Structural Insights into Escherichia coli Shiga Toxin (Stx) Glycosphingolipid Receptors of Porcine Renal Epithelial Cells and Inhibition of Stx-Mediated Cellular Injury Using Neoglycolipid-Spiked Glycovesicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of LLC-PK1 and PK-15 Cells
2.2. Preparation of the Neoglycolipids Gb3-PE and Gb4-PE
2.3. Production of Neoglycolipid- and Gb3Cer-Loaded Glycovesicles
2.4. Stx2e Cytotoxicity and Stx2e Inhibition Assay
2.5. Isolation of Neutral GSLs from LLC-PK1 and PK-15 Cells
2.6. Stx1a, Stx2a, Stx2e and Anti-Stx, Anti-GSL, and Secondary Antibodies
2.7. Thin-Layer Chromatography and Overlay Assay
2.8. Structural Characterization of GSLs and Neoglycolipids by Mass Spectrometry
3. Results
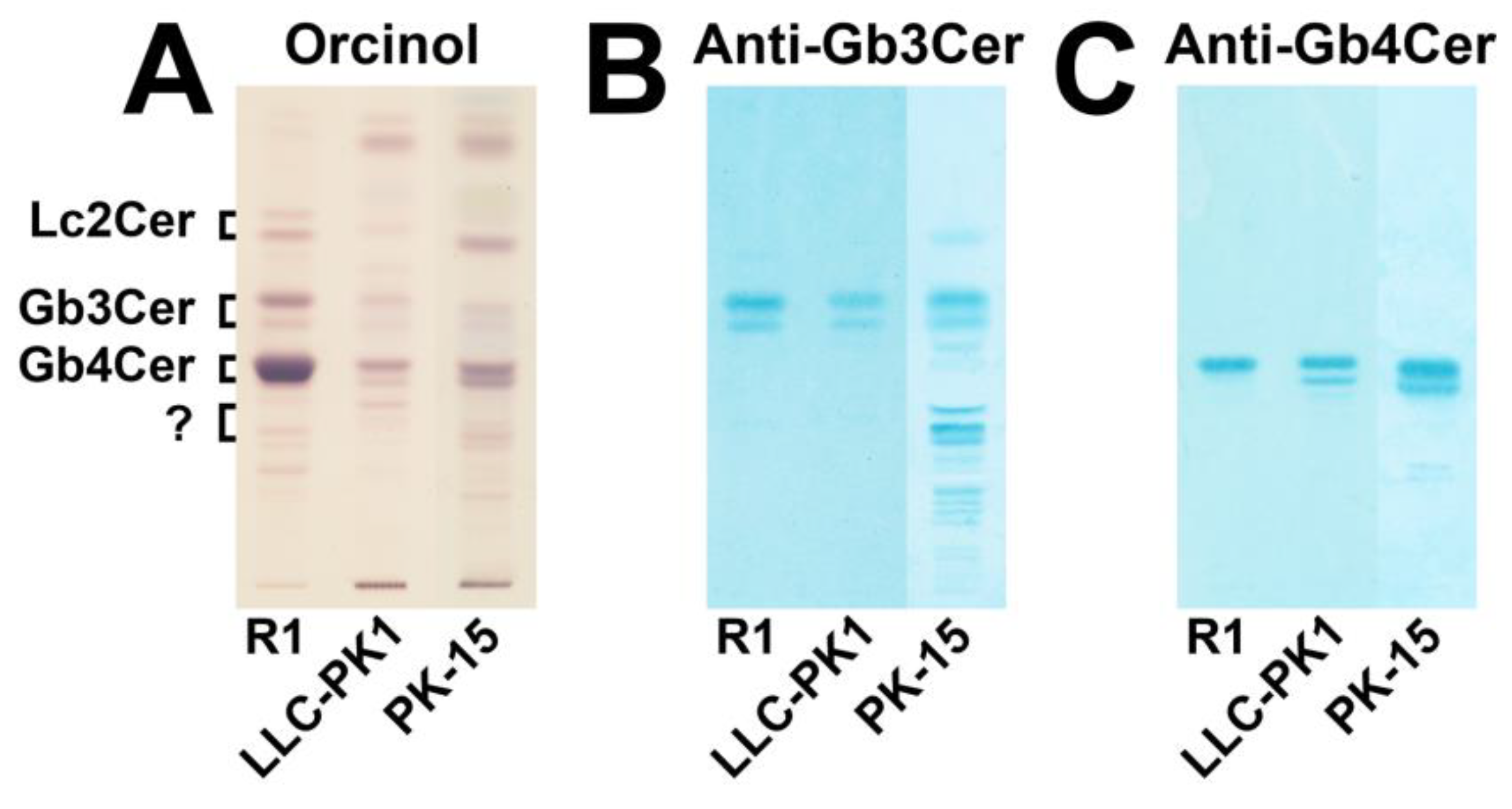
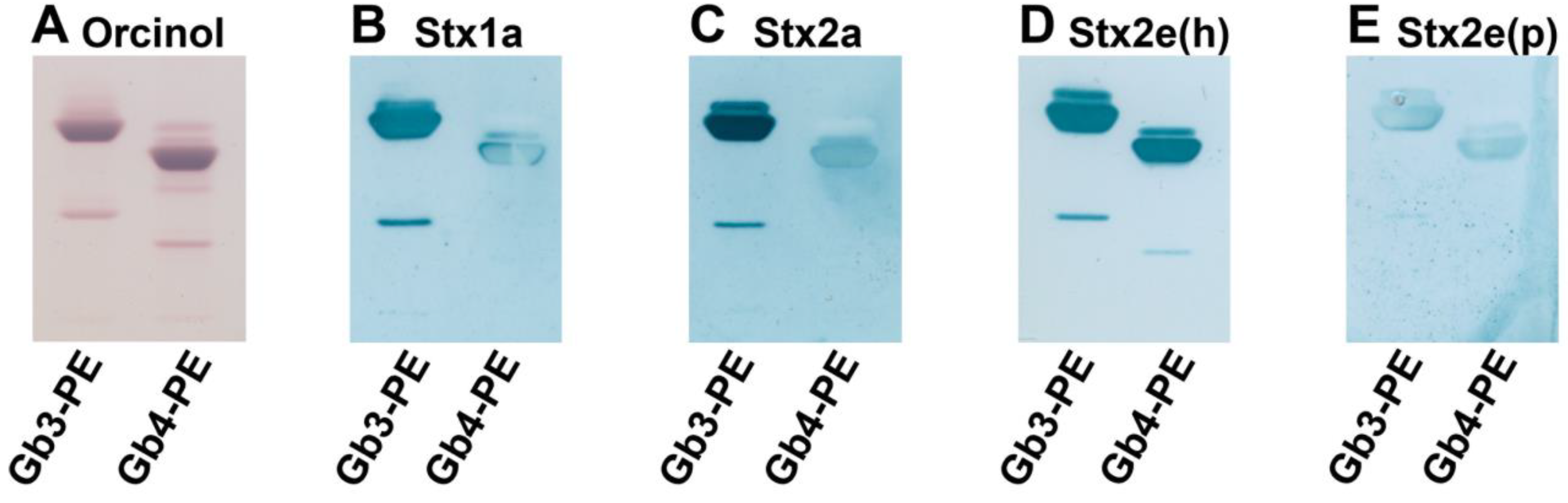
3.1. Immunochemical Detection of Gb3Cer and Gb4Cer in GSL Preparations of LLC-PK1 and PK-15 Cells
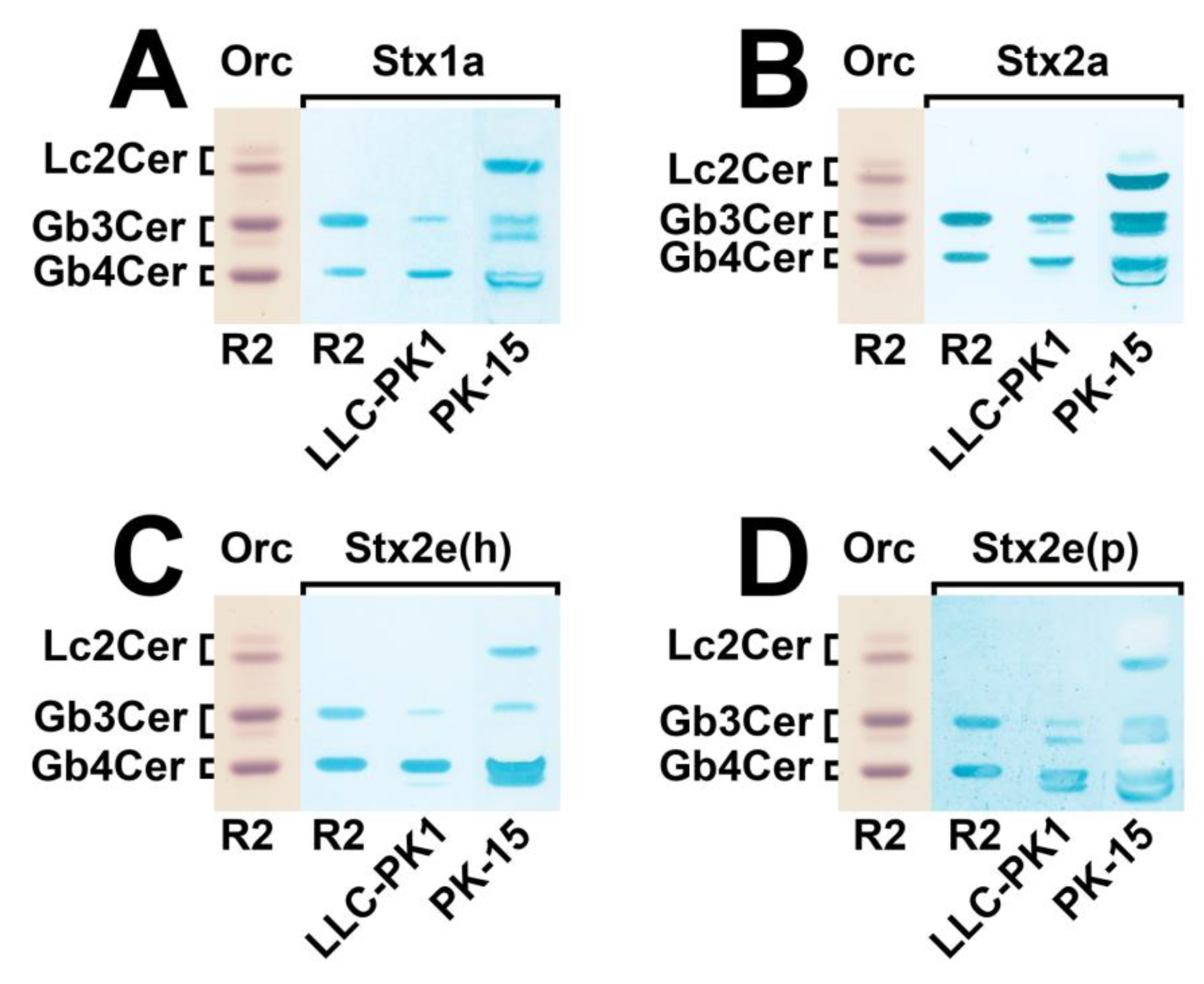
3.2. Identification of Stx-Binding GSLs Expressed by LLC-PK1 and PK-15 Cells
3.3. Structural Characterization of Stx Receptors Gb3Cer and Gb4Cer of LLC-PK1 and PK-15 Cells
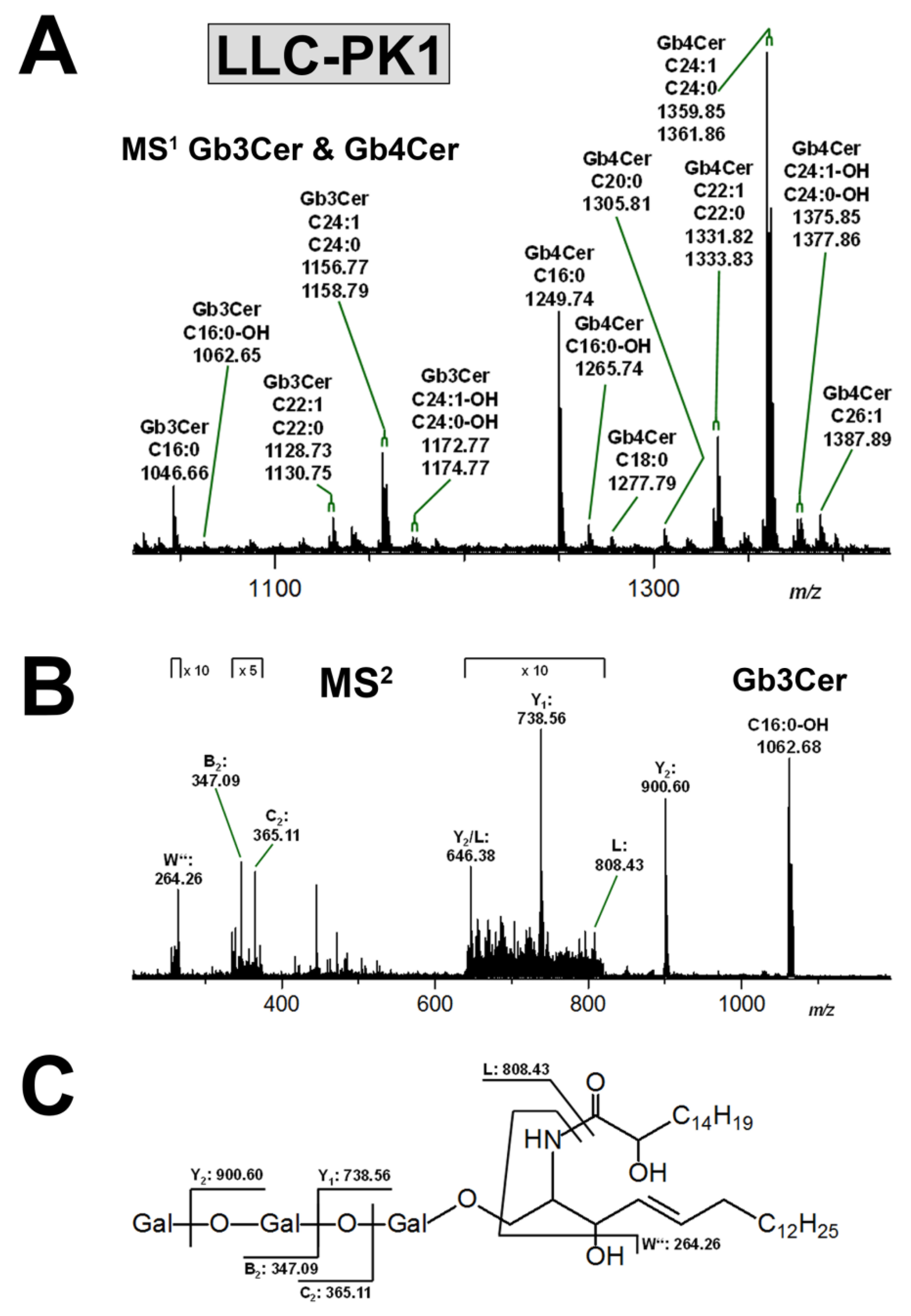
3.3.1. Gb3Cer and Gb4Cer Lipoforms of LLC-PK1 Cells
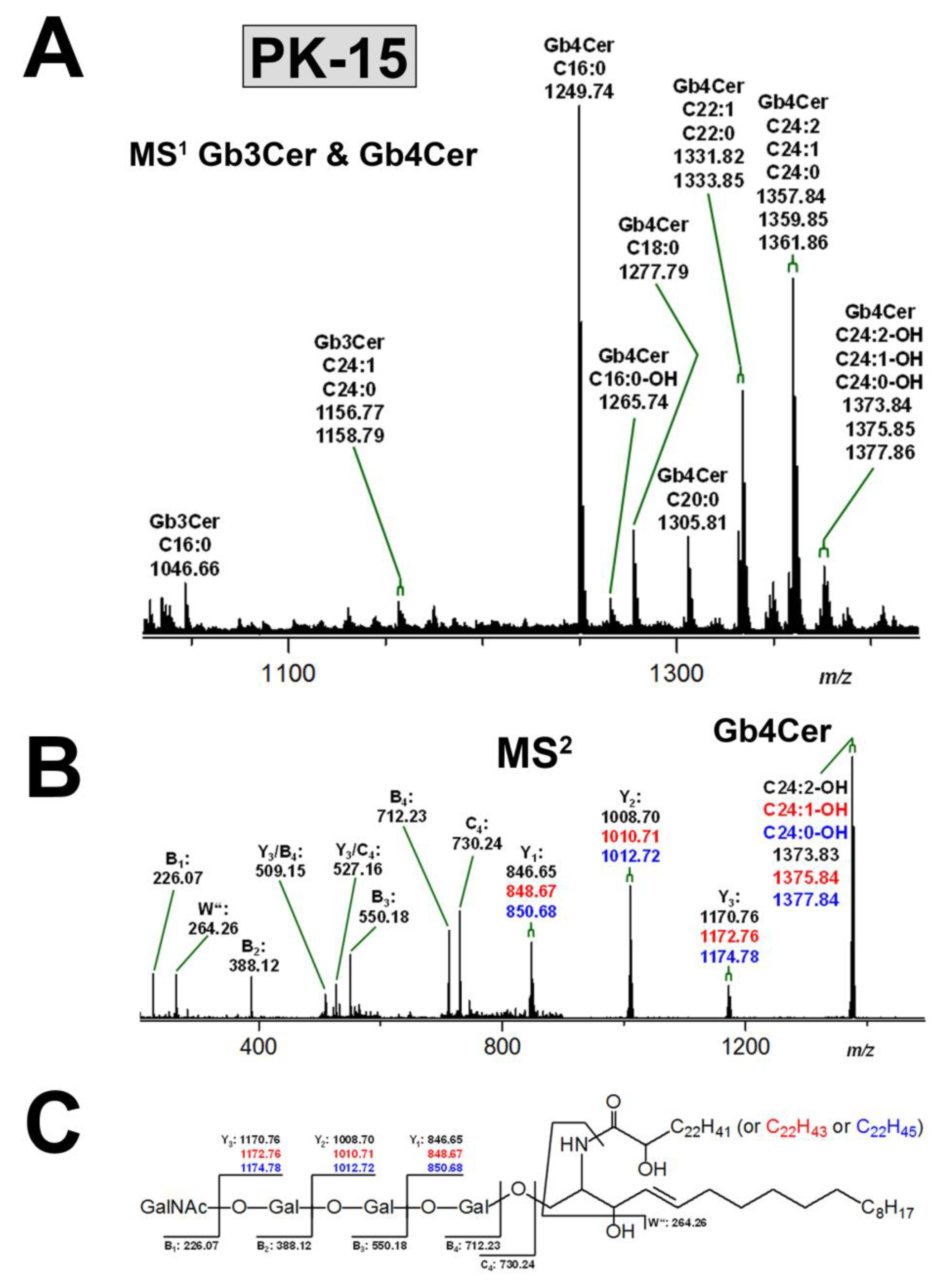
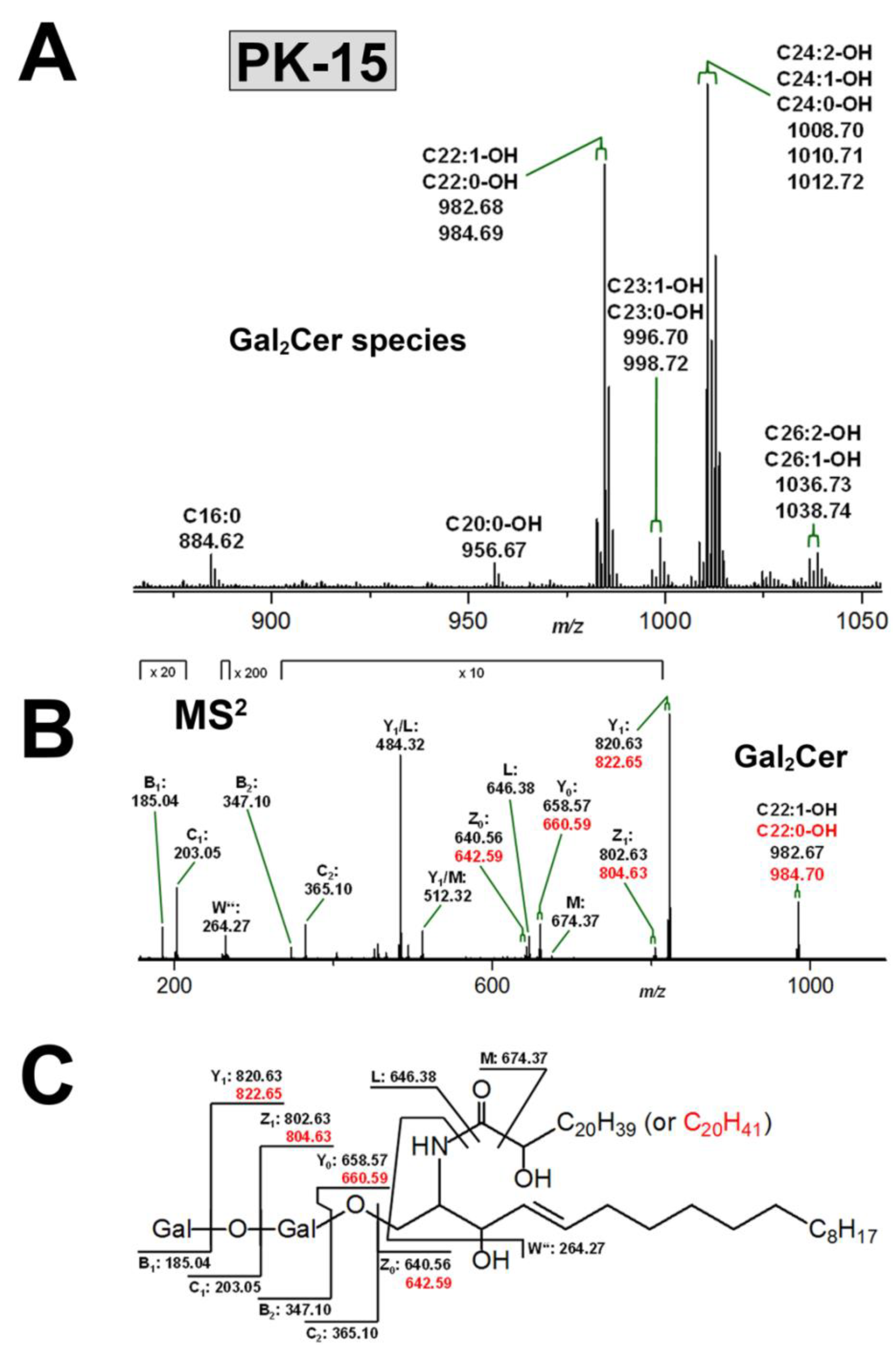
3.3.2. Gb3Cer and Gb4Cer Lipoforms of PK-15 Cells
3.4. Structural Characterization of Stx Receptor Galabiosylceramide of PK-15 Cells
3.5. Neoglycolipids as Receptors and Potential Inhibitors of Stxs
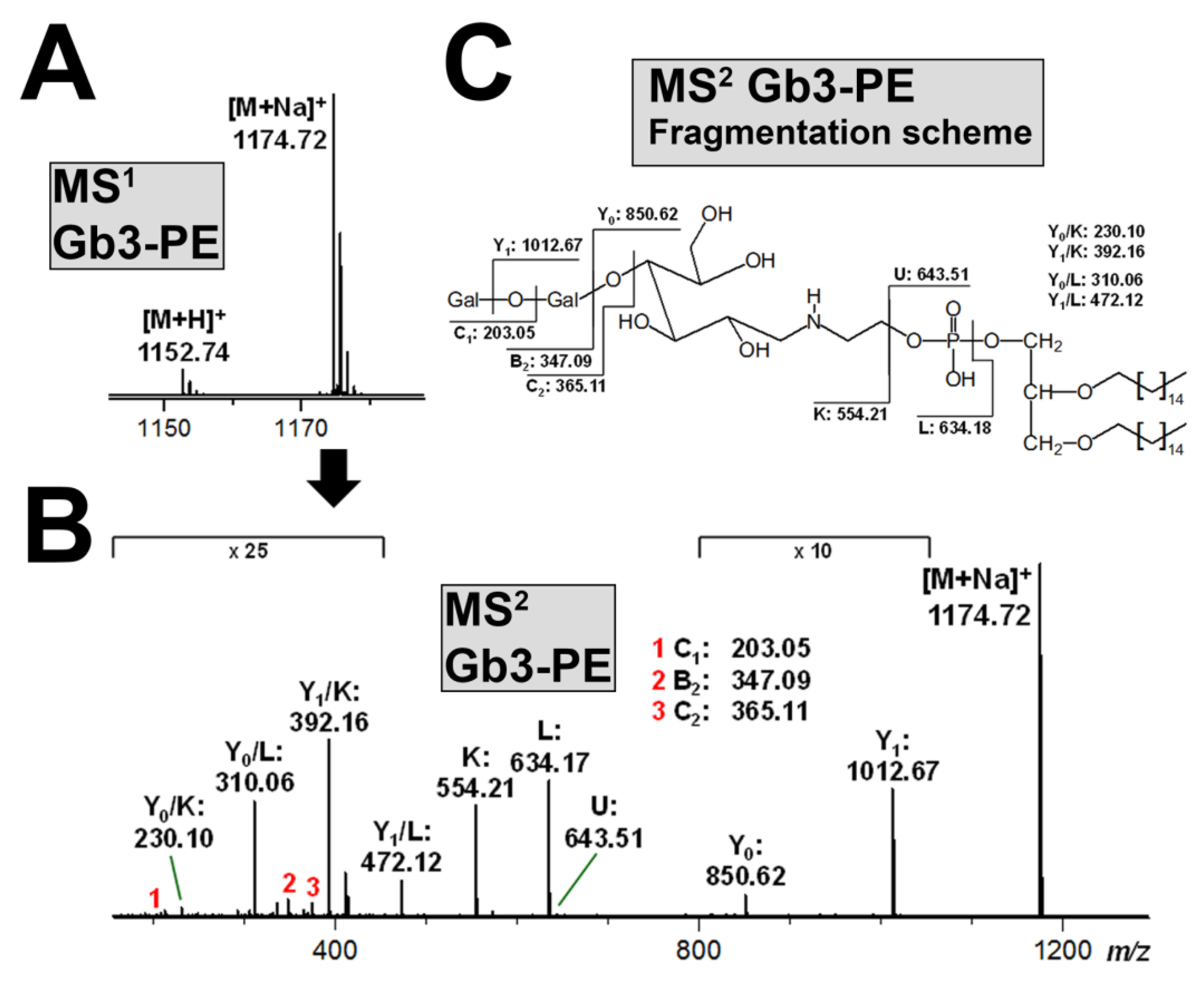
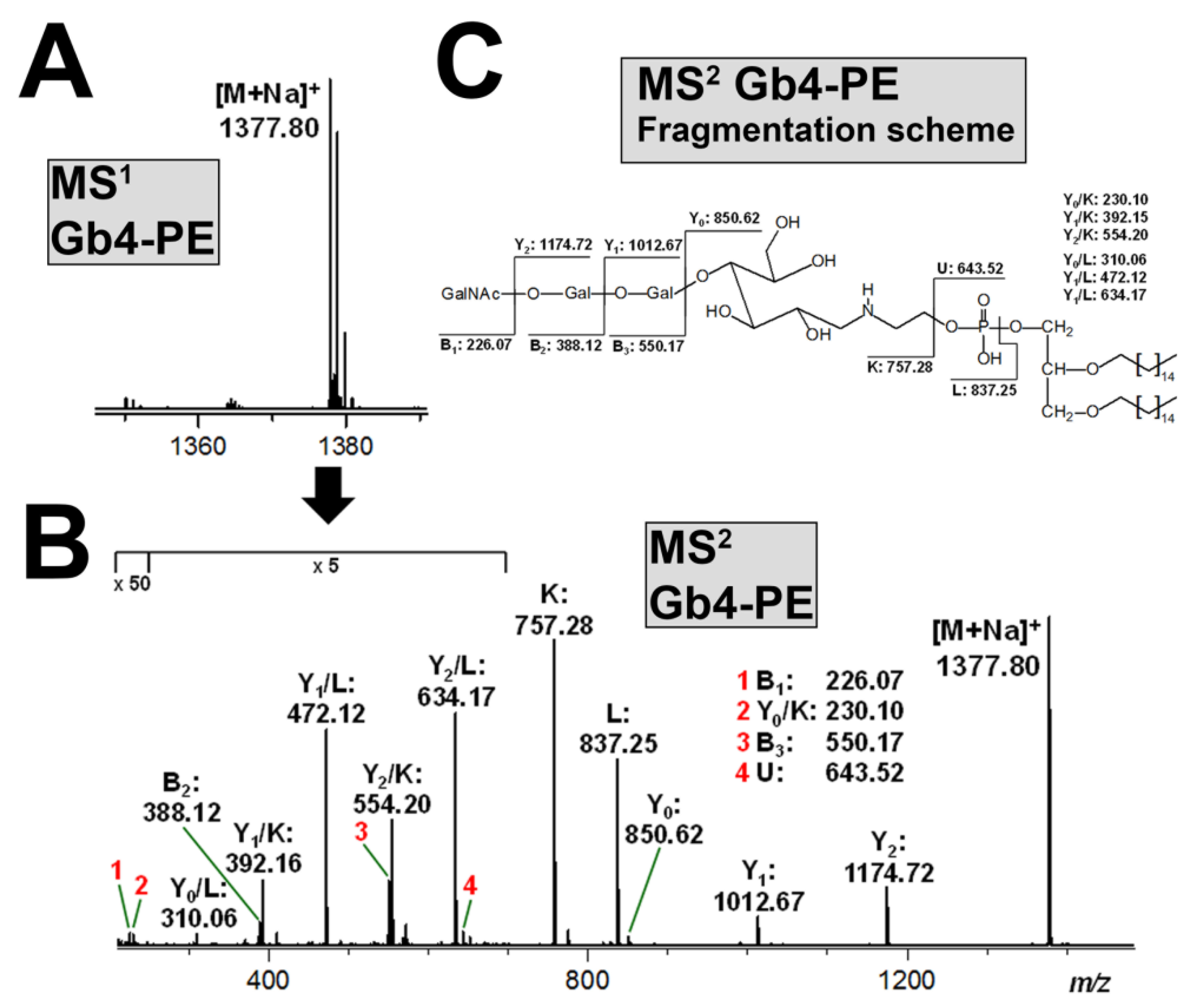
3.6. Structural Characterization of Stx-Binding Neoglycolipids Gb3-PE and Gb4-PE
3.7. Protection of Kidney Epithelial Cells from Stx2e-Mediated Damage by Gb3-PE and Gb4-PE
4. Discussion
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Imberechts, H.; De Greve, H.; Lintermans, P. The pathogenesis of edema disease in pigs. A review. Vet. Microbiol. 1992, 31, 221–233. [Google Scholar] [CrossRef]
- Van Beers-Schreurs, H.M.; Vellenga, L.; Wensing, T.; Breukin, H.J. The pathogenesis of the post-weaning syndrome in weaned piglets: A review. Vet. Q. 1992, 14, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Moxley, R.A. Edema disease. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 175–185. [Google Scholar] [CrossRef]
- Casanova, N.A.; Redondo, L.M.; Dailoff, G.C.; Arenas, D.; Fernández Miyakawa, M.E. Overview of the role of Shiga toxins in porcine edema disease pathogenesis. Toxicon 2018, 148, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Matise, I.; Sirinarumitr, T.; Bosworth, B.T.; Moon, H.W. Vascular ultrastructure and DNA fragmentation in swine infected with Shiga toxin-producing Escherichia coli. Vet. Pathol. 2000, 37, 318–327. [Google Scholar] [CrossRef]
- Benson, K.; Cramer, S.; Galla, H.J. Impedance-based monitoring: Barrier properties and beyond. Fluids Barriers CNS 2013, 10, 5. [Google Scholar] [CrossRef]
- Meisen, I.; Rosenbrück, R.; Galla, H.J.; Hüwel, S.; Kouzel, I.U.; Mormann, M.; Karch, H.; Müthing, J. Expression of Shiga toxin 2e glycosphingolipid receptors of primary porcine brain endothelial cells and toxin-mediated breakdown of the blood-brain barrier. Glycobiology 2013, 23, 745–759. [Google Scholar] [CrossRef]
- Moxley, R.A.; Duhamel, G.E. Comparative pathology of bacterial enteric diseases of swine. Adv. Exp. Med. Biol. 1999, 473, 83–101. [Google Scholar] [CrossRef]
- Friedrich, A.W.; Bielaszewska, M.; Zhang, W.L.; Pulz, M.; Kuczius, T.; Ammon, A.; Karch, H. Escherichia coli harboring Shiga toxin 2 gene variants: Frequency and association with clinical symptoms. J. Infect. Dis. 2002, 185, 74–84. [Google Scholar] [CrossRef]
- Beutin, L.; Krause, G.; Zimmermann, S.; Kaulfuss, S.; Gleier, K. Characterization of Shiga toxin-producing Escherichia coli strains isolated from human patients in Germany over a 3-year period. J. Clin. Microbiol. 2004, 42, 1099–1108. [Google Scholar] [CrossRef]
- Sonntag, A.K.; Bielaszewska, M.; Mellmann, A.; Dierksen, N.; Schierack, P.; Wieler, L.H.; Schmidt, M.A.; Karch, H. Shiga toxin 2e-producing Escherichia coli isolates from humans and pigs differ in their virulence profiles and interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 2005, 71, 8855–8863. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Cheasty, T.; Chart, H.; Rowe, B. Isolation of Vero cytotoxin-producing Escherichia coli serotypes O9ab:H- and O101:H- carrying VT2 variant gene sequences from a patient with haemolytic uraemic syndrome. Eur. J. Clin. Microbiol. Infect. Dis. 1994, 13, 1074–1076. [Google Scholar] [CrossRef] [PubMed]
- Fasel, D.; Mellmann, A.; Cernela, N.; Hächler, H.; Fruth, A.; Khanna, N.; Egli, A.; Beckmann, C.; Hirsch, H.H.; Goldenberger, D.; et al. Hemolytic uremic syndrome in a 65-year-old male linked to a very unusual type of stx2e- and eae-harboring O51:H49 Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 2014, 51, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Wieler, L.H.; Bauerfeind, R. STEC as a veterinary problem. Diagnostics and prophylaxis in animals. Methods Mol. Med. 2003, 73, 75–89. [Google Scholar]
- Zweifel, C.; Schumacher, S.; Beutin, L.; Blanco, J.; Stephan, R. Virulence profiles of Shiga toxin 2e-producing Escherichia coli isolated from healthy pig at slaughter. Vet. Microbiol. 2006, 117, 328–332. [Google Scholar] [CrossRef]
- Tseng, M.; Fratamico, P.M.; Manning, S.D.; Funk, J.A. Shiga toxin-producing Escherichia coli in swine: The public health perspective. Anim. Health Res. Rev. 2014, 15, 63–75. [Google Scholar] [CrossRef]
- Tseng, M.; Fratamico, P.M.; Bagi, L.; Manzinger, D.; Funk, J.A. Shiga toxin-producing E. coli (STEC) in swine: Prevalence over the finishing period and characteristics of the STEC isolates. Epidemiol. Infect. 2015, 143, 505–514. [Google Scholar] [CrossRef]
- Ercoli, L.; Farneti, S.; Ranucci, D.; Scuota, S.; Branclari, R. Role of verocytotoxigenic Escherichia coli in the swine production chain. Int. J. Food Saf. 2015, 4, 5156. [Google Scholar] [CrossRef]
- Bitzan, M.; Klemt, M.; Steffens, R.; Müller-Wiefel, D.E. Differences in verotoxin neutralizing activity of therapeutic immunoglobulins and sera from healthy controls. Infection 1993, 21, 140–145. [Google Scholar] [CrossRef]
- Gannon, V.P.; Gyles, C.L. Characteristics of the Shiga-like toxin produced by Escherichia coli associated with porcine edema disease. Vet. Microbiol. 1990, 24, 89–100. [Google Scholar] [CrossRef]
- Franke, S.; Gunzer, F.; Wieler, L.H.; Baljer, G.; Karch, H. Construction of recombinant Shiga-like toxin IIv (SLT-IIv) and ist use in monitoring the SLT-IIv antibody status of pigs. Vet. Microbiol. 1995, 43, 41–52. [Google Scholar] [CrossRef]
- Franke, S.; Harmsen, D.; Caprioli, A.; Pierard, D.; Wieler, L.H.; Karch, H. Clonal relatedness of Shiga-like toxin-producing Escherichia coli O101 strains of human and porcine origin. J. Clin. Microbiol. 1995, 33, 3174–3178. [Google Scholar] [PubMed]
- Boyd, B.; Tyrrell, G.; Maloney, M.; Gyles, C.; Brunton, J.; Lingwood, C. Alteration of the glycolipid binding specificity of the pig edema toxin from globotetraosyl to globotriaosyl ceramide alters in vivo tissue targeting and results in verotoxin 1-like disease in pigs. J. Exp. Med. 1993, 177, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Waddell, T.E.; Lingwood, C.A.; Gyles, C.L. Interaction of verotoxin 2e with pig intestine. Infect. Immun. 1996, 64, 1714–1719. [Google Scholar]
- Waddell, T.E.; Coomber, B.L.; Gyles, C.L. Localization of potential binding sites for the edema disease Verotoxin (VT2e) in pigs. Can. J. Vet. Res. 1998, 62, 81–86. [Google Scholar]
- Winter, K.R.K.; Stoffregen, W.C.; Dean-Nystrom, E.A. Shiga toxin binding to isolated porcine tissues and peripheral blood leukocytes. Infect. Immun. 2004, 72, 6680–6684. [Google Scholar] [CrossRef]
- Pohlenz, J.F.; Winter, K.R.; Dean-Nystrom, E.A. Shiga-toxigenic Escherichia coli-inoculated neonatal piglets develop kidney lesions that are comparable to those in humans with hemolytic-uremic syndrome. Infect. Immun. 2005, 73, 612–616. [Google Scholar] [CrossRef]
- DeGrandis, S.; Law, H.; Brunton, J.; Gyles, C.; Lingwood, C.A. Globotetraosylceramide is recognized by the pig edema disease toxin. J. Biol. Chem. 1989, 264, 12520–12525. [Google Scholar]
- Keusch, G.T.; Jacewicz, M.; Acheson, D.W.K.; Donohue-Rolfe, A.; Kane, A.V.; McCluer, R.H. Globotriaosylceramide, Gb3, is an alternative functional receptor for Shiga-like toxin 2e. Infect. Immun. 1995, 63, 1138–1141. [Google Scholar]
- Müthing, J.; Meisen, I.; Zhang, W.; Bielaszewska, M.; Mormann, M.; Bauerfeind, R.; Schmidt, M.A.; Friedrich, A.W.; Karch, H. Promiscuous Shiga toxin 2e and its intimate relationship to Forssman. Glycobiology 2012, 22, 849–862. [Google Scholar] [CrossRef]
- Steil, D.; Bonse, R.; Meisen, I.; Pohlentz, G.; Vallejo, G.; Karch, H.; Müthing, J. A topographical atlas of Shiga toxin 2e receptor distribution in the tissues of weaned piglets. Toxins 2016, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Steil, D.; Schepers, C.L.; Pohlentz, G.; Legros, N.; Runde, J.; Humpf, H.U.; Karch, H.; Müthing, J. Shiga toxin glycosphingolipid receptors of Vero-B4 kidney epithelial cells and their membrane microdomain lipid environment. J. Lipid Res. 2015, 56, 2322–2336. [Google Scholar] [CrossRef] [PubMed]
- Scheutz, F.; Teel, L.D.; Beutin, L.; Piérard, D.; Buvens, G.; Karch, H.; Mellmann, A.; Caprioli, A.; Tozzoli, R.; Morabito, S.; et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J. Clin. Microbiol. 2012, 50, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- Nakao, H.; Takeda, T. Escherichia coli Shiga toxin. J. Nat. Toxins 2000, 9, 299–313. [Google Scholar]
- Ray, P.E.; Liu, X.H. Pathogenesis of Shiga toxin-induced hemolytic uremic syndrome. Pediatr. Nephrol. 2001, 16, 823–839. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Karch, H. Consequences of enterohaemorrhagic Escherichia coli infection for the vascular endothelium. Thromb. Hemost. 2005, 94, 312–318. [Google Scholar] [CrossRef]
- Müthing, J.; Schweppe, C.H.; Karch, H.; Friedrich, A.W. Shiga toxins, glycosphingolipid diversity, and endothelial cell injury. Thromb. Haemost. 2009, 101, 252–264. [Google Scholar]
- Zoja, C.; Buelli, S.; Morigi, M. Shiga toxin-associated hemolytic uremic syndrome: Pathophysiology of endothelial dysfunction. Pediatr. Nephrol. 2010, 25, 2231–2240. [Google Scholar] [CrossRef]
- Melton-Celsa, A.; Mohawk, K.; Teel, L.; O’Brien, A. Pathogenesis of Shiga-toxin producing Escherichia coli. Curr. Top. Microbiol. Immunol. 2012, 357, 67–103. [Google Scholar] [CrossRef]
- Bauwens, A.; Betz, J.; Meisen, I.; Kemper, B.; Karch, H.; Müthing, J. Facing glycosphingolipid-Shiga toxin interaction: Dire straits for endothelial cells of the human vasculature. Cell. Mol. Life Sci. 2013, 70, 425–457. [Google Scholar] [CrossRef]
- Kiyokawa, N.; Taguchi, T.; Mori, T.; Uchida, H.; Sato, N.; Takeda, T.; Fujimoto, J. Induction of apoptosis in normal human renal tubular epithelial cells by Escherichia coli Shiga toxins 1 and 2. J. Infect. Dis. 1998, 178, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Karpman, D.; Håkansson, A.; Perez, M.T.; Isaksson, C.; Carlemalm, E.; Caprioli, A.; Svanborg, C. Apoptosis of renal cortical cells in the hemolytic-uremic syndrome: In vivo and in vitro studies. Infect. Immun. 1998, 66, 636–644. [Google Scholar] [PubMed]
- Kodama, T.; Nagayama, K.; Yamada, K.; Ohba, Y.; Akeda, Y.; Honda, T. Induction of apoptosis in human renal proximal tubular epithelial cells by Escherichia coli verocytotoxin 1 in vitro. Med. Microbiol. Immunol. 1999, 188, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.K.; Stricklett, P.K.; Schmid, D.; Kohan, D.E. Cytotoxic effect of Shiga toxin-1 on human glomerular epithelial cells. Kidney Int. 2000, 57, 2350–2359. [Google Scholar] [CrossRef]
- Kaneko, K.; Kiyokawa, N.; Ohtomo, Y.; Nagaoka, R.; Yamashiro, Y.; Taguchi, T.; Mori, T.; Fujimoto, J.; Takeda, T. Apoptosis of renal tubular cells in Shiga toxin-mediated hemolytic uremic syndrome. Nephron 2001, 87, 182–185. [Google Scholar] [CrossRef]
- Creydt, V.P.; Silberstein, C.; Zotta, E.; Ibarra, C. Cytotoxic effect of Shiga toxin-2 holotoxin and its B subunit on human renal tubular epithelial cells. Microbes Infect. 2006, 8, 410–419. [Google Scholar] [CrossRef]
- Silberstein, C.; Pistone Creydt, V.; Gerhardt, E.; Núñez, P.; Ibarra, C. Inhibition of water absorption in human proximal tubular epithelial cells in response to Shiga toxin-2. Pediatr. Nephrol. 2008, 23, 1981–1990. [Google Scholar] [CrossRef]
- Márquez, L.B.; Araoz, A.; Repetto, H.A.; Ibarra, F.R.; Silberstein, C. Effects of shiga toxin 2 on cellular regeneration mechanisms in primary and three-dimensional cultures of human renal tubular epithelial cells. Microb. Pathog. 2016, 99, 87–94. [Google Scholar] [CrossRef]
- Taguchi, T.; Uchida, H.; Kiyokawa, N.; Mori, T.; Sato, N.; Horie, H.; Takeda, T.; Fujimoto, J. Verotoxins induce apoptosis in human renal tubular epithelium derived cells. Kidney Int. 1998, 53, 1681–1688. [Google Scholar] [CrossRef]
- Bitzan, M.; Bickford, B.B.; Foster, G.H. Verotoxin (Shiga toxin) sensitizes renal epithelial cells to increased heme toxicity: Possible implications for the hemolytic uremic syndrome. J. Am. Soc. Nephrol. 2004, 15, 2334–2343. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Mathew, R.; Trachtman, H. Cytoprotective effect of curcumin in human proximal tubule epithelial cells exposed to Shiga toxin. Biochem. Biophys. Res. Commun. 2001, 283, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Nestoridi, E.; Kushak, R.I.; Duguerre, D.; Grabowski, E.F.; Ingelfinger, J.R. Up-regulation of tissue factor activity on human proximal tubular epithelial cells in response to Shiga toxin. Kidney Int. 2005, 67, 2254–2266. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Foster, G.H.; Bitzan, M. Silencing of Bak ameliorates apoptosis of human proximal tubular epithelial cells by Escherichia coli-derived Shiga toxin 2. Infection 2005, 33, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Lentz, E.K.; Leyva-Illades, D.; Lee, M.S.; Cherla, R.P.; Tesh, V.L. Differential response of the human renal proximal tubular epithelial cell line HK-2 to Shiga toxin types 1 and 2. Infect. Immun. 2011, 79, 3527–3540. [Google Scholar] [CrossRef] [PubMed]
- Psotka, M.A.; Obata, F.; Kolling, G.L.; Gross, L.K.; Saleem, M.A.; Satchell, S.C.; Mathieson, P.W.; Obrig, T.G. Shiga toxin 2 targets the murine renal collecting duct epithelium. Infect. Immun. 2009, 77, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, R.; Do, P.M.; Griffey, S.M.; Vilches-Moure, J.G.; Friedman, M. Ingested Shiga toxin 2 (Stx2) causes histopathological changes in kidney, spleen, and thymus tissues and mortality in mice. J. Agric. Food Chem. 2010, 58, 9281–9286. [Google Scholar] [CrossRef]
- Morace, I.; Pilz, R.; Federico, G.; Jennemann, R.; Krunic, D.; Nordström, V.; von Gerichten, J.; Marsching, C.; Schießl, I.M.; Müthing, J.; et al. Renal globotriaosylceramide facilitates tubular albumin absorption and its inhibition protects against acute kidney injury. Kidney Int. 2019, 96, 327–341. [Google Scholar] [CrossRef]
- Porubsky, S.; Federico, G.; Müthing, J.; Jennemann, R.; Gretz, N.; Büttner, S.; Obermüller, N.; Jung, O.; Hauser, I.A.; Gröne, E.; et al. Direct acute tubular damage contributes to Shigatoxin-mediated kidney failure. J. Pathol. 2014, 234, 120–133. [Google Scholar] [CrossRef]
- Handler, J.S.; Perkins, F.M.; Johnson, J.P. Studies of renal cell function using cell culture techniques. Am. J. Physiol. 1980, 238, F1–F9. [Google Scholar] [CrossRef]
- Toutain, H.; Morin, J.P. Renal proximal tubule cell cultures for studying drug-induced nephrotoxicity and modulation of phenotype expression by medium components. Ren. Fail. 1992, 14, 371–383. [Google Scholar] [CrossRef]
- Sun, M.; Liu, X.; Cao, S.; He, Q.; Zhou, R.; Ye, J.; Li, Y.; Chen, H. Inhibition of porcine circovirus type 1 and type 2 production in PK-15 cells by small interfering RNAs targeting the Rep gene. Vet. Microbiol. 2007, 123, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Chen, J.; Wei, Y.; Shi, H.; Zhang, X.; Yuan, J.; Shi, D.; Liu, J.; Zhu, X.; Wang, X.; et al. Porcine parvovirus induces activation of NF-κB signaling pathways in PK-15 cells mediated by toll-like receptors. Mol. Immunol. 2017, 85, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, J.; Zhang, X.; Zhao, Q.; Lu, M.; Lv, Y. RIG-1 and MDA-5 signaling pathways contribute to IFN-β production and viral replication in porcine circovirus virus type 2-infected PK-15 cells in vitro. Vet. Microbiol. 2017, 211, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Xu, C.M.; Song, Z.B.; Wang, M.; Liu, Q.Y.; Jiang, P.; Li, Y.F.; Bai, J.; Wang, X.W. Vimentin modulates infectious porcine circovirus type 2 in PK-15 cells. Virus Res. 2018, 243, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.N.; Cherry, W.R.; Weaver, G.W. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro 1976, 12, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Perantoni, A.; Berman, J.J. Properties of Wilms’ tumor line (TuWi) and pig kidney line (LLC-PK1) typical of normal kidney tubular epithelium. In Vitro 1979, 15, 446–454. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Fukase, M.; Takenaka, M.; Nakada, M.; Miyauchi, A.; Fujita, T. Calcitonin-responsive clonal cell line from porcine kidney (PK (15)) in serum-free medium. Endocrinol. Jpn. 1985, 32, 819–828. [Google Scholar] [CrossRef]
- Newman, J.T.; Smith, K.O. Characteristics of a swine papovavirus. Infect. Immun. 1972, 5, 961–967. [Google Scholar]
- Dulac, G.C.; Afshar, A. Porcine circovirus antigens in PK-15 cell line (ATCC CCL-33) and evidence of antibodies to circovirus in Canadian pigs. Can. J. Vet. Res. 1989, 53, 431–433. [Google Scholar]
- Kouzel, I.U.; Pohlentz, G.; Schmitz, J.S.; Steil, D.; Humpf, H.U.; Karch, H.; Müthing, J. Shiga toxin glycosphingolipid receptors in human Caco-2 and HCT-8 colon epithelial cell lines. Toxins 2017, 9, 338. [Google Scholar] [CrossRef]
- Betz, J.; Bielaszewska, M.; Thies, A.; Humpf, H.U.; Dreisewerd, K.; Karch, H.; Kim, K.S.; Friedrich, A.W.; Müthing, J. Shiga toxin glycosphingolipid receptors in microvascular and macrovascular endothelial cells: Differential association with membrane lipid raft microdomains. J. Lipid Res. 2011, 52, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Pohlentz, G.; Steil, D.; Rubin, D.; Mellmann, A.; Karch, H.; Müthing, J. Pectin-derived neoglycolipids: Tools for differentiation of Shiga toxin subtypes and inhibitors of Shiga toxin-mediated cellular injury. Carbohydr. Polym. 2019, 212, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Steil, D.; Pohlentz, G.; Legros, N.; Mormann, M.; Mellmann, A.; Karch, H.; Müthing, J. Combining mass spectrometry, surface acoustic wave interaction analysis, and cell viability assays for characterization of Shiga toxin subtypes of pathogenic Escherichia coli bacteria. Anal. Chem. 2018, 90, 8989–8997. [Google Scholar] [CrossRef] [PubMed]
- Legros, N.; Dusny, S.; Humpf, H.U.; Pohlentz, G.; Karch, H.; Müthing, J. Shiga toxin glycosphingolipid receptors and their lipid membrane ensemble in primary human blood-brain-barrier endothelial cells. Glycobiology 2017, 27, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Müthing, J.; Egge, H.; Kniep, B.; Mühlradt, P.F. Structural characterization of gangliosides from murine T lymphocytes. Eur. J. Biochem. 1987, 163, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Mellmann, A.; Bletz, S.; Böking, T.; Kipp, F.; Becker, K.; Schultes, A.; Prior, K.; Harmsen, D. Real-time genome sequencing of resistant bacteria provides precision infection control in an institutional setting. J. Clin. Microbiol. 2016, 54, 2874–2881. [Google Scholar] [CrossRef]
- Legros, N.; Pohlentz, G.; Steil, D.; Kouzel, I.U.; Liashkovich, I.; Mellmann, A.; Karch, H.; Müthing, J. Membrane assembly of Shiga toxin glycosphingolipid receptors and toxin refractiveness of MDCK II epithelial cells. J. Lipid Res. 2018, 59, 1383–1401. [Google Scholar] [CrossRef]
- Meisen, I.; Friedrich, A.W.; Karch, H.; Witting, U.; Peter-Katalinić, J.; Müthing, J. Application of combined high-performance thin-layer chromatography immunostaining and nanoESI-QTOF tandem mass spectrometry to the full structural characterization of high- and low-affinity binding ligands of Shiga toxin 1. Rapid Commun. Mass Spectrom. 2005, 19, 3659–3665. [Google Scholar] [CrossRef]
- Legros, N.; Ptascheck, S.; Pohlentz, G.; Karch, H.; Dobrindt, U.; Müthing, J. PapG subtype-specific binding characteristics of Escherichia coli towards globo-series glycosphingolipids of human kidney and bladder uroepithelial cells. Glycobiology 2019, 29, 789–802. [Google Scholar] [CrossRef]
- Legros, N.; Pohlentz, G.; Runde, J.; Dusny, S.; Humpf, H.U.; Karch, H.; Müthing, J. Colocalization of receptors for Shiga toxins with lipid rafts in primary human renal glomerular endothelial cells and influence of D-PDMP on synthesis and distribution of glycosphingolipid receptors. Glycobiology 2017, 27, 947–965. [Google Scholar] [CrossRef]
- Schweppe, C.H.; Hoffmann, P.; Nofer, J.R.; Pohlentz, G.; Mormann, M.; Karch, H.; Friedrich, A.W.; Müthing, J. Neutral glycosphingolipids in human blood: A precise mass spectrometry analysis with special reference to lipoprotein-associated Shiga toxin receptors. J. Lipid Res. 2010, 51, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–440. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. Structure elucidation of glycosphingolipids and gangliosides using high-performance tandem mass spectrometry. Biochemistry 1988, 27, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Müthing, J.; Distler, U. Advances on the compositional analysis of glycosphingolipids combining thin-layer chromatography with mass spectrometry. Mass Spectrom. Rev. 2010, 29, 425–479. [Google Scholar] [CrossRef] [PubMed]
- Meisen, I.; Mormann, M.; Müthing, J. Thin-layer chromatography, overlay technique and mass spectrometry: A versatile triad advancing glycosphingolipidomics. Biochim. Biophys. Acta 2011, 1811, 875–896. [Google Scholar] [CrossRef]
- Hsu, F.F.; Turk, J.; Stewart, M.E.; Downing, D.T. Structural studies on ceramides as lithiated adducts by low energy collisional-activated dissociation tandem mass spectrometry with electrospray ionization. J. Am. Soc. Mass Spectrom. 2002, 13, 680–695. [Google Scholar] [CrossRef][Green Version]
- Sonnino, S.; Prinetti, A. Membrane domains and the “lipid raft” concept. Curr. Med. Chem. 2013, 20, 4–21. [Google Scholar]
- Lingwood, C.A.; Binnington, B.; Manis, A.; Branch, D.R. Globotriaosyl ceramide receptor function—Where membrane structure and pathology intersect. FEBS Lett. 2010, 584, 1879–1886. [Google Scholar] [CrossRef]
- Sandvig, K.; Bergan, J.; Kavaliauskiene, S.; Skotland, T. Lipid requirement for entry of protein toxins into cells. Prog. Lipid Res. 2014, 54, 1–13. [Google Scholar] [CrossRef]
- Aigal, S.; Claudinon, J.; Römer, W. Plasma membrane reorganization: A glycolipid gateway for microbes. Biochim. Biophys. Acta 2015, 1853, 858–871. [Google Scholar] [CrossRef]
- Khan, F.; Proulx, F.; Lingwood, C.A. Detergent-resistant globotriaosyl ceramide may define verotoxin/glomeruli-restricted hemolytic uremic syndrome. Kidney Int. 2009, 75, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.E. Shiga-like toxins and HIV-1 ‘go through’ glycosphingolipids and lipid rafts in renal cells. Kidney Int. 2009, 75, 1135–1137. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology 2006, 21, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Simons, K. Detergent resistance as a tool in membrane research. Nat. Protoc. 2007, 2, 2159–2165. [Google Scholar] [CrossRef]
- Morris, R.J.; Jen, A.; Warley, A. Isolation of nano-meso scale detergent resistant membrane that has properties expected of lipid ‘rafts’. J. Neurochem. 2011, 116, 671–677. [Google Scholar] [CrossRef]
- Riske, K.A.; Domingues, C.C.; Casadei, B.R.; Mattei, B.; Caritá, A.C.; Lira, R.B.; Preté, P.S.C.; de Paula, E. Biophysical approaches in the study of membrane solubilization: Quantitative assessment and the role of lateral inhomogeneity. Biophys. Rev. 2017, 9, 649–667. [Google Scholar] [CrossRef]
- Legros, N.; Pohlentz, G.; Steil, D.; Müthing, J. Shiga toxin-glycosphingolipid interaction: Status quo of research with focus on primary human brain and kidney endothelial cells. Int. J. Med. Microbiol. 2018, 308, 1073–1084. [Google Scholar] [CrossRef]
- Johannes, L. Shiga toxin—A model for glycolipid-dependent and lectin-driven endocytosis. Toxins 2017, 9, 340. [Google Scholar] [CrossRef]
- Sandvig, K.; Garred, O.; Prydz, K.; Kozlov, J.V.; Hansen, S.H.; van Deurs, B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature 1992, 358, 510–512. [Google Scholar] [CrossRef]
- Sandvig, K.; Kavaliauskiene, S.; Skotland, T. Clathrin-independent endocytosis: An increasing degree of complexity. Histochem. Cell Biol. 2018, 150, 107–118. [Google Scholar] [CrossRef]
- Endo, Y.; Tsurugi, K.; Yutsudo, T.; Takeda, Y.; Ogasawara, T.; Igarashi, K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 1988, 171, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Brigotti, M.; Accorsi, P.; Carnicelli, D.; Rizzi, S.; González Vara, A.; Montanaro, L.; Sperti, S. Shiga toxin 1: Damage to DNA in vitro. Toxicon 2001, 39, 341–348. [Google Scholar] [CrossRef]
- Brigotti, M.; Alfieri, R.; Sestili, P.; Bonelli, M.; Petronini, P.G.; Guidarelli, A.; Barbieri, L.; Stirpe, F.; Sperti, S. Damage to nuclear DNA induced by Shiga toxin 1 and ricin in human endothelial cells. FASEB J. 2002, 16, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Koo, S.; Jeong, D.G.; Tesh, V.L. Shiga toxins as multi-functional proteins: Induction of host cellular stress responses, role in pathogenesis and therapeutic applications. Toxins 2016, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Toval, F.; Schiller, R.; Meisen, I.; Putze, J.; Kouzel, I.U.; Zhang, W.; Karch, H.; Bielaszewska, M.; Mormann, M.; Müthing, J.; et al. Characterization of urinary tract infection-associated Shiga toxin-producing Escherichia coli. Infect. Immun. 2004, 82, 4631–4642. [Google Scholar] [CrossRef]
- Totsika, M.; Moriel, D.G.; Idris, A.; Rogers, B.A.; Wurpel, D.J.; Phan, M.D.; Paterson, D.L.; Schembri, M.A. Uropathogenic Escherichia coli mediated urinary tract infection. Curr. Drug Targets 2012, 13, 1386–1399. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. Uropathogenic Escherichia coli (UPEC) infections: Virulence, factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef]
- Westerlund-Wikström, B.; Korhonen, T.K. Molecular structure of adhesin domains in Escherichia coli fimbriae. Int. J. Med. Microbiol. 2005, 295, 479–486. [Google Scholar] [CrossRef]
- Lane, M.C.; Mobley, H.L. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int. 2007, 72, 19–25. [Google Scholar] [CrossRef]
- Kamath, V.P.; Yeske, R.E.; Gregson, J.M.; Ratcliffe, R.M.; Fang, Y.R.; Palcic, M.M. Large-scale chemical and chemo-enzymatic synthesis of a spacer-containing Pk-trisaccharide. Carbohydr. Res. 2004, 339, 1141–1146. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Weiss, A.A.; Iyer, S.S. Glycan-based high-affinity ligands for toxins and pathogen receptors. Med. Res. Rev. 2010, 30, 327–393. [Google Scholar] [CrossRef] [PubMed]
- Melton-Celsa, A.R.; O’Brien, A.D. New therapeutic developments against Shiga toxin-producing Escherichia coli. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- MacConnachie, A.A.; Todd, W.T. Potential therapeutic agents for the prevention and treatment of haemolytic uraemic syndrome in Shiga toxin producing Escherichia coli infection. Curr. Opin. Infect. Dis. 2004, 17, 479–482. [Google Scholar] [CrossRef] [PubMed]
| Strain 1 | Stx Subtype | Reference Sequence (GenBank acc. no.) | SNP Position (Nucleotide Exchange) 2 | AA Codon Position (AA Exchange) 3 |
|---|---|---|---|---|
| 2074/97 | Stx1a | stx1a (M19473) | 200 (T → A) | 67 (Thr → Ser) |
| 03-06016 | Stx2a | stx2a (EF441599) | 170 (C → T) | 57 (Leu → Ser) |
| 2771/97 | Stx2e(h) | stx2e (FM998846) | 380 (T → C) | 127 (Thr → Ile) |
| 887 (C → A) | 296 (Thr → Lys) | |||
| S115G | Stx2e(p) | stx2e (FM998846) | 33 (C → T) | no AA exchange; |
| 380 (T → C) | 127 (Thr → Ile) | |||
| 937 (C → T) | 313 (Pro → Ser) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detzner, J.; Gloerfeld, C.; Pohlentz, G.; Legros, N.; Humpf, H.-U.; Mellmann, A.; Karch, H.; Müthing, J. Structural Insights into Escherichia coli Shiga Toxin (Stx) Glycosphingolipid Receptors of Porcine Renal Epithelial Cells and Inhibition of Stx-Mediated Cellular Injury Using Neoglycolipid-Spiked Glycovesicles. Microorganisms 2019, 7, 582. https://doi.org/10.3390/microorganisms7110582
Detzner J, Gloerfeld C, Pohlentz G, Legros N, Humpf H-U, Mellmann A, Karch H, Müthing J. Structural Insights into Escherichia coli Shiga Toxin (Stx) Glycosphingolipid Receptors of Porcine Renal Epithelial Cells and Inhibition of Stx-Mediated Cellular Injury Using Neoglycolipid-Spiked Glycovesicles. Microorganisms. 2019; 7(11):582. https://doi.org/10.3390/microorganisms7110582
Chicago/Turabian StyleDetzner, Johanna, Caroline Gloerfeld, Gottfried Pohlentz, Nadine Legros, Hans-Ulrich Humpf, Alexander Mellmann, Helge Karch, and Johannes Müthing. 2019. "Structural Insights into Escherichia coli Shiga Toxin (Stx) Glycosphingolipid Receptors of Porcine Renal Epithelial Cells and Inhibition of Stx-Mediated Cellular Injury Using Neoglycolipid-Spiked Glycovesicles" Microorganisms 7, no. 11: 582. https://doi.org/10.3390/microorganisms7110582
APA StyleDetzner, J., Gloerfeld, C., Pohlentz, G., Legros, N., Humpf, H.-U., Mellmann, A., Karch, H., & Müthing, J. (2019). Structural Insights into Escherichia coli Shiga Toxin (Stx) Glycosphingolipid Receptors of Porcine Renal Epithelial Cells and Inhibition of Stx-Mediated Cellular Injury Using Neoglycolipid-Spiked Glycovesicles. Microorganisms, 7(11), 582. https://doi.org/10.3390/microorganisms7110582





