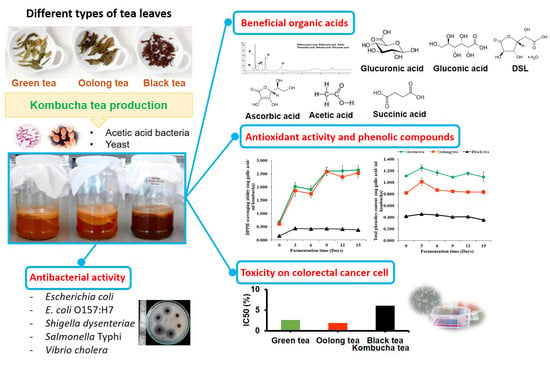
Efficacy of Kombucha Obtained from Green, Oolong, and Black Teas on Inhibition of Pathogenic Bacteria, Antioxidation, and Toxicity on Colorectal Cancer Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Starter Culture of Kombucha Tea
2.2. Preparation of Kombucha Tea Obtained from Camellia Sinensis Tea Leaves
2.3. pH and Total Acidity Determination
2.4. Total Soluble Solids (ºBrix) and Alcohol Content
2.5. Microbiological Determination
2.6. HPLC Analysis of Organic Acids
2.7. DPPH Radical Scavenging Assay of Kombucha Tea
2.8. Total Phenolic Content of Kombucha Tea
2.9. Pathogenic Enteric Bacteria Used in the Study
2.10. Antimicrobial Activity of Kombucha Tea
2.11. Cytotoxicity Test of Kombucha Tea on Human Colorectal Carcinoma (Caco-2) and NIH/3T3 Cells
2.12. Statistical Analysis
3. Results
3.1. Appearance of Kombucha Tea during Fermentation
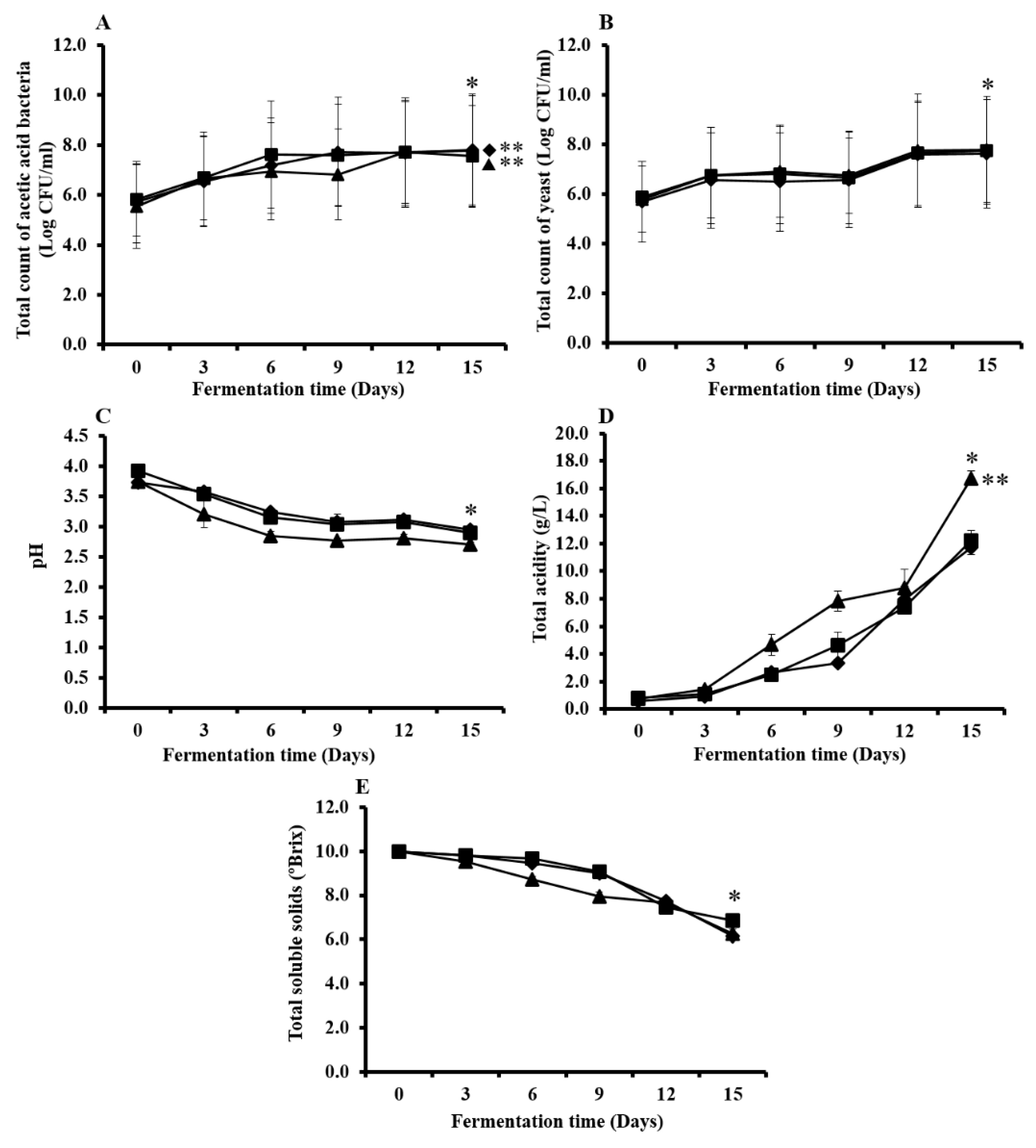
3.2. Microbial and Chemical Changes that Occur During Kombucha Tea Fermentation
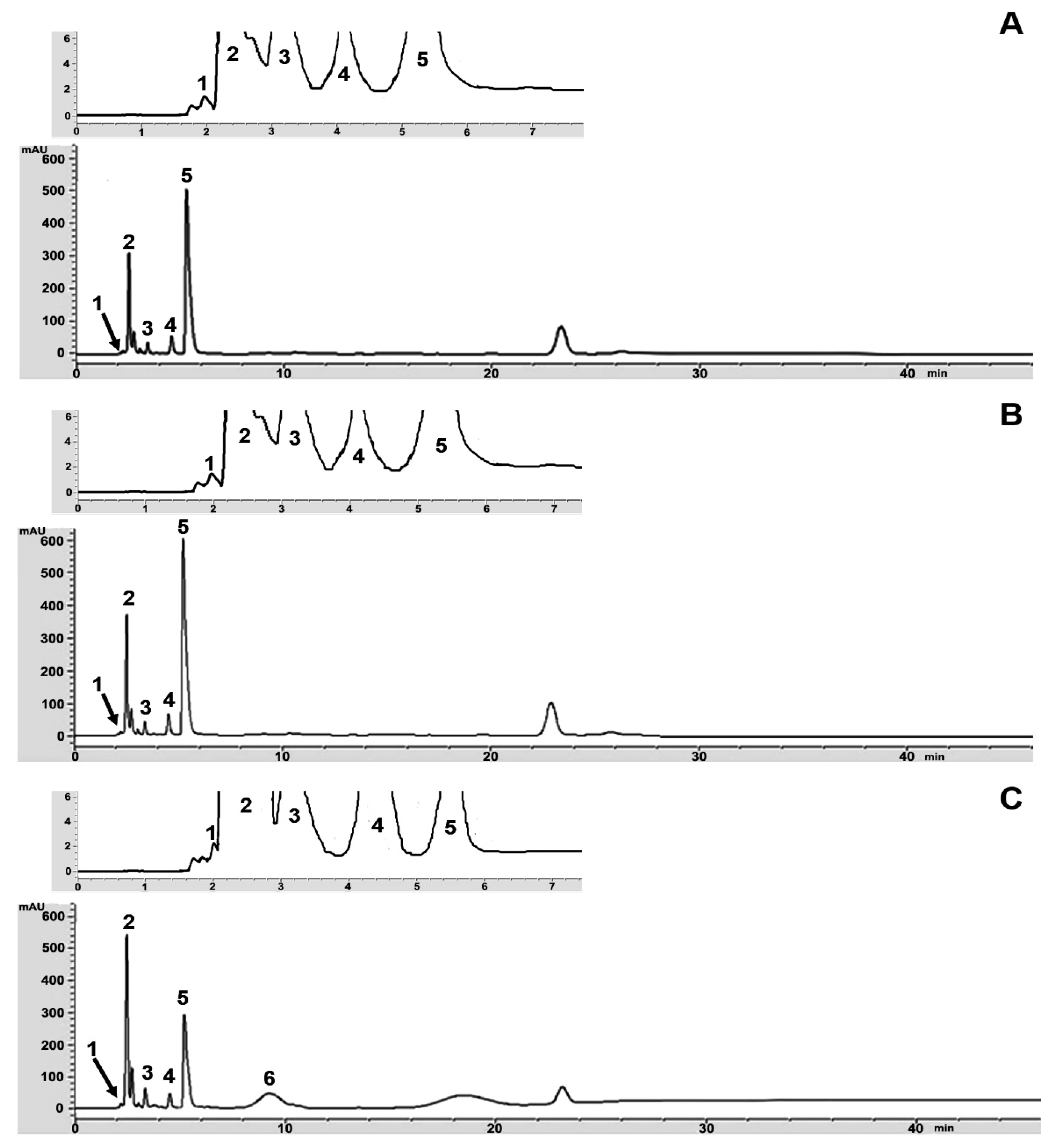
3.3. Organic Acids in Kombucha Tea
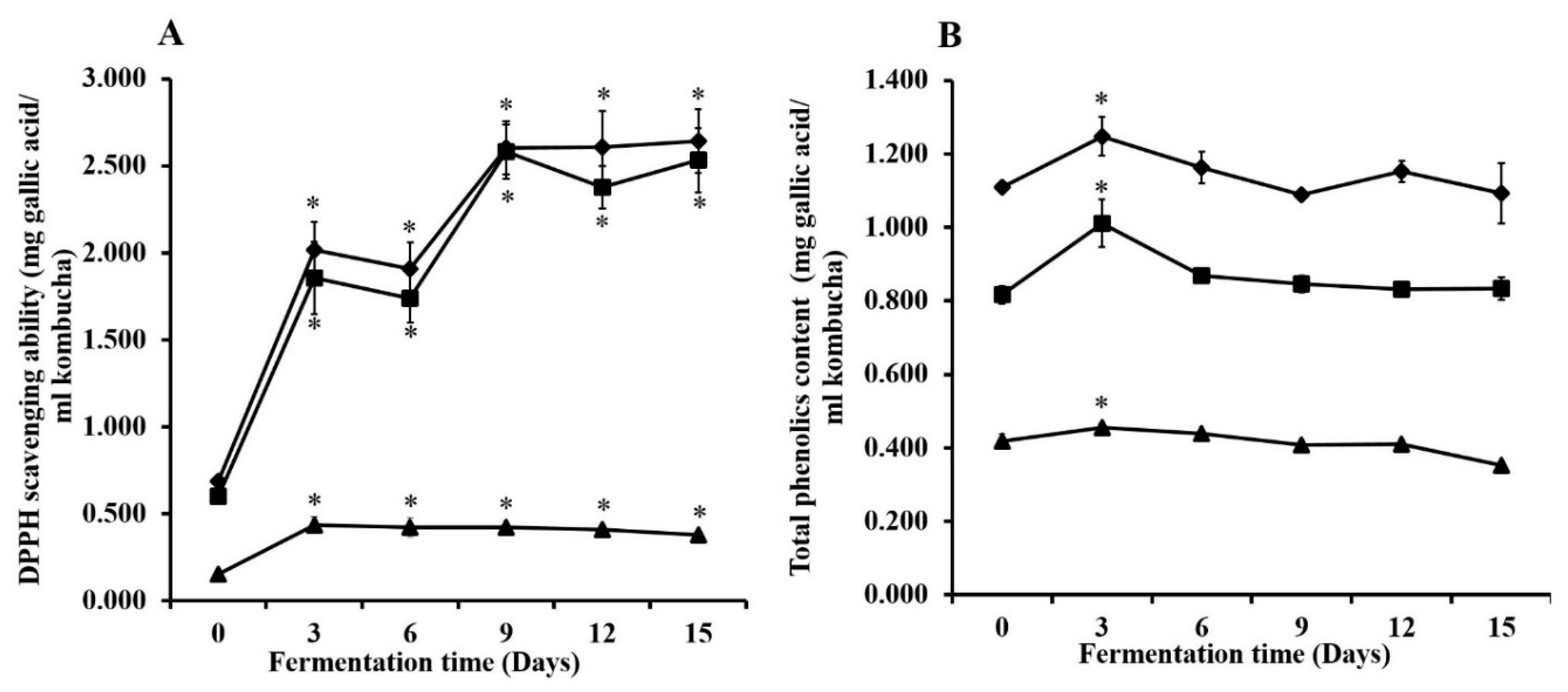
3.4. DPPH Scavenging Ability and Total Phenolic Content during Kombucha Fermentation
3.5. Total Acidity and Antibacterial Activity of Kombucha Tea
3.6. Cytotoxicity Test of Kombucha Tea Against Caco-2 and NIH/3T3 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teoh, A.L.; Heard, G.; Cox, J. Yeast ecology of kombucha fermentation. Int. J. Food Microbiol. 2004, 95, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Malbasa, R.V.; Loncar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha tea—Microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Dufresne, C.; Farnworth, E. Tea, Kombucha, and health. Food Res. Int. 2000, 33, 409–421. [Google Scholar] [CrossRef]
- Banerjee, D.; Hassarajani, S.A.; Maity, B.; Narayan, G.; Bandyopadhya, S.K.; Chattopadhyay, S. Comparative healing property of kombucha tea and black tea against indomethacin induced gastric ulceration in mice: Possible mechanism of action. J. Funct. Foods 2010, 1, 284–293. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Gachhui, R.; Sil, P.C. The prophylactic role of D-saccharic acid-1,4-lactone against hyperglycemia-induced hepatic apoptosis via inhibition of both extrinsic and intrinsic pathways in diabetic rats. J. Funct. Foods 2013, 4, 283–296. [Google Scholar] [CrossRef]
- Yang, Z.W.; Ji, B.P.; Zhou, F.; Li, B.; Luo, Y.; Yang, L.; Li, T. Hypocholesterolaemic and antioxidant effects of kombucha tea in high-cholesterol fed mice. J. Sci. Food Agric. 2009, 89, 150–156. [Google Scholar] [CrossRef]
- Jigisha, A.; Nishant, R.; Navin, K. Green tea: A magical herb with miraculous outcomes. Int. Res. J. Pharm. 2012, 3, 139–148. [Google Scholar]
- Peterson, J.; Dwyer, J.; Jacques, P.; Rand, W.; Prior, R.; Chui, K. Tea variety and brewing techniques influence flavonoid content of black tea. J. Food Compos. Anal. 2004, 17, 397–405. [Google Scholar] [CrossRef]
- Cooper, R.; Morre, D.J.; Morre, D.M. Medicinal benefits of green tea. J. Altern. Complement. Med. 2005, 11, 521–528. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Poonam, R.B.; Singhal, R.S. Tea polyphenols as nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2008, 7, 229–254. [Google Scholar] [CrossRef]
- Chu, S.C.; Chen, C. Effects of origins and fermentation time on the antioxidant activities of kombucha. Food Chem. 2005, 98, 502–507. [Google Scholar] [CrossRef]
- Talawat, S.; Ahantharik, P.; Laohawiwattanakul, S.; Premsuk, A.; Ratanapo, S. Efficacy of fermented teas in antibacterial activity. Kasetsart J. 2006, 40, 925–933. [Google Scholar]
- Srihari, T.; Satyanarayana, U. Changes in free radical scavenging activity of kombucha during fermentation. Int. J. Pharm. Sci. Res. 2012, 4, 1978–1981. [Google Scholar]
- Markov, S.L.; Malbasa, R.V.; Hauk, M.J.; Cvetkovic, D.D. Investigation of tea fungus microbe associations. The Yeasts. Acta Period. Technol. 2001, 32, 133–138. [Google Scholar]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Singhatong, S.; Leelarungrayub, D.; Chaiyasut, C. Antioxidant and toxicity activities of Artocarpus lakoocha Roxb. Heartwood extract. J. Med. Plants Res. 2010, 4, 947–953. [Google Scholar]
- Battikh, H.; Chaieb, K.; Bakhrouf, A.; Ammar, E. Antibacterial and antifungal activities of black and green kombucha teas. J. Food Biochem. 2013, 37, 231–236. [Google Scholar] [CrossRef]
- John, H.R.; Ferraro, M.J. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; pp. 12–34. [Google Scholar]
- Umthong, S.; Phuwapraisirisan, P.; Puthong, S. In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines. BMC Complement. Altern. Med. 2011, 11, 1–8. [Google Scholar] [CrossRef]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991, 55, 35–58. [Google Scholar]
- Blanc, P.J. Characterization of the tea fungus metabolites. Biotechnol. Lett. 1996, 18, 139–142. [Google Scholar] [CrossRef]
- Bauer-Petrovska, B.; Petrushevska-Tozi, L. Mineral and water soluble vitamin content in the Kombucha drink. Int. J. Food Sci. Technol. 2000, 35, 201–205. [Google Scholar] [CrossRef]
- Deghrigue, M.; Chriaa, J.; Battikh, H.; Abid, K.; Bakhrouf, A. Antiproliferative and antimicrobial activities of Kombucha tea. Afr. J. Microbiol. Res. 2013, 7, 3466–3470. [Google Scholar]
- Jain, A.; Manghani, C.; Kohli, S.; Nigam, D.; Rani, V. Tea and human health. Toxicol. Lett. 2013, 220, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; The Association of Official Analytical Chemists (AOAC International): Gaithersburg, MD, USA, 2000. [Google Scholar]
- Ilmara, V.; Raimonds, L.; Arturs, P.; Pavels, S. Glucuronic acid from fermented beverages: Biomedical function in humans and its role in health protection. IJRRAS 2013, 14, 218–230. [Google Scholar]
- Dipti, P.; Yogesh, B.; Kain, A.K.; Pauline, T.; Anju, B.; Sairam, M. Lead induced oxidative stress: Beneficial effects of kombucha tea. Biomed. Environ. Sci. 2003, 16, 276–282. [Google Scholar]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Duenas, M.; Hernandez, T.; Estrella, I. Changes in the content of bioactive polyphenolic compounds of lentils by the action of exogenous enzymes. Effect on their antioxidant activity. Food Chem. 2007, 101, 90–97. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. Value-added Tea (Camellia sinesis) as a functional food using the Kombucha ‘Tea Fungus’. Chiang Mai J. Sci. 2018, 45, 136–146. [Google Scholar]
- Hanausek, M.; Walasze, Z.; Slaga, T.J. Detoxifying cancer causing agents to prevent cancer. Integr. Cancer Ther. 2003, 2, 139–144. [Google Scholar] [CrossRef]
- Olas, B.; Saluk-Juszcak, J.; Nowak, P.; Glowacki, R.; Bald, E.; Wachowicz, B. Protective effects of d-glucaro 1,4-lactone against oxidative/nitrative modifications of plasma proteins. J. Nutr. 2007, 23, 164–171. [Google Scholar] [CrossRef]
- Walaszek, Z.; Szemraj, J.; Hanausek, M.; Adams, A.K.; Sherman, U. d-Glucaric acid content of various fruits and vegetables and cholesterol-lowering effects of dietary d-glucarate in the rat. Nutr. Res. 1996, 16, 673–681. [Google Scholar] [CrossRef]
- Saluk-Juszcak, J.; Olas, B.; Nowak, P.; Staron, A.; Wachowicz, B. Protective effects of d-glucaro 1,4-lactone against oxidative modifications in blood platelets. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Dibner, J.J.; Buttin, P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 2002, 11, 453–463. [Google Scholar] [CrossRef]
- Ludovico, P.; Sansonetty, F.; Silva, M.T.; Corte-Real, M. Acetic acid induces a programmed cell death process in the food spoilage yeast Zygosaccharomyces bailii. FEMS Microbiol. Lett. 2003, 3, 91–96. [Google Scholar] [CrossRef]
- Sreeramulu, G.; Zhu, Y.; Knol, W. Characterization of antimicrobial activity in kombucha fermentation. Acta Biotechnol. 2001, 21, 49–56. [Google Scholar] [CrossRef]
- Sreeramulu, G.; Zhu, Y.; Knol, W. Kombucha fermentation and its antimicrobial activity. J. Agric. Food Chem. 2000, 48, 2589–2594. [Google Scholar] [CrossRef]
- Battikh, H.; Bakhrouf, A.; Ammar, E. Antimicrobial effect of kombucha analogues. J. Food Sci. Technol. 2011, 47, 71–77. [Google Scholar] [CrossRef]
- Hoffmann, N. The Ubiquitous Co-Enzyme UDP Glucuronic Acid Detoxifying Agent in Kombucha Tea. 2000. Available online: www.bluemarble.de/Norbert/kombucha/Glucuron/body_glucuron.htm (accessed on 1 July 2002).
- Velicanski, A.S.; Cvetkovic, D.D.; Markov, S.L.; Tumbas, V.T.; Savatovic, S.M. Antimicrobial and antioxidant activity of lemon balm kombucha. Acta Period. Technol. 2007, 38, 165–172. [Google Scholar] [CrossRef]
- Cetojevic-Simin, D.D.; Bogdanovic, G.M.; Cvetkovic, D.D.; Velicanski, A.S. Antiproliferative and antimicrobial activity of traditional Kombucha and Satureja montana L. Kombucha. J. BUON 2008, 13, 395–401. [Google Scholar]
- Chen, Z.Y.; Zhu, Q.Y.; Tsang, D.; Huang, Y. Degradation of green tea catechins in tea drinks. J. Agric. Food Chem. 2001, 49, 477–482. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, H.Y. Cancer preventive mechanisms of the green tea polyphenol (-)-Epigallocatechin-3-gallate. Molecules 2007, 12, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Eun, J.S.; Kim, B.G.; Kim, S.Y.; Jeon, H. Vitexin, an HIf-1α alpha inhibitor, has anti-metastatic potential in PC12 cells. Mol. Cells 2006, 22, 291–299. [Google Scholar] [PubMed]
- Jayabalan, R.; Chen, P.N.; Hsieh, Y.S.; Prabhakaran, K.; Pitchai, P.; Marimuthu, S.; Thangaraj, P.; Swaminathan, K.; Yun, S.E. Effect of solvent fractions of kombucha tea on viability and invasiveness of cancer cells-characterization of dimethyl 2-(2-hydroxy-2-methoxypropylidine) malonate and vitexin. Indian J. Biotechnol. 2011, 10, 75–82. [Google Scholar]
- Srihari, T.; Arunkumar, R.; Arunakaran, J.; Satyanarayana, U. Downregulation of signalling molecules involved in angiogenesis of prostate cancer cell line (PC-3) by kombucha (lyophilized). Biomed. Prev. Nutr. 2013, 3, 53–58. [Google Scholar] [CrossRef]
- Conney, A.H.; Lu, Y.P.; Lou, Y.R.; Huang, M.T. Inhibitory effects of tea and caffeine on UV-induced carcinogenesis: Relationship to enhanced apoptosis and decreased tissue fat. Eur. J. Cancer Prev. 2002, 2, 28–36. [Google Scholar]
- Ioannides, C.; Yoxall, V. Antimutagenic activity of tea: Role of polyphenols. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 649–656. [Google Scholar] [CrossRef]
- Park, A.M.; Dong, Z. Signal transduction pathways: Targets for green and black tea polyphenols. J. Biochem. Mol. Biol. 2003, 6, 66–77. [Google Scholar]
- Zhao, X.; Song, J.L.; Kim, J.D.; Lee, J.S.; Park, K.Y. Fermented Pu-erh tea increases In Vitro anticancer activities in HT-29 cells and has antiangiogenetic effects on HUVECs. J. Environ. Pathol. Toxicol. Oncol. 2013, 32, 275–288. [Google Scholar] [CrossRef]
- Wang, K.; Gan, X.; Tang, X.; Wang, S.; Tan, H. Determination of D-saccharic acid-1,4-lactone from brewed kombucha broth by high performance capillary electrophoresis. J. Chromatogr. B 2010, 878, 371–374. [Google Scholar] [CrossRef]
- Hemila, H.; Herman, Z. Vitamin C and the common cold: A retrospective analysis of chalmers review. J. Am. Coll. Nutr. 1995, 14, 116–123. [Google Scholar] [CrossRef]
- Weisburger, J.H.; Hara, Y.; Dolan, L.; Luo, F.Q.; Pittman, B.; Zang, E. Tea polyphenols as inhibitors of mutagenicity of major classes of carcinogens. Mutat. Res. 1996, 371, 57–63. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, CDC. Unexplained severe illness possibly associated with consumption of kombucha tea—Iowa. MMWR-Morb. Mortal. Wkly. Rep. 1995, 44, 892–900. [Google Scholar]

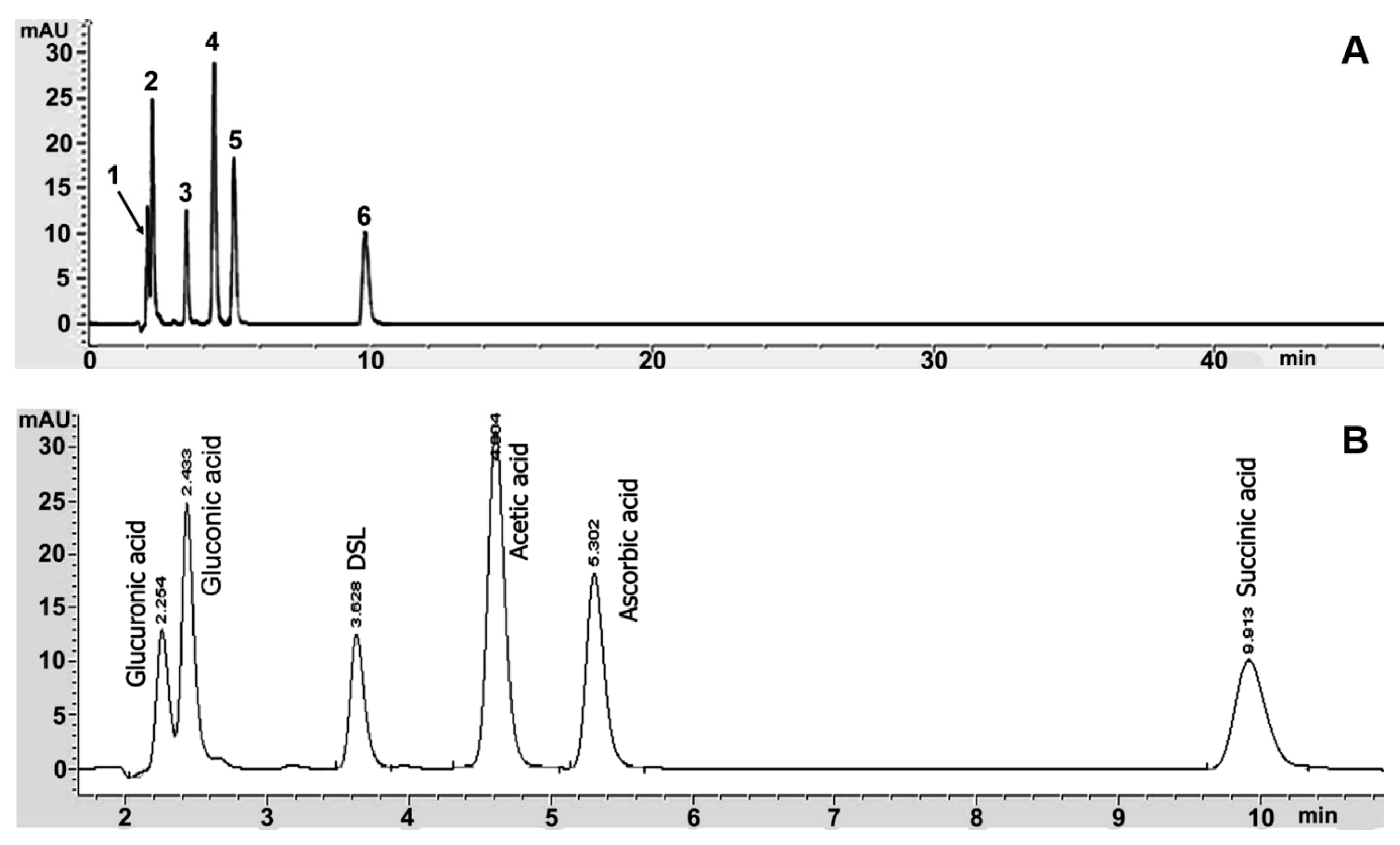
| Kombucha | Organic Acids Content (g/L) | |||||
|---|---|---|---|---|---|---|
| Glucuronic | Gluconic | DSL | Ascorbic | Acetic | Succinic | |
| Green tea | 1.37 ± 0.01 c | 41.42 ± 0.02 c | 3.44 ± 0.03 c | 0.61 ± 0.00 a | 10.42 ± 0.00 b | ND d |
| Oolong tea | 0.07 ± 0.01 b | 48.75 ± 0.03 b | 4.02 ± 0.02 b | 0.60 ± 0.00 a | 10.48 ± 0.00 ab | ND d |
| Black tea | 1.58 ± 0.01 a | 70.11 ± 0.01 a | 5.23 ± 0.01 a | 0.70 ± 0.00 a | 11.15 ± 0.00 a | 3.05 ± 0.01 a |
| Type of Camellia Sinensis | Tested Extract | a Inhibition Zone Diameter (mm) of Target Bacteria | ||||
|---|---|---|---|---|---|---|
| E. coli | E. coli O157: H7 | S. Dysenteriae | S. Typhi | V. Cholera | ||
| Green tea | b Fermented tea | 24.7 ± 0.6 | 24.3 ± 0.6 | 21.7 ± 0.6 | 23.7 ± 0.6 | 20.0 ± 0.0 |
| c Unfermented tea | 0 | 0 | 0 | 0 | 0 | |
| d Neutralized kombucha | 0 | 0 | 0 | 0 | 0 | |
| e Heat-denatured Kombucha M1 | 25.0 ± 0.0 | 23.0 ± 0.0 | 21.0 ± 0.0 | 23.0 ± 0.0 | 19.0 ± 0.0 | |
| f Heat-denatured Kombucha M2 | 15.3 ± 0.6 | 22.3 ± 0.6 | 19.7 ± 0.6 | 20.7 ± 0.6 | 17.3 ± 0.6 | |
| g Acetic acid (11.72 g/L) | 20.3 ± 0.6 | 22.0 ± 0.0 | 20.0 ± 0.0 | 21.0 ± 0.0 | 22.0 ± 0.0 | |
| Oolong tea | Fermented tea | 23.7 ± 0.6 | 20.3 ± 0.6 | 19.3 ± 0.6 | 24.7 ± 0.6 | 20.0 ± 0 |
| Unfermented tea | 0 | 0 | 0 | 0 | 0 | |
| Neutralized kombucha | 0 | 0 | 0 | 0 | 0 | |
| Heat-denatured Kombucha M1 | 20.0 ± 0.0 | 19.3 ± 0.6 | 19.3 ± 0.6 | 24.0 ± 0.0 | 16.7 ± 0.6 | |
| Heat-denatured Kombucha M2 | 15.7 ± 0.6 | 15.0 ± 0.0 | 17.0 ± 0 | 19.7 ± 0.6 | 15.7 ± 0.6 | |
| Acetic acid (12.24 g/L) | 21.0 ± 0.0 | 20.0 ± 0.0 | 21.0 ± 1.0 | 20.3 ± 0.6 | 22.0 ± 0.0 | |
| Black tea | Fermented tea | 21.0 ± 0.0 | 21.3 ± 0.6 | 21.0 ± 0.0 | 20.0 ± 0.0 | 21.0 ± 0.0 |
| Unfermented tea | 0 | 0 | 0 | 0 | 0 | |
| Neutralized kombucha | 0 | 0 | 0 | 0 | 0 | |
| Heat-denatured Kombucha M1 | 20.3 ± 0.6 | 20.3 ± 0.6 | 20.0 ± 0.0 | 18.0 ± 0.0 | 20.0 ± 0.0 | |
| Heat-denatured Kombucha M2 | 18.0 ± 0.0 | 16.3 ± 0.6 | 19.7 ± 0.6 | 17.3 ± 0.6 | 18.0 ± 0.0 | |
| Acetic acid (16.75 g/L) | 20.0 ± 0.0 | 20.0 ± 0.0 | 20.7 ± 0.6 | 21.0 ± 0.0 | 21.7 ± 0.6 | |
| Gentamycin (1 mg/mL) | 20.3 ± 0.6 | 19.3 ± 0.6 | 23.3 ± 0.6 | 22.3 ± 0.6 | 20.0 ± 0.0 | |
| Type of Camellia Sinensis | Tested Extract | a IC50 (%) | |
|---|---|---|---|
| Caco-2 Cells | NIH/3T3 Cells | ||
| Green tea | b Fermented tea | 2.603 ± 0.072 * | 2.851 ± 0.052 * |
| c Unfermented tea | 3.661 ± 2.228 | 3.819 ± 0.122 | |
| d Neutralized kombucha | 8.621 ± 0.685 | 8.915 ± 0.715 | |
| e Heat-denatured kombucha M1 | 1.758 ± 0.065 * | 1.916 ± 0.011* | |
| f Heat-denatured kombucha M2 | 1.309 ± 0.117 | 1.518 ± 1.202 | |
| g Acetic acid (11.72 g/L) | 33.155 ± 2.834 | 35.206 ± 0.625 | |
| Oolong tea | Fermented tea | 1.899 ± 0.242 | 1.922 ± 0.186 |
| Unfermented tea | 2.097 ± 0.305 | 2.519 ± 0.024 | |
| Neutralized kombucha | 4.194 ± 0.081 | 4.671 ± 1.205 | |
| Heat-denatured kombucha M1 | 1.179 ± 0.041 | 1.219 ± 0.012 | |
| Heat-denatured kombucha M2 | 1.515 ± 0.138 | 1.641 ± 0.220 | |
| Acetic acid (12.24 g/L) | 12.610 ± 0.341 | 12.817 ± 0.452 | |
| Black tea | Fermented tea | 6.077 ± 0.222 * | 6.697 ± 0.030 * |
| Unfermented tea | 6.082 ± 0.191 | 6.244 ± 0.659 | |
| Neutralized kombucha | 16.339 ± 0.833 | 17.066 ± 1.568 | |
| Heat-denatured kombucha M1 | 6.083 ± 0.053 * | 6.702 ± 0.156 * | |
| Heat-denatured kombucha M2 | 5.699 ± 0.008 | 5.819 ± 0.209 | |
| Acetic acid (16.75 g/L) | 8.720 ± 0.047 | 8.810 ± 0.142 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewkod, T.; Bovonsombut, S.; Tragoolpua, Y. Efficacy of Kombucha Obtained from Green, Oolong, and Black Teas on Inhibition of Pathogenic Bacteria, Antioxidation, and Toxicity on Colorectal Cancer Cell Line. Microorganisms 2019, 7, 700. https://doi.org/10.3390/microorganisms7120700
Kaewkod T, Bovonsombut S, Tragoolpua Y. Efficacy of Kombucha Obtained from Green, Oolong, and Black Teas on Inhibition of Pathogenic Bacteria, Antioxidation, and Toxicity on Colorectal Cancer Cell Line. Microorganisms. 2019; 7(12):700. https://doi.org/10.3390/microorganisms7120700
Chicago/Turabian StyleKaewkod, Thida, Sakunnee Bovonsombut, and Yingmanee Tragoolpua. 2019. "Efficacy of Kombucha Obtained from Green, Oolong, and Black Teas on Inhibition of Pathogenic Bacteria, Antioxidation, and Toxicity on Colorectal Cancer Cell Line" Microorganisms 7, no. 12: 700. https://doi.org/10.3390/microorganisms7120700
APA StyleKaewkod, T., Bovonsombut, S., & Tragoolpua, Y. (2019). Efficacy of Kombucha Obtained from Green, Oolong, and Black Teas on Inhibition of Pathogenic Bacteria, Antioxidation, and Toxicity on Colorectal Cancer Cell Line. Microorganisms, 7(12), 700. https://doi.org/10.3390/microorganisms7120700