Meta-Analysis and Meta-Regression Indicate Dynamic Prevalence and Moderators of Foodborne Pathogens in African Indigenous Fermented Milk
Abstract
1. Introduction
2. Materials and Methods
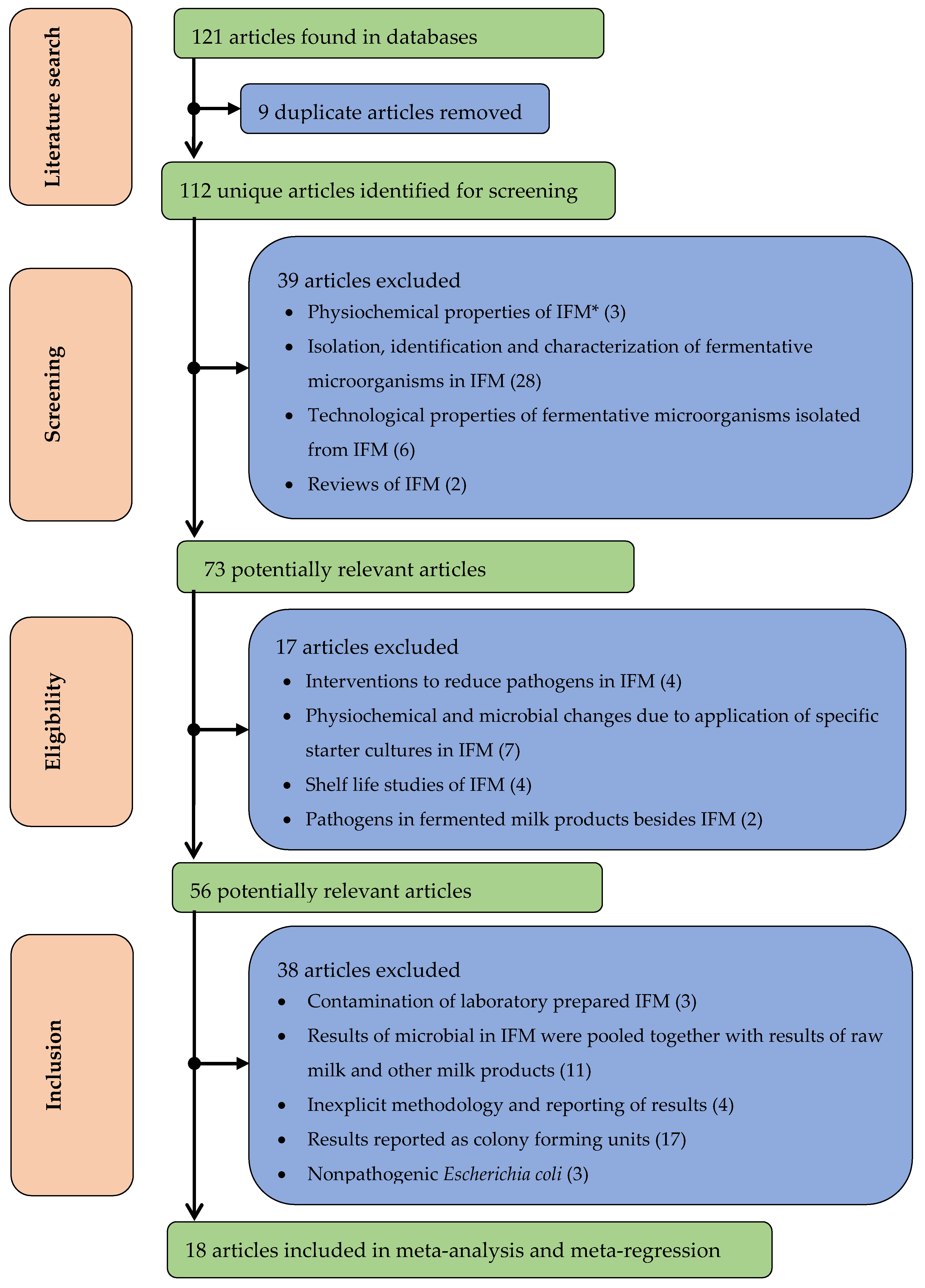
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Data Analysis
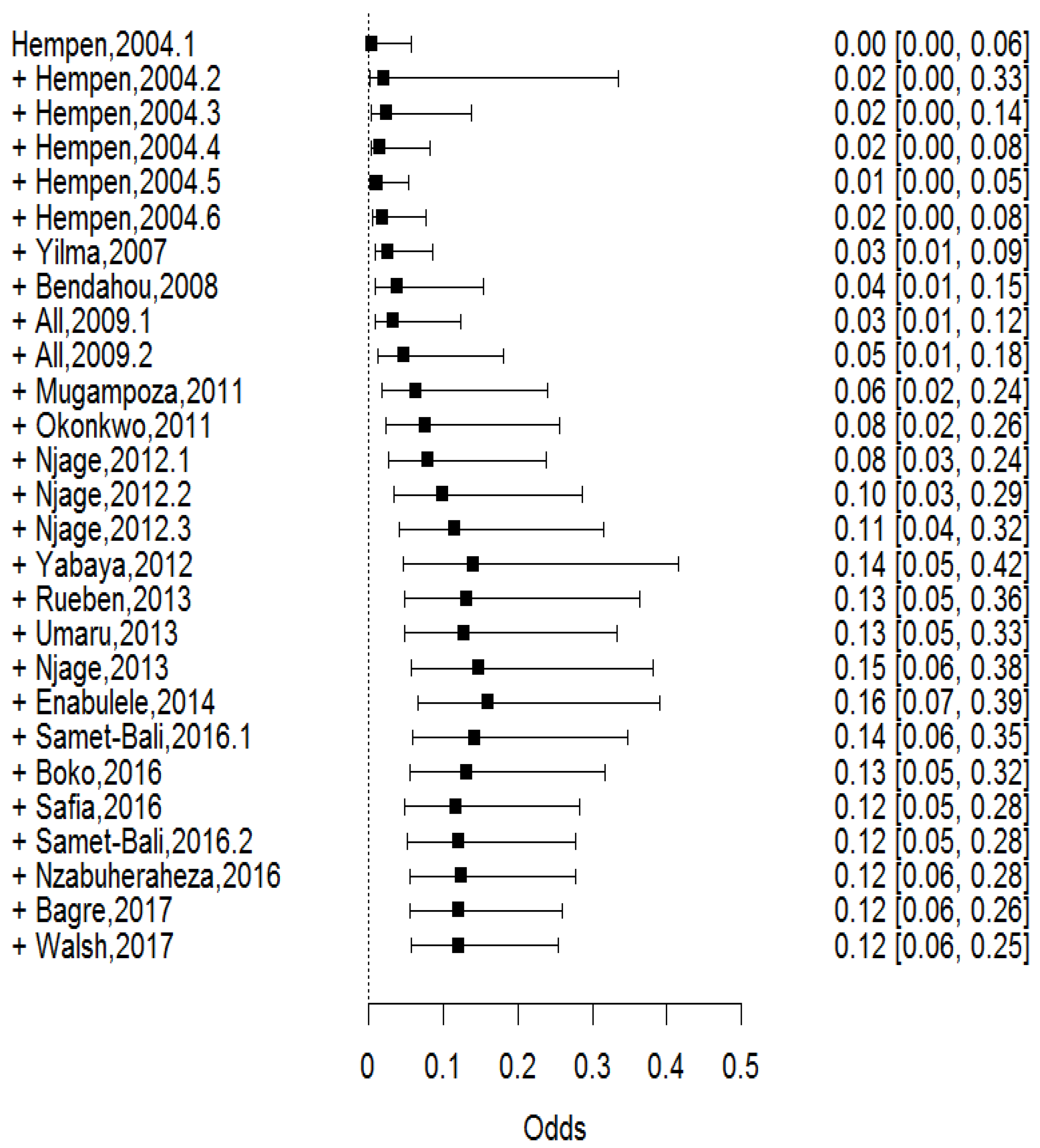
3. Results
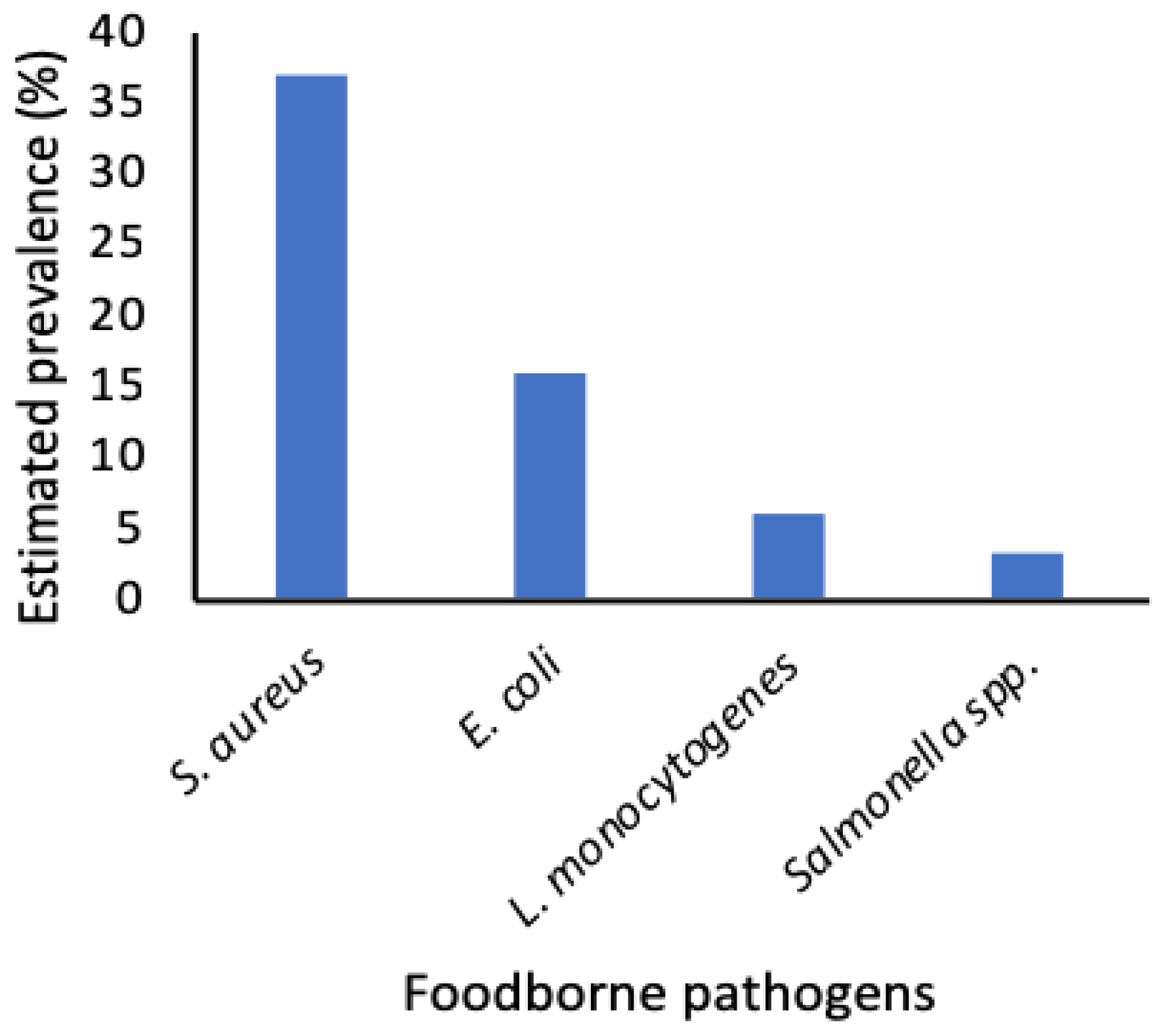
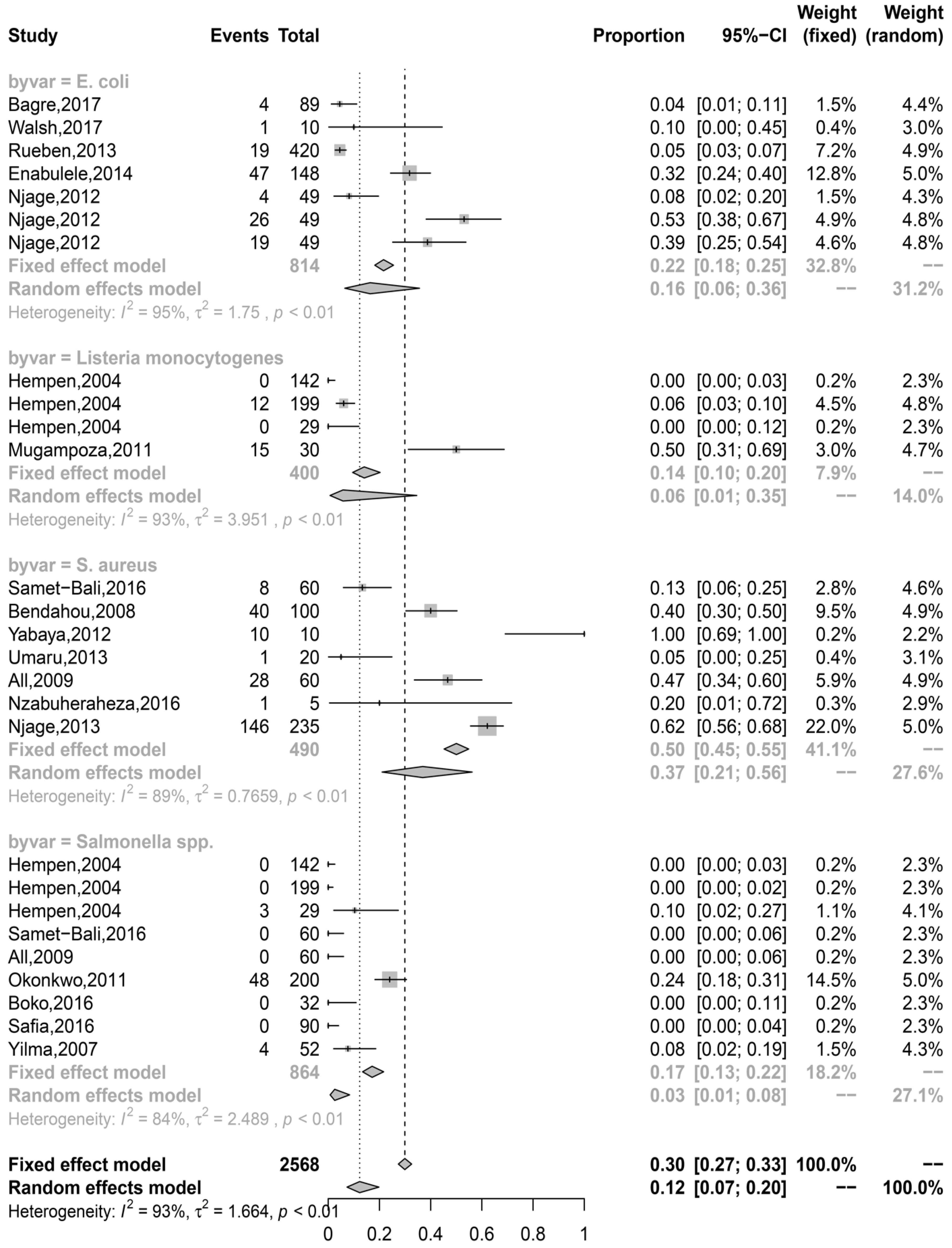
3.1. Staphylococcus spp., Pathogenic E. coli, L. Monocytogenes, and Salmonella spp. were the most Reported Foodborne Pathogens in IFM
3.2. Staphylococcus aureus and Pathogenic Escherichia coli were the Most Prevalent Pathogens in Published Studies
3.3. IFM Metadata in Published Studies
3.4. Prevalence Estimates were Highly Associated with the Point of Sampling, Country Clusters, and Pathogens in IFM
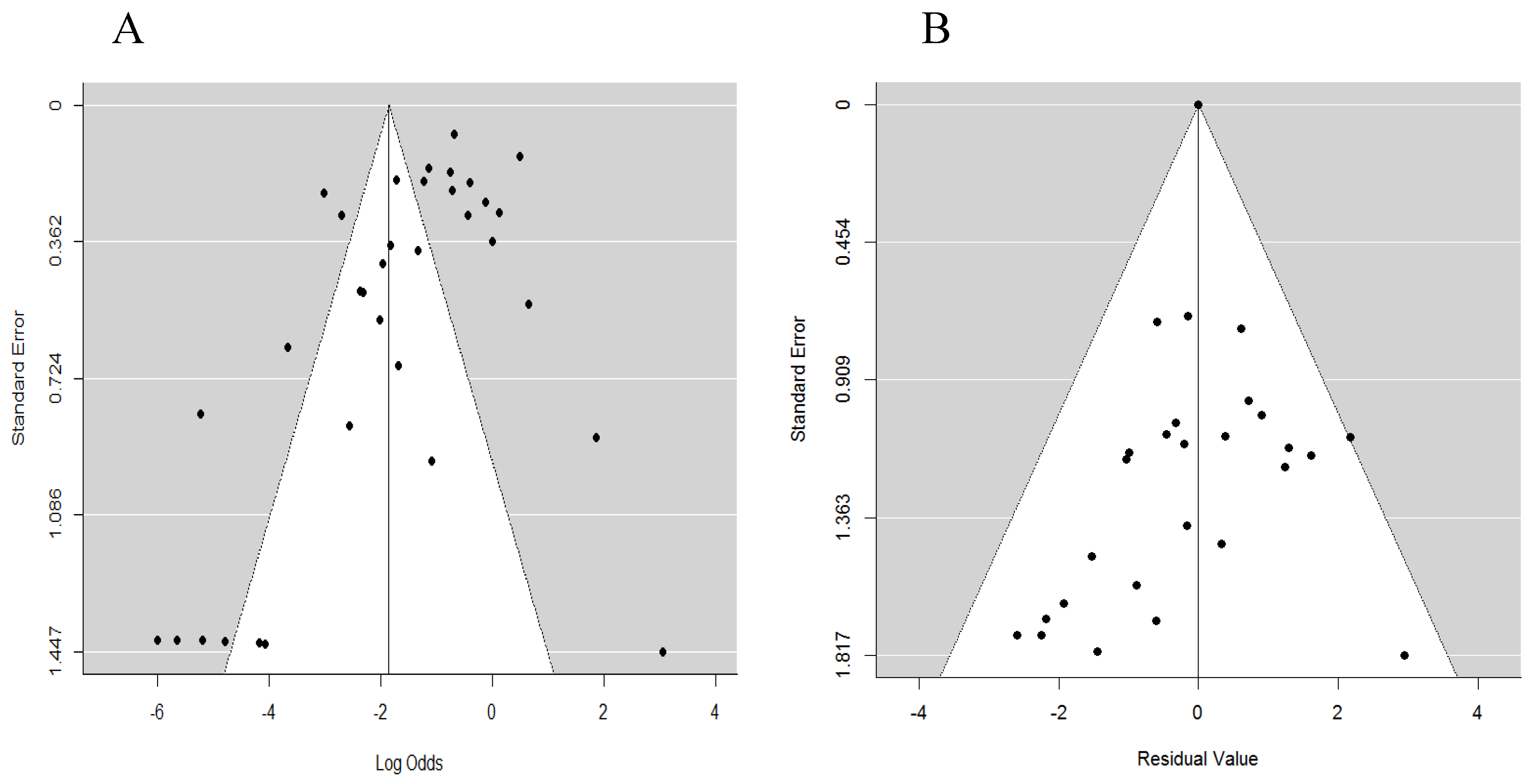
3.5. Publication and Heterogeneity Bias
3.6. The Prevalence of Foodborne Pathogens in IFM was Dynamic over Time
3.7. An Insight into the Antibiotic Resistance Risk from Pathogens in IFM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jans, C.; Bugnard, J.; Njage, P.M.K.; Lacroix, C.; Meile, L. Lactic Acid Bacteria diversity of African raw and fermented camel milk products reveals a highly competitive, potentially health-threatening predominant microflora. LWT-Food Sci. Technol. 2012, 47, 371–379. [Google Scholar] [CrossRef]
- Mathara, J.M.; Schillinger, U.; Kutima, P.M.; Mbugua, S.K.; Holzapfel, W.H. Isolation, identification and characterisation of the dominant microorganisms of kule naoto: The Maasai traditional fermented milk in Kenya. Int. J. Food Microbiol. 2004, 94, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.G.V.; Franz, C.M.A.P.; Schillinger, U.; Holzapfel, W.H. Lactobacillus spp. with in vitro probiotic properties from human faeces and traditional fermented products. Int. J. Food Microbiol. 2006, 109, 205–214. [Google Scholar] [CrossRef]
- Charlton, K.E.; Steyn, K.; Levitt, N.S.; Peer, N.; Jonathan, D.; Gogela, T.; Rossouw, K.; Gwebushe, N.; Lombard, C.J. A food-based dietary strategy lowers blood pressure in a low socio-economic setting: A randomised study in South Africa. Public Health Nutr. 2008, 11, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- All, A.A.A.A.; Dardir, H.A. Hygienic quality of local traditional fermented skimmed milk (laban rayb) sold in Egypt. World J. Dairy Food Sci. 2009, 4, 205–209. [Google Scholar]
- Horlbog, J.A.; Kent, D.; Stephan, R.; Guldimann, C. Surviving host - and food relevant stresses: Phenotype of L. monocytogenes strains isolated from food and clinical sources. Sci. Rep. 2018, 8, 12931. [Google Scholar] [CrossRef]
- Macuamule, C.L.S.; Wiid, I.J.; van Helden, P.D.; Tanner, M.; Witthuhn, R.C. Effect of milk fermentation by kefir grains and selected single strains of Lactic Acid Bacteria on the survival of Mycobacterium bovis BCG. Int. J. Food Microbiol. 2016, 217, 170–176. [Google Scholar] [CrossRef]
- De Campos, A.C.L.P.; Puño-Sarmiento, J.J.; Medeiros, L.P.; Gazal, L.E.S.; Maluta, R.P.; Navarro, A.; Kobayashi, R.K.T.; Fagan, E.P.; Nakazato, G. Virulence genes and antimicrobial resistance in Escherichia coli from cheese made from unpasteurized milk in Brazil. Foodborne Pathog. Dis. 2018, 15, 94–100. [Google Scholar] [CrossRef]
- Opiyo, B.A.; Wangoh, J.; Njage, P.M.K. Microbiological performance of dairy processing plants is influenced by scale of production and the implemented Food Safety Management System: A case study. J. Food Prot. 2013, 76, 975–983. [Google Scholar] [CrossRef]
- Njage, P.M.K.; Opiyo, B.; Wangoh, J.; Wambui, J. Scale of production and implementation of food safety programs influence the performance of current Food Safety Management Systems: Case of dairy processors. Food Control 2018, 85, 85–97. [Google Scholar] [CrossRef]
- Yang, H.; Bain, R.; Bartram, J.; Gundry, S.; Pedley, S.; Wright, J. Water safety and inequality in access to drinking-water between rich and poor households. Environ. Sci. Technol. 2013, 47, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Odongo, N.O.; Lamuka, P.O.; Matofari, J.W.; Abong, G.O. Risk factors associated with the post-harvest loss of milk along camel milk value chain in Isiolo County, Kenya. Afr. J. Agric. Res. 2016, 11, 674–682. [Google Scholar] [CrossRef]
- Ruangwittayanusorn, K.; Promket, D.; Chantiratikul, A. Monitoring the hygiene of raw milk from farms to milk retailers. Agric. Agric. Sci. Procedia 2016, 11, 95–99. [Google Scholar] [CrossRef]
- Lore, T.A.; Mbugua, S.K.; Wangoh, J. Enumeration and identification of microflora in suusac, a Kenyan traditional fermented camel milk product. LWT-Food Sci. Technol. 2005, 38, 125–130. [Google Scholar] [CrossRef]
- Maitha, I.M.; Kaindi, D.W.M.; Wangoh, J.; Mbugua, S. Microbial quality and safety of traditional fermented camel milk product suusac sampled from different regions in North Eastern, Kenya. Asian Food Sci. J. 2019, 1–9. [Google Scholar] [CrossRef]
- Njage, P.M.K.; Dolci, S.; Jans, C.; Wangoh, J.; Lacroix, C.; Meile, L. Phenotypic and genotypic antibiotic resistance patterns of Staphylococcus aureus from raw and spontaneously fermented camel milk. Br. J. Appl. Sci. Technol. 2013, 3, 87–98. [Google Scholar] [CrossRef]
- Njage, P.M.K.; Jans, C.; Wangoh, J.; Lacroix, C.; Meile, L. Detection, isolation and molecular characterisation of Shigatoxigenic O157 and non-O157 Escherichia coli in raw and fermented camel milk. Afr. J. Microbiol. Res. 2012, 6, 6031–6038. [Google Scholar]
- Larsson, S.C.; Crippa, A.; Orsini, N.; Wolk, A.; Michaëlsson, K. Milk consumption and mortality from all causes, cardiovascular disease, and cancer: A systematic review and meta-analysis. Nutrients 2015, 7, 7749–7763. [Google Scholar] [CrossRef]
- Franz, C.M.A.P.; Huch, M.; Mathara, J.M.; Abriouel, H.; Benomar, N.; Reid, G.; Galvez, A.; Holzapfel, W.H. African fermented foods and probiotics. Int. J. Food Microbiol. 2014, 190, 84–96. [Google Scholar] [CrossRef]
- Jans, C.; Meile, L.; Kaindi, D.W.M.; Kogi-Makau, W.; Lamuka, P.; Renault, P.; Kreikemeyer, B.; Lacroix, C.; Hattendorf, J.; Zinsstag, J.; et al. African fermented dairy products – overview of predominant technologically important microorganisms focusing on African Streptococcus infantarius variants and potential future applications for enhanced food safety and security. Int. J. Food Microbiol. 2017, 250, 27–36. [Google Scholar] [CrossRef]
- Paudyal, N.; Anihouvi, V.; Hounhouigan, J.; Matsheka, M.I.; Sekwati-Monang, B.; Amoa-Awua, W.; Atter, A.; Ackah, N.B.; Mbugua, S.; Asagbra, A.; et al. Prevalence of foodborne pathogens in food from selected African countries – a meta-analysis. Int. J. Food Microbiol. 2017, 249, 35–43. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Macro Indicators. Available online: http://www.fao.org/faostat/en/#data/MK (accessed on 9 May 2017).
- UNICEF. WHO/UNICEF JMP Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baseline. Available online: https://www.unicef.org/publications/files/Progress_on_Drinking_Water_Sanitation_and_Hygiene_2017.pdf (accessed on 9 May 2017).
- Viechtbauer, W. Conducting meta-analysis in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Schwarzer, G. Meta: An R package for meta-analysis. R News 2007, 7, 40–45. [Google Scholar]
- Enabulele, S.A.; Eghafona, N.O.; Dahiru, M. Molecular characterisation and verotoxigenic potentials of Enterohaemorrhagic Escherichia coli O157:H7 isolated from fermented fresh cow milk (nunu) sold in selected cities in Nigeria. J. Basic Appl. Sci. 2014, 1, 51–62. [Google Scholar]
- Reuben, C.R.; Okolocha, E.C.; Bello, M.; Tanimu, H. Occurrence and antibiogram of Escherichia coli O157:H7 in locally fermented milk (nono) sold under market conditions in Nasarawa State, Nigeria. Int. J. Sci. Eng. Res. 2013, 1, 591–598. [Google Scholar]
- Bagre, T.S.; Sambe-Ba, B.; Ibrahim, H.B.; Tchamba, G.B.; Dembele, R.; Wane, A.A.; Bako, E.; Traore, A.S.; Barro, N.; Gassama-Sow, A. Isolation and characterization of Enteropathogenic and Enterotoxinogenic Escherichia coli from dairy products consumed in Burkina Faso. Afr. J. Microbiol. Res. 2017, 11, 537–545. [Google Scholar]
- Walsh, A.M.; Crispie, F.; Daari, K.; O’Sullivan, O.; Martin, J.C.; Arthur, C.T.; Claesson, M.J.; Scott, K.P.; Cotter, P.D. Strain-level metagenomic analysis of the fermented dairy beverage nunu highlights potential food safety risks. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef]
- Hempen, M.; Unger, F.; Münstermann, S.; Seck, M.T.; Niamy, V. The Hygienic Status of Raw and Sour Milk from Smallholder Dairy Farms and Local Markets and Potential Risk for Public Health in The Gambia, Senegal and Guinea; International Trypanotolerance Centre: Banjul, Gambia, 2004. [Google Scholar]
- Mugampoza, D.; Muyanja, C.M.B.K.; Ogwok, P.; Serunjogi, M.L.; Nasinyama, G.W. Occurrence of Listeria monocytogenes in bulked raw milk and traditionally fermented dairy products in Uganda. Afr. J. Food Agric. Nutr. Dev. 2011, 11, 4610–4622. [Google Scholar] [CrossRef]
- Bendahou, A.; Lebbadi, M.; Ennanei, L.; Essadqui, F.Z.; Abid, M. Characterization of Staphylococcus species isolated from raw milk and milk products (lben and jben) in North Morocco. J. Infect. Dev. Ctries. 2008, 2, 218–225. [Google Scholar]
- Nzabuheraheza, F.; Nyiramugwera, A. Microbiological analysis of traditionally fermented milk sold in Kinigi Sector of Musanze District in Rwanda. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 10841–10852. [Google Scholar] [CrossRef]
- Samet-Bali, O.; Felfoul, I.; Lajnaf, R.; Attia, H.; Ayadi, M.A. Hygienic quality of “rayeb”, a traditional Tunisian fermented cow’s milk. Int. Food Res. J. 2016, 23, 366–369. [Google Scholar]
- Umaru, G.A.; Kabir, J.; Umoh, V.J.; Bello, M.; Kwaga, J.K.P. Methicillin-Resistant Staphylococcus aureus (MRSA) in fresh and fermented milk in Zaria and Kaduna, Nigeria. Int. J. Drug Res. Technol. 2013, 3, 67–75. [Google Scholar]
- Yabaya, A.; Manga, S.S.; Lucy, M.; Alhassan, H.M. Bacteriological quality of fermented milk sold locally in Samaru and Sabongari Market, Zaria – Nigeria. Cont. J. Microbiol. 2012, 6, 14–18. [Google Scholar]
- Boko, C.; Adje, Y.; Atindogbe, G.; Hounmanou, Y.M.G.; Dossa, F.; Sessou, P.; Farougou, S. Evaluation of the production technologies and the microbial and physico-chemical qualities of curdled milk produced in Benin. J. Appl. Biosci. 2016, 104, 10019–10033. [Google Scholar] [CrossRef]
- Okonkwo, O.I. Microbiological analyses and safety evaluation of nono: A fermented milk product consumed in most parts of Northern Nigeria. Int. J. Dairy Sci. 2011, 6, 181–189. [Google Scholar] [CrossRef][Green Version]
- Safia, C.N.; Benalia, Y.; Ahcène, H.; Redha, M.C.; Feriha, T.; Yacine, T.; Amel, C.; Abdelghani, Z. The bacteriological quality of unpasteurized milk and traditional dairy products sold via informal circuit in Djelfa City (Algeria). J. Microbiol. Biotechnol. Res. 2016, 6, 9–16. [Google Scholar]
- Yilma, Z.; Faye, B.; Loiseau, G. Occurrence and distribution of species of Enterobacteriaceae in selected Ethiopian traditional dairy products: A contribution to epidemiology. Food Control 2007, 18, 1397–1404. [Google Scholar] [CrossRef]
- Islam, M.Z.; Musekiwa, A.; Islam, K.; Ahmed, S.; Chowdhury, S.; Ahad, A.; Biswas, P.K. Regional variation in the prevalence of E. coli O157 in cattle: A meta-analysis and meta-regression. PLoS ONE 2014, 9, e93299. [Google Scholar] [CrossRef]
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Gonfa, A.; Foster, H.A.; Holzapfel, W.H. Field survey and literature review on traditional fermented milk products of Ethiopia. Int. J. Food Microbiol. 2001, 68, 173–186. [Google Scholar] [CrossRef]
- John, M.N.; Joseph, W.M.; Zacchaeus, O.N.; Moses, B.S. Spontaneously fermented Kenyan milk products: A review of the current state and future perspectives. Afr. J. Food Sci. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Uthman, O.A.; Uthman, M.B. Geography of Africa biomedical publications: An analysis of 1996–2005 PubMed papers. Int. J. Health Geogr. 2007, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Manguiat, L.S.; Fang, T.J. Microbiological quality of chicken- and pork-based street-vended foods from Taichung, Taiwan, and Laguna, Philippines. Food Microbiol. 2013, 36, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Muloi, D.; Alarcon, P.; Ombui, J.; Ngeiywa, K.J.; Abdullahi, B.; Muinde, P.; Karani, M.K.; Rushton, J.; Fèvre, E.M. Value chain analysis and sanitary risks of the camel milk system supplying Nairobi City, Kenya. Prev. Vet. Med. 2018, 159, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Odongo, N.; Matofari, J.; Abong’, G.; Lamuka, P.; Abey, K. Knowledge and practices of food hygiene and safety among camel milk handlers in the pastoral camel value chain in Kenya. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 11803–11821. [Google Scholar]
- Kivaria, F.M.; Noordhuizen, J.P.T.M.; Kapaga, A.M. Risk indicators associated with subclinical mastitis in smallholder dairy cows in Tanzania. Trop. Anim. Health Prod. 2004, 36, 581–592. [Google Scholar] [CrossRef]
- Almaw, G.; Zerihun, A.; Asfaw, Y. Bovine mastitis and its association with selected risk factors in smallholder dairy farms in and around Bahir Dar, Ethiopia. Trop. Anim. Health Prod. 2008, 40, 427–432. [Google Scholar] [CrossRef]
- Logue, C.M.; Wannemuehler, Y.; Nicholson, B.A.; Doetkott, C.; Barbieri, N.L.; Nolan, L.K. Comparative analysis of phylogenetic assignment of human and avian ExPEC and fecal commensal Escherichia coli using the (previous and revised) Clermont phylogenetic typing methods and its impact on avian pathogenic Escherichia coli (APEC) classification. Front. Microbiol. 2017, 8, 283. [Google Scholar] [CrossRef]
- Amenu, K.; Grace, D.; Nemo, S.; Wieland, B. bacteriological quality and safety of ready-to-consume milk and naturally fermented milk in Borana Pastoral Area, Southern Ethiopia. Trop. Anim. Health Prod. 2019, 51, 2079–2084. [Google Scholar] [CrossRef]
- Sonnier, J.L.; Karns, J.S.; Lombard, J.E.; Kopral, C.A.; Haley, B.J.; Kim, S.-W.; Van Kessel, J.A.S. Prevalence of Salmonella enterica, Listeria monocytogenes, and pathogenic Escherichia coli in bulk tank milk and milk filters from US dairy operations in the National Animal Health Monitoring System Dairy 2014 study. J. Dairy Sci. 2018, 101, 1943–1956. [Google Scholar] [CrossRef]
- Owusu-Kwarteng, J.; Wuni, A.; Akabanda, F.; Jespersen, L. Prevalence and characteristics of Listeria monocytogenes isolates in raw milk, heated milk and nunu, a spontaneously fermented milk beverage, in Ghana. Beverages 2018, 4, 40. [Google Scholar] [CrossRef]
- Seyoum, E.T.; Woldetsadik, D.A.; Mekonen, T.K.; Gezahegn, H.A.; Gebreyes, W.A. Prevalence of Listeria monocytogenes in raw bovine milk and milk products from Central Highlands of Ethiopia. J. Infect. Dev. Ctries. 2015, 9, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Parry-Hanson Kunadu, A.; Holmes, M.; Miller, E.L.; Grant, A.J. Microbiological quality and antimicrobial resistance characterization of Salmonella spp. in fresh milk value chains in Ghana. Int. J. Food Microbiol. 2018, 277, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Matofari, J.W.; Shitandi, A.; Shalo, P.L.; Nanua, N.J.; Younan, M. A survey of Salmonella enterica contamination of camel milk in Kenya. Afr. J. Microbiol. Res. 2007, 1, 46–050. [Google Scholar]
- Omiccioli, E.; Amagliani, G.; Brandi, G.; Magnani, M. A new platform for Real-Time PCR detection of Salmonella Spp., Listeria monocytogenes and Escherichia coli O157 in milk. Food Microbiol. 2009, 26, 615–622. [Google Scholar] [CrossRef]
- Martínez-Suárez, J.V.; Ortiz, S.; López-Alonso, V. Potential impact of the resistance to quaternary ammonium disinfectants on the persistence of Listeria monocytogenes in food processing environments. Front. Microbiol. 2016, 7, 638. [Google Scholar] [CrossRef]
- Nyambane, B.; Thari, W.M.; Wangoh, J.; Njage, P.M.K. Lactic Acid Bacteria and yeasts involved in the fermentation of amabere amaruranu, a Kenyan fermented milk. Food Sci. Nutr. 2014, 2, 692–699. [Google Scholar] [CrossRef]
- Kebede, A.; Viljoen, B.C.; Gadaga, T.H.; Narvhus, J.A.; Lourens-Hattingh, A. The effect of container type on the growth of yeast and Lactic Acid Bacteria during production of sethemi, South African spontaneously fermented milk. Food Res. Int. 2007, 40, 33–38. [Google Scholar] [CrossRef]
- Beukes, E.M.; Bester, B.H.; Mostert, J.F. The microbiology of South African traditional fermented milks. Int. J. Food Microbiol. 2001, 63, 189–197. [Google Scholar] [CrossRef]
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes persistence in food-associated environments: Epidemiology, strain characteristics, and implications for public health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef]
- Jans, C.; Kaindi, D.W.M.; Böck, D.; Njage, P.M.K.; Kouamé-Sina, S.M.; Bonfoh, B.; Lacroix, C.; Meile, L. Prevalence and comparison of Streptococcus infantarius subsp. infantarius and Streptococcus gallolyticus subsp. macedonicus in raw and fermented dairy products from East and West Africa. Int. J. Food Microbiol. 2013, 167, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Jans, C.; de Wouters, T.; Bonfoh, B.; Lacroix, C.; Kaindi, D.W.M.; Anderegg, J.; Böck, D.; Vitali, S.; Schmid, T.; Isenring, J.; et al. Phylogenetic, epidemiological and functional analyses of the Streptococcus bovis/Streptococcus equinus complex through an overarching MLST scheme. BMC Microbiol. 2016, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Corsello, G.; Nocerino, R.; Carta, M.; Marinello, R.; Picca, M.; De Marco, G.; Micillo, M.; Ferrara, D.; Vigneri, P.; et al. Effect of fermented milk with Lactobacillus paracasei CBA l74 on gastrointestinal and respiratory infections in children: Multicenter randomized controlled trial. Dig. Liver Dis. 2016, 48, e280–e281. [Google Scholar] [CrossRef]
- Van den Nieuwboer, M.; Klomp-Hogeterp, A.; Verdoorn, S.; Metsemakers-Brameijer, L.; Vriend, T.M.; Claassen, E.; Larsen, O.F.A. Improving the bowel habits of elderly residents in a nursing home using probiotic fermented milk. Benef. Microbes 2015, 6, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, A.; Bi, X.; Nguyen, L.; Matsuda, K.; Pham, H.; Phan, C.; Khu, D.; Ichimura, H. Effects of short-term probiotic ingestion on immune profiles and microbial translocation among HIV-1-infected Vietnamese children. Int. J. Mol. Sci. 2017, 18, 2185. [Google Scholar] [CrossRef] [PubMed]
- Johler, S.; Weder, D.; Bridy, C.; Huguenin, M.-C.; Robert, L.; Hummerjohann, J.; Stephan, R. Outbreak of Staphylococcal food poisoning among children and staff at a Swiss boarding school due to soft cheese made from raw milk. J. Dairy Sci. 2015, 98, 2944–2948. [Google Scholar] [CrossRef] [PubMed]
- Johler, S.; Tichaczek-Dischinger, P.S.; Rau, J.; Sihto, H.-M.; Lehner, A.; Adam, M.; Stephan, R. Outbreak of Staphylococcal food poisoning due to SEA-producing Staphylococcus aureus. Foodborne Pathog. Dis. 2013, 10, 777–781. [Google Scholar] [CrossRef]
- Fetsch, A.; Contzen, M.; Hartelt, K.; Kleiser, A.; Maassen, S.; Rau, J.; Kraushaar, B.; Layer, F.; Strommenger, B. Staphylococcus aureus food-poisoning outbreak associated with the consumption of ice-cream. Int. J. Food Microbiol. 2014, 187, 1–6. [Google Scholar] [CrossRef]
- Nema, V.; Agrawal, R.; Kamboj, D.V.; Goel, A.K.; Singh, L. Isolation and characterization of heat resistant enterotoxigenic Staphylococcus aureus from a food poisoning outbreak in Indian subcontinent. Int. J. Food Microbiol. 2007, 117, 29–35. [Google Scholar] [CrossRef]
- Mungai, E.A.; Behravesh, C.B.; Gould, L.H. Increased outbreaks associated with nonpasteurized milk, United States, 2007–2012. Emerg. Infect. Dis. 2015, 21, 119–122. [Google Scholar] [CrossRef]
- Heiman, K.E.; Mody, R.K.; Johnson, S.D.; Griffin, P.M.; Gould, L.H. Escherichia coli O157 outbreaks in the United States, 2003–2012. Emerg. Infect. Dis. 2015, 21, 1293. [Google Scholar] [CrossRef] [PubMed]
- Currie, A.; Galanis, E.; Chacon, P.A.; Murray, R.; Wilcott, L.; Kirkby, P.; Honish, L.; Franklin, K.; Farber, J.; Parker, R.; et al. Outbreak of Escherichia coli O157:H7 infections linked to aged raw milk gouda cheese, Canada, 2013. J. Food Prot. 2018, 81, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.A.; Gould, L.H.; Hunter, J.C.; Kucerova, Z.; Jackson, B. Listeriosis outbreaks associated with soft cheeses, United States, 1998-20141. Emerg. Infect. Dis. 2018, 24, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Costard, S.; Espejo, L.; Groenendaal, H.; Zagmutt, F.J. Outbreak-related disease burden associated with consumption of unpasteurized cow’s milk and cheese, United States, 2009-2014. Emerg. Infect. Dis. 2017, 23, 957–964. [Google Scholar] [CrossRef]
- Ung, A.; Baidjoe, A.Y.; Van Cauteren, D.; Fawal, N.; Fabre, L.; Guerrisi, C.; Danis, K.; Morand, A.; Donguy, M.-P.; Lucas, E.; et al. Disentangling a complex nationwide Salmonella dublin outbreak associated with raw-milk cheese consumption, France, 2015 to 2016. Euro Surveill. 2019, 24. [Google Scholar] [CrossRef]
- Lupindu, A.M. Epidemiology of Shiga Toxin-producing Escherichia coli O157:H7 in Africa in review. South. Afr. J. Infect. Dis. 2018, 33, 24–30. [Google Scholar] [CrossRef]
- Gilchrist, J.J.; MacLennan, C.A. Invasive nontyphoidal Salmonella disease in Africa. EcoSal Plus 2019, 8. [Google Scholar] [CrossRef]
- Olanya, O.M.; Hoshide, A.K.; Ijabadeniyi, O.A.; Ukuku, D.O.; Mukhopadhyay, S.; Niemira, B.A.; Ayeni, O. Cost estimation of listeriosis (Listeria monocytogenes) occurrence in South Africa in 2017 and its food safety implications. Food Control 2019, 102, 231–239. [Google Scholar] [CrossRef]
- Njage, P.M.K.; Dolci, S.; Jans, C.; Wangoh, J.; Lacroix, C.; Meile, L. Characterization of yeasts associated with camel milk using phenotypic and molecular identification techniques. Res. J. Microbiol. 2011, 6, 678–692. [Google Scholar] [CrossRef]
- Kaindi, D.; Schelling, E.; Wangoh, J.; Imungi, J.; Farah, Z.; Meile, L. Microbiological quality of raw camel milk across the Kenyan market chain. Food 2011, 5, 79–83. [Google Scholar]
- King, T.; Lucchini, S.; Hinton, J.C.D.; Gobius, K. Transcriptomic analysis of Escherichia coli O157:H7 and K-12 cultures exposed to inorganic and organic acids in stationary phase reveals acidulant- and strain-specific acid tolerance responses. Appl. Environ. Microbiol. 2010, 76, 6514–6528. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-W.; Yu, R.-C.; Chou, C.-C. Acid adaptation affects the viability of Salmonella typhimurium during the lactic fermentation of skim milk and product storage. Int. J. Food Microbiol. 2007, 114, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Abdelwaheb, C.; Ahmed, L. Acid pre-adaptation enhances virulence of Salmonella enterica serovar typhimurium dam mutant. Pathol. Biol. 2009, 57, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Gahan, C.G.M.; O’Driscoll, B.; Hill, C. Acid adaptation of Listeria monocytogenes can enhance survival in acidic foods and during milk fermentation. Appl. Environ. Microbiol. 1996, 62, 3128–3132. [Google Scholar] [PubMed]
- Cotter, P.D.; Hill, C. Surviving the acid test: Responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 2003, 67, 429–453. [Google Scholar] [CrossRef]
| Pathogen | IFM Samples | Positive Samples | Prevalence (%) | Country | Reference |
|---|---|---|---|---|---|
| Pathogenic E. coli* | 148 | 47 | 31.8 | Nigeria | [26] |
| Pathogenic E. coli | 49 | 4 | 8.2 | Kenya | [17] |
| Pathogenic E. coli | 49 | 26 | 53.1 | Kenya | [17] |
| Pathogenic E. coli | 49 | 19 | 38.8 | Kenya | [17] |
| Pathogenic E. coli | 420 | 19 | 4.6 | Nigeria | [27] |
| Pathogenic E. coli | 89 | 4 | 4.5 | Burkina Faso | [28] |
| Pathogenic E. coli | 10 | 1 | 10.0 | Ghana | [29] |
| L. monocytogenes | 142 | 0 | 0.0 | Gambia | [30] |
| L. monocytogenes | 199 | 12 | 6.0 | Guinea | [30] |
| L. monocytogenes | 29 | 0 | 0.0 | Senegal | [30] |
| L. monocytogenes | 30 | 15 | 50.0 | Uganda | [31] |
| S. aureus | 60 | 28 | 46.7 | Egypt | [5] |
| S. aureus | 100 | 40 | 40.0 | Morocco | [32] |
| S. aureus | 235 | 146 | 62.1 | Kenya | [16] |
| S. aureus | 5 | 1 | 20.0 | Rwanda | [33] |
| S. aureus | 60 | 8 | 13.3 | Tunisia | [34] |
| S. aureus | 20 | 1 | 5.0 | Nigeria | [35] |
| S. aureus | 10 | 10 | 100.0 | Nigeria | [36] |
| Salmonella spp. | 60 | 0 | 0.0 | Egypt | [5] |
| Salmonella spp. | 32 | 0 | 0.0 | Benin | [37] |
| Salmonella spp. | 142 | 0 | 0.0 | Gambia | [30] |
| Salmonella spp. | 199 | 0 | 0.0 | Guinea | [30] |
| Salmonella spp. | 29 | 3 | 10.3 | Senegal | [30] |
| Salmonella spp. | 200 | 48 | 24.0 | Nigeria | [38] |
| Salmonella spp. | 90 | 0 | 0.0 | Algeria | [39] |
| Salmonella spp. | 60 | 0 | 0.0 | Tunisia | [34] |
| Salmonella spp. | 52 | 4 | 7.7 | Ethiopia | [40] |
| Pathogen | Sampling Point | Microbial Confirmation | Mean pH | Mean Temperature (°C) | Reference |
|---|---|---|---|---|---|
| Pathogenic E. coli* | Market and retail | PCR | [26] | ||
| Pathogenic E. coli | Market and retail | [28] | |||
| Pathogenic E. coli | Production | PCR | [17] | ||
| Pathogenic E. coli | Collection | PCR | [17] | ||
| Pathogenic E. coli | Market and retail | PCR | [17] | ||
| Pathogenic E. coli | Market and retail | Biochemically | [27] | ||
| Pathogenic E. coli | Production | Biochemically | [29] | ||
| L. monocytogenes | Market and retail | Biochemically | 4.2 | 28.6 | [30] |
| L. monocytogenes | Market and retail | Biochemically | 4.1 | 29.2 | [30] |
| L. monocytogenes | Market and retail | Biochemically | 4.6 | 31.7 | [30] |
| L. monocytogenes | Collection | Biochemically | 4.4 | 8.6 | [31] |
| S. aureus | Market and retail | Biochemically | 3.9 | [5] | |
| S. aureus | Market and retail | PCR | [32] | ||
| S. aureus | Market and retail | PCR | [16] | ||
| S. aureus | Market and retail | Biochemically | [33] | ||
| S. aureus | Market and retail | Biochemically | 3.9 | [34] | |
| S. aureus | Household | Biochemically | [35] | ||
| S. aureus | Market and retail | Biochemically | 3.9 | [36] | |
| Salmonella spp. | Market and retail | Biochemically | [5] | ||
| Salmonella spp. | Production | Biochemically | 3.8 | [37] | |
| Salmonella spp. | Market and retail | Biochemically | [30] | ||
| Salmonella spp. | Market and retail | Biochemically | [30] | ||
| Salmonella spp. | Market and retail | Biochemically | [30] | ||
| Salmonella spp. | Market and retail | Biochemically | 4.3 | [38] | |
| Salmonella spp. | Market and retail | Biochemically | [39] | ||
| Salmonella spp. | Market and retail | Biochemically | 3.9 | [34] | |
| Salmonella spp. | Production | Biochemically | [40] |
| Univariate Analysis | Multivariate Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables and Covariates | Estimated Prev Dif | SE | 95% CI (LB) | 95% CI (UB) | p | Estimated Prev Dif | SE | 95% CI (LB) | 95% CI (UB) | p |
| Intercept | −69.86 | 193.90 | −449.88 | 310.17 | 0.72 | −26.10 | 190.28 | −399.04 | 346.85 | 0.89 |
| Confirmation method | ||||||||||
| Biochemical tests (ref) | - | - | - | - | - | - | - | - | - | - |
| PCR | −0.05 | 0.79 | −1.59 | 1.50 | 0.95 | |||||
| Point of sampling | ||||||||||
| Collection point (ref) | - | - | - | - | - | - | - | - | - | - |
| Household | −6.56 | 2.03 | −10.55 | −2.57 | 0.00 | −6.11 | 1.29 | −8.51 | −3.46 | 0.00 |
| Market and retail | −2.81 | 1.325 | −5.42 | −0.205 | 0.04 | −3.735 | 1.19 | −5.88 | −1.21 | 0.01 |
| Production | −2.94 | 1.31 | −5.52 | −0.37 | 0.03 | −2.80 | 1.13 | −4.89 | −0.45 | 0.02 |
| Pathogen | ||||||||||
| E. coli (ref) | - | - | - | - | - | - | - | - | - | - |
| L. monocytogenes | −0.95 | 1.22 | −3.35 | 1.44 | 0.44 | −1.25 | 1.15 | −3.51 | 1.00 | 0.28 |
| S. aureus | 3.01 | 0.95 | 1.15 | 4.87 | 0.00 | 2.23 | 0.79 | 0.68 | 3.78 | 0.00 |
| Salmonella spp. | −0.80 | 1.01 | −2.77 | 1.18 | 0.43 | −1.18 | 0.86 | −2.86 | 0.50 | 0.17 |
| Cluster | ||||||||||
| Cluster 1 (ref) | - | - | - | - | - | - | - | - | - | - |
| Cluster 2 | 2.09 | 1.74 | −1.32 | 5.51 | 0.03 | |||||
| Cluster 3 | 0.21 | 1.70 | −3.12 | 3.54 | 0.90 | |||||
| Cluster 4 | −1.08 | 1.23 | −3.49 | 1.33 | 0.38 | |||||
| Cluster 5 | 1.86 | 0.91 | 0.07 | 3.65 | 0.04 | |||||
| Year | 0.03 | 0.10 | −0.15 | 0.22 | 0.72 | 0.01 | 0.09 | −0.17 | 0.20 | 0.89 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wambui, J.; Njage, P.M.K.; Tasara, T.; Buys, E.M. Meta-Analysis and Meta-Regression Indicate Dynamic Prevalence and Moderators of Foodborne Pathogens in African Indigenous Fermented Milk. Microorganisms 2019, 7, 563. https://doi.org/10.3390/microorganisms7110563
Wambui J, Njage PMK, Tasara T, Buys EM. Meta-Analysis and Meta-Regression Indicate Dynamic Prevalence and Moderators of Foodborne Pathogens in African Indigenous Fermented Milk. Microorganisms. 2019; 7(11):563. https://doi.org/10.3390/microorganisms7110563
Chicago/Turabian StyleWambui, Joseph, Patrick Murigu Kamau Njage, Taurai Tasara, and Elna Maria Buys. 2019. "Meta-Analysis and Meta-Regression Indicate Dynamic Prevalence and Moderators of Foodborne Pathogens in African Indigenous Fermented Milk" Microorganisms 7, no. 11: 563. https://doi.org/10.3390/microorganisms7110563
APA StyleWambui, J., Njage, P. M. K., Tasara, T., & Buys, E. M. (2019). Meta-Analysis and Meta-Regression Indicate Dynamic Prevalence and Moderators of Foodborne Pathogens in African Indigenous Fermented Milk. Microorganisms, 7(11), 563. https://doi.org/10.3390/microorganisms7110563





