Functional Characterization and Structural Analysis of NADH Oxidase Mutants from Thermus thermophilus HB27: Role of Residues 166, 174, and 194 in the Catalytic Properties and Thermostability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cloning and Expression of the Tt-NOX Variants
2.2.1. Bacterial Strain and Growth Conditions
2.2.2. Site-directed Mutagenesis to Create Tt-NOX Variants
2.3. Expression of the Recombinant NOX Variants in E. coli
2.4. Purification of the Tt-NOX Variants
2.5. Determination of Enzyme Activity and Kinetics Parameters
2.6. Effects of Temperature and pH on the Activity of Tt-NOX Variants
2.7. Thermal-stability Assays and Melting-point Determination
2.8. SDS-PAGE Analysis
2.9. Quantification of FMN and FAD Bound to the Enzyme
2.10. Computational Details
3. Results and Discussion
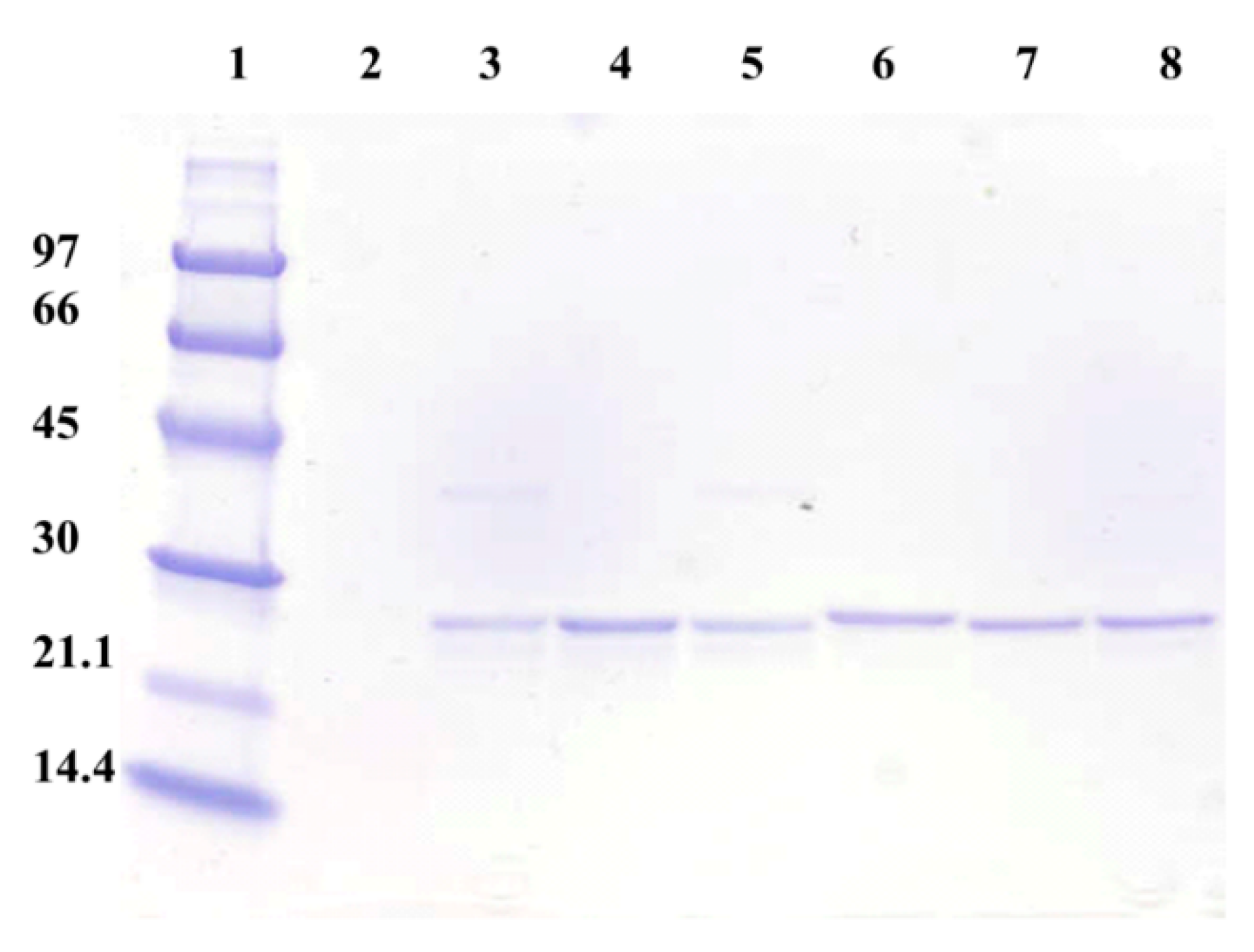
3.1. Expression of the Different Recombinant Tt27-NOX Variants and Sequence Analysis
- (i)
- The R166/R174/H194 variant corresponds to the gene bank accession number CAA42707.1, and it is the variant previously described by Park et al. [3];
- (ii)
- The R166/H174/H194 variant corresponds to the sequence that appears in the BacMap genomic atlas (Available online: http://wishart.biology.ualberta.ca/BacMap/) [32];
- (iii)
- The K166/H174/H194 variant corresponds to the published genome sequence of T. thermophilus HB27 by Henne et al. [21] and as it appears in the Kyoto Encyclopedia of Genes and Genomes (KEGG) (Available online: http://www.genome.jp/kegg/kegg2.html);
- (iv)
- The K166/R174/Y194 and (v) R166/H174/Y194 variants correspond to two other mutants that were constructed to identify other possible alterations in the properties of the enzyme.
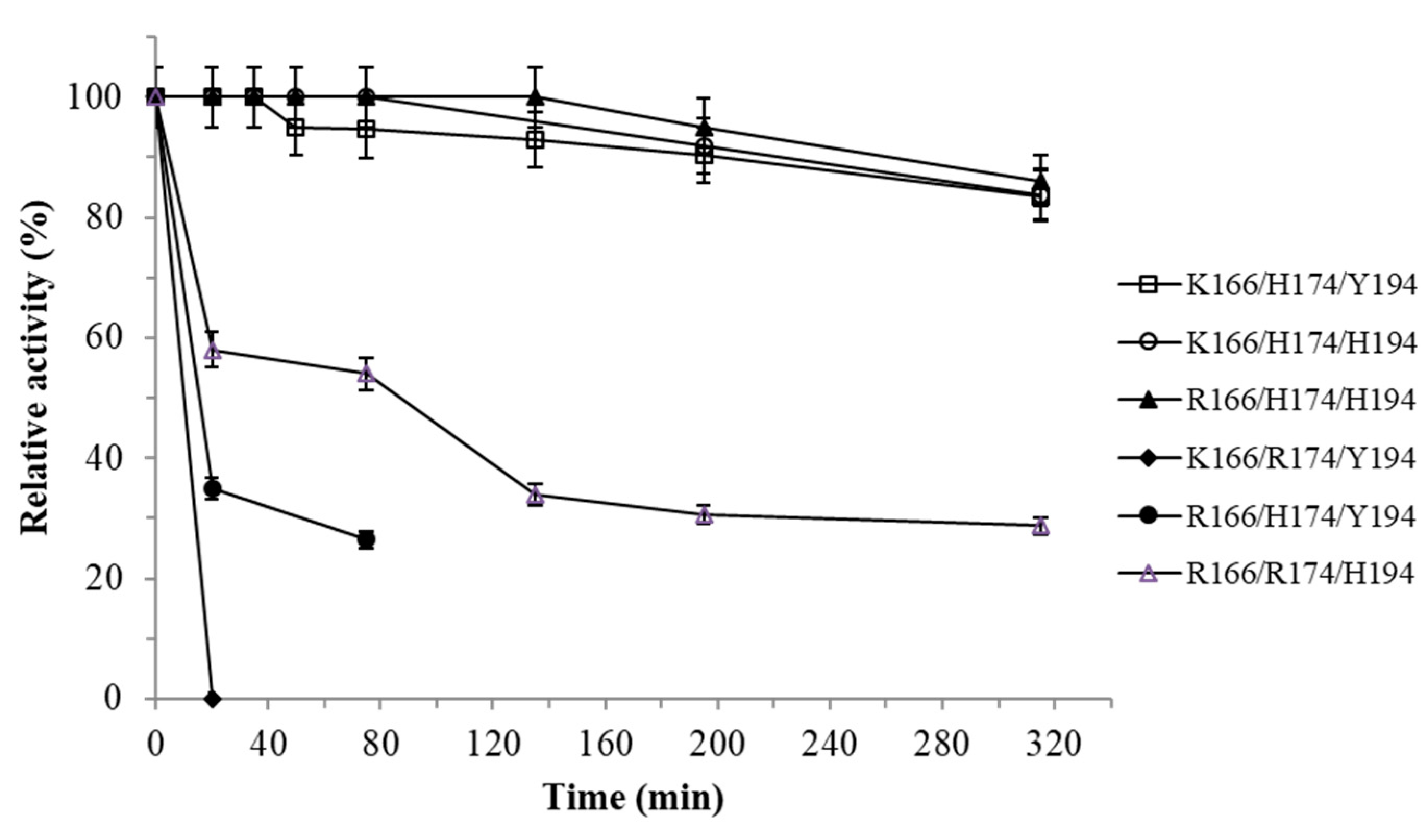
3.2. Thermostability Analysis of Tt27-NOX Variants and its Influence on the Temperature-based Purification Process
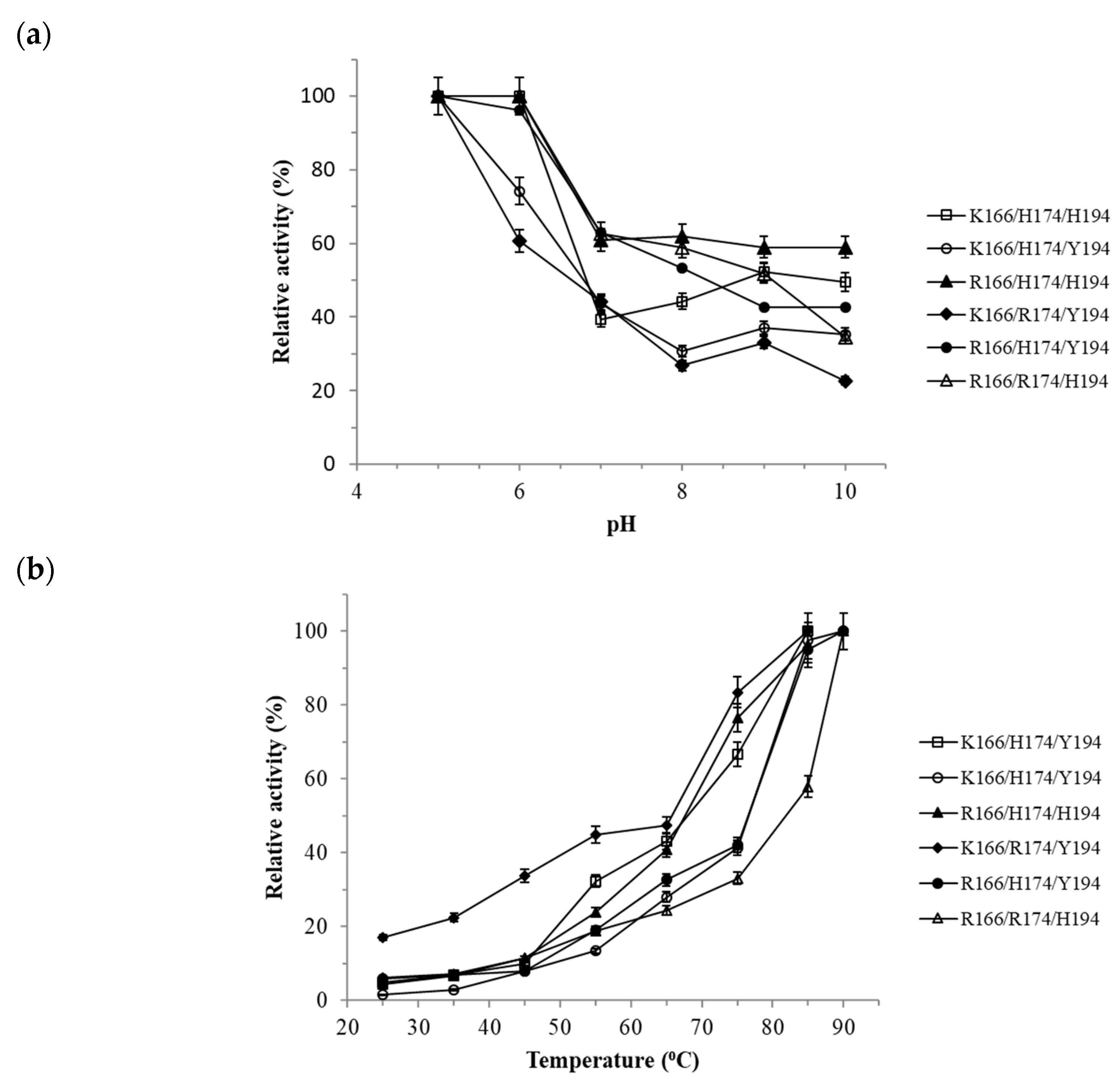
3.3. Functional Characterization of Tt27-NOX Variants
3.4. Catalytic Properties Analysis of Tt27-NOX Variants
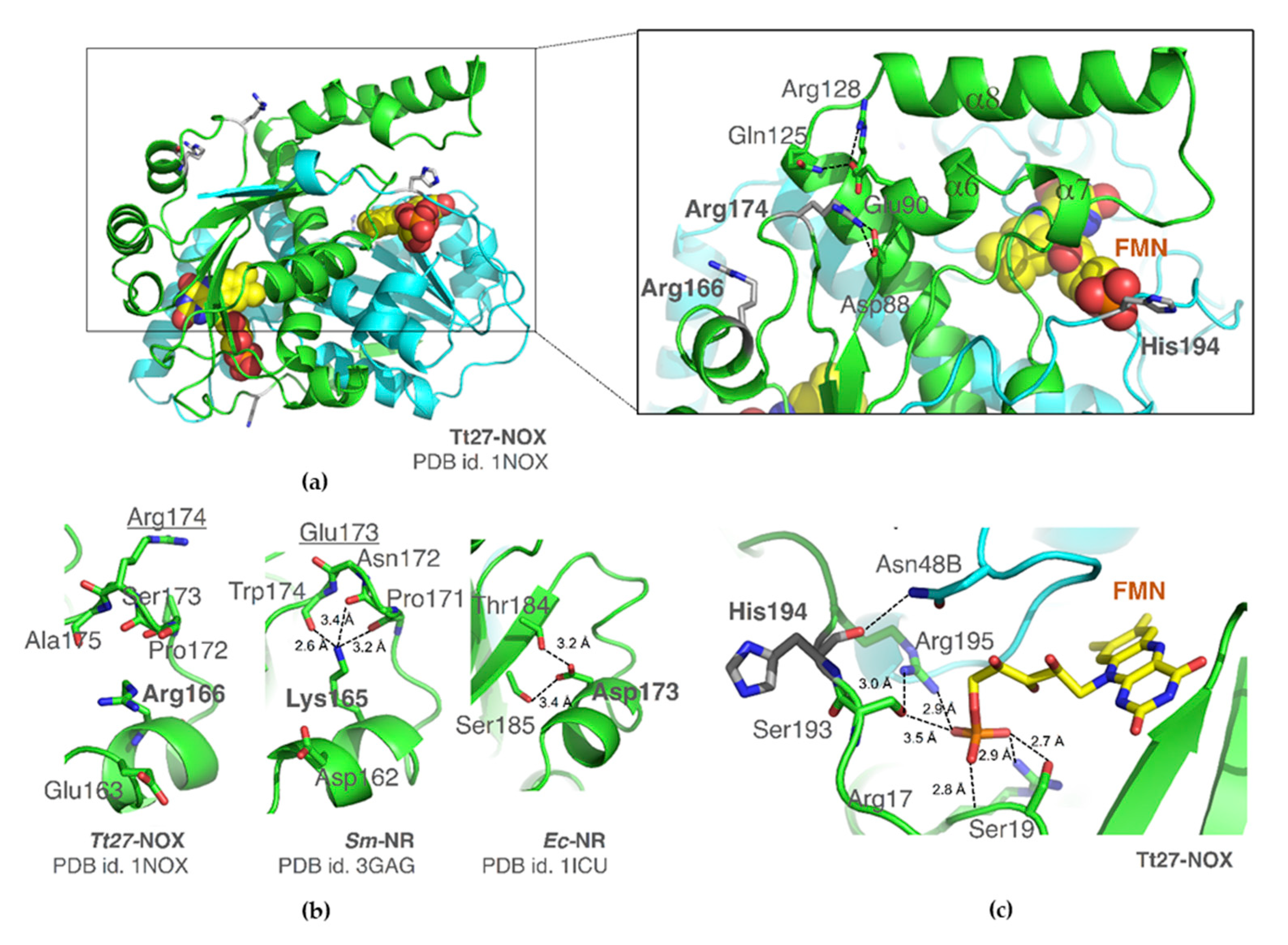
3.5. Structural Evidences
3.5.1. Structural Superimposition. Residue 166
3.5.2. Residue 174
3.5.3. Residue 194
3.5.4. Unfolding of Tt27-NOX by Means of Unbiased Extensive Molecular Dynamic Simulations
3.5.5. Cofactor Specificity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, R.A.D.; Da Costa, M.S. The genus Thermus and related microorganisms. In The Prokaryotes: A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications; Balows, A., Trüper, H.G., Dworkin, M., Harder, W., Schleifer, K.-H., Eds.; Springer: New York, NY, USA, 1992; pp. 3745–3753. [Google Scholar]
- Cava, F.; Hidalgo, A.; Berenguer, J. Thermus thermophilus as biological model. Extremophiles 2009, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Reiser, C.O.A.; Kondruweit, S.; Erdmann, H.; Schmid, R.D.; Sprinzl, M. Purification and characterization of a NADH oxidase from the thermophile Thermus thermophilus HB8. Eur. J. Biochem. 1992, 205, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Hecht, H.J.; Erdmann, H.; Park, H.J.; Sprinzl, M.; Schmid, R.D. Crystal structure of NADH oxidase from Thermus thermophilus. Nat. Struct. Biol. 1995, 2, 1109. [Google Scholar] [CrossRef] [PubMed]
- Hritz, J.; Žoldák, G.; Sedlák, E. Cofactor assisted gating mechanism in the active site of NADH oxidase from Thermus thermophilus. Proteins Struct. Funct. Bioinform. 2006, 64, 465–476. [Google Scholar] [CrossRef]
- Žoldák, G.; Šut’ák, R.; Antalík, M.; Sprinzl, M.; Sedlák, E. Role of conformational flexibility for enzymatic activity in NADH oxidase from Thermus thermophilus. Eur. J. Biochem. 2003, 270, 4887–4897. [Google Scholar] [CrossRef]
- Žoldák, G.; Sprinzl, M.; Sedlák, E. Modulation of activity of NADH oxidase from Thermus thermophilus through change in flexibility in the enzyme active site induced by Hofmeister series anions. Eur. J. Biochem. 2004, 271, 48–57. [Google Scholar] [CrossRef]
- Tóth, K.; Sedlák, E.; Sprinzl, M.; Žoldák, G. Flexibility and enzyme activity of NADH oxidase from Thermus thermophilus in the presence of monovalent cations of Hofmeister series. Biochim. Biophys. Acta 2008, 1784, 789–795. [Google Scholar] [CrossRef]
- Merkley, E.D.; Daggett, V.; Parson, W.W. A temperature-dependent conformational change of NADH oxidase from Thermus thermophilus HB8. Proteins Struct. Funct. Bioinform. 2012, 80, 546–555. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; Vega, D.; Bolivar, J.M.; Godoy, C.A.; Hidalgo, A.; Berenguer, J.; Guisán, J.M.; López-Gallego, F. New biotechnological perspectives of a NADH oxidase variant from Thermus thermophilus HB27 as NAD+-recycling enzyme. BMC Biotechnol. 2011, 11, 101. [Google Scholar] [CrossRef]
- Rocha-Martin, J.; Acosta, A.; Guisan, J.M.; López-Gallego, F. Immobilizing systems biocatalysis for the selective oxidation of glycerol coupled to in situ cofactor recycling and hydrogen peroxide elimination. ChemCatChem 2015, 7, 1939–1947. [Google Scholar] [CrossRef]
- Rocha-Martin, J.; Acosta, A.; Berenguer, J.; Guisan, J.M.; Lopez-Gallego, F. Selective oxidation of glycerol to 1,3-dihydroxyacetone by covalently immobilized glycerol dehydrogenases with higher stability and lower product inhibition. Bioresour. Technol. 2014, 170, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Martin, J.; Velasco-Lozano, S.; Guisan, J.M.; Lopez-Gallego, F. Oxidation of phenolic compounds catalyzed by immobilized multi-enzyme systems with integrated hydrogen peroxide production. Green Chem. 2014, 16, 303–311. [Google Scholar] [CrossRef]
- Alonso-de Castro, S.; Cortajarena, A.L.; López-Gallego, F.; Salassa, L. Bioorthogonal catalytic activation of platinum and ruthenium anticancer complexes by FAD and flavoproteins. Angew. Chem. 2018, 130, 3197–3201. [Google Scholar] [CrossRef]
- Radoi, A.; Compagnone, D.; Devic, E.; Palleschi, G. Low potential detection of NADH with Prussian Blue bulk modified screen-printed electrodes and recombinant NADH oxidase from Thermus thermophilus. Sens. Actuators B Chem. 2007, 121, 501–506. [Google Scholar] [CrossRef]
- Creanga, C.; El Murr, N. Development of new disposable NADH biosensors based on NADH oxidase. J. Electroanal. Chem. 2011, 656, 179–184. [Google Scholar] [CrossRef]
- Serban, S.; El Murr, N. Redox-flexible NADH oxidase biosensor: A platform for various dehydrogenase bioassays and biosensors. Electrochim. Acta 2006, 51, 5143–5149. [Google Scholar] [CrossRef]
- Leca, B.; Marty, J.-L. Reusable ethanol sensor based on a NAD+-dependent dehydrogenase without coenzyme addition. Anal. Chim. Acta 1997, 340, 143–148. [Google Scholar] [CrossRef]
- Ghica, M.E.; Pauliukaite, R.; Marchand, N.; Devic, E.; Brett, C.M.A. An improved biosensor for acetaldehyde determination using a bienzymatic strategy at poly (neutral red) modified carbon film electrodes. Anal. Chim. Acta 2007, 591, 80–86. [Google Scholar] [CrossRef]
- Campàs, M.; Olteanu, M.G.; Marty, J.-L. Enzymatic recycling for signal amplification: Improving microcystin detection with biosensors. Sens. Actuators B Chem. 2008, 129, 263–267. [Google Scholar]
- Henne, A.; Brüggemann, H.; Raasch, C.; Wiezer, A.; Hartsch, T.; Liesegang, H.; Johann, A.; Lienard, T.; Gohl, O.; Martinez-Arias, R. The genome sequence of the extreme thermophile Thermus thermophilus. Nat. Biotechnol. 2004, 22, 547. [Google Scholar] [CrossRef]
- Koyama, Y.; Hoshino, T.; Tomizuka, N.; Furukawa, K. Genetic transformation of the extreme thermophile Thermus thermophilus and of other Thermus spp. J. Bacteriol. 1986, 166, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Arcos, S.; Fernández-Herrero, L.A.; Berenguer, J. A thermophilic nitrate reductase is responsible for the strain specific anaerobic growth of Thermus thermophilus HB8. Biochim. Biophys. Acta Gene Struct. Expr. 1998, 1396, 215–227. [Google Scholar] [CrossRef]
- Lennox, E.S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1955, 1, 190–206. [Google Scholar] [CrossRef]
- Sambrook, J.; Faruquz, E. Molecular Clonning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Cleland, W.W. Determining the chemical mechanisms of enzyme-catalyzed reactions by kinetic studies. Adv. Enzymol. Relat. Areas Mol. Biol. 1977, 45, 273–387. [Google Scholar]
- Forneris, F.; Orru, R.; Bonivento, D.; Chiarelli, L.R.; Mattevi, A. ThermoFAD, a Thermofluor®-adapted flavin ad hoc detection system for protein folding and ligand binding. Febs. J. 2009, 276, 2833–2840. [Google Scholar] [CrossRef]
- Toomey, D.; Mayhew, S.G. Purification and characterisation of NADH oxidase from Thermus aquaticus YT-1 and evidence that it functions in a peroxide-reduction system. Eur. J. Biochem. 1998, 251, 935–945. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Kräutler, V.; van Gunsteren, W.F.; Hünenberger, P.H. A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J. Comput. Chem. 2001, 22, 501–508. [Google Scholar] [CrossRef]
- Cruz, J.; Liu, Y.; Liang, Y.; Zhou, Y.; Wilson, M.; Dennis, J.J.; Stothard, P.; Van Domselaar, G.; Wishart, D.S. BacMap: An up-to-date electronic atlas of annotated bacterial genomes. Nucleic. Acids. Res. 2012, 40, D599–D604. [Google Scholar] [CrossRef]
- Rocha-Martin, J.; Vega, D.E.; Cabrera, Z.; Bolivar, J.M.; Fernandez-Lafuente, R.; Berenguer, J.; Guisan, J.M. Purification, immobilization and stabilization of a highly enantioselective alcohol dehydrogenase from Thermus thermophilus HB27 cloned in E. coli. Process Biochem. 2009, 44, 1004–1012. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; Vega, D.; Bolivar, J.M.; Hidalgo, A.; Berenguer, J.; Guisán, J.M.; López-Gallego, F. Characterization and further stabilization of a new anti-prelog specific alcohol dehydrogenase from Thermus thermophilus HB27 for asymmetric reduction of carbonyl compounds. Bioresour. Technol. 2012, 103, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Abian, O.; Fernandez-Lafuente, R.; Guisan, J.M. Reversible enzyme immobilization via a very strong and nondistorting ionic adsorption on support–polyethylenimine composites. Biotechnol. Bioeng. 2000, 68, 98–105. [Google Scholar] [CrossRef]
- Fuentes, M.; Pessela, B.C.C.; Maquiese, J.V.; Ortiz, C.; Segura, R.L.; Palomo, J.M.; Abian, O.; Torres, R.; Mateo, C.; Fernández-Lafuente, R.; et al. Reversible and strong immobilization of proteins by ionic exchange on supports coated with sulfate-dextran. Biotechnol. Prog. 2004, 20, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Tung, J.-S.; Knight, C.A. Relative importance of some factors affecting the electrophoretic migration of proteins in sodium dodecyl sulfate-polyacrylamide gels. Anal. Biochem. 1972, 48, 153–163. [Google Scholar] [CrossRef]
- Rover, L., Jr.; Fernandes, J.C.; de Oliveira Neto, G.; Kubota, L.T.; Katekawa, E.; Serrano, S.H.P. Study of NADH stability using ultraviolet-visible spectrophotometric analysis and factorial design. Anal. Biochem. 1998, 260, 50–55. [Google Scholar] [CrossRef]
- Yang, X.; Ma, K. Characterization of an exceedingly active NADH oxidase from the anaerobic hyperthermophilic bacterium Thermotoga maritima. J. Bacteriol. 2007, 189, 3312–3317. [Google Scholar] [CrossRef]
- Maeda, K.; Truscott, K.; Liu, X.L.; Scopes, R.K. A thermostable NADH oxidase from anaerobic extreme thermophiles. Biochem. J. 1992, 284, 551–555. [Google Scholar] [CrossRef]
- Kengen, S.W.M.; Van Der Oost, J.; De Vos, W.M. Molecular characterization of H2O2-forming NADH oxidases from Archaeogiobus fulgidus. Eur. J. Biochem. 2003, 270, 2885–2894. [Google Scholar] [CrossRef]
- Wu, X.; Kobori, H.; Orita, I.; Zhang, C.; Imanaka, T.; Xing, X.-H.; Fukui, T. Application of a novel thermostable NAD(P)H oxidase from hyperthermophilic archaeon for the regeneration of both NAD+ and NADP+. Biotechnol. Bioeng. 2012, 109, 53–62. [Google Scholar] [CrossRef]
- Jia, B.; Park, S.-C.; Lee, S.; Pham, B.P.; Yu, R.; Le, T.L.; Han, S.W.; Yang, J.-K.; Choi, M.-S.; Baumeister, W.; et al. Hexameric ring structure of a thermophilic archaeon NADH oxidase that produces predominantly H2O. Febs. J. 2008, 275, 5355–5366. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.E.; Donnelly, C.J.; Mullendore, M.E.; van der Oost, J.; de Vos, W.M.; Crane, E.J., III. The NADH oxidase from Pyrococcus Furiosus. Eur. J. Biochem. 2001, 268, 5816–5823. [Google Scholar] [CrossRef] [PubMed]
- Haynes, C.A.; Koder, R.L.; Miller, A.-F.; Rodgers, D.W. Structures of nitroreductase in three states: Effects of inhibitor binding and reduction. J. Biol. Chem. 2002, 277, 11513–11520. [Google Scholar] [CrossRef] [PubMed]
- Lovering, A.L.; Hyde, E.I.; Searle, P.F.; White, S.A. The structure of Escherichia coli nitroreductase complexed with nicotinic acid: Three crystal forms at 1.7 Å, 1.8 Å and 2.4 Å resolution. J. Mol. Biol. 2001, 309, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Merkley, E.D.; Parson, W.W.; Daggett, V. Temperature dependence of the flexibility of thermophilic and mesophilic flavoenzymes of the nitroreductase fold. Protein Eng. Des. Sel. 2010, 23, 327–336. [Google Scholar] [CrossRef]
- Miletti, T.; Di Trani, J.; Levros, L.-C.; Mittermaier, A. Conformational plasticity surrounding the active site of NADH oxidase from Thermus thermophilus. Protein Sci. 2015, 24, 1114–1128. [Google Scholar] [CrossRef] [PubMed]


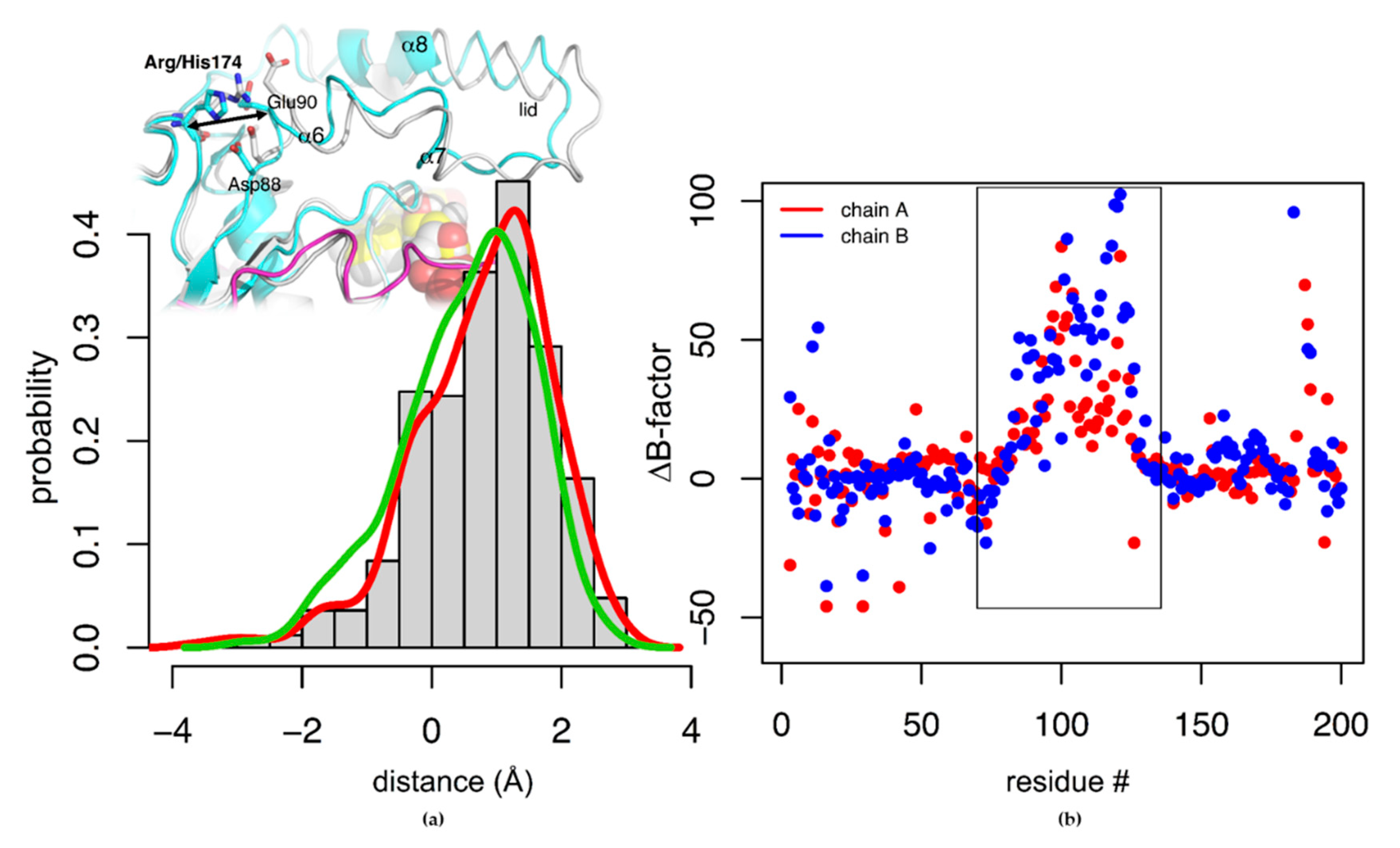
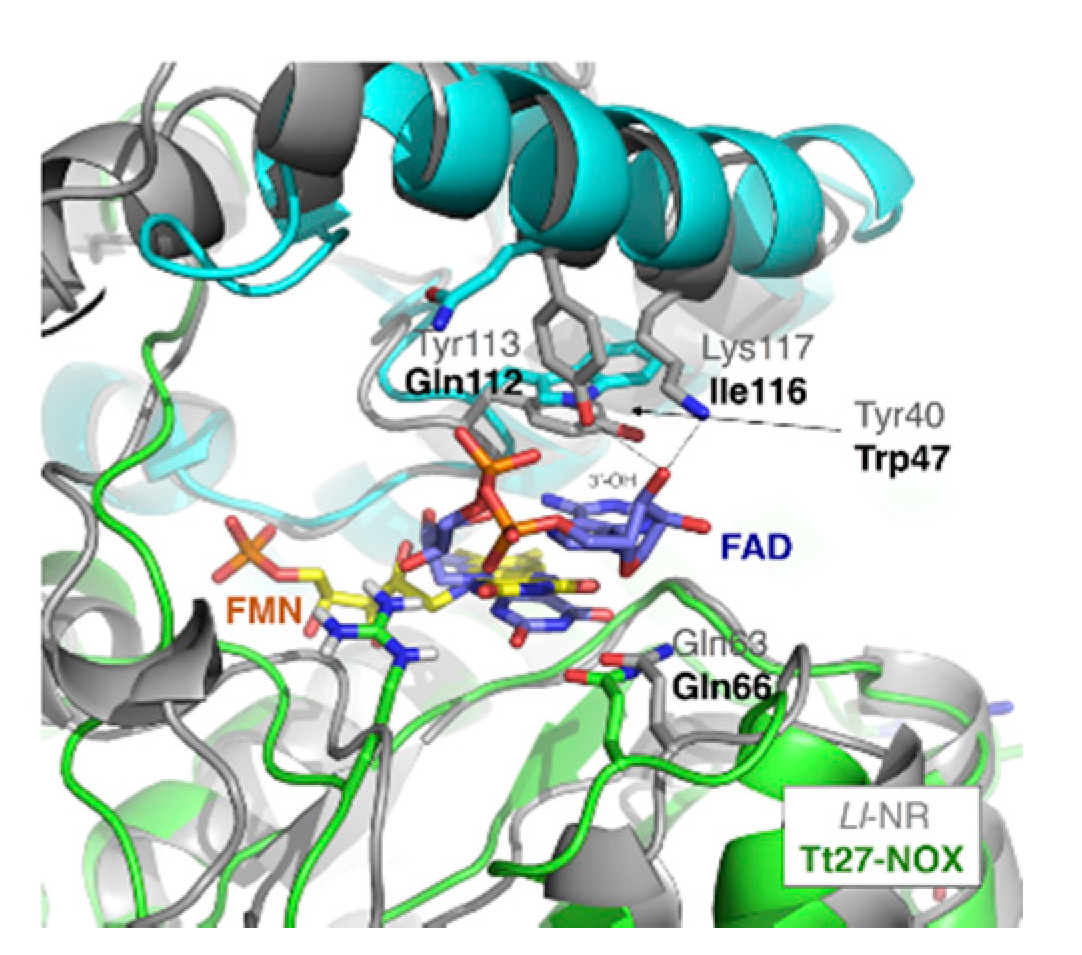
| Tt-NOX Variants | Tm No flavin Cofactor (°C) | Tm FMN (°C) | Tm FAD (°C) |
|---|---|---|---|
| K166/R174//Y194 | 44.1 | 43.5 | 39.8 |
| K166/H174/Y194 | 87.1 | 91.8 | 90.8 |
| Tt-NOX Variants | KM NADH (µM) 1 | KM FMN (µM) 2 | Km FAD (µM) 2 | KM Riboflavin (µM) 2 | Specific Activityc (U/mg) 3 | kcat NADH (s−1) | kcat NADH/KM NADH (106 M−1·s−1) |
|---|---|---|---|---|---|---|---|
| K166/H174/Y194 | 1.6 ± 0.1 | 43 ± 2.2 | 34 ± 1.7 | 69.7 ± 3.5 | 68 ± 3.3 | 23.3 ± 1.2 | 14.6 ± 0.07 |
| K166/H174/H194 | 1.3 ± 0.06 | 45 ± 2.3 | 46 ± 2.3 | 68.7 ± 3.4 | 75 ± 6 | 27.2 ± 1.4 | 21 ± 1.1 |
| R166/H174/H194 | 1.3 ± 0.06 | 47 ± 2.3 | 29 ± 1.5 | 50.7 ± 2.6 | 50 ± 2.5 | 17.9 ± 0.9 | 13.8 ± 0.7 |
| K166/R174/Y194 | 1.9 ± 0.1 | 15 ± 0.8 | 127 ± 6.4 | 60 ± 3 | 24 ± 1.2 | 8.6 ± 0.4 | 4.5 ± 0.22 |
| R166/H174/Y194 | 4.6 ± 0.2 | 27 ± 1.4 | 30 ± 1.5 | 44 ± 2.2 | 44.3 ± 2.2 | 15.9 ± 0.8 | 3.5 ± 0.18 |
| R166/R174/H194 | 1.8 ± 0.1 | 23.6 ± 1 | 31.9 ± 1.4 | 50.8 ± 2.6 | 42 ± 2.3 | 15.1 ± 0.8 | 8.4 ± 0.42 |
| NOX Origin | NOX Type 1=H2O2; 2=H2O | Specific Activity NADH (U/mg) | KM NADH (µM) | pH Optimum | Temperature Optimum (°C) | Reference |
|---|---|---|---|---|---|---|
| T. thermophilus HB27 (K166/H174/H194) | 2 | 75 (25 °C) | 1.3 | 5 | 90 | This work |
| T. thermophilus HB8 | 2 | 5.2 (25 °C) | 4.14 | 5 | 80 | [3] |
| Thermotoga. maritima | 2 | 230 (80 °C) | 42 | n.d. | 80 | [39] |
| Clostridium thermosaccharolyticum | 2 | 23 (25 °C) | 19 | 7 | n.d. | [40] |
| Archaeogiobus fulgidus (NoxA-1) | 2 | 5.82 (70 °C) | 0.13 | 8 | 80 | [41] |
| Thermococcus kodakarensis KOD1 | 2 | 0.38 (25 °C) | 49 | 3.5 | > 90 | [42] |
| Thermococcus profundus | 1, 2 | 7 (75 °C) | 53.1 | 7.5–8.0 | 70 | [43] |
| Pyrococcus furiosus | 1, 2 | 20 (75 °C) | < 4 | 5.5–8.5 | 85 | [44] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha-Martin, J.; Sánchez-Murcia, P.A.; López-Gallego, F.; Hidalgo, A.; Berenguer, J.; Guisan, J.M. Functional Characterization and Structural Analysis of NADH Oxidase Mutants from Thermus thermophilus HB27: Role of Residues 166, 174, and 194 in the Catalytic Properties and Thermostability. Microorganisms 2019, 7, 515. https://doi.org/10.3390/microorganisms7110515
Rocha-Martin J, Sánchez-Murcia PA, López-Gallego F, Hidalgo A, Berenguer J, Guisan JM. Functional Characterization and Structural Analysis of NADH Oxidase Mutants from Thermus thermophilus HB27: Role of Residues 166, 174, and 194 in the Catalytic Properties and Thermostability. Microorganisms. 2019; 7(11):515. https://doi.org/10.3390/microorganisms7110515
Chicago/Turabian StyleRocha-Martin, Javier, Pedro A. Sánchez-Murcia, Fernando López-Gallego, Aurelio Hidalgo, José Berenguer, and José M. Guisan. 2019. "Functional Characterization and Structural Analysis of NADH Oxidase Mutants from Thermus thermophilus HB27: Role of Residues 166, 174, and 194 in the Catalytic Properties and Thermostability" Microorganisms 7, no. 11: 515. https://doi.org/10.3390/microorganisms7110515
APA StyleRocha-Martin, J., Sánchez-Murcia, P. A., López-Gallego, F., Hidalgo, A., Berenguer, J., & Guisan, J. M. (2019). Functional Characterization and Structural Analysis of NADH Oxidase Mutants from Thermus thermophilus HB27: Role of Residues 166, 174, and 194 in the Catalytic Properties and Thermostability. Microorganisms, 7(11), 515. https://doi.org/10.3390/microorganisms7110515








