Red-Brown Pigmentation of Acidipropionibacterium jensenii Is Tied to Haemolytic Activity and cyl-Like Gene Cluster
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Isolation of DNA, Genome Sequencing, and Assembly
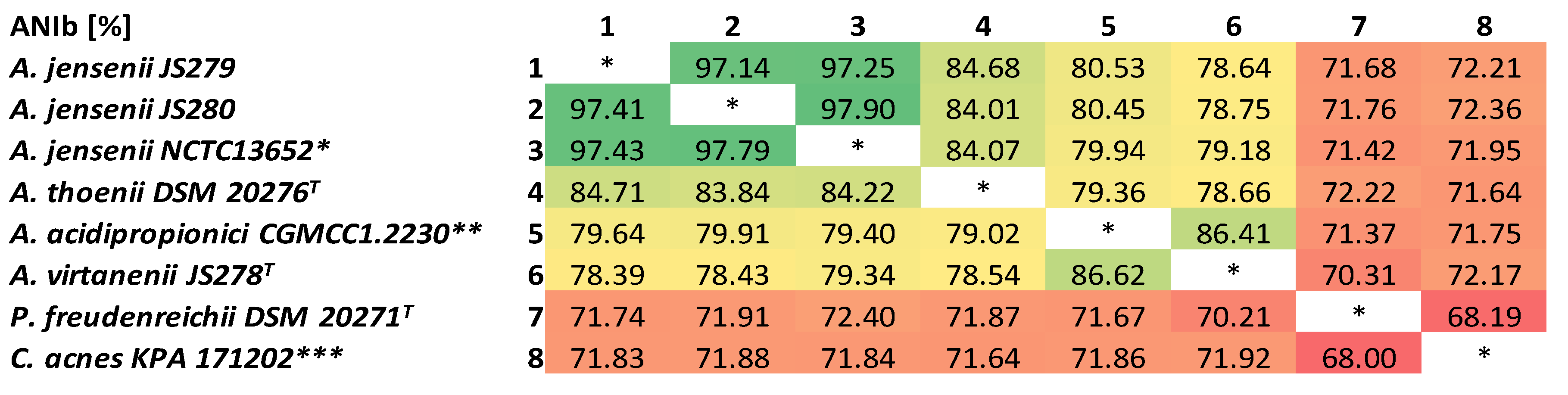
2.3. Bioinformatics Analyses and Classification of the Strains
2.4. Haemolytic Activity
2.4.1. Detection of Haemolysis and Pigmentation
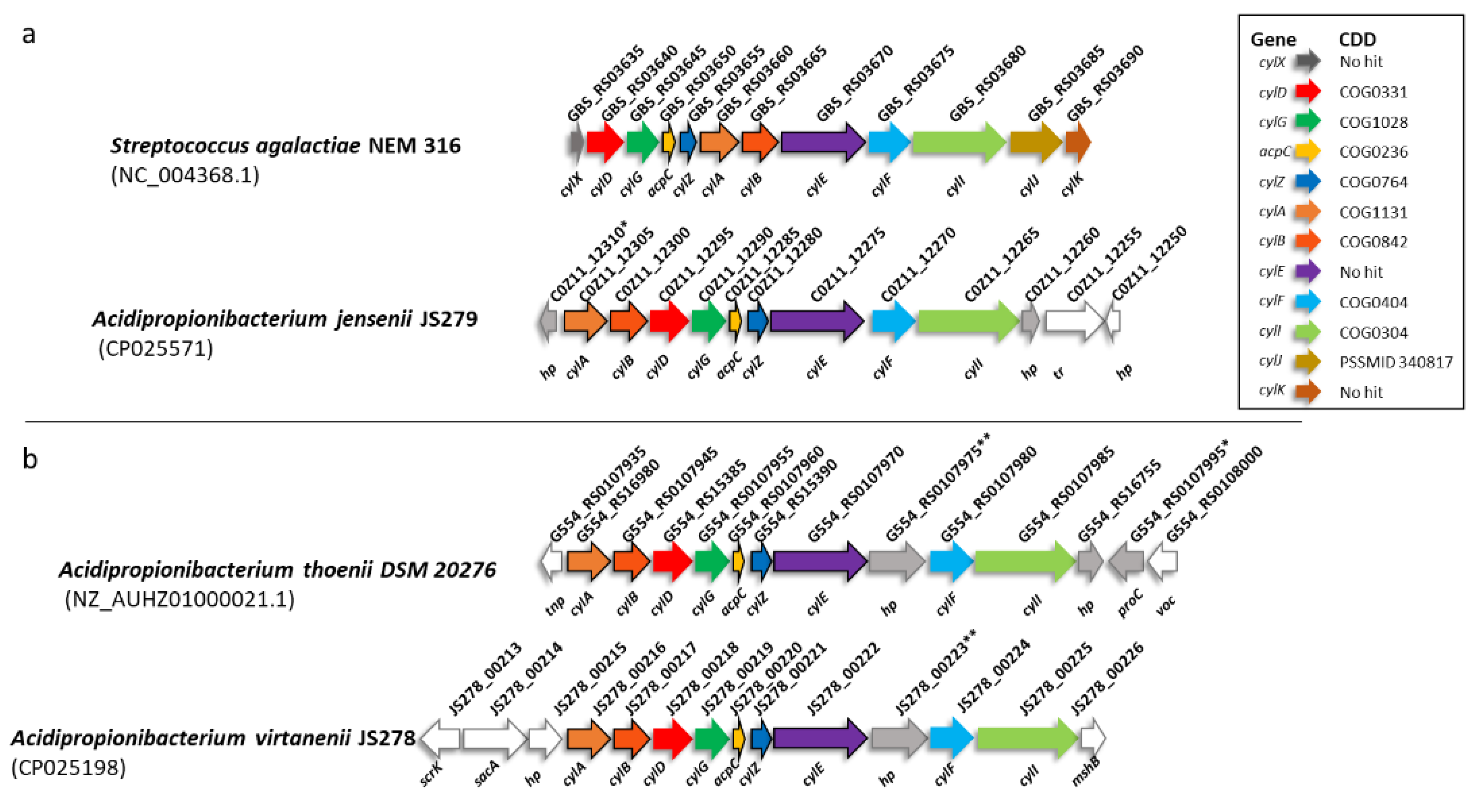
2.4.2. PCR Amplification of the cylG Gene Fragment
2.5. Further Characterisation of the Strains JS279 and JS280
2.5.1. Detection of Defence Mechanisms and Mobile Genetic Elements
2.5.2. Phenotypic Characterisation
3. Results and Discussion
3.1. Classification of Strains JS279 and JS280 as A. jensenii
3.2. Haemolytic Activity, Pigmentation and cylG Gene Detection by PCR
3.3. Further Characterisation of the Strains JS279 and JS280
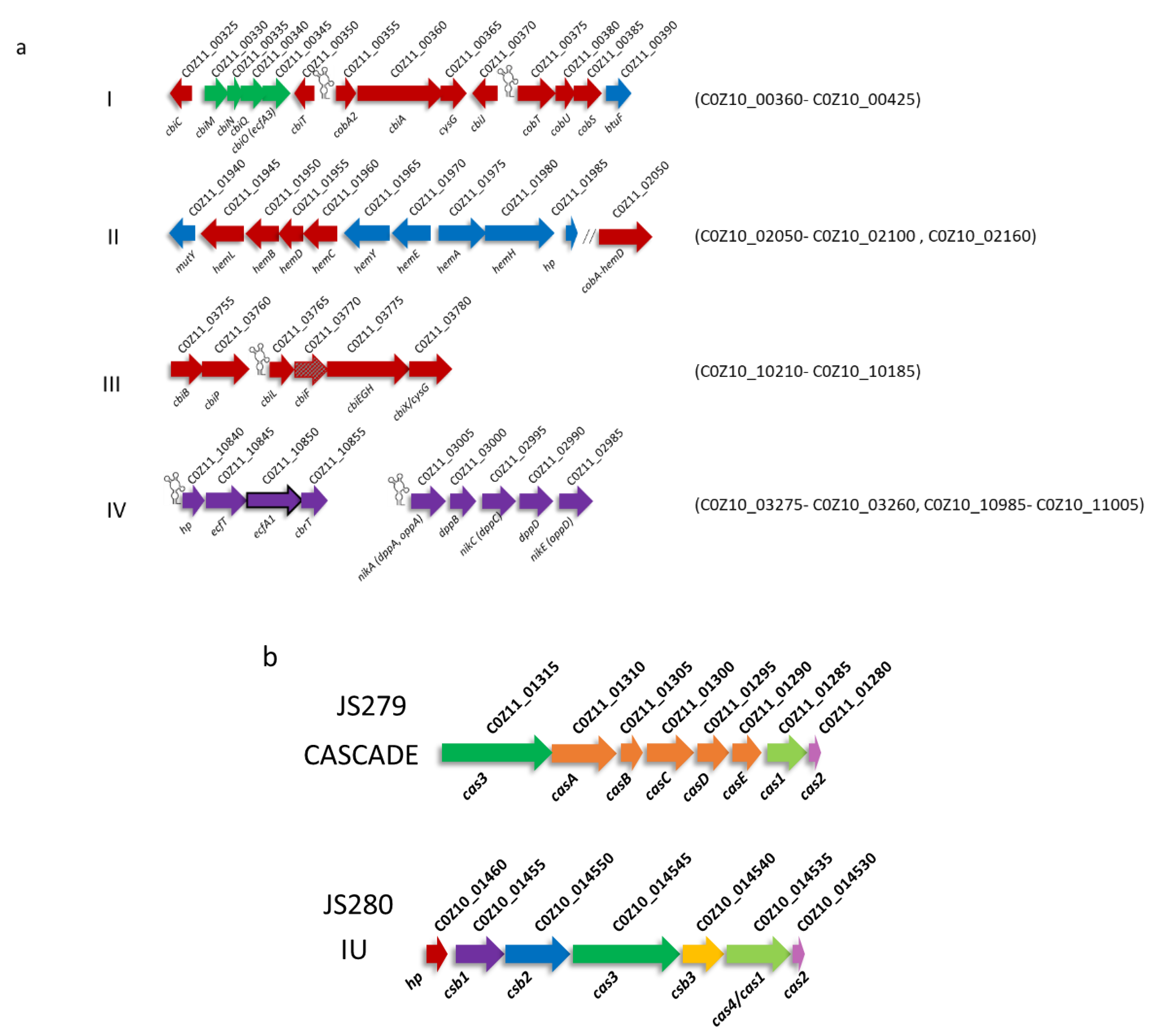
3.3.1. Biosynthesis of Vitamin B12
3.3.2. Mobile Elements and Defence Mechanisms
3.3.3. Phenotypes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scholz, C.F.P.; Kilian, M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 4422–4432. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, C.B. The propionic acid bacteria. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 1928. [Google Scholar]
- Stackebrandt, E.; Cummins, C.S.; Johnson, J.L. Family Propionibacteriaceae: The Genus Propionibacterium. In The Prokaryotes: Volume 3: Archaea. Bacteria: Firmicutes, Actinomycetes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer New York: New York, NY, USA, 2006; pp. 400–418. [Google Scholar]
- Britz, T.J.; Riedel, K.H.J. Propionibacterium species diversity in leerdammer cheese. Int. J. Food Microbiol. 1994, 22, 257–267. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Racioppo, A.; Sinigaglia, M.; Speranza, B.; Campaniello, D.; Corbo, M.R. A low-power ultrasound attenuation improves the stability of biofilm and hydrophobicity of Propionibacterium freudenreichii subsp. freudenreichii DSM 20271 and Acidipropionibacterium jensenii DSM 20535. Food Microbiol. 2019, 78, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Rabah, H.; Rosa do Carmo, F.; Jan, G. Dairy Propionibacteria: Versatile Probiotics. Microorganisms 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. Probiotic viability and physico-chemical and sensory properties of plain and stirred fruit yogurts made from goat’s milk. Food Chem. 2012, 135, 1411–1418. [Google Scholar] [CrossRef]
- Sabater, C.; Fara, A.; Palacios, J.; Corzo, N.; Requena, T.; Montilla, A.; Zárate, G. Synthesis of prebiotic galactooligosaccharides from lactose and lactulose by dairy propionibacteria. Food Microbiol. 2019, 77, 93–105. [Google Scholar] [CrossRef]
- Zárate, G.; Chaia, A.P. Propionibacteria also have Probiotic Potential. In Probiotics and Prebiotics: Current Research and Future Trends; 2016; pp. 69–92. Available online: https://doi.org/10.21775/9781910190098.05 (accessed on 6 September 2019).
- Liu, L.; Guan, N.; Zhu, G.; Li, J.; Shin, H.; Du, G.; Chen, J. Pathway engineering of Propionibacterium jensenii for improved production of propionic acid. Sci. Rep. 2016, 6, 19963. [Google Scholar] [CrossRef]
- Zhuge, X.; Liu, L.; Shin, H.D.; Li, J.; Du, G.; Chen, J. Improved propionic acid production from glycerol with metabolically engineered Propionibacterium jensenii by integrating fed-batch culture with a pH-shift control strategy. Bioresour. Technol. 2014, 152, 519–525. [Google Scholar] [CrossRef]
- Grinstead, D.A.; Barefoot, S.F. Jenseniin G, a heat-stable bacteriocin produced by Propionibacterium jensenii P126. Appl. Environ. Microbiol. 1992, 58, 215–220. [Google Scholar]
- Wang, G.; Abercrombie, J.G.; Huang, G.; Jeremy Tzeng, T.R. Enhanced fed-batch production, partial purifcation, characterization of jenseniin p, and discovery of a new bacteriocin-like substance produced by Propionibacterium jensenii b1264. Eur. Food Res. Technol. 2014, 239, 79–86. [Google Scholar] [CrossRef]
- Miescher, S.; Stierli, M.P.; Teuber, M.; Meile, L. Propionicin SM1, a Bacteriocin from Propionibacterium jensenii DF1: Isolation and Characterization of the Protein and its Gene. Syst. Appl. Microbiol. 2000, 23, 174–184. [Google Scholar] [CrossRef]
- Garnier, L.; Mounier, J.; Lê, S.; Pawtowski, A.; Pinon, N.; Camier, B.; Chatel, M.; Garric, G.; Thierry, A.; Coton, E.; et al. Development of antifungal ingredients for dairy products: From in vitro screening to pilot scale application. Food Microbiol. 2019, 81, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Meile, L.; Le, G.; Thierry, A. Safety assessment of dairy microorganisms. J. Dairy Sci. 2008, 126, 316–320. [Google Scholar]
- Patrick, S.; McDowell, A. Propionibacterium. In Bergey’s Manual of Systematics of Archaea and Bacteria; Goodfellow, M., Kämpfer, P., Busse, H.-J., Trujillo, M.E., Suzuki, K.-I., Ludwig, W., Whitman, W.B., Eds.; John Wiley & Sons, Ltd.: New York, NY, USA, 2012; pp. 1138–1154. [Google Scholar]
- Laulund, S.; Wind, A.; Derkx, P.; Zuliani, V. Regulatory and Safety Requirements for Food Cultures. Microorganisms 2017, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Vedamuthu, E.R.; Washam, C.J.; Reinbold, G.W. Isolation of inhibitory factor in raw milk whey active against propionibacteria. Appl. Microbiol. 1971, 22, 552–556. [Google Scholar]
- Vanberg, C.; Lutnaes, B.F.; Langsrud, T.; Nes, I.F.; Holo, H. Propionibacterium jensenii produces the polyene pigment granadaene and has hemolytic properties similar to those of Streptococcus agalactiae. Appl. Environ. Microbiol. 2007, 73, 5501–5506. [Google Scholar] [CrossRef]
- Baer, A.; Ryba, I. Serological identification of propionibacteria in milk and cheese samples. Int. Dairy J. 1992, 2, 299–310. [Google Scholar] [CrossRef]
- de Carvalho, A.; Guezenec, S.; Gautier, M.; Grimont, P.A. Reclassification of “Propionibacterium rubrum” as P. jensenii. Res. Microbiol. 1995, 146, 51–58. [Google Scholar] [CrossRef]
- Dasen, G.; Smutny, J.; Teuber, M.; Meile, L. Classification and identification of propionibacteria based on ribosomal RNA genes and PCR. Syst. Appl. Microbiol. 1998, 21, 251–259. [Google Scholar] [CrossRef]
- Rossi, F.; Torriani, S.; Dellaglio, F. Genus- and species-specific PCR-based detection of dairy propionibacteria in environmental samples by using primers targeted to the genes encoding 16S rRNA. Appl. Environ. Microbiol. 1999, 65, 4241–4244. [Google Scholar]
- Rosa-Fraile, M.; Dramsi, S.; Spellerberg, B. Group B streptococcal haemolysin and pigment, a tale of twins. FEMS Microbiol. Rev. 2014, 38, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Whidbey, C.; Harrell, M.I.; Burnside, K.; Ngo, L.; Becraft, A.K.; Iyer, L.M.; Aravind, L.; Hitti, J.; Adams Waldorf, K.M.; Rajagopal, L. A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. J. Exp. Med. 2013, 210, 1265–1281. [Google Scholar] [CrossRef] [PubMed]
- Vedamuthu, E.; Reinbold, G.; Hammond, E.G. Inhibitory Activity of Acid and Rennet Whey on Propionibacteria. J. Dairy Sci. 1968, 51, 503–510. [Google Scholar] [CrossRef]
- Wilson, A.T.; Rosenblum, H. The antistreptococcal property of milk: I. Some characteristics of the activity of lactenin in vitro. The effect of lactenin on hemolytic streptococci of the several serological groups. J. Exp. Med. 1952, 95, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Garrity, G.M. List of new names and new combinations previously effectively, but not validly, published. Int. J. Syst. Evol. Microbiol. 2018, 68, 2707–2709. [Google Scholar] [CrossRef]
- Saito, M.; Shinozaki-Kuwahara, N.; Tsudukibashi, O.; Hashizume-Takizawa, T.; Kobayashi, R.; Kurita-Ochiai, T. Pseudopropionibacterium rubrum sp. nov., a novel red-pigmented species isolated from human gingival sulcus. Microbiol. Immunol. 2018, 62, 388–394. [Google Scholar] [CrossRef]
- Dekio, I.; McDowell, A.; Sakamoto, M.; Tomida, S.; Ohkuma, M. Proposal of new combination, Cutibacterium acnes subsp. elongatum comb. nov., and emended descriptions of the genus Cutibacterium, Cutibacterium acnes subsp. acnes and Cutibacterium acnes subsp. defendens. Int. J. Syst. Evol. Microbiol. 2019, 69, 1087–1092. [Google Scholar] [CrossRef]
- Sörensen, M.; Mak, T.N.; Hurwitz, R.; Ogilvie, L.A.; Mollenkopf, H.J.; Meyer, T.F.; Brüggemann, H. Mutagenesis of Propionibacterium acnes and analysis of two CAMP factor knock-out mutants. J. Microbiol. Methods 2010, 83, 211–216. [Google Scholar] [CrossRef]
- Achermann, Y.; Goldstein, E.J.C.; Coenye, T.; Shirtliffa, M.E. Propionibacterium acnes: From Commensal to opportunistic biofilm-associated implant pathogen. Clin. Microbiol. Rev. 2014, 27, 419–440. [Google Scholar] [CrossRef]
- Malik, A.C.; Reinbold, G.W.; Vedamuthu, E.R. An evaluation of the taxonomy of Propionibacterium. Can. J. Microbiol. 1968, 14, 1185–1191. [Google Scholar] [CrossRef]
- Deptula, P.; Smolander, O.-P.; Laine, P.; Roberts, R.J.; Edelmann, M.; Peltola, P.; Piironen, V.; Paulin, L.; Storgårds, E.; Savijoki, K.; et al. Acidipropionibacterium virtanenii sp. nov., isolated from malted barley. Int. J. Syst. Evol. Microbiol. 2018, 68, 3175–3183. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, P.; Deptula, P.; Smolander, O.-P.; Tamene, F.; Kammonen, J.; Savijoki, K.; Paulin, L.; Piironen, V.; Auvinen, P.; Varmanen, P. Complete genome sequence of Propionibacterium freudenreichii DSM 20271T. Stand. Genomic Sci. 2015, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Rosselló-Móra, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.E.; Mau, B.; Perna, N.T. Progressivemauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef]
- Shaw, L.N.; Jonsson, I.M.; Singh, V.K.; Tarkowski, A.; Stewart, G.C. Inactivation of traP has no effect on the Agr quorum-sensing system or virulence of Staphylococcus aureus. Infect. Immun. 2007, 75, 4519–4527. [Google Scholar] [CrossRef]
- Altschul, S.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Grissa, I.; Vergnaud, G.; Pourcel, C. CRISPRFinder: A web tool to identify clustered regularly interspace short palindromic repeats. Nucleic Acids Res. 2007, 35, 52–57. [Google Scholar] [CrossRef]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S.L. IslandViewer 4: Expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef]
- Lima-Mendez, G.; Van Helden, J.; Toussaint, A.; Leplae, R. Prophinder: A computational tool for prophage prediction in prokaryotic genomes. Bioinformatics 2008, 24, 863–865. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.J.; Vincze, T.; Posfai, J.; Macelis, D. REBASE-a database for DNA restriction and modification: Enzymes, genes and genomes. Nucleic Acids Res. 2015, 43, D298–D299. [Google Scholar] [CrossRef]
- Deptula, P.; Laine, P.K.; Roberts, R.J.; Smolander, O.-P.; Vihinen, H.; Piironen, V.; Paulin, L.; Jokitalo, E.; Savijoki, K.; Auvinen, P.; et al. De novo assembly of genomes from long sequence reads reveals uncharted territories of Propionibacterium freudenreichii. BMC Genomics 2017, 18, 790. [Google Scholar] [CrossRef] [PubMed]
- Guan, N.; Du, B.; Li, J.; Shin, H.; Chen, R.R.; Du, G.; Chen, J.; Liu, L. Comparative genomics and transcriptomics analysis-guided metabolic engineering of Propionibacterium acidipropionici for improved propionic acid production. Biotechnol. Bioeng. 2017, 115, 483–494. [Google Scholar] [CrossRef]
- Brüggemann, H.; Henne, A.; Hoster, F.; Liesegang, H.; Wiezer, A.; Strittmatter, A.; Hujer, S.; Dürre, P.; Gottschalk, G. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science 2004, 305, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Spellerberg, B.; Martin, S.; Brandt, C.; Lütticken, R. The cyl genes of Streptococcus agalactiae are involved in the production of pigment. FEMS Microbiol. Lett. 2000, 188, 125–128. [Google Scholar] [CrossRef][Green Version]
- Spellerberg, B.; Pohl, B.; Haase, G.; Martin, S.; Weber-Heynemann, J.; Lütticken, R. Identification of Genetic Determinants for the Hemolytic Activity of Streptococcus agalactiae by ISS1Transposition. J. Bacteriol. 1999, 181, 3212–3219. [Google Scholar]
- Forquin, M.P.; Tazi, A.; Rosa-Fraile, M.; Poyart, C.; Trieu-Cuot, P.; Dramsi, S. The Putative Glycosyltransferase-Encoding Gene cylJ and the Group B Streptococcus (GBS)-Specific Gene cylK Modulate Hemolysin Production and Virulence of GBS. Infect. Immun. 2007, 75, 2063–2066. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef]
- Deptula, P.; Kylli, P.; Chamlagain, B.; Holm, L.; Kostiainen, R.; Piironen, V.; Savijoki, K.; Varmanen, P. BluB/CobT2 fusion enzyme activity reveals mechanisms responsible for production of active form of vitamin B12 by Propionibacterium freudenreichii. Microb. Cell Fact. 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Parizzi, L.P.; Grassi, M.C.B.; Llerena, L.A.; Carazzolle, M.F.; Queiroz, V.L.; Lunardi, I.; Zeidler, A.F.; Teixeira, P.J.P.L.; Mieczkowski, P.; Rincones, J.; et al. The genome sequence of Propionibacterium acidipropionici provides insights into its biotechnological and industrial potential. BMC Genomics 2012, 13, 562. [Google Scholar] [CrossRef] [PubMed]
- Chamlagain, B.; Deptula, P.; Edelmann, M.; Kariluoto, S.; Grattepanche, F.; Lacroix, C.; Varmanen, P.; Piironen, V. Effect of the lower ligand precursors on vitamin B12 production by food-grade Propionibacteria. LWT Food Sci. Technol. 2016, 72. [Google Scholar] [CrossRef]
- Goldfarb, T.; Sberro, H.; Weinstock, E.; Cohen, O.; Doron, S.; Charpak-Amikam, Y.; Afik, S.; Ofir, G.; Sorek, R. BREX is a novel phage resistance system widespread in microbial genomes. EMBO J. 2015, 34, 169–183. [Google Scholar] [CrossRef]
- Scholz, C.F.P.; Brüggemann, H.; Lomholt, H.B.; Tettelin, H.; Kilian, M. Genome stability of Propionibacterium acnes: A comprehensive study of indels and homopolymeric tracts. Sci. Rep. 2016, 6, 20662. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Coda, R.; Wang, Y.; Verni, M.; Kajala, I.; Katina, K.; Laitila, A. Characterization of indigenous Pediococcus pentosaceus, Leuconostoc kimchii, Weissella cibaria and Weissella confusa for faba bean bioprocessing. Int. J. Food Microbiol. 2018, 302, 24–34. [Google Scholar] [CrossRef]
- Koussémon, M.; Combet-Blanc, Y.; Patel, B.K.; Cayol, J.L.; Thomas, P.; Garcia, J.L.; Ollivier, B. Propionibacterium microaerophilum sp. nov., a microaerophilic bacterium isolated from olive mill wastewater. Int. J. Syst. Evol. Microbiol. 2001, 51, 1373–1382. [Google Scholar] [CrossRef]
- Lucena-Padros, H.; Gonzalez, J.M.; Caballero-Guerrero, B.; Ruiz-Barba, J.L.; Maldonado-Barragan, A. Propionibacterium olivae sp. nov. and Propionibacterium damnosum sp. nov., isolated from spoiled packaged Spanish-style green olives. Int. J. Syst. Evol. Microbiol. 2014, 64, 2980–2985. [Google Scholar] [CrossRef]
- Ekinci, F.; Gurel, M. Effect of Using Propionic Acid Bacteria as an Adjunct Culture in Yogurt Production. J. Dairy Sci. 2008, 91, 892–899. [Google Scholar] [CrossRef]
- Schwenninger, S.M.; Meile, L. A Mixed Culture of Propionibacterium jensenii and Lactobacillus paracasei subsp. paracasei Inhibits Food Spoilage Yeasts. Syst. Appl. Microbiol. 2004, 27, 229–237. [Google Scholar] [CrossRef]
- Suomalainen, T.H.; Mäyrä-Makinen, A.M. Propionic acid bacteria as protective cultures in fermented milks and breads. Lait 1999, 79, 165–174. [Google Scholar] [CrossRef]
- Huang, Y.; Adams, M.C. An in vitro model for investigating intestinal adhesion of potential dairy propionibacteria probiotic strains using cell line C2BBe1. Lett. Appl. Microbiol. 2003, 36, 213–216. [Google Scholar] [CrossRef] [PubMed]

| Strain | Strain Information | Source/Reference |
|---|---|---|
| Acidipropionibacterium jensenii | ||
| JS279; VTT E-113203 | Isolated from malted barley | VTT Culture Collection |
| JS280; VTT E-113204 | Isolated from malted barley | VTT Culture Collection |
| DSM 20535 | Type strain; Isolated from buttermilk | German collection of microorganisms and cell cultures (DSMZ) |
| DSM 20275 | Isolated from buttermilk | DSMZ |
| DSM 20278 | Isolated from buttermilk | DSMZ |
| HAMBI 243; DSM 20274 | Microbial Domain Biological Resource Centre HAMBI (HAMBI) | |
| HAMBI 245; DSM 20279 | Isolated from cheese | HAMBI |
| Acidipropionibacterium thoenii | ||
| HAMBI 247; DSM 20276 | Type strain; Isolated from cheese | HAMBI |
| DSM 20277 | Isolated from cheese | DSMZ |
| Acidipropionibacterium virtanenii | ||
| JS278; DSM 106790 | Type strain; Isolated from malted barley | [35] |
| Propionibacterium freudenreichii | ||
| DSM 20271 | Type strain; Isolated from cheese | DSMZ |
| Strain | Genome Size (bp) | G+C mol% | Genes | CDS | rRNAs (5S,16S,23S) | tRNAs | Refseq | Genome Status | Reference |
|---|---|---|---|---|---|---|---|---|---|
| A. jensenii JS279 | 3032477 | 68.6 | 2672 | 2610 | 3,3,3 | 50 | CP025571 | complete | (this study) |
| A. jensenii JS280 | 3044937 | 68.8 | 2699 | 2637 | 3,3,3 | 50 | CP025570 | complete | (this study) |
| A. jensenii NCTC13652 T | 3180547 | 68.5 | 2839 | 2684 | 4,4,4 | 50 | NZ_LR134473.1 | complete | N/A * |
| A. thoenii DSM 20276 T | 2938072 | 68 | 2678 | 2617 | 3,4,1 ** | 50 | NZ_KE384018.1 | draft | N/A |
| A. acidipropionici CGMCC1.2230 T | 3651382 | 68.8 | 3318 | 3162 | 4,4,4 | 53 | NZ_CP013126.1 | complete | [49] |
| A. virtanenii JS278 T | 3432872 | 68.4 | 3152 | 3086 | 3,3,3 | 56 | CP025198 | complete | [35] |
| P. freudenreichii DSM 20271 T | 2649166 | 67.3 | 2333 | 2280 | 2,2,2 | 44 | NZ_CP010341.1 | complete | [36] |
| C. acnes KPA 171202 | 2560265 | 60 | 2565 | 2416 | 3,3,3 | 45 | AE017283 | complete | [50] |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| Source | malted barley | malted barley | buttermilk | malted barley | dairy product | cheese | olive waste-water | spoiled green olives | spoiled green olives |
| Colony Colour | orange/red-brown | cream/yellow | cream/yellow | cream/orange >7days | cream | orange/red-brown | white | cream | white to cream |
| Haemolysis | + | − | − | + | − | + | N/A | N/A | N/A |
| Cell Size (µm) | 1–2 | 1–2 | N/A | 1–5 | N/A | N/A | 2–3.5 | 1.4–4 | 5–30 |
| Catalase test | + | + | − | + | − | + | − | + | − |
| Conditions for growth | |||||||||
| Temperature (°C) | 12–37 | 12–42 | N/A | 12–37 | N/A | N/A | 20–45 | 20–42 | 20–42 |
| pH | 4.5–9 | 4.5–9 | N/A | 5–9 | N/A | N/A | 4.5–9.5 | 4–10 | 4.5–8 |
| Maximum NaCl (%) | 6.5 | 6.5 | N/A | 6.5 | N/A | N/A | 2 | 4 | 4 |
| Fermentation Profile Determined by API Test | |||||||||
| d-Arabinose | − | − | − | +§ | + | − | − | + | − |
| l-Arabinose | - | − | − | + | + | − | + | + | + |
| d-Xylose | − | − | − | − | − | − | − | + | − |
| Sorbose | − | − | − | − | − | − | + | − | − |
| Rhamnose | − | − | − | − | + | − | + | − | + |
| Inositol | + | + | + | + | + | − | + | N/A | N/A |
| Mannitol | + | + | + | + | + | − | + | N/A | N/A |
| Sorbitol | − | − | − | + | + | + | + | N/A | N/A |
| Methyl-α-d-mannopyranoside | − | − | − | − | − | + | − | N/A | N/A |
| Methyl-α-d-Glucopyranoside | +§ | + | − | + | + | + | + | N/A | N/A |
| N-Acetyl Glucosamine | − | − | v | + | + | + | + | − | + |
| Amygdalin | − | − | v | v | + | − | − | − | − |
| Arbutin | − | − | − | + | + | N/A | N/A | + | − |
| Esculin | + | + | +§ | + | + | + | − | N/A | N/A |
| Salicin | − | +§ | +§ | +§ | + | N/A | N/A | N/A | N/A |
| d-Cellobiose | − | − | + | + | + | N/A | + | + | + |
| Maltose | + | + | + | + | + | N/A | N/A | N/A | N/A |
| Lactose | − | − | − | +§ | + | + | − | − | + |
| Melibiose | + | + | + | + | − | − | − | − | + |
| Saccharose | + | + | + | + | + | N/A | N/A | N/A | N/A |
| Trehalose | + | + | + | + | + | N/A | N/A | N/A | N/A |
| Melezitose | + | + | +§ | − | + | − | + | − | − |
| Raffinose | + | + | + | + | − | − | − | − | − |
| Amidon (starch) | +§ | + | − | + | + | + | + | − | − |
| Glycogen | − | − | − | − | − | − | + | − | − |
| Xylitol | + | + | − | + | + | N/A | N/A | N/A | N/A |
| Gentiobiose | − | − | + | +§ | + | N/A | N/A | N/A | N/A |
| d-Fucose | − | − | − | − | − | − | − | N/A | N/A |
| Potassium Gluconate | V § | + | +§ | + | + | − | + | − | − |
| Reference | (this study) | (this study) | [35] | [35] | [35] | [61] | [61] | [62] | [62] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deptula, P.; Loivamaa, I.; Smolander, O.-P.; Laine, P.; Roberts, R.J.; Piironen, V.; Paulin, L.; Savijoki, K.; Auvinen, P.; Varmanen, P. Red-Brown Pigmentation of Acidipropionibacterium jensenii Is Tied to Haemolytic Activity and cyl-Like Gene Cluster. Microorganisms 2019, 7, 512. https://doi.org/10.3390/microorganisms7110512
Deptula P, Loivamaa I, Smolander O-P, Laine P, Roberts RJ, Piironen V, Paulin L, Savijoki K, Auvinen P, Varmanen P. Red-Brown Pigmentation of Acidipropionibacterium jensenii Is Tied to Haemolytic Activity and cyl-Like Gene Cluster. Microorganisms. 2019; 7(11):512. https://doi.org/10.3390/microorganisms7110512
Chicago/Turabian StyleDeptula, Paulina, Iida Loivamaa, Olli-Pekka Smolander, Pia Laine, Richard J. Roberts, Vieno Piironen, Lars Paulin, Kirsi Savijoki, Petri Auvinen, and Pekka Varmanen. 2019. "Red-Brown Pigmentation of Acidipropionibacterium jensenii Is Tied to Haemolytic Activity and cyl-Like Gene Cluster" Microorganisms 7, no. 11: 512. https://doi.org/10.3390/microorganisms7110512
APA StyleDeptula, P., Loivamaa, I., Smolander, O.-P., Laine, P., Roberts, R. J., Piironen, V., Paulin, L., Savijoki, K., Auvinen, P., & Varmanen, P. (2019). Red-Brown Pigmentation of Acidipropionibacterium jensenii Is Tied to Haemolytic Activity and cyl-Like Gene Cluster. Microorganisms, 7(11), 512. https://doi.org/10.3390/microorganisms7110512





