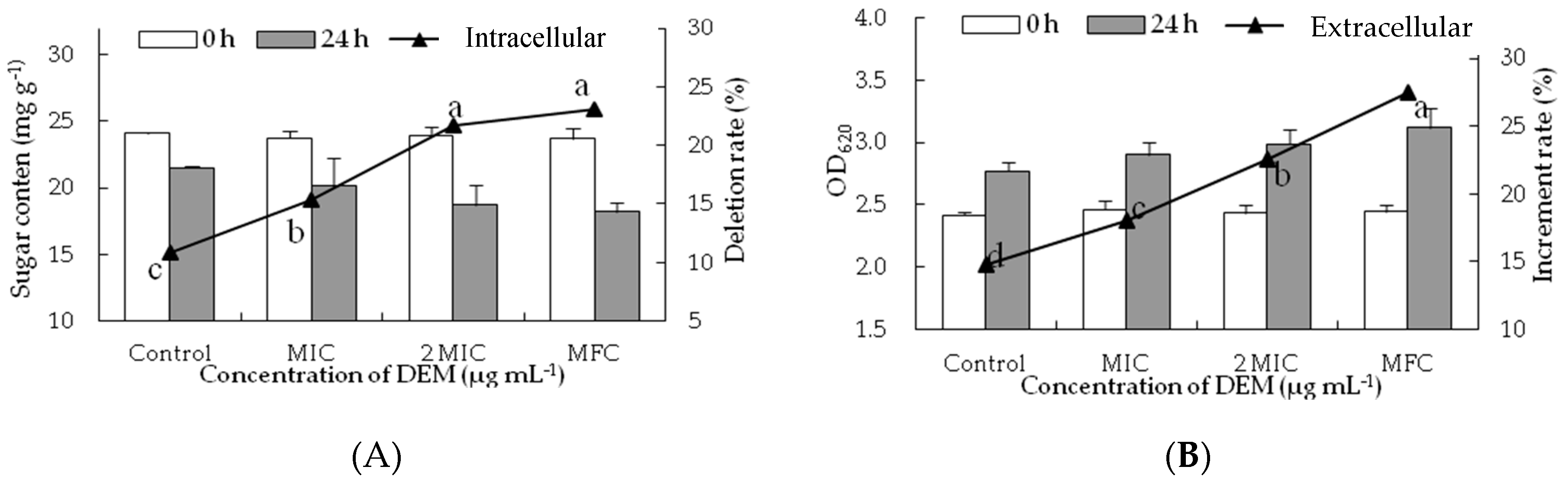
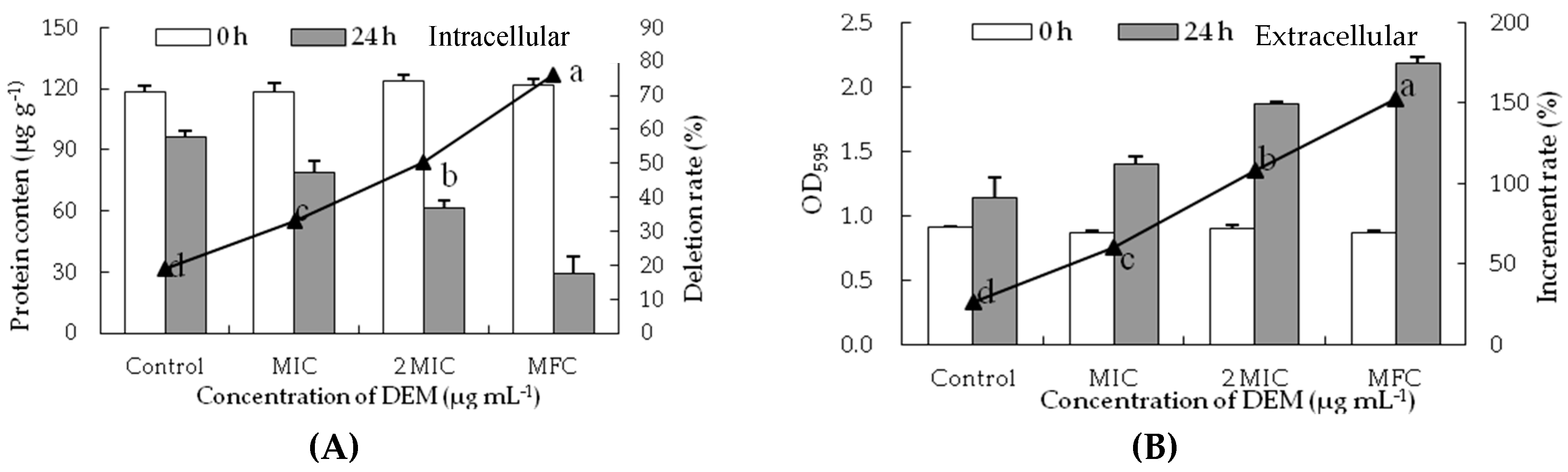
3.4. Intracellular and Extracellular of Cytoplasmic Constituents
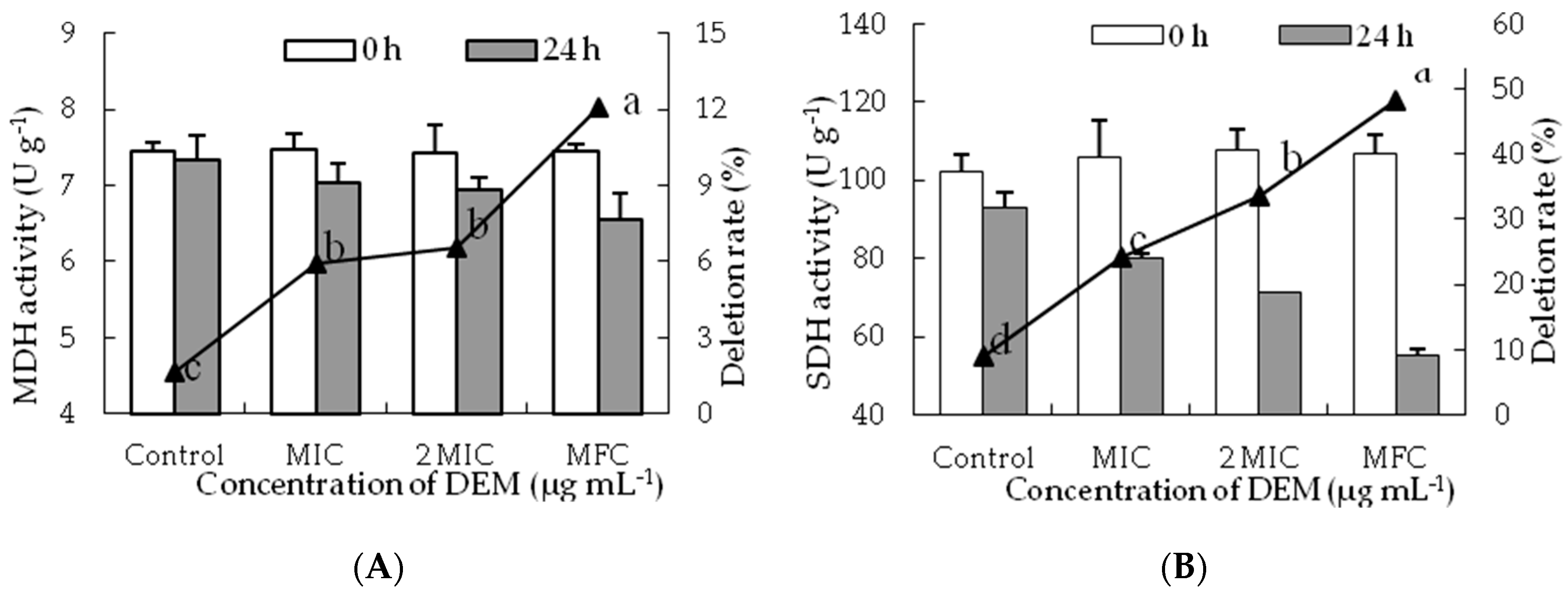
3.5. Activities of MDH and SDH
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, Y.Z.; Han, R.; Cao, Q.W.; Yu, J.H.; Mao, J.L.; Zhang, T.F.; Wang, S.Q.; Niu, Y.H.; Liu, D.L. Pharmacophore-based virtual screening of novel inhibitors and docking analysis for CYP51A from Penicillium italicum. Mar. Drugs 2017, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Talibi, I.; Boubaker, H.; Boudyach, E.H.; Ait Ben Aoumar, A. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Cruz Cabral, L.; Fernández Pinto, V.; Patriarca, A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Karim, H.; Boubaker, H.; Askarne, L.; Cherifi, K.; Lakhtar, H.; Msanda, F.; Boudyach, E.H.; Ait Ben Aoumar, A. Use of Cistus aqueous extracts as botanical fungicides in the control of citrus sour rot. Microb. Pathog. 2017, 104, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS, plant- and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol. Technol. 2016, 122, 41–52. [Google Scholar] [CrossRef]
- Tripathi, P.; Dubey, N.K. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Wan, C.P.; Li, P.; Chen, C.Y.; Peng, X.; Li, M.X.; Chen, M.; Wang, J.S.; Chen, J.Y. Antifungal activity of Ramulus cinnamomi explored by 1H-NMR based metabolomics approach. Molecules 2017, 22, 2237. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.; Jia, L.; Zhou, H.; He, X. Effect of octanal on the mycelial growth of Penicillium italicum and P. digitatum. World J. Microbiol. Biotechnol. 2014, 30, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.; OuYang, Q.; Jia, L. Citral inhibits mycelial growth of Penicillium italicum by a membrane damage mechanism. Food Control 2014, 41, 116–121. [Google Scholar] [CrossRef]
- Yang, S.Z.; Zhou, J.; Li, D.M.; Shang, C.Y.; Peng, L.T.; Pan, S.Y. The structure-antifungal activity relationship of 5,7-dihydroxyflavonoids against Penicillium italicum. Food Chem. 2017, 224, 26–31. [Google Scholar] [CrossRef]
- Peng, L.T.; Yang, S.Z.; Cheng, Y.J.; Chen, F.; Pan, S.Y.; Fan, G. Antifungal activity and action mode of pinocembrin from propolis against Penicillium italicum. Food Sci. Biotechnol. 2012, 21, 1533–1539. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, W.D.; Liu, R.H.; Hu, X.J.; Su, J.; Zheng, Z.G.; Zhang, W. Advances in studies on chemical constituents and pharmacology of Cynanchum atratum. J. Pharm. Pract. 2007, 25, 6–10. [Google Scholar]
- Chen, C.Y.; Peng, X.; Chen, J.Y.; Wan, C.P. Antifungal activity of Cynanchum atratum alkaloids against citrus postharvest blue mould. J. Fruit Sci. 2019, 36. in press. [Google Scholar] [CrossRef]
- You, Y.C.; Mi, H.K.; Lee, H.; Ahn, K.S.; Um, J.Y.; Lee, S.; Kim, J.; Yang, W.M. Cynanchum atratum inhibits the development of atopic dermatitis in 2,4-dinitrochlorobenzene-induced mice. Biomed. Pharmacoth. 2017, 90, 321–327. [Google Scholar] [CrossRef]
- Liu, H.W.; Xiong, Z.L.; Li, F.M.; Qu, G.X.; Kobayashi, H.; Yao, X.S. Two new pregnane glycosides from Dioscorea futschauensis R. Kunth. Chem. Pharm. Bull. 2003, 51, 1089–1091. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Chen, C.Y.; Chen, J.Y.; Wan, C.P. Antioxidant and antifungal activity of Cynanchum atratum Bunge extracts. Jiangsu Agric. Sci. 2017, 45, 140–144. [Google Scholar] [CrossRef]
- Fu, Y.W.; Zhang, Q.Z.; Xu, D.H.; Liang, J.H.; Bin, W. Antiparasitic effect of Cynatratoside-C from Cynanchum atratum against Ichthyophthirius multifiliis on grass carp. J. Agric. Food Chem. 2014, 62, 7183–7189. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.X.; Liu, K.X.; Huang, T.; Yan, C.; Huang, L.J.; Liu, S.; Mu, S.Z.; Hao, X.J. Seco-pregnane steroidal glycosides from the roots of Cynanchum atratum and their anti-TMV activity. Fitoterapia 2014, 97, 50–63. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Ding, M.L.; Tao, L.J.; Zhang, M.; Xu, X.H.; Zhang, C.F. Immunosuppressive C21 steroidal glycosides from the root of Cynanchum atratum. Fitoterapia 2015, 105, 194–201. [Google Scholar] [CrossRef]
- Jin, Q.H.; Han, X.H.; Yun, C.Y.; Lee, C.; Lee, J.W.; Lee, D.H.; Lee, M.K.; Jung, S.H.; Hong, J.T.; Kim, Y.; et al. Melanogenesis inhibitory pregnane glycosides from Cynanchum atratum. Bioorg. Med. Chem. Lett. 2018, 28, 1252–1256. [Google Scholar] [CrossRef]
- Peng, X.; Wan, C.P.; Chen, C.Y.; Chen, J.Y. Effects of the complex coating of chitosan with extract of Cynanchum atratum on cold storage of navel orange. J. Hunan Agric. Univ. 2017, 43, 26–30. [Google Scholar] [CrossRef]
- Zheng, J.P.; Gan, W.; Chen, Y.H.; Chen, C.Y.; Chen, J.Y. Control effects of Cynanchum atratum extracts on postharvest blue mold in Xinyu tangerine. Acta Agric. Univ. Jxsis 2017, 39, 1119–1125. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wan, C.P.; Peng, X.; Chen, Y.H.; Chen, M.; Chen, J.Y. Optimization of antifungal extracts from Ficus hirta fruits using response surface methodology and antifungal activity tests. Molecules 2015, 20, 19647–19659. [Google Scholar] [CrossRef] [PubMed]
- Talibi, I.; Askarne, L.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Ait Ben Aoumar, A. Antifungal activity of some Moroccan plants against Geotrichum candidum, the causal agent of postharvest citrus sour rot. Crop Prot. 2012, 35, 41–46. [Google Scholar] [CrossRef]
- Dou, S.W.; Liu, S.Q.; Xu, X.Y.; OuYang, Q.L.; Tao, N.G. Octanal inhibits spore germination of Penicillium digitatum involving membrane peroxidation. Protoplasma 2017, 254, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Jing, G.X.; Tao, N.G.; Jia, L.; Zhou, H.E. Influence of α-terpineol on the growth and morphogenesis of Penicillium digitatum. Bot. Stud. 2015, 56, 35–40. [Google Scholar] [CrossRef]
- Wu, Y.L.; Duan, X.F.; Jing, G.X.; OuYang, Q.L.; Tao, N.G. Cinnamaldehyde inhibits the mycelial growth of Geotrichum citri-aurantii and induces defense responses against sour rot in citrus fruit. Postharvest Biol. Technol. 2017, 129, 23–28. [Google Scholar] [CrossRef]
- Xu, L.C.; Tao, N.G.; Yang, W.H.; Jing, G.X. Cinnamaldehyde damaged the cell membrane of Alternaria alternata and induced the degradation of mycotoxins in vivo. Ind. Crop. Prod. 2018, 112, 427–433. [Google Scholar] [CrossRef]
- Zhou, H.E.; Tao, N.G.; Jia, L. Antifungal activity of citral, octanal and α-terpineol against Geotrichum citri-aurantii. Food Control 2014, 37, 277–283. [Google Scholar] [CrossRef]
- Zheng, S.J.; Jing, G.X.; Wang, X.; Ouyang, Q.L.; Jia, L.; Tao, N.G. Citral exerts its antifungal activity against Penicillium digitatum by affecting the mitochondrial morphology and function. Food Chem. 2015, 178, 76–81. [Google Scholar] [CrossRef]
- Wu, Y.L.; OuYang, Q.L.; Tao, N.G. Plasma membrane damage contributes to antifungal activity of citronellal against Penicillium digitatum. J. Food Sci. Technol. 2016, 53, 3853–3858. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.P.; Li, Y.C.; Li, H.Y.; Yu, T.; Zheng, X.D. Antifungal activity of thyme oil against Geotrichum citri-aurantii in vitro and in vivo. J. Appl. Microbiol. 2009, 107, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Shao, X.F.; Li, Y.H.; Wei, Y.Y.; Xu, F.; Wang, H.F. Metabolomic analysis and mode of action of metabolites of tea tree oil involved in the suppression of Botrytis cinerea. Front. Microbiol. 2017, 8, 1017–1027. [Google Scholar] [CrossRef]
- Scorzoni, L.; Sangalli-Leite, F.; de Lacorte Singulani, J.; de Paula e Silva, A.C.A.; Costa-Orlandi, C.B.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Searching new antifungals: The use of in vitro and in vivo methods for evaluation of natural compounds. J. Microbiol. Meth. 2016, 123, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Wu, M.; Liu, Z.H.; Su, B.; Li, L.; Liu, Y.X.; Huang, R.Q.; Liu, Y.; Wang, Q.M. Bioactivity of phenanthroindolizidine alkaloids and their salt derivatives. Chin. J. Pestic. Sci. 2010, 12, 507–510. [Google Scholar] [CrossRef]
- Yang, S.Z.; Liu, L.M.; Li, D.M.; Xia, H.; Su, X.J.; Peng, L.T.; Pan, S.Y. Use of active extracts of poplar buds against Penicillium italicum and possible modes of action. Food Chem. 2016, 196, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, G.M.; Cerioni, L.; González, M.M.; Cabrerizo, F.M.; Rapisarda, V.A.; Volentini, S.I. Antifungal activity of β-carbolines on Penicillium digitatum and Botrytis cinerea. Food Microbiol. 2017, 62, 9–14. [Google Scholar] [CrossRef]
- Morales, J.; Mendoza, L.; Cotoras, M. Alteration of oxidative phosphorylation as a possible mechanism of the antifungal action of p-coumaric acid against Botrytis cinerea. J. Appl. Microbiol. 2017, 123, 969–976. [Google Scholar] [CrossRef]
- Simionato, A.S.; Navarro, M.O.P.; de Jesus, M.L.A.; Barazetti, A.R.; da Silva, C.S.; Simões, G.C.; Balbi-Peña, M.I.; de Mello, J.C.P.; Panagio, L.A.; de Almeida, R.S.C.; et al. The effect of phenazine-1-carboxylic acid on mycelial growth of Botrytis cinerea produced by Pseudomonas aeruginosa LV strain. Front. Microbiol. 2017, 8, 1102–1110. [Google Scholar] [CrossRef]
- Xu, D.D.; Deng, Y.Z.; Han, T.Y.; Jiang, L.Q.; Xi, P.G.; Wang, Q.; Jiang, Z.D.; Gao, L.W. In vitro and in vivo effectiveness of phenolic compounds for the control of postharvest gray mold of table grapes. Postharvest Biol. Technol. 2018, 139, 106–114. [Google Scholar] [CrossRef]
- Zhang, M.L.; Xu, L.Y.; Zhang, L.Y.; Guo, Y.H.; Qi, X.; He, L. Effects of quercetin on postharvest blue mold control in kiwifruit. Sci. Hortic. 2018, 228, 18–25. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, K.W.; Yang, H.H.; Yuan, Y.H.; Yue, T.L. Antifungal mechanism of cinnamaldehyde and citral combination against Penicillium expansum based on FT-IR fingerprint, plasma membrane, oxidative stress and volatile profile. RSC Adv. 2018, 8, 5806–5815. [Google Scholar] [CrossRef]
- Gong, L.; Tan, H.B.; Chen, F.; Li, T.T.; Zhu, J.Y.; Jian, Q.J.; Yuan, D.B.; Xu, L.X.; Hu, W.Z.; Jiang, Y.M.; et al. Novel synthesized 2,4-DAPG analogues: Antifungal activity, mechanism and toxicology. Sci. Rep. 2016, 6, 32266–32274. [Google Scholar] [CrossRef] [PubMed]
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, K.W.; Yang, H.H.; Zhang, Z.W.; Yuan, Y.H.; Yue, T.L. Effect of cinnamaldehyde and citral combination on transcriptional profile, growth, oxidative damage and patulin biosynthesis of Penicillium expansum. Front. Microbiol. 2018, 9, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Shao, X.F.; Xu, J.Y.; Wei, Y.Y.; Xu, F.; Wang, H.F. Tea tree oil exhibits antifungal activity against Botrytis cinerea by affecting mitochondria. Food Chem. 2017, 234, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Sun, C.; Fu, D.; Yu, T. Test for l-glutamate inhibition of growth of Alternaria alternata by inducing resistance in tomato fruit. Food Chem. 2017, 230, 145–153. [Google Scholar] [CrossRef]
| Concentrations (µg mL−1) | Mycelial Growth 1 | Spore Germination 2 | ||
|---|---|---|---|---|
| Mycelial Diameter (mm) | MGI (%) | Germination Rate (%) | SGI (%) | |
| 0 | 55.50 ± 1.29 a | 0 e | 94.77 ± 2.63 a | 0 f |
| 0.78 | 38.25 ± 0.96 b | 30.08 ± 2.27 d | 71.68 ± 2.42 b | 23.36 ± 2.55 e |
| 1.56 | 24.00 ± 1.41 c | 56.77 ± 2.55 c | 49.55 ± 3.01 c | 47.72 ± 3.18 d |
| 3.13 | 9.75 ± 1.71 d | 82.43 ± 3.08 b | 26.16 ± 2.75 d | 72.40 ± 2.90 c |
| 6.25 | 0 e | 100 ± 0.00 a | 4.37 ± 0.18 e | 95.39 ± 0.19 b |
| 12.50 | 0 e | 100 ± 0.00 a | 0 f | 100 ± 0.00 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Qi, W.; Peng, X.; Chen, J.; Wan, C. Inhibitory Effect of 7-Demethoxytylophorine on Penicillium italicum and its Possible Mechanism. Microorganisms 2019, 7, 36. https://doi.org/10.3390/microorganisms7020036
Chen C, Qi W, Peng X, Chen J, Wan C. Inhibitory Effect of 7-Demethoxytylophorine on Penicillium italicum and its Possible Mechanism. Microorganisms. 2019; 7(2):36. https://doi.org/10.3390/microorganisms7020036
Chicago/Turabian StyleChen, Chuying, Wenwen Qi, Xuan Peng, Jinyin Chen, and Chunpeng Wan. 2019. "Inhibitory Effect of 7-Demethoxytylophorine on Penicillium italicum and its Possible Mechanism" Microorganisms 7, no. 2: 36. https://doi.org/10.3390/microorganisms7020036
APA StyleChen, C., Qi, W., Peng, X., Chen, J., & Wan, C. (2019). Inhibitory Effect of 7-Demethoxytylophorine on Penicillium italicum and its Possible Mechanism. Microorganisms, 7(2), 36. https://doi.org/10.3390/microorganisms7020036






