Serine Protease Autotransporters of the Enterobacteriaceae (SPATEs): Out and About and Chopping It Up
Abstract
1. Introduction
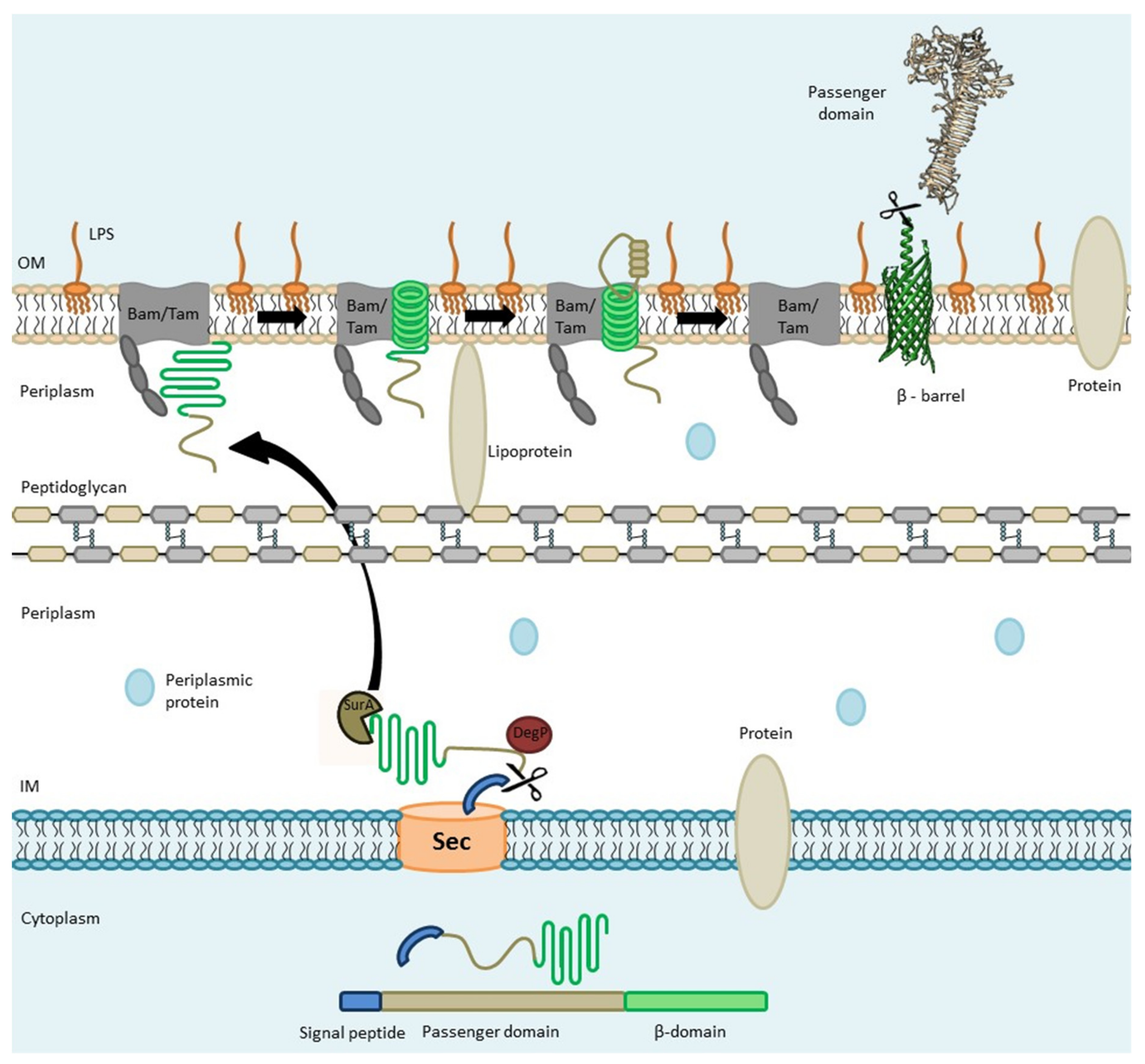
2. The Autotransporter Secretion Pathway
2.1. Sec-Dependent Export of AT Proteins through the Inner Membrane
2.2. AT Protein Transit through the Periplasm
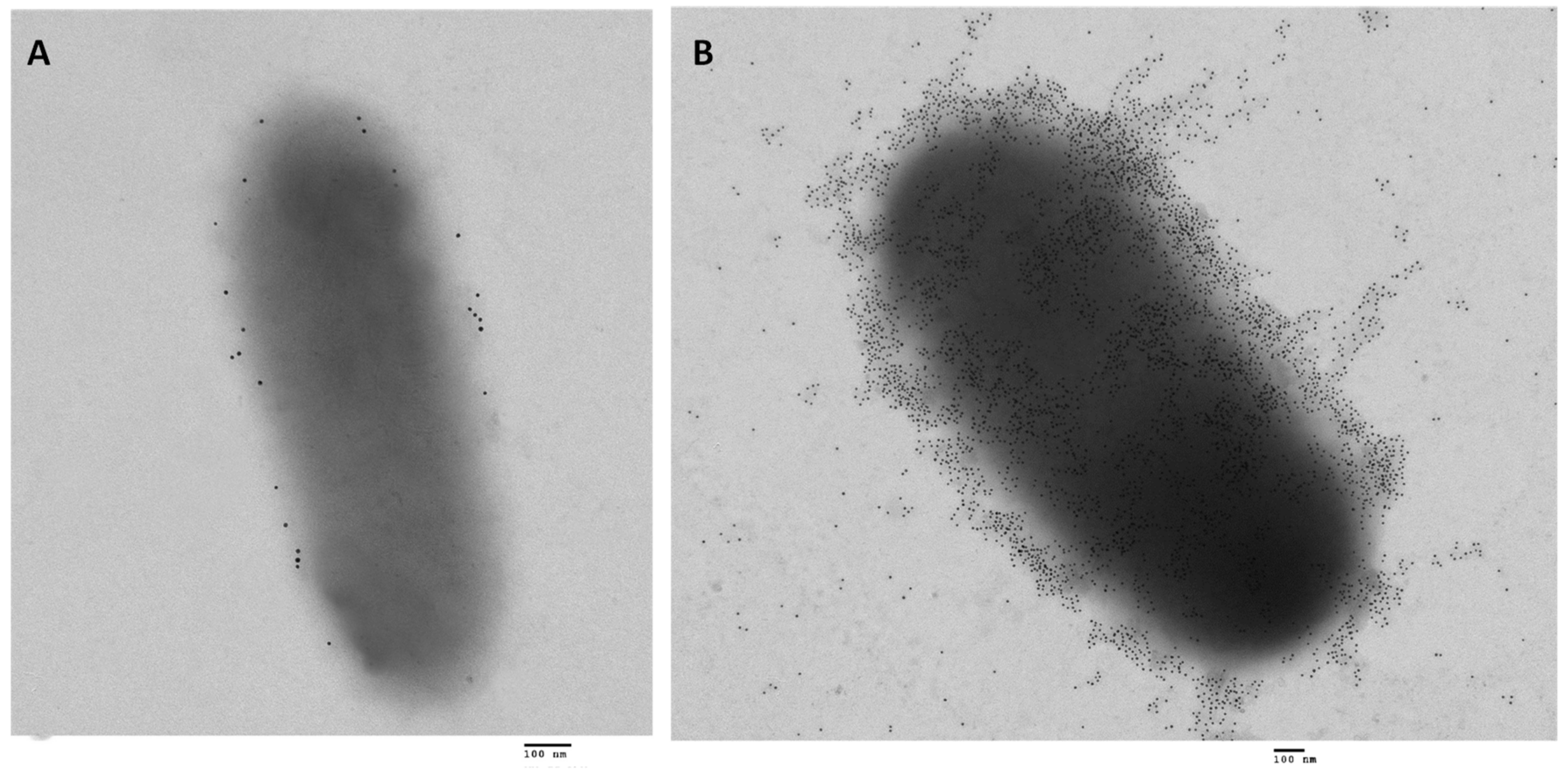
2.3. Transport of ATs through the Outer Membrane (The Hybrid-Barrel Model)
2.4. Passenger Domain Cleavage
2.5. A Cleavage Site is Located in the “Linker Domain” of SPATE Proteins
3. SPATEs
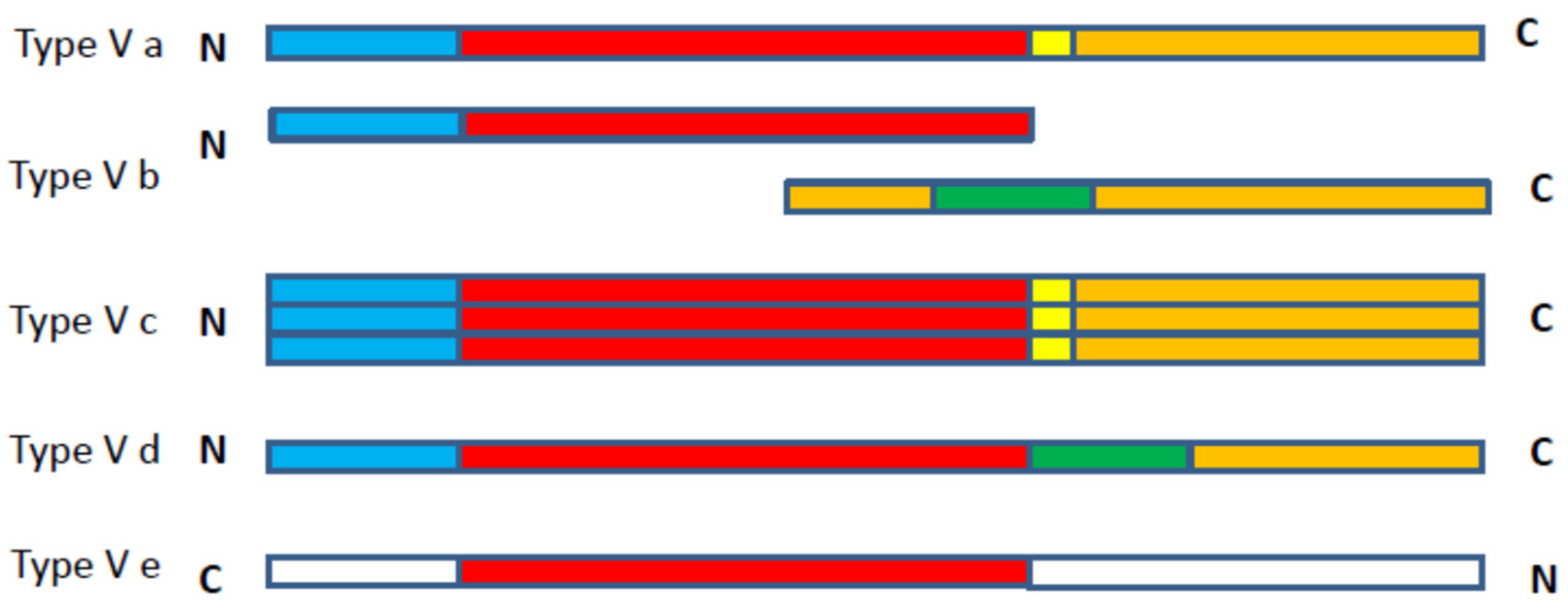
3.1. Classification of SPATES
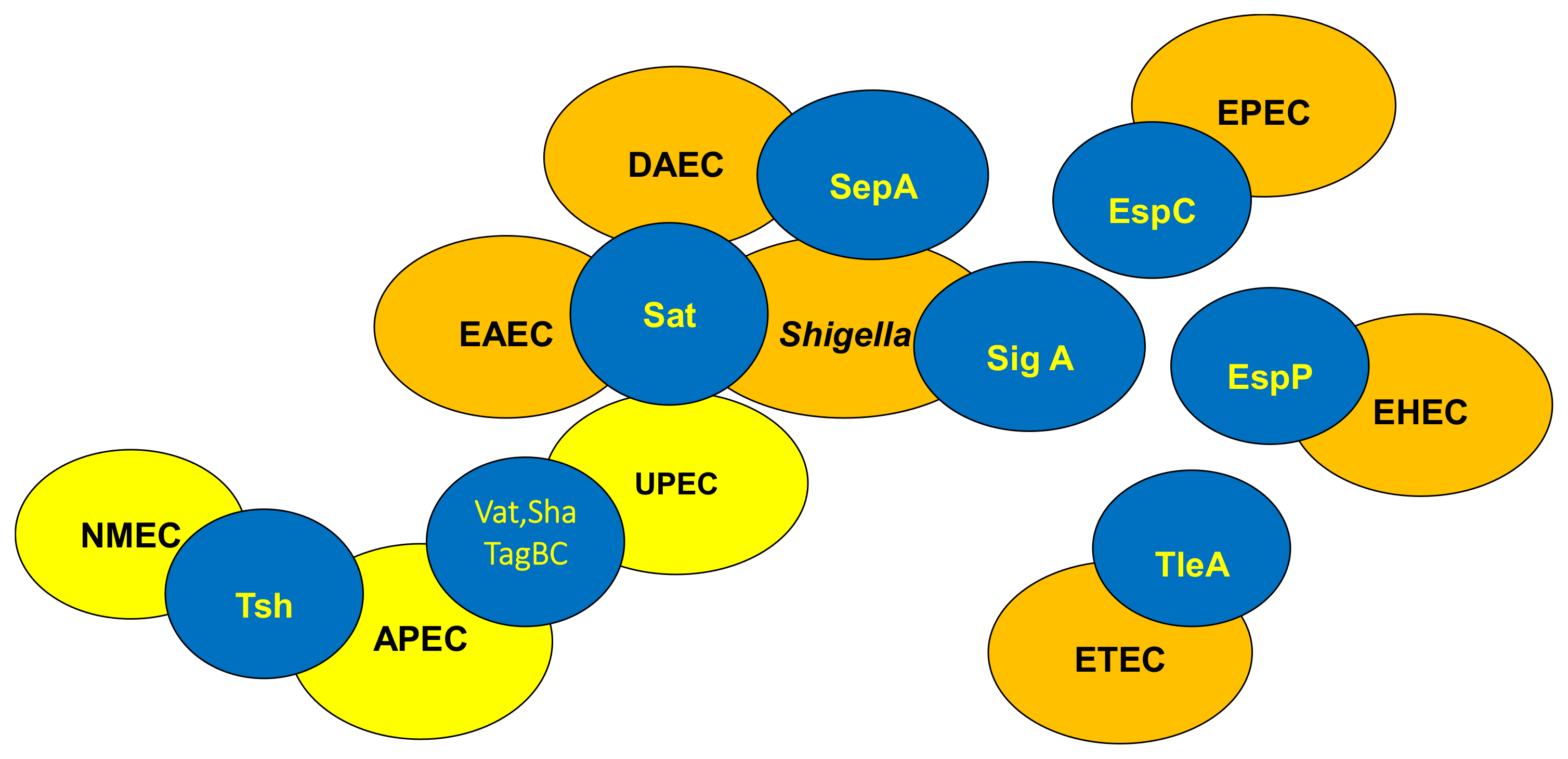
3.2. Distribution of SPATEs among Intestinal and Extra-Intestinal Pathogenic E. coli
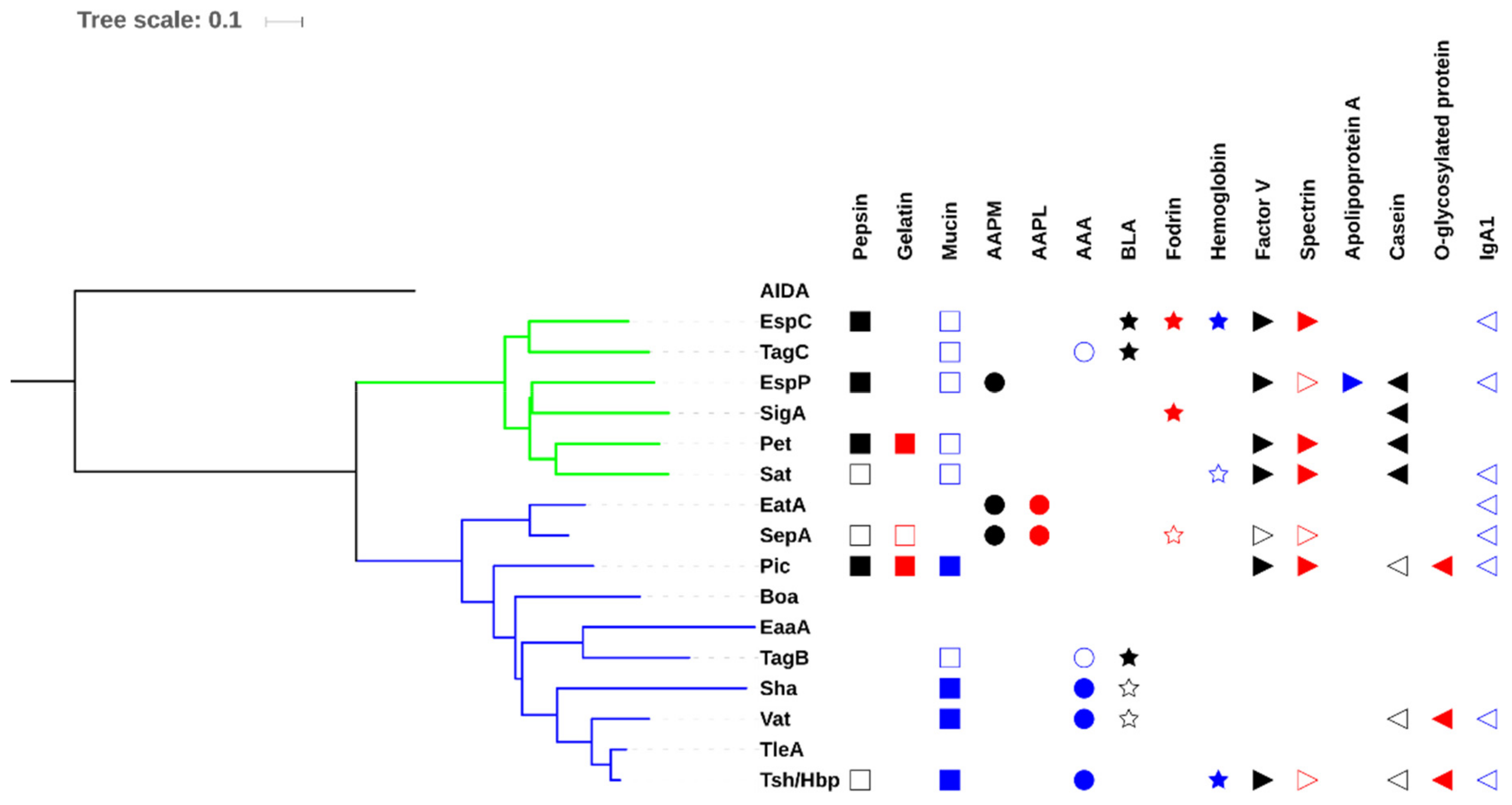
3.3. Allelic Variation
3.4. Confusion Due to Improper Annotation of Uncharacterized SPATE Encoding Proteins
4. SPATEs Demonstrate a Diversity of Biological Activities Associated with Virulence
4.1. EaaA/EaaC
4.2. EatA
4.3. EspC
4.4. Pet
4.5. Pic
4.6. EspP
4.7. Tsh/Hbp
4.8. TleA
4.9. Vat
4.10. Sha
4.11. Sat
4.12. SepA
4.13. SigA
4.14. Boa
4.15. TagBC
5. Regulation of Expression of SPATEs
5.1. Regulation by LER
5.2. Regulation by H-NS
5.3. Regulation of Vat by the MarR-Related Protein VatX
5.4. Co-Regulation of SPATEs by CRP and FIS Proteins
6. Some SPATEs Can Also Mediate Degradation of Bacterial Protein Targets
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henderson, I.R.; Navarro-Garcia, F.; Desvaux, M.; Fernandez, R.C.; Ala’Aldeen, D. Type V protein secretion pathway: The autotransporter story. Microbiol. Mol. Biol. Rev. 2004, 68, 692–744. [Google Scholar] [CrossRef] [PubMed]
- Linke, D.; Riess, T.; Autenrieth, I.B.; Lupas, A.; Kempf, V.A. Trimeric autotransporter adhesins: Variable structure, common function. Trends Microbiol. 2006, 14, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Salacha, R.; Kovačić, F.; Brochier-Armanet, C.; Wilhelm, S.; Tommassen, J.; Filloux, A.; Voulhoux, R.; Bleves, S. The Pseudomonas aeruginosa patatin-like protein PlpD is the archetype of a novel Type V secretion system. Environ. Microbiol. 2010, 12, 1498–1512. [Google Scholar] [PubMed]
- Leo, J.C.; Grin, I.; Linke, D. Type V secretion: Mechanism (s) of autotransport through the bacterial outer membrane. Phil. Trans. R. Soc. B 2012, 367, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Dubuisson, F.; Locht, C.; Antoine, R. Two-partner secretion in Gram-negative bacteria: A thrifty, specific pathway for large virulence proteins. Mol. Microbiol. 2001, 40, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Mian, H.S.; Sandercock, L.E.; Chirgadze, N.Y.; Pai, E.F. Crystal structure of the passenger domain of the Escherichia coli autotransporter EspP. J. Mol. Biol. 2011, 413, 985–1000. [Google Scholar] [CrossRef]
- Otto, B.R.; Sijbrandi, R.; Luirink, J.; Oudega, B.; Heddle, J.G.; Mizutani, K.; Park, S.-Y.; Tame, J.R. Crystal structure of hemoglobin protease, a heme binding autotransporter protein from pathogenic Escherichia coli. J. Biol. Chem. 2005, 280, 17339–17345. [Google Scholar] [CrossRef]
- Meza-Aguilar, J.D.; Fromme, P.; Torres-Larios, A.; Mendoza-Hernández, G.; Hernandez-Chiñas, U.; Campos, C.A.E.; Fromme, R. X-ray crystal structure of the passenger domain of plasmid encoded toxin (Pet), an autotransporter enterotoxin from enteroaggregative Escherichia coli (EAEC). Biochem. Biophys. Res. Commun. 2014, 445, 439–444. [Google Scholar] [CrossRef]
- Veiga, E.; Sugawara, E.; Nikaido, H.; de Lorenzo, V.; Fernández, L.A. Export of autotransported proteins proceeds through an oligomeric ring shaped by C-terminal domains. EMBO J. 2002, 21, 2122–2131. [Google Scholar] [CrossRef]
- Selkrig, J.; Mosbahi, K.; Webb, C.T.; Belousoff, M.J.; Perry, A.J.; Wells, T.J.; Morris, F.; Leyton, D.L.; Totsika, M.; Phan, M.-D. Discovery of an archetypal protein transport system in bacterial outer membranes. Nat. Struct. Mol. Biol. 2012, 19, 506. [Google Scholar] [CrossRef]
- Ruiz-Perez, F.; Henderson, I.R.; Leyton, D.L.; Rossiter, A.E.; Zhang, Y.; Nataro, J.P. Roles of periplasmic chaperone proteins in the biogenesis of serine protease autotransporters of Enterobacteriaceae. J. Bacteriol. 2009, 191, 6571–6583. [Google Scholar] [CrossRef]
- Sijbrandi, R.; Urbanus, M.L.; Corinne, M.; Bernstein, H.D.; Oudega, B.; Otto, B.R.; Luirink, J. Signal recognition particle (SRP)-mediated targeting and Sec-dependent translocation of an extracellular Escherichia coli protein. J. Biol. Chem. 2003, 278, 4654–4659. [Google Scholar] [CrossRef] [PubMed]
- Purdy, G.E.; Fisher, C.R.; Payne, S.M. IcsA surface presentation in Shigella flexneri requires the periplasmic chaperones DegP, Skp, and SurA. J. Bacteriol. 2007, 189, 5566–5573. [Google Scholar] [CrossRef] [PubMed]
- Sauri, A.; Soprova, Z.; Wickström, D.; de Gier, J.-W.; Van der Schors, R.C.; Smit, A.B.; Jong, W.S.; Luirink, J. The Bam (Omp85) complex is involved in secretion of the autotransporter haemoglobin protease. Microbiology 2009, 155, 3982–3991. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.H.; Tian, P.; Ieva, R.; Dautin, N.; Bernstein, H.D. Secretion of a bacterial virulence factor is driven by the folding of a C-terminal segment. Proc. Natl. Acad. Sci. USA 2010, 107, 17739–17744. [Google Scholar] [CrossRef]
- Michaelis, S.; Hunt, J.; Beckwith, J. Effects of signal sequence mutations on the kinetics of alkaline phosphatase export to the periplasm in Escherichia coli. J. Bacteriol. 1986, 167, 160–167. [Google Scholar] [CrossRef]
- Martoglio, B.; Dobberstein, B. Signal sequences: More than just greasy peptides. Trends Cell Biol. 1998, 8, 410–415. [Google Scholar] [CrossRef]
- Randall, L.; Hardy, S. SecB, one small chaperone in the complex milieu of the cell. Cell. Mol. Life Sci. Cmls 2002, 59, 1617–1623. [Google Scholar] [CrossRef]
- Valent, Q.A.; Scotti, P.A.; High, S.; de Gier, J.W.L.; von Heijne, G.; Lentzen, G.; Wintermeyer, W.; Oudega, B.; Luirink, J. The Escherichia coli SRP and SecB targeting pathways converge at the translocon. EMBO J. 1998, 17, 2504–2512. [Google Scholar] [CrossRef]
- Bredemeier, R.; Schlegel, T.; Ertel, F.; Vojta, A.; Borissenko, L.; Bohnsack, M.T.; Groll, M.; Von Haeseler, A.; Schleiff, E. Functional and phylogenetic properties of the pore-forming β-barrel transporters of the Omp85 family. J. Biol. Chem. 2007, 282, 1882–1890. [Google Scholar] [CrossRef]
- Fleming, P.J.; Patel, D.S.; Wu, E.L.; Qi, Y.; Yeom, M.S.; Sousa, M.C.; Fleming, K.G.; Im, W. BamA POTRA domain interacts with a native lipid membrane surface. Biophys. J. 2016, 110, 2698–2709. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Albrecht, R.; Schütz, M.; Oberhettinger, P.; Faulstich, M.; Bermejo, I.; Rudel, T.; Diederichs, K.; Zeth, K. Structure of BamA, an essential factor in outer membrane protein biogenesis. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014, 70, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Gruss, F.; Zähringer, F.; Jakob, R.P.; Burmann, B.M.; Hiller, S.; Maier, T. The structural basis of autotransporter translocation by TamA. Nat. Struct. Mol. Biol. 2013, 20, 1318. [Google Scholar] [CrossRef] [PubMed]
- Noinaj, N.; Kuszak, A.J.; Balusek, C.; Gumbart, J.C.; Buchanan, S.K. Lateral opening and exit pore formation are required for BamA function. Structure 2014, 22, 1055–1062. [Google Scholar] [CrossRef]
- Eslava, C.; Navarro-García, F.; Czeczulin, J.R.; Henderson, I.R.; Cravioto, A.; Nataro, J.P. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect. Immun. 1998, 66, 3155–3163. [Google Scholar]
- Brunder, W.; Schmidt, H.; Karch, H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157: H7 cleaves human coagulation factor V. Mol. Microbiol. 1997, 24, 767–778. [Google Scholar] [CrossRef]
- Dautin, N.; Barnard, T.J.; Anderson, D.E.; Bernstein, H.D. Cleavage of a bacterial autotransporter by an evolutionarily convergent autocatalytic mechanism. EMBO J. 2007, 26, 1942–1952. [Google Scholar] [CrossRef]
- Dautin, N.; Bernstein, H.D. Residues in a conserved α-helical segment are required for cleavage but not secretion of an Escherichia coli serine protease autotransporter passenger domain. J. Bacteriol. 2011, 193, 3748–3756. [Google Scholar] [CrossRef]
- Kostakioti, M.; Stathopoulos, C. Role of the α-helical linker of the C-terminal translocator in the biogenesis of the serine protease subfamily of autotransporters. Infect. Immun. 2006, 74, 4961–4969. [Google Scholar] [CrossRef]
- Leyton, D.L.; Adams, L.M.; Kelly, M.; Sloan, J.; Tauschek, M.; Robins-Browne, R.M.; Hartland, E.L. Contribution of a novel gene, rpeA, encoding a putative autotransporter adhesin to intestinal colonization by rabbit-specific enteropathogenic Escherichia coli. Infect. Immun. 2007, 75, 4664–4669. [Google Scholar] [CrossRef]
- Habouria, H.; Pokharel, P.; Maris, S.; Garénaux, A.; Bessaiah, H.; Houle, S.; Veyrier, F.J.; Guyomard-Rabenirina, S.; Talarmin, A.; Dozois, C.M. Three new serine-protease autotransporters of Enterobacteriaceae (SPATEs) from extra-intestinal pathogenic Escherichia coli and combined role of SPATEs for cytotoxicity and colonization of the mouse kidney. Virulence 2019, 10, 568–587. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Beppu, T.; Horinouchi, S. Two genes encoding serine protease homologues in Serratia marcescens and characterization of their products in Escherichia coli. J. Biochem. 1997, 121, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Parham, N.J.; Pollard, S.J.; Desvaux, M.; Scott-Tucker, A.; Liu, C.; Fivian, A.; Henderson, I.R. Distribution of the serine protease autotransporters of the Enterobacteriaceae among extraintestinal clinical isolates of Escherichia coli. J. Clin. Microbiol. 2005, 43, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.; Santiago, A.; Smith, R.; Smith, M.; Robins-Browne, R.M.; Nataro, J.P.; Ruiz-Perez, F. Role of class 1 serine protease autotransporter in the pathogenesis of Citrobacter rodentium colitis. Infect. Immun. 2014, 82, 2626–2636. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Zhou, H.-Z.; Jin, Q.-W.; Zhang, J. The serine protease autotransporter Tsh contributes to the virulence of Edwardsiella tarda. Vet. Microbiol. 2016, 189, 68–74. [Google Scholar] [CrossRef]
- Dautin, N. Serine protease autotransporters of enterobacteriaceae (SPATEs): Biogenesis and function. Toxins 2010, 2, 1179–1206. [Google Scholar] [CrossRef]
- Dozois, C.M.; Dho-Moulin, M.; Brée, A.; Fairbrother, J.M.; Desautels, C.; Curtiss, R. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the Tsh genetic region. Infect. Immun. 2000, 68, 4145–4154. [Google Scholar] [CrossRef]
- Stein, M.; Kenny, B.; Stein, M.A.; Finlay, B.B. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J. Bacteriol. 1996, 178, 6546–6554. [Google Scholar] [CrossRef]
- Henderson, I.R.; Czeczulin, J.; Eslava, C.; Noriega, F.; Nataro, J.P. Characterization of Pic, a Secreted Protease ofShigella flexneri and Enteroaggregative Escherichia coli. Infect. Immun. 1999, 67, 5587–5596. [Google Scholar]
- Peterson, J.H.; Szabady, R.L.; Bernstein, H.D. An unusual signal peptide extension inhibits the binding of bacterial presecretory proteins to the signal recognition particle, trigger factor, and the SecYEG complex. J. Biol. Chem. 2006, 281, 9038–9048. [Google Scholar] [CrossRef]
- Dutta, P.R.; Cappello, R.; Navarro-García, F.; Nataro, J.P. Functional comparison of serine protease autotransporters of Enterobacteriaceae. Infect. Immun. 2002, 70, 7105–7113. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Perez, F.; Wahid, R.; Faherty, C.S.; Kolappaswamy, K.; Rodriguez, L.; Santiago, A.; Murphy, E.; Cross, A.; Sztein, M.B.; Nataro, J.P. Serine protease autotransporters from Shigella flexneri and pathogenic Escherichia coli target a broad range of leukocyte glycoproteins. Proc. Natl. Acad. Sci. USA 2011, 108, 12881–12886. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Dotson, J.; Allen, K.P.; Fleckenstein, J.M. Identification and molecular characterization of EatA, an autotransporter protein of enterotoxigenic Escherichia coli. Infect. Immun. 2004, 72, 1786–1794. [Google Scholar] [CrossRef]
- Otto, B.R.; Van Dooren, S.J.; Nuijens, J.H.; Luirink, J.; Oudega, B. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli strain EB1. J. Exp. Med. 1998, 188, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Guyer, D.M.; Henderson, I.R.; Nataro, J.P.; Mobley, H.L. Identification of sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 2000, 38, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Parreira, V.; Gyles, C. A novel pathogenicity island integrated adjacent to the thrW tRNA gene of avian pathogenic Escherichia coli encodes a vacuolating autotransporter toxin. Infect. Immun. 2003, 71, 5087–5096. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Perez, F.; Nataro, J.P. Bacterial serine proteases secreted by the autotransporter pathway: Classification, specificity, and role in virulence. Cell. Mol. Life Sci. 2014, 71, 745–770. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Bioinformatics 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Ciccarelli, F.D.; Doerks, T.; Von Mering, C.; Creevey, C.J.; Snel, B.; Bork, P. Toward automatic reconstruction of a highly resolved tree of life. Science 2006, 311, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Heimer, S.R.; Rasko, D.A.; Lockatell, C.V.; Johnson, D.E.; Mobley, H.L. Autotransporter genes pic and tsh are associated with Escherichia coli strains that cause acute pyelonephritis and are expressed during urinary tract infection. Infect. Immun. 2004, 72, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.A.; Burland, V.; Plunkett, G.; Redford, P.; Roesch, P.; Rasko, D.; Buckles, E.; Liou, S.-R.; Boutin, A.; Hackett, J. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 2002, 99, 17020–17024. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Kansal, R.; Bartels, S.R.; Hamilton, D.J.; Shaaban, S.; Fleckenstein, J.M. Adhesin degradation accelerates delivery of heat-labile toxin by enterotoxigenic E. coli. J. Biol. Chem. 2011, 286, 29771–29779. [Google Scholar] [CrossRef]
- Navarro-García, F.; Canizalez-Roman, A.; Sui, B.Q.; Nataro, J.P.; Azamar, Y. The serine protease motif of EspC from enteropathogenic Escherichia coli produces epithelial damage by a mechanism different from that of Pet toxin from enteroaggregative E. coli. Infect. Immun. 2004, 72, 3609–3621. [Google Scholar] [CrossRef]
- Serapio-Palacios, A.; Navarro-Garcia, F. EspC, an autotransporter protein secreted by enteropathogenic Escherichia coli, causes apoptosis and necrosis through caspase and calpain activation, including direct procaspase-3 cleavage. MBio 2016, 7, e00479-16. [Google Scholar] [CrossRef]
- Elisa Drago-Serrano, M.; Gavilanes Parra, S.; Angel Manjarrez-Hernández, H. EspC, an autotransporter protein secreted by enteropathogenic Escherichia coli (EPEC), displays protease activity on human hemoglobin. FEMS Microbiol. Lett. 2006, 265, 35–40. [Google Scholar] [CrossRef]
- Navarro-Garcia, F.; Serapio-Palacios, A.; Vidal, J.E.; Salazar, M.I.; Tapia-Pastrana, G. EspC promotes epithelial cell detachment by enteropathogenic Escherichia coli via sequential cleavages of a cytoskeletal protein and then focal adhesion proteins. Infect. Immun. 2014, 82, 2255–2265. [Google Scholar] [CrossRef]
- Henderson, I.R.; Hicks, S.; Navarro-Garcia, F.; Elias, W.P.; Philips, A.D.; Nataro, J.P. Involvement of the Enteroaggregative Escherichia coli Plasmid-Encoded Toxin in Causing Human Intestinal Damage. Infect. Immun. 1999, 67, 5338–5344. [Google Scholar]
- Navarro-García, F.; Sears, C.; Eslava, C.; Cravioto, A.; Nataro, J.P. Cytoskeletal effects induced by pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect. Immun. 1999, 67, 2184–2192. [Google Scholar]
- Canizalez-Roman, A.; Navarro-García, F. Fodrin CaM-binding domain cleavage by Pet from enteroaggregative Escherichia coli leads to actin cytoskeletal disruption. Mol. Microbiol. 2003, 48, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Parham, N.J.; Srinivasan, U.; Desvaux, M.; Foxman, B.; Marrs, C.F.; Henderson, I.R. PicU, a second serine protease autotransporter of uropathogenic Escherichia coli. FEMS Microbiol. Lett. 2004, 230, 73–83. [Google Scholar] [CrossRef]
- Ayala-Lujan, J.L.; Vijayakumar, V.; Gong, M.; Smith, R.; Santiago, A.E.; Ruiz-Perez, F. Broad spectrum activity of a lectin-like bacterial serine protease family on human leukocytes. PLoS ONE 2014, 9, e107920. [Google Scholar] [CrossRef] [PubMed]
- Brockmeyer, J.; Bielaszewska, M.; Fruth, A.; Bonn, M.L.; Mellmann, A.; Humpf, H.-U.; Karch, H. Subtypes of the plasmid-encoded serine protease EspP in Shiga toxin-producing Escherichia coli: Distribution, secretion, and proteolytic activity. Appl. Environ. Microbiol. 2007, 73, 6351–6359. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Zhang, W.-L.; Hemmrich, U.; Jelacic, S.; Brunder, W.; Tarr, P.; Dobrindt, U.; Hacker, J.; Karch, H. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect. Immun. 2001, 69, 6863–6873. [Google Scholar] [CrossRef] [PubMed]
- Orth, D.; Ehrlenbach, S.; Brockmeyer, J.; Khan, A.B.; Huber, G.; Karch, H.; Sarg, B.; Lindner, H.; Würzner, R. EspP, a serine protease of enterohemorrhagic Escherichia coli, impairs complement activation by cleaving complement factors C3/C3b and C5. Infect. Immun. 2010, 78, 4294–4301. [Google Scholar] [CrossRef]
- Otto, B.R.; Van Dooren, S.J.; Dozois, C.M.; Luirink, J.; Oudega, B. Escherichia coli hemoglobin protease autotransporter contributes to synergistic abscess formation and heme-dependent growth of Bacteroides fragilis. Infect. Immun. 2002, 70, 5–10. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Pardo, M.; Montero, D.; Oñate, A.; Farfán, M.J.; Ruiz-Pérez, F.; Del Canto, F.; Vidal, R. TleA, a Tsh-like autotransporter identified in a human enterotoxigenic Escherichia coli strain. Infect. Immun. 2015, 83, 1893–1903. [Google Scholar] [CrossRef]
- Guyer, D.M.; Radulovic, S.; Jones, F.-E.; Mobley, H.L. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect. Immun. 2002, 70, 4539–4546. [Google Scholar] [CrossRef]
- Benjelloun-Touimi, Z.; Sansonetti, P.J.; Parsot, C. SepA, the major extracellular protein of Shigella flexneri: Autonomous secretion and involvement in tissue invasion. Mol. Microbiol. 1995, 17, 123–135. [Google Scholar] [CrossRef]
- Benjelloun-Touimi, Z.; Tahar, M.S.; Montecucco, C.; Sansonetti, P.J.; Parsot, C. SepA, the 110 kDa protein secreted by Shigella flexneri: Two-domain structure and proteolytic activity. Microbiology 1998, 144, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasani, K.; Henderson, I.R.; Sakellaris, H.; Rajakumar, K.; Grant, T.; Nataro, J.P.; Robins-Browne, R.; Adler, B. The sigA gene which is borne on the shepathogenicity island of Shigella flexneri 2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect. Immun. 2000, 68, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasani, K.; Navarro-Garcia, F.; Huerta, J.; Sakellaris, H.; Adler, B. The immunogenic SigA enterotoxin of Shigella flexneri 2a binds to HEp-2 cells and induces fodrin redistribution in intoxicated epithelial cells. PLoS ONE 2009, 4, e8223. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.T.; Kostakioti, M.; Henderson, I.R.; Stathopoulos, C. Common themes and variations in serine protease autotransporters. Trends Microbiol. 2008, 16, 370–379. [Google Scholar] [CrossRef]
- Sandt, C.H.; Hill, C.W. Four Different Genes Responsible for Nonimmune Immunoglobulin-Binding Activities within a Single Strain of Escherichia coli. Infect. Immun. 2000, 68, 2205–2214. [Google Scholar] [CrossRef]
- Restieri, C.; Garriss, G.; Locas, M.-C.; Dozois, C.M. Autotransporter-encoding sequences are phylogenetically distributed among Escherichia coli clinical isolates and reference strains. Appl. Environ. Microbiol. 2007, 73, 1553–1562. [Google Scholar] [CrossRef]
- Kumar, P.; Luo, Q.; Vickers, T.J.; Sheikh, A.; Lewis, W.G.; Fleckenstein, J.M. EatA, an immunogenic protective antigen of enterotoxigenic Escherichia coli, degrades intestinal mucin. Infect. Immun. 2014, 82, 500–508. [Google Scholar] [CrossRef]
- Del Canto, F.; Valenzuela, P.; Cantero, L.; Bronstein, J.; Blanco, J.E.; Blanco, J.; Prado, V.; Levine, M.; Nataro, J.; Sommerfelt, H. Distribution of classical and nonclassical virulence genes in enterotoxigenic Escherichia coli isolates from Chilean children and tRNA gene screening for putative insertion sites for genomic islands. J. Clin. Microbiol. 2011, 49, 3198–3203. [Google Scholar] [CrossRef]
- Andrade, F.B.; Abreu, A.G.; Nunes, K.O.; Gomes, T.A.; Piazza, R.M.; Elias, W.P. Distribution of serine protease autotransporters of Enterobacteriaceae in typical and atypical enteroaggregative Escherichia coli. Infect. Genet. Evol. 2017, 50, 83–86. [Google Scholar] [CrossRef]
- Mellies, J.L.; Navarro-Garcia, F.; Okeke, I.; Frederickson, J.; Nataro, J.P.; Kaper, J.B. espC pathogenicity island of enteropathogenic Escherichia coli encodes an enterotoxin. Infect. Immun. 2001, 69, 315–324. [Google Scholar] [CrossRef]
- Vidal, J.E.; Navarro-García, F. Efficient translocation of EspC into epithelial cells depends on enteropathogenic Escherichia coli and host cell contact. Infect. Immun. 2006, 74, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.E.; Navarro-García, F. EspC translocation into epithelial cells by enteropathogenic Escherichia coli requires a concerted participation of type V and III secretion systems. Cell. Microbiol. 2008, 10, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Guignot, J.; Segura, A.; Van Nhieu, G.T. The serine protease EspC from enteropathogenic Escherichia coli regulates pore formation and cytotoxicity mediated by the type III secretion system. PLoS Pathog. 2015, 11, e1005013. [Google Scholar] [CrossRef] [PubMed]
- Garmendia, J.; Frankel, G.; Crepin, V.F. Enteropathogenic and enterohemorrhagic Escherichia coli infections: Translocation, translocation, translocation. Infect. Immun. 2005, 73, 2573–2585. [Google Scholar] [CrossRef]
- Nemec, K.N.; Scaglione, P.; Navarro-García, F.; Huerta, J.; Tatulian, S.A.; Teter, K. A host-specific factor is necessary for efficient folding of the autotransporter plasmid-encoded toxin. Biochimie 2010, 92, 171–177. [Google Scholar] [CrossRef]
- Wagner, J.K.; Heindl, J.E.; Gray, A.N.; Jain, S.; Goldberg, M.B. Contribution of the periplasmic chaperone Skp to efficient presentation of the autotransporter IcsA on the surface of Shigella flexneri. J. Bacteriol. 2009, 191, 815–821. [Google Scholar] [CrossRef]
- Navarro-García, F.; Canizalez-Roman, A.; Luna, J.; Sears, C.; Nataro, J.P. Plasmid-Encoded Toxin of Enteroaggregative Escherichia coli is Internalized by Epithelial Cells. Infect. Immun. 2001, 69, 1053–1060. [Google Scholar] [CrossRef]
- Navarro-García, F.; Canizalez-Roman, A.; Burlingame, K.E.; Teter, K.; Vidal, J.E. Pet, a non-AB toxin, is transported and translocated into epithelial cells by a retrograde trafficking pathway. Infect. Immun. 2007, 75, 2101–2109. [Google Scholar] [CrossRef]
- Dutta, P.R.; Sui, B.Q.; Nataro, J.P. Structure-function analysis of the enteroaggregative Escherichia coli plasmid-encoded toxin autotransporter using scanning linker mutagenesis. J. Biol. Chem. 2003, 278, 39912–39920. [Google Scholar] [CrossRef]
- Nava-Acosta, R.; Navarro-Garcia, F. Cytokeratin 8 is an epithelial cell receptor for Pet, a cytotoxic serine protease autotransporter of Enterobacteriaceae. MBio 2013, 4, e00838-13. [Google Scholar] [CrossRef]
- Chavez-Dueñas, L.; Serapio-Palacios, A.; Nava-Acosta, R.; Navarro-Garcia, F. The subdomain 2 of the autotransporter Pet is the ligand site for recognizing Pet receptor on the epithelial cell surface. Infect. Immun. 2016, 84, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Villaseca, J.M.; Navarro-García, F.; Mendoza-Hernández, G.; Nataro, J.P.; Cravioto, A.; Eslava, C. Pet toxin from enteroaggregative Escherichia coli produces cellular damage associated with fodrin disruption. Infect. Immun. 2000, 68, 5920–5927. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; O’Brien, G.A.; Nishioka, W.K.; McGahon, A.J.; Mahboubi, A.; Saido, T.C.; Green, D.R. Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J. Biol. Chem. 1995, 270, 6425–6428. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.K.; Posmantur, R.; Nath, R.; McGinnis, K.; Whitton, M.; Talanian, R.V.; Glantz, S.B.; Morrow, J.S. Simultaneous degradation of αII-and βII-spectrin by caspase 3 (CPP32) in apoptotic cells. J. Biol. Chem. 1998, 273, 22490–22497. [Google Scholar] [CrossRef]
- Sanchez-Villamil, J.I.; Navarro-Garcia, F.; Castillo-Romero, A.; Gutierrez-Gutierrez, F.; Tapia-Pastrana, G. Curcumin blocks cytotoxicity of enteroaggregative and enteropathogenic Escherichia coli by blocking Pet and EspC proteolytic release from bacterial outer membrane. Front. Cell. Infect. Microbiol. 2019, 9, 334. [Google Scholar] [CrossRef]
- Abreu, A.G.; Abe, C.M.; Nunes, K.O.; Moraes, C.T.; Chavez-Dueñas, L.; Navarro-Garcia, F.; Barbosa, A.S.; Piazza, R.M.; Elias, W.P. The serine protease Pic as a virulence factor of atypical enteropathogenic Escherichia coli. Gut Microbes 2016, 7, 115–125. [Google Scholar] [CrossRef]
- Harrington, S.M.; Sheikh, J.; Henderson, I.R.; Ruiz-Perez, F.; Cohen, P.S.; Nataro, J.P. The Pic protease of enteroaggregative Escherichia coli promotes intestinal colonization and growth in the presence of mucin. Infect. Immun. 2009, 77, 2465–2473. [Google Scholar] [CrossRef]
- Navarro-Garcia, F.; Gutierrez-Jimenez, J.; Garcia-Tovar, C.; Castro, L.A.; Salazar-Gonzalez, H.; Cordova, V. Pic, an autotransporter protein secreted by different pathogens in the Enterobacteriaceae family, is a potent mucus secretagogue. Infect. Immun. 2010, 78, 4101–4109. [Google Scholar] [CrossRef]
- Abreu, A.G.; Fraga, T.R.; Granados Martínez, A.P.; Kondo, M.Y.; Juliano, M.A.; Juliano, L.; Navarro-Garcia, F.; Isaac, L.; Barbosa, A.S.; Elias, W.P. The serine protease Pic from enteroaggregative Escherichia coli mediates immune evasion by the direct cleavage of complement proteins. J. Infect. Dis. 2015, 212, 106–115. [Google Scholar] [CrossRef]
- Bhullar, K.; Zarepour, M.; Yu, H.; Yang, H.; Croxen, M.; Stahl, M.; Finlay, B.B.; Turvey, S.E.; Vallance, B.A. The serine protease autotransporter pic modulates citrobacter rodentium pathogenesis and its innate recognition by the host. Infect. Immun. 2015, 83, 2636–2650. [Google Scholar] [CrossRef][Green Version]
- Brockmeyer, J.; Spelten, S.; Kuczius, T.; Bielaszewska, M.; Karch, H. Structure and function relationship of the autotransport and proteolytic activity of EspP from Shiga toxin-producing Escherichia coli. PLoS ONE 2009, 4, e6100. [Google Scholar] [CrossRef] [PubMed]
- Djafari, S.; Ebel, F.; Deibel, C.; Krämer, S.; Hudel, M.; Chakraborty, T. Characterization of an exported protease from Shiga toxin-producing Escherichia coli. Mol. Microbiol. 1997, 25, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Boisen, N.; Ruiz-Perez, F.; Scheutz, F.; Krogfelt, K.A.; Nataro, J.P. High prevalence of serine protease autotransporter cytotoxins among strains of enteroaggregative Escherichia coli. Am. J. Trop. Med. Hyg. 2009, 80, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Velarde, J.J.; Nataro, J.P. Hydrophobic residues of the autotransporter EspP linker domain are important for outer membrane translocation of its passenger. J. Biol. Chem. 2004, 279, 31495–31504. [Google Scholar] [CrossRef]
- Ruiz-Perez, F.; Henderson, I.R.; Nataro, J.P. Interaction of FkpA, a peptidyl-prolyl cis/trans isomerase with EspP autotransporter protein. Gut Microbes 2010, 1, 339–344. [Google Scholar] [CrossRef]
- Xicohtencatl-Cortes, J.; Saldaña, Z.; Deng, W.; Castañeda, E.; Freer, E.; Tarr, P.I.; Finlay, B.B.; Puente, J.L.; Girón, J.A. Bacterial macroscopic rope-like fibers with cytopathic and adhesive properties. J. Biol. Chem. 2010, 285, 32336–32342. [Google Scholar] [CrossRef]
- Khan, A.B.; Naim, A.; Orth, D.; Grif, K.; Mohsin, M.; Prager, R.; Dierich, M.P.; Würzner, R. Serine protease espP subtype α, but not β or γ, of Shiga toxin-producing Escherichia coli is associated with highly pathogenic serogroups. Int. J. Med Microbiol. 2009, 299, 247–254. [Google Scholar] [CrossRef]
- Weiss, A.; Joerss, H.; Brockmeyer, J. Structural and functional characterization of cleavage and inactivation of human serine protease inhibitors by the bacterial SPATE protease EspPα from enterohemorrhagic E. coli. PLoS ONE 2014, 9, e111363. [Google Scholar] [CrossRef]
- Tse, C.; In, J.; Yin, J.; Donowitz, M.; Doucet, M.; Foulke-Abel, J.; Ruiz-Perez, F.; Nataro, J.; Zachos, N.; Kaper, J. Enterohemorrhagic E. coli (EHEC)—Secreted Serine Protease EspP Stimulates Electrogenic Ion Transport in Human Colonoid Monolayers. Toxins 2018, 10, 351. [Google Scholar] [CrossRef]
- Provence, D.L.; Curtiss, R. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect. Immun. 1994, 62, 1369–1380. [Google Scholar]
- Nicholson, B.A.; West, A.C.; Mangiamele, P.; Barbieri, N.; Wannemuehler, Y.; Nolan, L.K.; Logue, C.M.; Li, G. Genetic characterization of ExPEC-like virulence plasmids among a subset of NMEC. PLoS ONE 2016, 11, e0147757. [Google Scholar] [CrossRef] [PubMed]
- Kostakioti, M.; Stathopoulos, C. Functional analysis of the Tsh autotransporter from an avian pathogenic Escherichia coli strain. Infect. Immun. 2004, 72, 5548–5554. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, C.; Provence, D.L.; Curtiss, R. Characterization of the Avian Pathogenic Escherichia coli Hemagglutinin Tsh, a Member of the Immunoglobulin A Protease-Type Family of Autotransporters. Infect. Immun. 1999, 67, 772–781. [Google Scholar] [PubMed]
- Maluta, R.P.; Gatti, M.S.V.; Joazeiro, P.P.; De Paiva, J.B.; Rojas, T.C.G.; Silveira, F.; Houle, S.; Kobayashi, R.K.T.; Dozois, C.M.; Dias da Silveira, W. Avian extraintestinal Escherichia coli exhibits enterotoxigenic-like activity in the in vivo rabbit ligated ileal loop assay. Foodborne Pathog. Dis. 2014, 11, 484–489. [Google Scholar] [CrossRef]
- Nichols, K.B.; Totsika, M.; Moriel, D.G.; Lo, A.W.; Yang, J.; Wurpel, D.J.; Rossiter, A.E.; Strugnell, R.A.; Henderson, I.R.; Ulett, G.C. Molecular characterization of the vacuolating autotransporter toxin in uropathogenic Escherichia coli. J. Bacteriol. 2016, 198, 1487–1498. [Google Scholar] [CrossRef]
- Subashchandrabose, S.; Smith, S.N.; Spurbeck, R.R.; Kole, M.M.; Mobley, H.L. Genome-wide detection of fitness genes in uropathogenic Escherichia coli during systemic infection. PLoS Pathog. 2013, 9, e1003788. [Google Scholar] [CrossRef]
- Gibold, L.; Garenaux, E.; Dalmasso, G.; Gallucci, C.; Cia, D.; Mottet-Auselo, B.; Faïs, T.; Darfeuille-Michaud, A.; Nguyen, H.T.T.; Barnich, N. The Vat-AIEC protease promotes crossing of the intestinal mucus layer by Crohn’s disease-associated Escherichia coli. Cell. Microbiol. 2016, 18, 617–631. [Google Scholar] [CrossRef]
- Tapader, R.; Chatterjee, S.; Singh, A.; Dayma, P.; Haldar, S.; Pal, A.; Basu, S. The high prevalence of serine protease autotransporters of Enterobacteriaceae (SPATEs) in Escherichia coli causing neonatal septicemia. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2015–2024. [Google Scholar] [CrossRef]
- Liévin-Le Moal, V.; Comenge, Y.; Ruby, V.; Amsellem, R.; Nicolas, V.; Servin, A.L. Secreted autotransporter toxin (Sat) triggers autophagy in epithelial cells that relies on cell detachment. Cell. Microbiol. 2011, 13, 992–1013. [Google Scholar] [CrossRef]
- Maroncle, N.M.; Sivick, K.E.; Brady, R.; Stokes, F.-E.; Mobley, H.L. Protease activity, secretion, cell entry, cytotoxicity, and cellular targets of secreted autotransporter toxin of uropathogenic Escherichia coli. Infect. Immun. 2006, 74, 6124–6134. [Google Scholar] [CrossRef]
- Guignot, J.; Chaplais, C.; Coconnier-Polter, M.H.; Servin, A.L. The secreted autotransporter toxin, Sat, functions as a virulence factor in Afa/Dr diffusely adhering Escherichia coli by promoting lesions in tight junction of polarized epithelial cells. Cell. Microbiol. 2007, 9, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Taddei, C.R.; Fasano, A.; Ferreira, A.J.; Trabulsi, L.R.; Martinez, M.B. Secreted autotransporter toxin produced by a diffusely adhering Escherichia coli strain causes intestinal damage in animal model assays. FEMS Microbiol. Lett. 2005, 250, 263–269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Toloza, L.; Giménez, R.; Fábrega, M.J.; Alvarez, C.S.; Aguilera, L.; Cañas, M.A.; Martín-Venegas, R.; Badia, J.; Baldomà, L. The secreted autotransporter toxin (Sat) does not act as a virulence factor in the probiotic Escherichia coli strain Nissle 1917. BMC Microbiol. 2015, 15, 250. [Google Scholar] [CrossRef] [PubMed]
- Coron, E.; Flamant, M.; Aubert, P.; Wedel, T.; Pedron, T.; Letessier, E.; Galmiche, J.P.; Sansonetti, P.J.; Neunlist, M. Characterisation of early mucosal and neuronal lesions following Shigella flexneri infection in human colon. PLoS ONE 2009, 4, e4713. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Contreras, A.; Birtley, J.R.; Boll, E.; Zhao, Y.; Mumy, K.L.; Toscano, J.; Ayehunie, S.; Reinecker, H.-C.; Stern, L.J.; McCormick, B.A. Shigella depends on SepA to destabilize the intestinal epithelial integrity via cofilin activation. Gut Microbes 2017, 8, 544–560. [Google Scholar] [CrossRef]
- Huang, T.Y.; DerMardirossian, C.; Bokoch, G.M. Cofilin phosphatases and regulation of actin dynamics. Curr. Opin. Cell Biol. 2006, 18, 26–31. [Google Scholar] [CrossRef]
- Scorza, F.B.; Colucci, A.M.; Maggiore, L.; Sanzone, S.; Rossi, O.; Ferlenghi, I.; Pesce, I.; Caboni, M.; Norais, N.; Di Cioccio, V. High yield production process for Shigella outer membrane particles. PLoS ONE 2012, 7, e35616. [Google Scholar]
- Oany, A.R.; Pervin, T.; Mia, M.; Hossain, M.; Shahnaij, M.; Mahmud, S.; Kibria, K. Vaccinomics approach for designing potential peptide vaccine by targeting Shigella spp. serine protease autotransporter subfamily protein SigA. J. Immunol. Res. 2017, 2017, 6412353. [Google Scholar] [CrossRef]
- Fookes, M.; Schroeder, G.N.; Langridge, G.C.; Blondel, C.J.; Mammina, C.; Connor, T.R.; Seth-Smith, H.; Vernikos, G.S.; Robinson, K.S.; Sanders, M. Salmonella bongori provides insights into the evolution of the Salmonellae. PLoS Pathog. 2011, 7, e1002191. [Google Scholar] [CrossRef]
- Ebel, F.; Deibel, C.; Kresse, A.U.; Guzman, C.A.; Chakraborty, T. Temperature-and medium-dependent secretion of proteins by Shiga toxin-producing Escherichia coli. Infect. Immun. 1996, 64, 4472–4479. [Google Scholar]
- Brockmeyer, J.; Aldick, T.; Soltwisch, J.; Zhang, W.; Tarr, P.I.; Weiss, A.; Dreisewerd, K.; Müthing, J.; Bielaszewska, M.; Karch, H. Enterohaemorrhagic Escherichia coli haemolysin is cleaved and inactivated by serine protease EspPα. Environ. Microbiol. 2011, 13, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, D.; Umanski, T.; Fang, Y.; Rosenshine, I. Hierarchy in the expression of the locus of enterocyte effacement genes of enteropathogenic Escherichia coli. Mol. Microbiol. 1999, 34, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.J.; Sperandio, V.; Girón, J.A.; Shin, S.; Mellies, J.L.; Wainwright, L.; Hutcheson, S.W.; McDaniel, T.K.; Kaper, J.B. The Locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE-and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 2000, 68, 6115–6126. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K.; Rouviere-Yaniv, J. Histonelike proteins of bacteria. Microbiol. Rev. 1987, 51, 301. [Google Scholar]
- Workman, J.; Kingston, R. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu. Rev. Biochem. 1998, 67, 545–579. [Google Scholar] [CrossRef]
- Anuchin, A.; Goncharenko, A.; Demidenok, O.; Kaprelyants, A. Histone-like proteins of bacteria (review). Appl. Biochem. Microbiol. 2011, 47, 580–585. [Google Scholar] [CrossRef]
- Allsopp, L.P.; Beloin, C.; Ulett, G.C.; Valle, J.; Totsika, M.; Sherlock, O.; Ghigo, J.-M.; Schembri, M.A. Molecular characterization of UpaB and UpaC, two new autotransporter proteins of uropathogenic Escherichia coli CFT073. Infect. Immun. 2012, 80, 321–332. [Google Scholar] [CrossRef]
- Allsopp, L.P.; Beloin, C.; Moriel, D.G.; Totsika, M.; Ghigo, J.-M.; Schembri, M.A. Functional heterogeneity of the UpaH autotransporter protein from uropathogenic Escherichia coli. J. Bacteriol. 2012, 194, 5769–5782. [Google Scholar] [CrossRef]
- Totsika, M.; Wells, T.J.; Beloin, C.; Valle, J.; Allsopp, L.P.; King, N.P.; Ghigo, J.-M.; Schembri, M.A. Molecular characterization of the EhaG and UpaG trimeric autotransporter proteins from pathogenic Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 2179–2189. [Google Scholar] [CrossRef]
- Münch, R.; Hiller, K.; Grote, A.; Scheer, M.; Klein, J.; Schobert, M.; Jahn, D. Virtual Footprint and PRODORIC: An integrative framework for regulon prediction in prokaryotes. Bioinformatics 2005, 21, 4187–4189. [Google Scholar] [CrossRef]
- George, A.; Levy, S. Gene in the major cotransduction gap of the Escherichia coli K-12 linkage map required for the expression of chromosomal resistance to tetracycline and other antibiotics. J. Bacteriol. 1983, 155, 541–548. [Google Scholar] [PubMed]
- Cohen, S.P.; McMurry, L.; Hooper, D.; Wolfson, J.; Levy, S. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: Decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob. Agents Chemother. 1989, 33, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- George, A.M.; Levy, S.B. Amplifiable resistance to tetracycline, chloramphenicol, and other antibiotics in Escherichia coli: Involvement of a non-plasmid-determined efflux of tetracycline. J. Bacteriol. 1983, 155, 531–540. [Google Scholar] [PubMed]
- Asako, H.; Nakajima, H.; Kobayashi, K.; Kobayashi, M.; Aono, R. Organic solvent tolerance and antibiotic resistance increased by overexpression of marA in Escherichia coli. Appl. Environ. Microbiol. 1997, 63, 1428–1433. [Google Scholar] [PubMed]
- Ariza, R.; Cohen, S.; Bachhawat, N.; Levy, S.; Demple, B. Repressor mutations in the marRAB operon that activate oxidative stress genes and multiple antibiotic resistance in Escherichia coli. J. Bacteriol. 1994, 176, 143–148. [Google Scholar] [CrossRef]
- Cohen, S.P.; Hächler, H.; Levy, S. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J. Bacteriol. 1993, 175, 1484–1492. [Google Scholar] [CrossRef]
- Simms, A.N.; Mobley, H.L. PapX, a P fimbrial operon-encoded inhibitor of motility in uropathogenic Escherichia coli. Infect. Immun. 2008, 76, 4833–4841. [Google Scholar] [CrossRef][Green Version]
- Gosset, G.; Zhang, Z.; Nayyar, S.; Cuevas, W.A.; Saier, M.H. Transcriptome analysis of Crp-dependent catabolite control of gene expression in Escherichia coli. J. Bacteriol. 2004, 186, 3516–3524. [Google Scholar] [CrossRef]
- Green, J.; Stapleton, M.R.; Smith, L.J.; Artymiuk, P.J.; Kahramanoglou, C.; Hunt, D.M.; Buxton, R.S. Cyclic-AMP and bacterial cyclic-AMP receptor proteins revisited: Adaptation for different ecological niches. Curr. Opin. Microbiol. 2014, 18, 1–7. [Google Scholar] [CrossRef]
- Won, H.-S.; Lee, Y.-S.; Lee, S.-H.; Lee, B.-J. Structural overview on the allosteric activation of cyclic AMP receptor protein. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2009, 1794, 1299–1308. [Google Scholar] [CrossRef]
- Rossiter, A.E.; Browning, D.F.; Leyton, D.L.; Johnson, M.D.; Godfrey, R.E.; Wardius, C.A.; Desvaux, M.; Cunningham, A.F.; Ruiz-Perez, F.; Nataro, J.P. Transcription of the plasmid-encoded toxin gene from Enteroaggregative Escherichia coli is regulated by a novel co-activation mechanism involving CRP and Fis. Mol. Microbiol. 2011, 81, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Kahmann, R. Purification and properties of the Escherichia coli host factor required for inversion of the G segment in bacteriophage Mu. J. Biol. Chem. 1986, 261, 15673–15678. [Google Scholar] [PubMed]
- Travers, A.; Schneider, R.; Muskhelishvili, G. DNA supercoiling and transcription in Escherichia coli: The FIS connection. Biochimie 2001, 83, 213–217. [Google Scholar] [CrossRef]
- Kahramanoglou, C.; Seshasayee, A.S.; Prieto, A.I.; Ibberson, D.; Schmidt, S.; Zimmermann, J.; Benes, V.; Fraser, G.M.; Luscombe, N.M. Direct and indirect effects of H-NS and Fis on global gene expression control in Escherichia coli. Nucleic Acids Res. 2010, 39, 2073–2091. [Google Scholar] [CrossRef] [PubMed]
- Rossiter, A.E.; Godfrey, R.E.; Connolly, J.A.; Busby, S.J.; Henderson, I.R.; Browning, D.F. Expression of different bacterial cytotoxins is controlled by two global transcription factors, CRP and Fis, that co-operate in a shared-recruitment mechanism. Biochem. J. 2015, 466, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Beutin, L.; Karch, H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157: H7 strain EDL 933. Infect. Immun. 1995, 63, 1055–1061. [Google Scholar] [PubMed]
- Welch, R. RTX toxin structure and function: A story of numerous anomalies and few analogies in toxin biology. In Pore-Forming Toxins; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
| SPATEs | Organism a | Biological Functions | References |
|---|---|---|---|
| EatA | ETEC | Enterotoxin | [54] |
| EspC | EPEC | Cytotoxin, Enterotoxin Cleavage of fodrin, hemoglobin, pepsin, coagulation factor V, translocator components (EspA/EspD) of T3SSCell rounding and cell detachment | [41,55,56,57,58] |
| Pet | EAEC | Mucosal cytoxicity, Cleavage of spectrin, pepsin, factor V | [25,41,59,60,61] |
| Pic | Shigella, EAEC | Serum resistance Mucinase, Hemagglutination Colonization, Cleavage of gelatin, factor V, O-glycans: PSGL-1, CD44, CD45, CD93 and CX3CL1 | [39,41,42,52,62,63] |
| EspP | EHEC, STEC | Cleaved pepsin, factor V, apolipoprotein, complement factors: C3/C3b and C5 | [26,41,64,65,66] |
| Tsh/Hbp | APEC | Hemagglutinin, Binding to Caco-2 cells and to EMPs (laminin, fibronectin, and collagen IV) and heme Cleavage of mucin, factor V and O-glycosylated proteins in leukocyte | [37,41,63,67] |
| Sha | APEC, UPEC | Autoaggregation, hemagglutination, biofilm formation, proteolytic activity on synthetic peptide: N-Succinyl-Ala-Ala-Ala-p-nitroanilide, adherence and cytopathic effects on bladder epithelial cell line | [31] |
| TleA | Binding to Caco-2 cells Cleavage of bovine submaxillary mucin, leukocyte surface glycoproteins CD45 and P-selectin glycoprotein ligand 1 | [68] | |
| Vat | APEC, UPEC | Vacuolating cytotoxin, Agglutinate leukocyte Cleavage of O-glycosylated proteins in leukocyte | [46,63] |
| Sat | UPEC | Vacuolating cytotoxin on HK-2, HEp-2 and Vero monkey kidney cells Cleavage of casein, factor V and spectrin | [41,45,69] |
| SepA | Shigella flexneri | Intestinal inflammation, proteolytic activity toward synthetic peptides: Suc-Ala-Ala-Pro-Phe-pNA, Suc-Val-Pro-Phe-pNA and Suc-Phe-Leu-Phe-pNA | [70,71] |
| SigA | Shigella flexneri | Cytotoxin, Cleavage of casein, recombinant human α II spectrin Cell rounding and cell detachment | [72,73] |
| Boa | Salmonella bongori | Unknown | [74] |
| TagBC | UPEC, APEC | Autoaggregation, proteolytic effect on synthetic peptide: N-Benzoyl-L-arginine 4-nitroanilide cytopathic effect on human bladder cell lines | [31] |
| SPATEs | Potential H-NS Binding Sites |
|---|---|
| boa | −97GCAATAAACC−88 (−), −96GCAATAAAAT−87 (−),−80GCTATAAAAA−71 (−) |
| sigA | −179TGGTTAGATA−170 (−),−170GTGATTGATT−161 (−), −19CCGATATTTC−10 (−) |
| pic | −159CAGATAAAAC−150 (+), −109TGCATTAATG−100 (−), −35GGGATATAAA−26 (−) |
| sepA | −176ATGATAAAAA−167(+), −35AAGATTAATT−26 (−) |
| tsh/hbp | −164CACATAAAGT−155 (−), −28AAAATAAAAT−19 (−), −10GTAATTAAAA−1 (+) |
| espC | −300ACCATTAAAA−291 (+), −299CCATTAAAAT−290 (+), −111GCCACAAACT−102 (−) |
| espP | −280TCGATTGTTA−271 (−), −96CAGATAAATG−87 (−), −46CTGATACATT−37 (+) |
| pet | −177ATGATTAATT−168 (+),−42AGGATTAAGA−33 (−),−24TCAATAAATG−15 (+) |
| sat | −177ACGATCAATT−168 (+),−166ACGATCAATT−157 (+),−24TCAATAAATG−15 (+) |
| eatA | −88GCTATCTATT−79 (+),−71ACAATAAATG−62 (+),−40TCCACACAAC−31 (−) |
| eaaA | −314ACCATACAGC−305 (−),−124GCGGTAAAAA−115 (−) |
| tagB | −304ACGAAAAAAA−295 (−),−161CTGATAAATA−152 (−),−128TCGATAAATG−119 (+) |
| tagC | −256GCAATTAATA−247 (+),−62TCGCTATATT−53 (+),−56ACTATAAATA−47 (−) |
| sha | −187CCCACAAATC−178 (−),−48TCCTTATATT−39 (+),−32TCAATAGATA−23 (−) |
| vat | −296TCCATATATC−287 (+),−295TGGATATATG−286 (−),−107GCTATATAAT−98 (−) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokharel, P.; Habouria, H.; Bessaiah, H.; Dozois, C.M. Serine Protease Autotransporters of the Enterobacteriaceae (SPATEs): Out and About and Chopping It Up. Microorganisms 2019, 7, 594. https://doi.org/10.3390/microorganisms7120594
Pokharel P, Habouria H, Bessaiah H, Dozois CM. Serine Protease Autotransporters of the Enterobacteriaceae (SPATEs): Out and About and Chopping It Up. Microorganisms. 2019; 7(12):594. https://doi.org/10.3390/microorganisms7120594
Chicago/Turabian StylePokharel, Pravil, Hajer Habouria, Hicham Bessaiah, and Charles M. Dozois. 2019. "Serine Protease Autotransporters of the Enterobacteriaceae (SPATEs): Out and About and Chopping It Up" Microorganisms 7, no. 12: 594. https://doi.org/10.3390/microorganisms7120594
APA StylePokharel, P., Habouria, H., Bessaiah, H., & Dozois, C. M. (2019). Serine Protease Autotransporters of the Enterobacteriaceae (SPATEs): Out and About and Chopping It Up. Microorganisms, 7(12), 594. https://doi.org/10.3390/microorganisms7120594







