A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches
Abstract
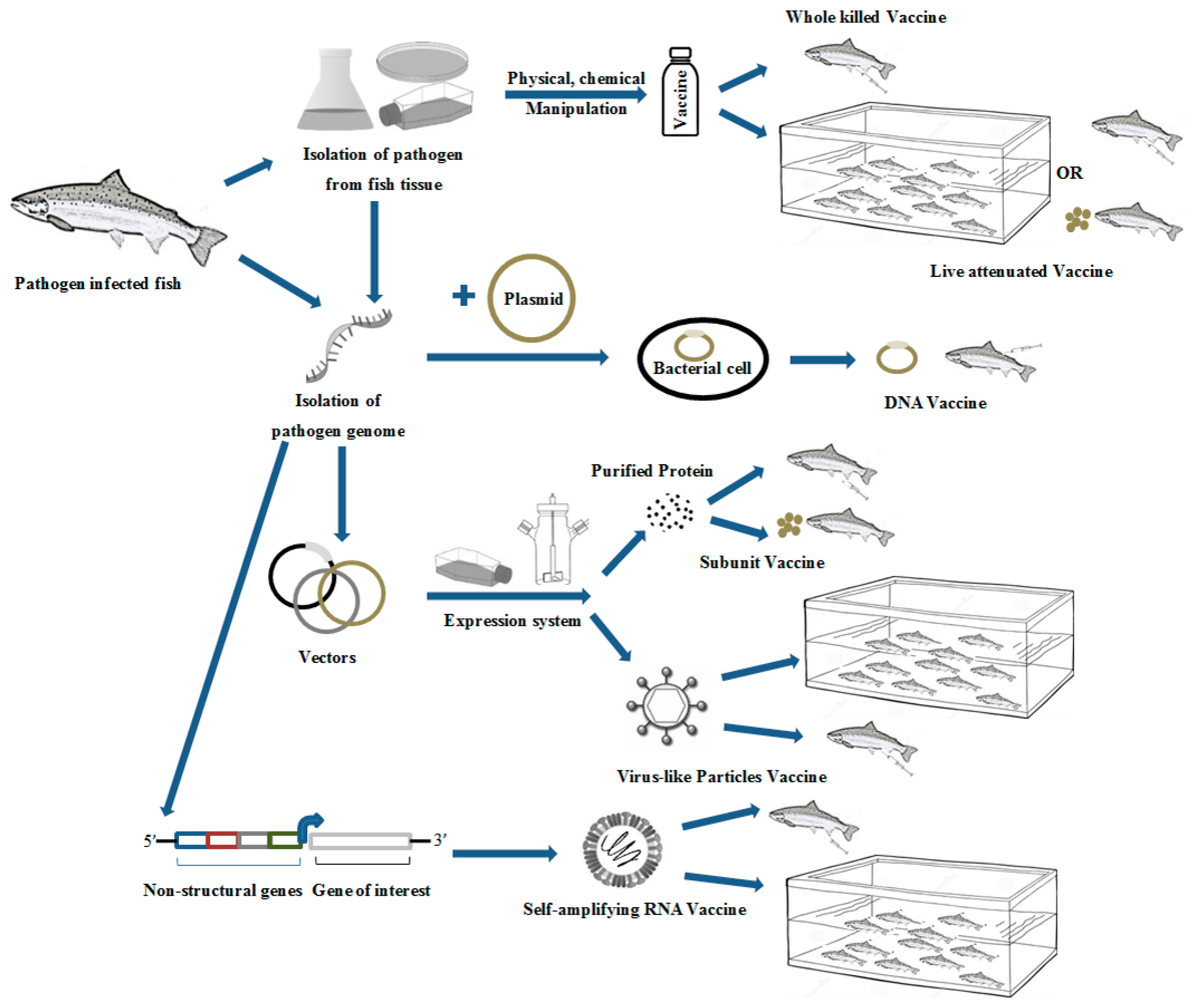
1. Introduction
2. Conventional Fish Vaccines
2.1. Inactivated/Killed Vaccine
2.2. Live Vaccines
3. Alternative Vaccine Technology
3.1. Subunit Vaccines
3.2. Nucleic Acid Vaccines
3.2.1. DNA Vaccines
3.2.2. RNA-Based Vaccines
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Sneeringer, S.; Bowman, M.; Clancy, M. The US and EU Animal Pharmaceutical Industries in the Age of Antibiotic Resistance; USDA Economic Research Service Report Number 264; USDA: Washington, DC, USA, May 2019. [Google Scholar]
- Snieszko, S.F.; Friddle, S.B. Prophylaxis of furunculosis in brook trout (Salvelinus Fontinalis) by oral immunization and sulfamerazine. Prog. Fish-Cult. 1949, 11, 161–168. [Google Scholar] [CrossRef]
- Gudding, R.; Goodrich, T. The History of fish vaccination. In Fish Vaccination, 1st ed.; Gudding, R., Lillehaug, A., Evensen, Ø., Eds.; John. Wiley & Sons, Inc.: New. York, NY, USA, 2014; pp. 1–11. [Google Scholar]
- Rodger, H.D. Fish disease causing economic impact in global aquaculture. In Fish Vaccines, 1st ed.; Adams, A., Ed.; Springer: Basel, Switzerland, 2016; pp. 1–34. [Google Scholar]
- Horzinek, M.C.; Schijns, V.E.C.J.; Denis, M.; Desmettre, P.; Babiuk, L.A. General description of vaccines. In Veterinary Vaccinology; Pastoret, P.P., Blancou, J., Vannier, P., Verschueren, C., Eds.; Elsevier Press: Amsterdam, The Netherlands, 1997; pp. 132–152. [Google Scholar]
- Sudheesh, P.S.; Cain, K.D. Prospects and challenges of developing and commercializing immersion vaccines for aquaculture. Int. Biol. Rev. 2017, 1, 1–20. [Google Scholar]
- Thompson, K.D.; Roberts, R.J. Fish Vaccines, 1st ed.; Adams, A., Ed.; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Gudding, R.; Van Muiswinkel, W.B. A history of fish vaccination: Science-based disease prevention in aquaculture. Fish Shellfish Immunol. 2013, 35, 1683–1688. [Google Scholar] [CrossRef]
- Plant, K.P.; LaPatra, S.E. Advances in fish vaccine delivery. Dev. Comp. Immunol. 2011, 35, 1256–1262. [Google Scholar] [CrossRef]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A. Adjuvants and immunostimulants in fish vaccines: Current knowledge and future perspectives. Fish Shellfish Immunol. 2013, 35, 1740–1750. [Google Scholar] [CrossRef]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A.; Munang′andu, H.M.; Evensen, Ø. Adjuvants in fish vaccines. In Fish Vaccination, 1st ed.; Gudding, R., Lillehaug, A., Evensen, Ø., Eds.; John. Wiley & Sons, Inc.: New. York, NY, USA, 2014; pp. 68–84. [Google Scholar]
- Sommerset, I.; Krossøy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef]
- Brudeseth, B.E.; Wiulsrød, R.; Fredriksen, B.N.; Lindmo, K.; Løkling, K.E.; Bordevik, M.; Steine, N.; Klevan, A.; Gravningen, K. Status and future perspectives of vaccines for industrialised fin-fish farming. Fish Shellfish Immunol. 2013, 35, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Adams, A. Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol. 2019, 90, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Van Oirschot, J.T. Classical attenuated vaccines. In Veterinary Vaccinology; Pastoret, P.P., Blancou, J., Vannier, P., Verschueren, C., Eds.; Elsevier Press: Amsterdam, The Netherlands, 1997; pp. 258–260. [Google Scholar]
- Brun, A.; Bárcena, J.; Blanco, E.; Borrego, B.; Dory, D.; Escribano, J.M.; Le Gall-Reculé, G.; Ortego, J.; Dixon, L.K. Current strategies for subunit and genetic viral veterinary vaccine development. Virus Res. 2011, 157, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, Y.K.; Kang, S.C.; Han, B.K.; Choi, K.M. Recent vaccine technology in industrial animals. Clin. Exp. Vaccine Res. 2016, 5, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Frietze, K.M.; Peabody, D.S.; Chackerian, B. Engineering virus-like particles as vaccine platforms. Curr. Opin. Virol. 2016, 18, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.F.; Rappuoli, R. Reverse vaccinology and vaccines for serogroup B Neisseria meningitidis. In Hot Topics in Infection and Immunity in Children, 2nd ed.; Pollard, A.J., Finn, A., Eds.; Springer: Boston, MA, USA, 2005; pp. 217–223. [Google Scholar]
- Cimica, V.; Galarza, J.M. Adjuvant formulations for virus-like particle (VLP) based vaccines. J. Clin. Immunol. 2017, 183, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Allnutt, F. Challenges and opportunities in developing oral vaccines against viral diseases of fish. J. Mar. Sci. Res. Dev. 2011, 2, 1–6. [Google Scholar] [CrossRef]
- Dhar, A.K.; Manna, S.K.; Allnutt, F.T. Viral vaccines for farmed finfish. Virus Dis. 2014, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.; Stone, D.A. Spring viraemia of carp Fish Viruses Bact. Pathobiol. Prot. 2017, 26, 79–90. [Google Scholar]
- Salonius, K.; Siderakis, C.; MacKinnon, A.M.; Griffiths, S.G. Use of Arthrobacter davidanieli as a live vaccine against Renibacterium salmoninarum and Piscirickettsia salmonis in salmonids. Dev. Biol. 2005, 121, 189–197. [Google Scholar]
- Shoemaker, C.A.; Klesius, P.H.; Drennan, J.D.; Evans, J.J. Efficacy of a modified live Flavobacterium columnare vaccine in fish. Fish Shellfish Immunol. 2011, 30, 304–308. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Mason, P.W.; Geall, A.; Mandl, C.W. RNA-based vaccines. Vaccine 2012, 30, 4414–4418. [Google Scholar] [CrossRef]
- Tizard, I. Grease, anthraxgate, and kennel cough: A revisionist history of early veterinary vaccines. In Veterinary Vaccines and Diagnostics; Schulz, R.D., Ed.; Academic Press: San Diego, CA, USA, 1998; Volume 41, pp. 7–24. [Google Scholar]
- Shoemaker, C.A.; Klesius, P.H.; Evans, J.J.; Arias, C.R. Use of modified live vaccines in aquaculture. J. World Aquacult. Soc. 2009, 40, 573–585. [Google Scholar] [CrossRef]
- Tlaxca, J.L.; Ellis, S.; Remmele, R.L., Jr. Live attenuated and inactivated viral vaccine formulation and nasal delivery: Potential and challenges. Adv. Drug Deliv. Rev. 2015, 93, 56–78. [Google Scholar] [CrossRef]
- Biering, E.; Villoing, S.; Sommerset, I.; Christie, K.E. Update on viral vaccines for fish. Dev. Biol. 2005, 121, 97–113. [Google Scholar]
- Baxter, D. Active and passive immunity, vaccine types, excipients and licensing. Occup. Med. 2007, 57, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, A.D.; Preiss, S.; Silva, F.T.; Garcon, N. Vaccine adjuvants: From 1920 to 2015 and beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef] [PubMed]
- Bricknell, I.R.; Bowden, T.J.; Lomax, J.; Ellis, A.E. Antibody response and protection of Atlantic salmon (Salmo salar) immunised with an extracellular polysaccharide of Aeromonas salmonicida. Fish Shellfish Immunol. 1997, 7, 1–16. [Google Scholar] [CrossRef]
- Evensen, Ø. Development of fish vaccines: Focusing on methods. In Fish Vaccines; Adams, A., Ed.; Springer: Basel, Switzerland, 2016; pp. 53–74. [Google Scholar]
- Yanong, R.P. Use of Vaccines in Finfish Aquaculture; Report FA156; US Department of Agriculture, Cooperative Extension Service: Washington, DC, USA; University of Florida, IFAS, Florida A. & M. University Cooperative Extension Program, and Boards of County Commissioner: Gainesville, FL, USA, 2011. [Google Scholar]
- Seder, R.A.; Hill, A.V. Vaccines against intracellular infections requiring cellular immunity. Nature 2000, 406, 793. [Google Scholar] [CrossRef] [PubMed]
- Nusbaum, K.E.; Morrison, E.E. Entry of 35 S-labeled Edwardsiella ictaluri into channel catfsh (Ictalutus punctatus). J. Aquat. Anim. Health 1996, 8, 146–149. [Google Scholar] [CrossRef]
- Evensen, Ø.; Leong, J.A.C. DNA vaccines against viral diseases of farmed fish. Fish Shellfish Immunol. 2013, 35, 1751–1758. [Google Scholar] [CrossRef]
- Tobar., I.; Arancibia., S.; Torres., C.; Vera., V.; Soto., P.; Carrasco., C.; Alvarado, M.; Neira, E.; Arcos, S.; Tobar, J.A. Successive oral immunizations against Piscirickettsia salmonis and infectious salmon anemia virus are required to maintain a long-term protection in farmed salmonids. Front. Immunol. 2015, 6, 244. [Google Scholar] [CrossRef]
- Desmettre, P.; Martinod, S. Research and development. In Veterinary Vaccinology; Pastoret, P.P., Blancou, J., Vannier, P., Verschueren, C., Eds.; Elsevier Press: Amsterdam, The Netherlands, 1997; pp. 175–194. [Google Scholar]
- Levine, M.M.; Sztein, M.B. Vaccine development strategies for improving immunization: The role of modern immunology. Nat. Immunol. 2004, 5, 460. [Google Scholar] [CrossRef]
- Klesius, P.H.; Pridgeon, J.W. Vaccination against enteric septicemia of catfish. In Fish Vaccination; John Wiley & Sons: Chichester, UK, 2014; pp. 211–225. [Google Scholar]
- Gomez-Casado, E.; Estepa, A.; Coll, J.M. A comparative review on European-farmed finfish RNA viruses and their vaccines. Vaccine 2011, 29, 2657–2671. [Google Scholar] [CrossRef]
- Fuchs, W.; Fichtner, D.; Bergmann, S.M.; Mettenleiter, T.C. Generation and characterization of koi herpesvirus recombinants lacking viral enzymes of nucleotide metabolism. Arch. Virol. 2011, 156, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, C.A.; Klesius, P.H. Protective immunity against enteric septicemia in channel catfish, Ictalurus punctatus (Rafinesque), following controlled exposure to Edwardsiella ictaluri. J. Fish Dis. 1997, 20, 361–368. [Google Scholar] [CrossRef]
- Shoemaker, C.A.; Klesius, P.H.; Bricker, J.M. Efficacy of a modified live Edwardsiella ictaluri vaccine in channel catfish as young as seven days post hatch. Aquacult 1999, 176, 189–193. [Google Scholar] [CrossRef]
- Shoemaker, C.A.; Klesius, P.H.; Evans, J.J. Immunization of eyed channel catfish, Ictalurus punctatus, eggs with monovalent Flavobacterium columnare vaccine and bivalent F. columnare and Edwardsiella ictaluri vaccine. Vaccine 2007, 25, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Norqvist, A.; Hagstrom, A.; Wolf-Watz, H.A.N.S. Protection of rainbow trout against vibriosis and furunculosis by the use of attenuated strains of Vibrio anguillarum. Appl. Environ. Microbiol. 1989, 55, 1400–1405. [Google Scholar] [PubMed]
- LaFrentz, B.R.; LaPatra, S.E.; Call, D.R.; Cain, K.D. Isolation of rifampicin resistant Flavobacterium psychrophilum strains and their potential as a live attenuated vaccine candidates. Vaccine 2008, 26, 5582–5589. [Google Scholar] [CrossRef] [PubMed]
- LaFrentz, B.R.; LaPatra, S.E.; Call, D.R.; Wiens, F.D.; Cain, K.D. Proteomic analysis of Flavobacterium psychrophilum cultured in vivo and in iron-limited media. Dis. Aquat. Organ. 2009, 87, 171–182. [Google Scholar] [CrossRef]
- Long, A.; Fehringer, T.R.; Swain, M.A.; LaFrentz, B.R.; Call, D.R.; Cain, K.D. Enhanced efficacy of an attenuated Flavobacterium psychrophilum strain cultured under iron-limited conditions. Fish Shellfish Immunol. 2013, 35, 1477–1482. [Google Scholar] [CrossRef]
- Makesh, M.; Sudheesh, P.S.; Cain, K.D. Systemic and mucosal immune response of rainbow trout to immunization with an attenuated Flavobacterium psychrophilum vaccine strain by different routes. Fish Shellfish Immunol. 2015, 44, 156–163. [Google Scholar] [CrossRef]
- Sudheesh, P.S.; Zimmerman, J.K.; Cain, K.D. Dietary effects on immunity, stress, and efficacy of two live attenuated Flavobacterium psychrophilum vaccine formulations. Aquacult 2016, 454, 35–43. [Google Scholar] [CrossRef]
- Sudheesh, P.S.; LaFrentz, B.R.; Call, D.R.; Siems, W.F.; LaPatra, S.E.; Wiens, G.D.; Cain, K.D. Identification of potential vaccine target antigens by immunoproteomic analysis of a virulent and a non-virulent strain of the fish pathogen Flavobacterium Psychrophilum. Dis. Aquat. Organ. 2007, 74, 37–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, J.; Bruce, T.J.; Sudheesh, P.S.; Knupp, C.; Loch, T.P.; Faisal, M.; Cain, K.D. Assessment of cross protection to heterologous strains of Flavobacterium psychrophilum following vaccination with a live-attenuated coldwater disease immersion vaccine. J. Fish Dis. 2019, 42, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Pridgeon, J.W.; Klesius, P.H. Development and efficacy of novobiocin and rifampicin-resistant Aeromonas hydrophila as novel vaccines in channel catfish and Nile tilapia. Vaccine 2011, 29, 7896–7904. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Olivares-Fuster, O.; LaFrentz, S.; Arias, C.R. New attenuated vaccine against columnaris disease in fish: Choosing the right parental strain is critical for vaccine efficacy. Vaccine 2013, 31, 5276–5280. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Fuster, O.; Arias, C.R. Development and characterization of rifampicin-resistant mutants from high virulent strains of Flavobacterium columnare. J. Fish Dis. 2011, 34, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lin, Q.; Fu, X.; Guo, H.; Liu, L.; Wu, S. Development and efficacy of a novel streptomycin-resistant Flavobacterium johnsoniae vaccine in grass carp (Ctenopharyngodon idella). Aquaculture 2015, 448, 93–97. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, C.; Zhao, Y.; Kong, X.; Pei, C.; Li, L.; Nie, G.; Li, X. Immune effects of the vaccine of live attenuated Aeromonas hydrophila screened by rifampicin on common carp (Cyprinus carpio. L). Vaccine 2016, 34, 3087–3092. [Google Scholar] [CrossRef]
- Evans, J.J.; Klesius, P.H.; Shoemaker, C.A. Modified Live Edwardsiella Tarda Vaccine for Aquatic Animals. U.S. Patent No. 7,067,122, 27 June 2006. [Google Scholar]
- Sun, Y.; Liu, C.S.; Sun, L. Isolation and analysis of the vaccine potential of an attenuated Edwardsiella tarda strain. Vaccine 2010, 28, 6344–6350. [Google Scholar] [CrossRef]
- Yu, L.P.; Hu, Y.H.; Sun, B.G.; Sun, L. C312M: An attenuated Vibrio anguillarum strain that induces immunoprotection as an oral and immersion vaccine. Dis. Aquat. Organ. 2012, 102, 33–42. [Google Scholar] [CrossRef]
- Laith, A.A.; Abdullah, M.A.; Nurhafizah, W.W.I.; Hussein, H.A.; Aya, J.; Effendy, A.W.M.; Najiah, M. Efficacy of live attenuated vaccine derived from the Streptococcus agalactiae on the immune responses of Oreochromis niloticus. Fish Shellfish Immunol. 2019, 90, 235–243. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Klesius, P.H. Major bacterial diseases in aquaculture and their vaccine development. In Animal Science Review; Hemming, D., Bodinham, M., Eds.; CABI: Boston, MA, USA, 2012; pp. 141–156. [Google Scholar]
- Griffiths, S.G.; Melville, K.J.; Salonius, K. Reduction of Renibacterium salmoninarum culture activity in. Atlantic salmon following vaccination with avirulent strains. Fish Shellfish Immunol. 1998, 8, 607–619. [Google Scholar] [CrossRef]
- Swain, P.; Behera, T.; Mohapatra, D.; Nanda, P.K.; Nayak, S.K.; Meher, P.K.; Das, B.K. Derivation of rough attenuated variants from smooth virulent Aeromonas hydrophila and their immunogenicity in fish. Vaccine 2010, 28, 4626–4631. [Google Scholar] [CrossRef] [PubMed]
- Itano, T.; Kawakami, H.; Kono, T.; Sakai, M. Live vaccine trials against nocardiosis in yellowtail Seriola quinqueradiata. Aquaculture 2006, 261, 1175–1180. [Google Scholar] [CrossRef]
- Perelberg, A.; Ronen, A.; Hutoran, M.; Smith, Y.; Kotler, M. Protection of cultured Cyprinus carpio against a lethal viral disease by an attenuated virus vaccine. Vaccine 2005, 23, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.G.; Hanson, L.A. Deletion of thymidine kinase gene attenuates channel catfish herpesvirus while maintaining infectivity. Virology 1995, 209, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Schröder, L.; Klafack, S.; Bergmann, S.M.; Fichtner, D.; Jin, Y.; Lee, P.Y.; Höper, D.; Mettenleiter, T.C.; Fuchs, W. Generation of a potential koi herpesvirus live vaccine by simultaneous deletion of the viral thymidine kinase and dUTPase genes. J. Gen. Virol. 2018, 100, 642–655. [Google Scholar] [CrossRef]
- Lawrence, M.L.; Banes, M.M.; Williams, M.L. Phenotype and virulence of a transposon-derived lipopolysaccharide O side-chain mutant strain of Edwarsiella ictaluri. J. Aquat. Anim. Health 2001, 13, 291–299. [Google Scholar] [CrossRef]
- Lawrence, M.L.; Banes, M.M. Tissue persistence and vaccine efficacy of an O polysaccharide mutant strain of Edwardsiella ictaluri. J. Aquat. Anim. Health 2005, 17, 228–232. [Google Scholar] [CrossRef]
- Abdelhamed, H.; Ibrahim, I.; Baumgartner, W.; Lawrence, M.L.; Karsi, A. The virulence and immune protection of Edwardsiella ictaluri HemR mutants in catfish. Fish Shellfish Immunol. 2018, 72, 153–160. [Google Scholar] [CrossRef]
- Triet, T.H.; Tinh, B.T.; Hau, L.V.; Huong, T.V.; Binh, N.Q. Development and potential use of an Edwardsiella ictaluri wzz mutant as a live attenuated vaccine against enteric septicemia in Pangasius hypophthalmus (Tra catfish). Fish Shellfish Immunol. 2019, 87, 87–95. [Google Scholar] [CrossRef]
- Igarashi, A.; Iida, T. A vaccination trial using live cells of Edwardsiella tarda in tilapia. Fish Pathol. 2002, 37, 145–148. [Google Scholar] [CrossRef][Green Version]
- Xiao, J.; Chen, T.; Wang, Q.; Liu, Q.; Wang, X.; Lv, Y.; Wu, H.; Zhang, Y. Search for live attenuated vaccine candidate against edwardsiellosis by mutating virulence-related genes of fish pathogen Edwardsiella tarda. Lett. Appl. Microbiol. 2011, 53, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Yang, G.; Xu, R.; Liu, X.; Zhang, Y.; Ma, Y.; Wang, Q. Pattern analysis of conditional essentiality (PACE)-based heuristic identification of an in vivo colonization determinant as a novel target for the construction of a live attenuated vaccine against Edwardsiella piscicida. Fish Shellfish Immunol. 2019, 90, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, J.T.; Stannard, J.A.; Lauth, X.; Ostland, V.E.; Powell, H.C.; Westerman, M.E.; Nizet, V. Streptococcus iniae phosphoglucomutase is a virulence factor and a target for vaccine development. Infect. Immun. 2005, 73, 6935–6944. [Google Scholar] [CrossRef]
- Locke, J.B.; Vicknair, M.R.; Ostland, V.E.; Nizet, V.; Buchanan, J.T. Evaluation of Streptococcus iniae killed bacterin and live attenuated vaccines in hybrid striped bass through injection and bath immersion. Dis. Aquat. Organ. 2010, 89, 117–123. [Google Scholar] [CrossRef]
- Soto, E.; Wiles, J.; Elzer, P.; Macaluso, K.; Hawke, J.P. Attenuated Francisella asiatica iglC mutant induces protective immunity to francisellosis in tilapia. Vaccine 2011, 29, 593–598. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, E.; Geng, Y.; Wang, K.; Chen, D.; Huang, X.; Ping, O.; Zhicai, Z.; Changliang, H.; Li, T.; et al. Multiples genome editing by natural transformation in Vibrio mimicus with potential application in attenuated vaccine development. Fish Shellfish Immunol. 2019, 92, 377–383. [Google Scholar] [CrossRef]
- Pang, H.; Qiu, M.; Zhao, J.; Hoare, R.; Monaghan, S.J.; Song, D.; Chang, Y.; Jian, J. Construction of a Vibrio alginolyticus hopPmaJ (hop) mutant and evaluation of its potential as a live attenuated vaccine in orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol. 2018, 76, 93–100. [Google Scholar] [CrossRef]
- Hansson, M.; Nygren, P.A.K.; Stahl, S. Design and production of recombinant subunit vaccines. Biotechnol. Appl. Biochem. 2000, 32, 95–107. [Google Scholar] [CrossRef]
- Holten-Andersen., L.; Doherty, T.M.; Korsholm, K.S.; Andersen, P. Combination of the cationic surfactant dimethyl dioctadecyl ammonium bromide and synthetic mycobacterial cord factor as an efficient adjuvant for tuberculosis subunit vaccines. Infect. Immun. 2004, 72, 1617. [Google Scholar] [CrossRef]
- Rappuoli, R.; Pizza, M.; Masignani, V.; Vadivelu, K. Meningococcal. B vaccine (4CMenB): The journey from research to real world experience. Expert Rev. Vaccines 2018, 17, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, N.; Olesen, N.J. Multiplication of. VHS virus in insect cells. Vet. Res. 1995, 26, 428–432. [Google Scholar] [PubMed]
- Lecocq-Xhonneux, F.; Thiry, M.; Dheur, I.; Rossius, M.; Vanderheijden, N.; Martial, J.; De Kinkelin, P. A recombinant viral haemorrhagic septicaemia virus glycoprotein expressed in insect cells induces protective immunity in rainbow trout. J. Gen. Virol. 1994, 75, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Cain, K.D.; Byrne, K.M.; Brassfield, A.L.; LaPatra, S.E.; Ristow, S.S. Temperature dependent characteristics of a recombinant infectious hematopoietic necrosis virus glycoprotein produced in insect cells. Dis. Aquat. Organ. 1999, 36, 1–10. [Google Scholar] [CrossRef]
- Crane, M.; Hyatt, A. Viruses of fish: an overview of significant pathogens. Viruses 2011, 3, 2025–2046. [Google Scholar] [CrossRef]
- Estepa, A.; Thiry, M.; Coll, J.M. Recombinant protein fragments from haemorrhagic septicaemia rhabdovirus stimulate trout leukocyte anamnestic responses in vitro. J. Gen. Virol. 1994, 75, 1329–1338. [Google Scholar] [CrossRef]
- Shivappa, R.N.; McAllister, P.E.; Edwards, G.H.; Santi, N. Using a baculovirus insect/larvae. Dev. Biol. 2005, 121, 165–174. [Google Scholar]
- Xu, C.; Mutoloki, S.; Evensen, Ø. Superior protection conferred by inactivated whole virus vaccine over subunit and. DNA vaccines against salmonid alphavirus infection in Atlantic salmon (Salmo salar L.). Vaccine 2012, 30, 3918–3928. [Google Scholar] [CrossRef]
- Rahman, M.H.; Ototake, M.; Iida, Y.; Yokomizo, Y.; Nakanishi, T. Efficacy of oil-adjuvanted vaccine for coldwater disease in ayu Plecoglossus altivelis. Fish Pathol. 2000, 35, 199–203. [Google Scholar] [CrossRef]
- Högfors, E.; Pullinen, K.R.; Madetoja, J.; Wiklund, T. Immunization of rainbow trout, Oncorhynchus mykiss (Walbaum), with a low molecular mass fraction isolated from Flavobacterium psychrophilum. J. Fish Dis. 2008, 31, 899–911. [Google Scholar] [CrossRef]
- Leong, J.C.; Anderson, E.; Bootland, L.M.; Chiou, P.W.; Johnson, M.; Kim, C.; Mourich, D.; Trobridge, G. Fish vaccine antigens produced or delivered by recombinant DNA technologies. Dev. Biol. Stand. 1997, 90, 267–277. [Google Scholar] [PubMed]
- Barnes, A. Prevention of disease by vaccination. In Aquaculture: Farming. Aquatic. Animals and Plants, 3rd ed.; Lucas, J.S., Southgate, P.C., Tucker, C.S., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 249–272. [Google Scholar]
- Rosenthal, J.A.; Chen, L.; Baker, J.L.; Putnam, D.; DeLisa, M.P. Pathogen-like particles: Biomimetic vaccine carriers engineered at the nanoscale. Curr. Opin. Biotechnol. 2014, 28, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Noad, R.; Roy, P. Virus-like particles as immunogens. Trends Microbiol. 2003, 11, 438–444. [Google Scholar] [CrossRef]
- Keller, S.A.; Bauer, M.; Manolova, V.; Muntwiler, S.; Saudan, P.; Bachmann, M.F. Cutting edge: Limited specialization of dendritic cell subsets for. MHC class. II-associated presentation of viral particles. J. Immunol. 2010, 184, 26–29. [Google Scholar] [CrossRef]
- Grgacic, E.V.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods 2006, 40, 60–65. [Google Scholar] [CrossRef]
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 2012, 31, 58–83. [Google Scholar] [CrossRef]
- Lin, C.S.; Lu, M.W.; Tang, L.; Liu, W.; Chao, C.B.; Lin, C.J.; Krishna, N.K.; Johnson, J.E.; Scneemann, A. Characterization of virus-like particles assembled in a recombinant baculovirus system expressing the capsid protein of a fish nodavirus. Virology 2001, 290, 50–58. [Google Scholar] [CrossRef]
- Lin, C.F.; Jiang, H.K.; Chen, N.C.; Wang, T.Y.; Chen, T.Y. Novel subunit vaccine with linear array epitope protect giant grouper against nervous necrosis virus infection. Fish Shellfish Immunol. 2018, 74, 551–558. [Google Scholar] [CrossRef]
- Liu, W.; Hsu, C.H.; Chang, C.Y.; Chen, H.H.; Lin, C.S. Immune response against grouper nervous necrosis virus by vaccination of virus-like particles. Vaccine 2006, 24, 6282–6287. [Google Scholar] [CrossRef]
- Lai, Y.X.; Jin, B.L.; Xua, Y.; Huanga, L.J.; Huanga, R.Q.; Zhang, Y.; Kwang, J.; He, J.G.; Xie, J.F. Immune responses of orange-spotted grouper, Epinephelus coioides, against virus-like particles of betanodavirus produced in Escherichia coli. Vet. Immunol. Immunopathol. 2014, 157, 87–96. [Google Scholar] [CrossRef]
- Marsian, J.; Hurdiss, D.L.; Ranson, N.A.; Ritala, A.; Paley, R.; Cano, I.; Lomonossoff, G.P. Plant-made nervous necrosis virus-like particles protect fish against disease. Front. Plant Sci. 2019, 10, 880. [Google Scholar] [CrossRef] [PubMed]
- Luu, V.T.; Moon, H.Y.; Hwang, J.Y.; Kang, B.K.; Hyun, A.K. Development of recombinant Yarrowia lipolytica producing virus-like particles of a fish nervous necrosis virus. J. Microbiol. 2017, 55, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.H.; Wu, S.Y.; Lin, C.H. Oral immunization with cell-free self-assembly virus-like particles against orange-spotted grouper nervous necrosis virus in grouper larvae, Epinephelus coioides. Vet. Immunol. Immunopathol. 2018, 197, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Kim, H.J.; Lan, N.T.; Han, H.J.; Lee, D.C.; Hwang, J.Y.; Kwon, M.G.; Kang, B.K.; Han, S.Y.; Moon, H.; et al. Oral vaccination through voluntary consumption of the convict grouper Epinephelus septemfasciatus with yeast producing the capsid protein of red-spotted grouper nervous necrosis virus. Vet. Microbiol. 2017, 204, 159–164. [Google Scholar] [CrossRef]
- Dhar, A.K.; Bowers, R.M.; Rowe, C.G.; Allnutt, F.T. Expression of a foreign epitope on infectious pancreatic necrosis virus VP2 capsid protein subviral particle (SVP) and immunogenicity in rainbow trout. Antivir. Res. 2010, 85, 525–531. [Google Scholar] [CrossRef]
- Guo, M.; Shi, W.; Wang, Y.; Wang, Y.; Chen, Y.; Li, D.; Ren, X.; Hua, X.; Tang, L.; Li, Y.; et al. Recombinant infectious hematopoietic necrosis virus expressing infectious pancreatic necrosis virus VP2 protein induces immunity against both pathogens. Fish Shellfish Immunol. 2018, 78, 187–194. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Geall, A.J. Recent innovations in mRNA vaccines. Curr. Opin. Immunol. 2016, 41, 18–22. [Google Scholar] [CrossRef]
- Kurath, G. Biotechnology and DNA vaccines for aquatic animals. Rev. Sci. Tech. Off. Int. Des. Epizoot. 2008, 27, 175–196. [Google Scholar] [CrossRef]
- Hølvold, L.B.; Myhr, A.I.; Dalmo, R.A. Strategies and hurdles using DNA vaccines to fish. Vet. Res. 2014, 45, 21. [Google Scholar] [CrossRef]
- Pasnik, D.J.; Smith, S.A. Immune and histopathologic responses of. DNA-vaccinated hybrid striped bass. Morone saxatilis× M. chrysops after acute. Mycobacterium marinum infection. Dis. Aquat. Org. 2006, 73, 33–41. [Google Scholar] [CrossRef]
- Pasnik, D.J.; Smith, S.A. Immunogenic and protective effects of a DNA vaccine for Mycobacterium marinum in fish. Vet. Immunol. Immunopathol. 2005, 103, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.D.; Mourich, D.V.; Fahrenkrug, S.C.; LaPatra, S.; Shepherd, J.; Leong, J.A. Genetic immunization of rainbow trout (Oncorhynchus mykiss) against infectious hematopoietic necrosis virus. Mol. Mar. Biol. Biotechnol. 1996, 5, 114–122. [Google Scholar] [PubMed]
- Dalmo, R.A. DNA vaccines for fish: Review and perspectives on correlates of protection. J. Fish Dis. 2018, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Heppell, J.; Lorenzen, N.; Armstron, N.K.; Wu, T.; Lorenzen, E.; Einer-Jensen, K.; Schorr, J.; Davis, H.L. Development of DNA vaccines for fish: Vector design, intramuscular injection and antigen expression using viral haemorrhagic septicaemia virus genes as model. Fish Shellfish Immunol. 1998, 8, 271–286. [Google Scholar] [CrossRef]
- Biering, E.; Salonius, K. DNA vaccines. In Fish Vaccination, 1st ed.; Gudding, R., Lillehaug, A., Evensen, Ø., Eds.; John. Wiley & Sons: Chichester, UK, 2014; pp. 47–55. [Google Scholar]
- Weiner, D.B. DNA vaccines: Crossing a line in the sand introduction to special issue. Vaccine 2008, 26, 5073. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Restifo, N.P.; Ying, H.; Hwang, L.; Leitner, W.W. The promise of nucleic acid vaccines. Gene 2000, 7, 89–92. [Google Scholar] [CrossRef]
- Geall, A.J.; Blagbrough, I.S. Rapid and sensitive ethidium bromide fluorescence quenching assay of polyamine conjugate-DNA interactions for the analysis of lipoplex formation in gene therapy. J. Pharm. Biomed. Anal. 2000, 22, 849–859. [Google Scholar] [CrossRef]
- Brito, L.A.; Kommareddy, S.; Maione, D.; Uematsu, Y.; Giovani, C.; Scorza, F.B.; Otten, G.R.; Yu, D.; Mandl, C.W.; Mason, P.W.; et al. Self-amplifying mRNA vaccines. In Advances in Genetics, 1st ed.; Friedmann, T., Dunlap, J.C., Goodwin, S.F., Eds.; Academic Press: San Diego, CA, USA, 2015; Volume 92, pp. 179–233. [Google Scholar]
- Perri, S.; Greer, C.E.; Thudium, K.; Doe, B.; Legg, H.; Liu, H.; Romero, R.E.; Tang, Z.; Bin, Q.; Dubensky, T.W.; et al. An alphavirus replicon particle chimera derived from Venezuelan equine encephalitis and sindbis viruses is a potent gene-based vaccine delivery vector. J. Virol. 2003, 77, 10394–10403. [Google Scholar] [CrossRef]
- Polo, J.M.; Belli, B.A.; Driver, D.A.; Frolov, I.; Sherrill, S.; Hariharan, M.J.; Townsend, K.; Perri, S.; Mento, S.J.; Jolly, D.J.; et al. Stable alphavirus packaging cell lines for Sindbis virus-and. Semliki Forest virus-derived vectors. Proc. Natl. Acad. Sci. USA 1999, 96, 4598–4603. [Google Scholar] [CrossRef]
- Weaver, S.C.; Kang, W.; Shirako, Y.; Rumenapf, T.; Strauss, E.G.; Strauss, J.H. Recombinational history and molecular evolution of western equine encephalomyelitis complex alphaviruses. J. Virol. 1997, 71, 613–623. [Google Scholar] [PubMed]
- Leitner, W.W.; Ying, H.; Restifo, N.P. DNA and. RNA-based vaccines: Principles, progress and prospects. Vaccine 1999, 18, 765–777. [Google Scholar] [CrossRef]
- Vander Veen, R.L.; Harris, D.H.; Kamrud, K.I. Alphavirus replicon vaccines. Anim. Health Res. Rev. 2012, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sandbulte, M.; Spickler, A.; Zaabel, P.; Roth, J. Optimal use of vaccines for control of influenza A virus in swine. Vaccines 2015, 3, 22–73. [Google Scholar] [CrossRef] [PubMed]
- Mogler, M.A.; Gander, J.; Harris, D.L.H. Development of an alphavirus RNA particle vaccine against porcine epidemic diarrhea virus. In Proceedings of the American Association of Swine Veterinarians Annual Meeting, Perry, IA, USA, 1–4 March 2014. [Google Scholar]
- Lundstrom, K. Replicon RNA viral vectors as vaccines. Vaccines 2016, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Murgia, M.V.; Mogler, M.; Certoma, A.; Green, D.; Monaghan, P.; Williams, D.T.; Rowland, R.R.; Gaudreault, N.N. Evaluation of an. African swine fever (ASF) vaccine strategy incorporating priming with an alphavirus-expressed antigen followed by boosting with attenuated ASF virus. Arch. Virol. 2019, 164, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Loy, J.D.; Gander, J.; Mogler, M.; Vander Veen, R.; Ridpath, J.; Harris, D.H.; Kamrud, K. Development and evaluation of a replicon particle vaccine expressing the E2 glycoprotein of bovine viral diarrhea virus (BVDV) in cattle. Virol. J. 2013, 10, 35. [Google Scholar] [CrossRef]
- Karlsen, M.; Villoing, S.; Rimstad, E.; Nylund, A. Characterization of untranslated regions of the salmonid alphavirus 3 (SAV3) genome and construction of a SAV3 based replicon. Virol. J. 2009, 6, 173. [Google Scholar] [CrossRef]
- Wolf, A.; Hodneland, K.; Frost, P.; Braaen, S.; Rimstad, E. A hemagglutinin-esterase-expressing salmonid alphavirus replicon protects Atlantic salmon (Salmo salar) against infectious salmon anemia (ISA). Vaccine 2013, 31, 661–669. [Google Scholar] [CrossRef]
- Wolf, A.; Hodneland, K.; Frost, P.; Hoeijmakers, M.; Rimstad, E. Salmonid alphavirus-based replicon vaccine against infectious salmon anemia (ISA): Impact of immunization route and interactions of the replicon vector. Fish Shellfish Immunol. 2014, 36, 383–392. [Google Scholar] [CrossRef]
| Disease | Pathogen | Major Fish Host | Vaccine Type | Antigens/Targets | Delivery Methods | Country/Region* | Further Information |
|---|---|---|---|---|---|---|---|
| Viral Diseases | |||||||
| Infectious hematopoietic necrosis | IHNV Rhabdovirus | Salmonids | DNA | G Glycoprotein | IM | Canada | https://www.dfo-mpo.gc.ca/aquaculture/rp-pr/acrdp-pcrda/projects-projets/P-07-04-010-eng.html |
| Infectious pancreatic necrosis | IPNV Birnavirus | Salmonids, sea bass, sea bream, turbot, Pacific cod | Inactivated | Inactivated IPNV | IP | Norway, Chile, UK | www.pharmaq.no |
| Subunit | VP2 and VP3 Capsid Proteins | Oral | Canada, USA | www.aquavac-vaccines.com | |||
| Subunit | VP2 Proteins | IP | Canada, Chile, Norway | http://www.msd-animal-health.no/ | |||
| Infectious salmon anemia | ISAV Orthomyxovirus | Atlantic salmon | Inactivated | Inactivated ISAV | IP | Norway, Chile, Ireland, Finland, Canada | www.pharmaq.no |
| Pancreatic disease virus | SAV alphaviruses | Salmonids | Inactivated | Inactivated SAV | IP | Norway, Chile, UK | https://www.merck-animal-health.co |
| Spring viremia of carp virus | SVCV Rhabdovirus | Carp | Subunit | G Glycoprotein | IP | Belgium | [22] |
| Inactivated | Inactivated SVCV | IP | Czech Republic | [23] | |||
| Koi herpesvirus disease | KHV Herpesvirus | Carp | Attenuated | Attenuated KHV | IMM or IP | Israel | [22] |
| Infectious spleen and kidney necrosis | ISKNV Iridovirus | Asian seabass, grouper, Japanese yellowtail | Inactivated | Inactivated ISKNV | IP | Singapore | https://www.aquavac-vaccines.com/ |
| Bacterial diseases | |||||||
| Enteric redmouth disease (ERM) | Yersinia ruckeri | Salmonids | Inactivated | Inactivated Y. ruckeri | IMM or oral | USA, Canada, Europe | http://www.msd-animal-health.ie/products_ni_vet/aquavac-erm-oral/overview.aspx; https://www.msd-animal-health-hub.co.uk |
| Vibriosis | Vibrio anguillarum; Vibrio ordalii; Vibrio salmonicida | Salmonids, ayu, grouper, sea bass, sea bream, yellowtail, cod, halibut | Inactivated | Inactivated Vibriosis spp. | IP or IMM | USA, Canada, Japan, Europe, Australia | https://www.merck-animal-health.com/species/aquaculture/trout.aspx; |
| Furunculosis | Aeromonas salmonicida subsp. salmonicida | Salmonids | Inactivated | Inactivated A. salmonicida spp. | IP or IMM | USA, Canada, Chile, Europe, Australia | https://www.msd-animal-health-me.com/species/aqua.aspx |
| Bacterial kidney disease (BKD) | Renibacterium salmoninarum | Salmonids | Avirulent live culture | Arthrobacter davidanieli | IP | Canada, Chile, USA | [24] |
| Enteric septicemia of catfish (ESC) | Edwarsiella ictaluri | Catfish | Inactivated | Inactivated E. ictaluri | IP | Vietnam | https://www.pharmaq.no/ |
| Columnaris disease | Flavobacterium columnaris | All freshwater finfish species, bream, bass, turbot, salmon | Attenuated | Attenuated F. columnare | IMM | USA | [25] |
| Pasteurellosis | Pasteurela piscicida | Sea bass, sea bream, sole | Inactivated | Inactivated P. pscicida | IMM | USA, Europe, Taiwan, Japan | ALPHA JECT 2000 |
| Lactococciosis | Lactococcus garviae | Rainbow trout, amberjack, yellowtail | Inactivated | Inactivated L. garviae | IP | Spain | https://www.hipra.com/ |
| Streptococcus infections | Streptococcus spp. | Tilapia, yellow tail, rainbow trout, ayu, sea bass, sea bream | Inactivated | Inactivated S. agalactiae (biotype 1) | IP | Taiwan Province of China, Japan, Brazil, Indonesia | https://www.aquavac-vaccines.com/products/aquavac-strep-sa1/ |
| Inactivated S. agalactiae (biotype 2) | IP | https://www.aquavac-vaccines.com/products/aquavac-strep-sa/ | |||||
| Inactivated S. iniae | IP or IMM | https://www.aquavac-vaccines.com/products/aquavac-strep-si/ | |||||
| Salmonid rickettsial septicemia | Piscirickettsia salmonis | Salmonids | Inactivated | Inactivated P. salmonis | IP | Chile | Evensen, 2016; https://www.pharmaq.no/products/injectable/ |
| Motile Aeromonas septicemia (MAS) | Aeromonas spp. | Striped catfish | Inactivated | A. hydrophila (serotype A and B) | IP | Vietnam | https://www.pharmaq.no/; ALPHAJECT Panga 2 |
| Wound Disease | Moritella viscosa | Salmonids | Inactivated | Inactivated M. viscosa | IP | Norway, UK, Ireland, Iceland | https://www.pharmaq.no |
| Tenacibaculosis | Tenacibaculum maritimum | Turbot | Inactivated | Inactivated T. maritimum | IP | Spain | https://www.hipra.com/ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches. Microorganisms 2019, 7, 569. https://doi.org/10.3390/microorganisms7110569
Ma J, Bruce TJ, Jones EM, Cain KD. A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches. Microorganisms. 2019; 7(11):569. https://doi.org/10.3390/microorganisms7110569
Chicago/Turabian StyleMa, Jie, Timothy J. Bruce, Evan M. Jones, and Kenneth D. Cain. 2019. "A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches" Microorganisms 7, no. 11: 569. https://doi.org/10.3390/microorganisms7110569
APA StyleMa, J., Bruce, T. J., Jones, E. M., & Cain, K. D. (2019). A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches. Microorganisms, 7(11), 569. https://doi.org/10.3390/microorganisms7110569






