Defective RNA of a Novel Mycovirus with High Transmissibility Detrimental to Biocontrol Properties of Trichoderma spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates/Strains and Mycelial Growth
2.2. Extraction of Nucleic acids
2.3. cDNA Cloning, Sequencing, and Sequence Analysis of ThHV1 and ThHV1-S
2.4. Northern Hybridization
2.5. Real-Time Semi-Quantitative PCR
2.6. Vertical and Horizontal Transmission of ThHV1 and ThHV1-S
2.7. The Mycoparasitism and Antifungal Activity Assay of Trichoderma
2.8. Suppression of B. cinerea Sporulation on Oilseed Rape Tissues
2.9. Detection of ThHV1 and ThHV1-S in Trichoderma Population
2.10. Statistical Data Analysis
3. Results
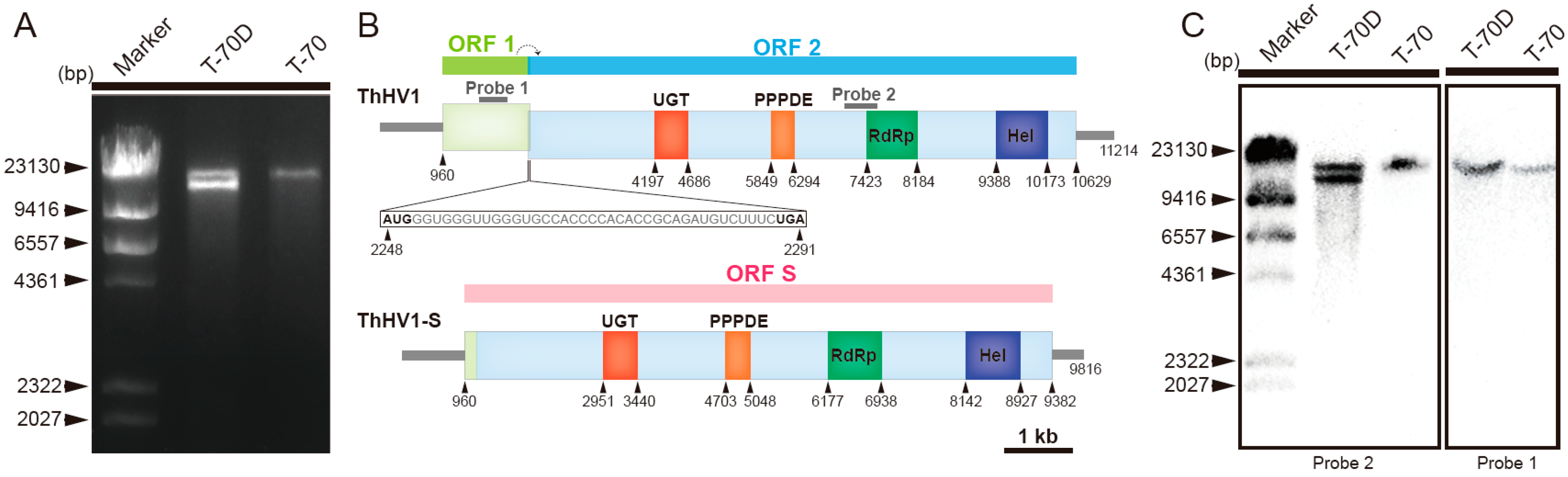
3.1. Nucleotide Sequences and Putative Polypeptides of ThHV1 and ThHV1-S
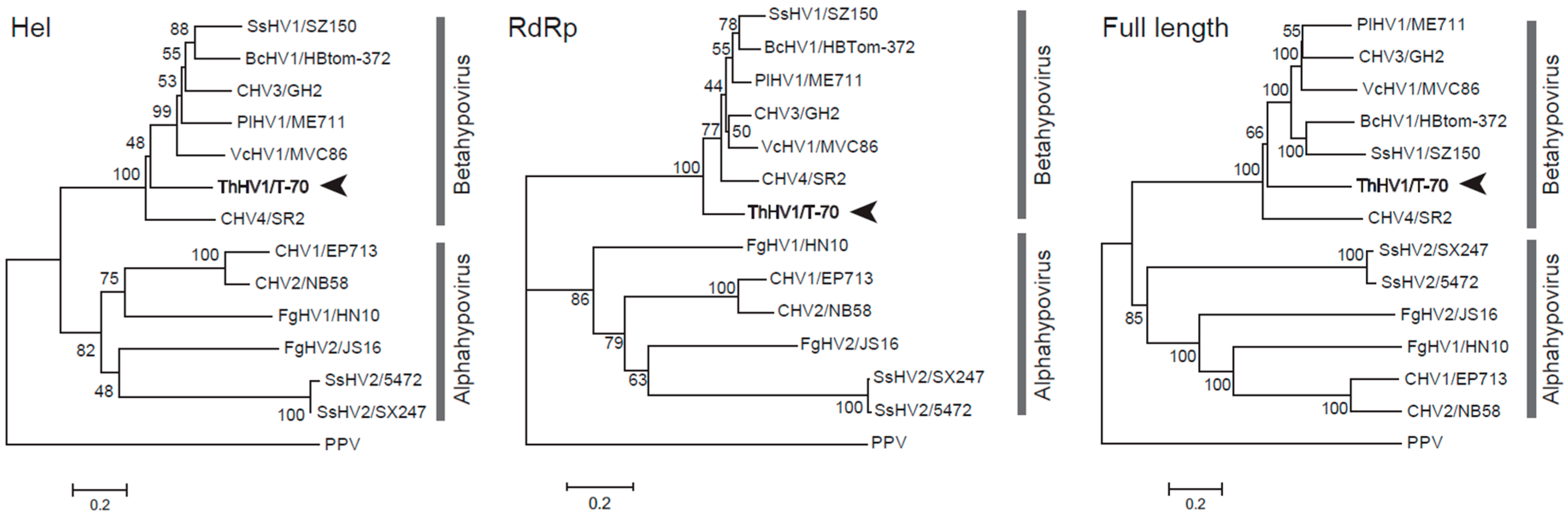
3.2. Phylogenetic Analysis
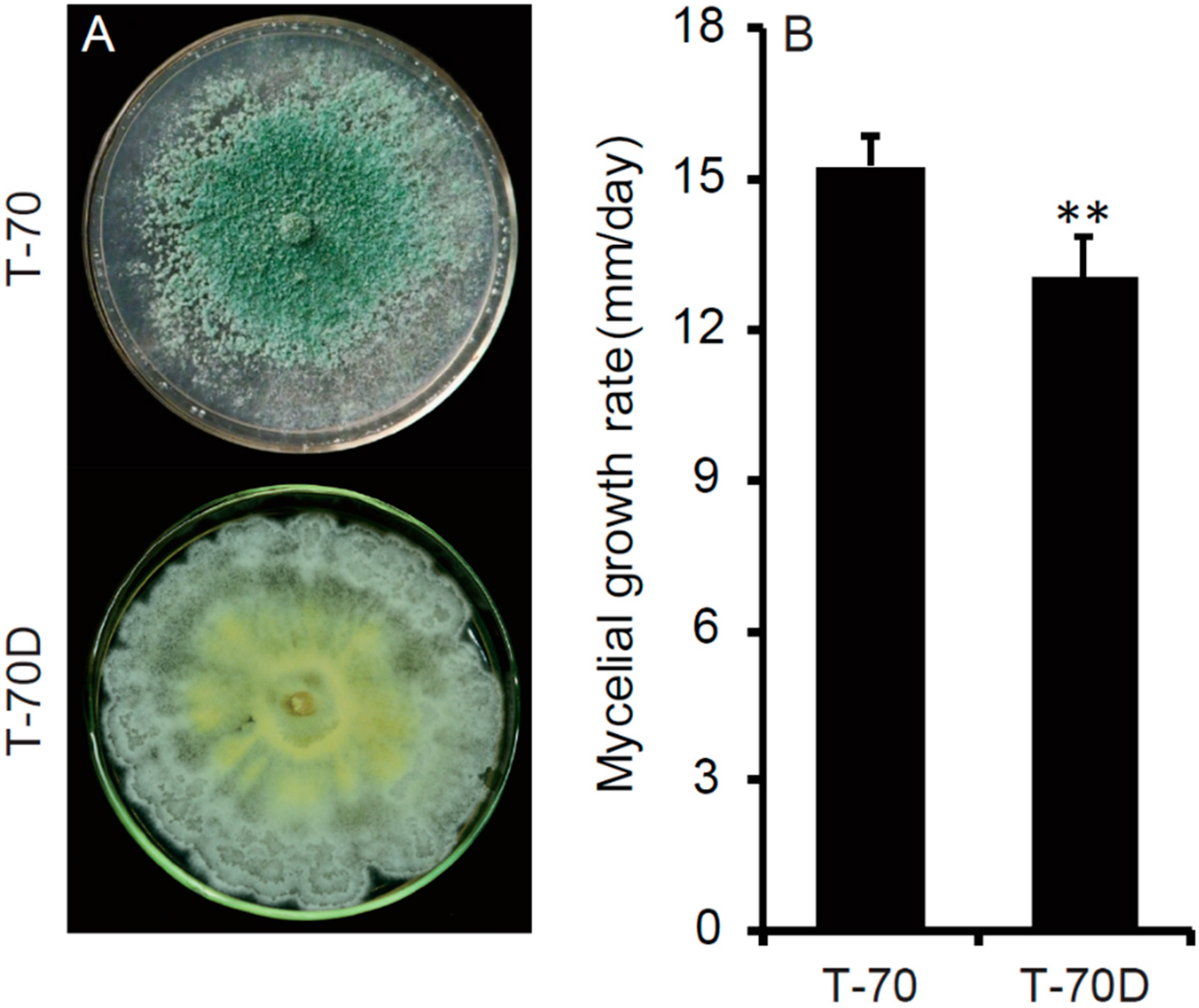
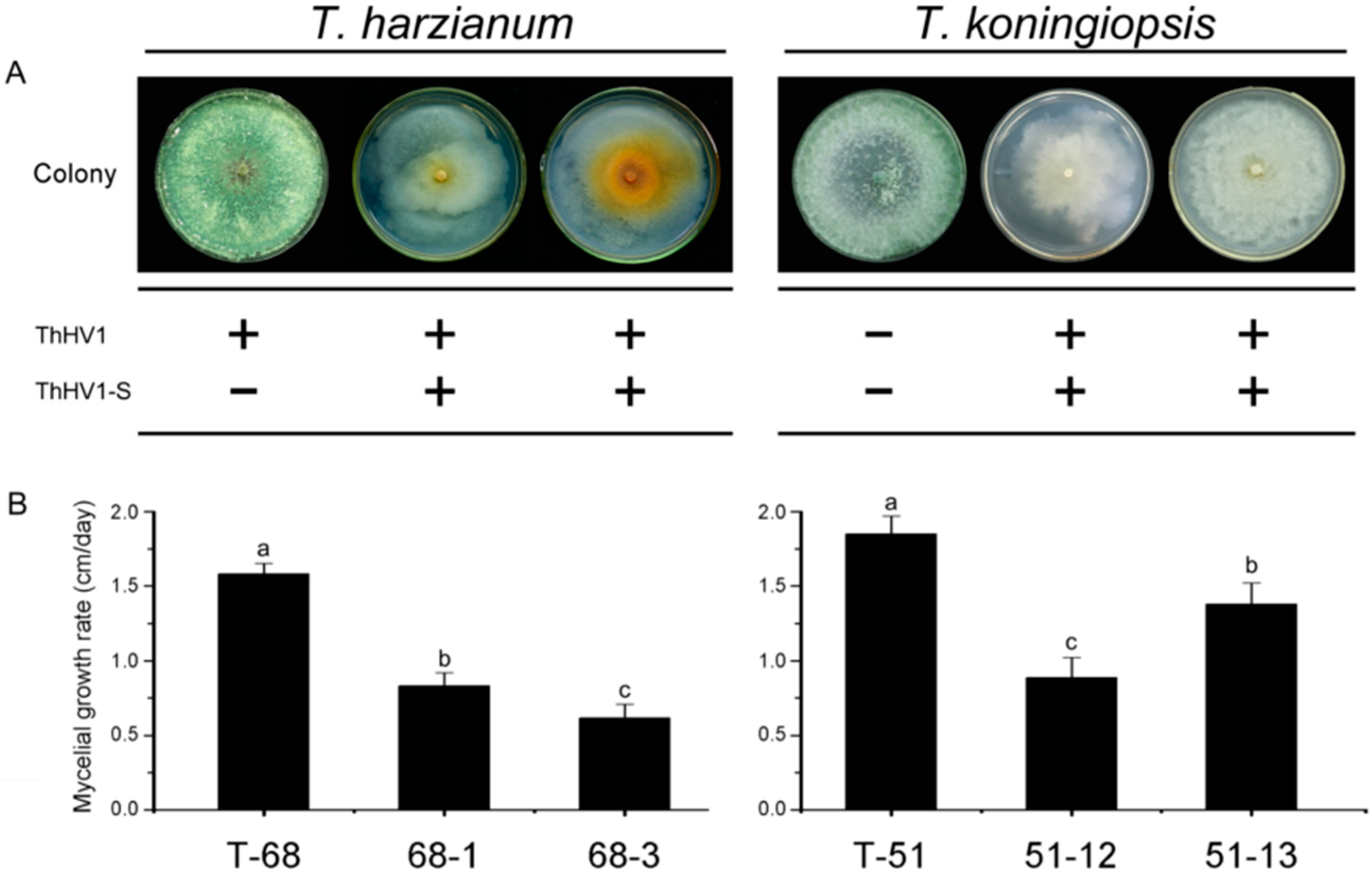
3.3. Debilitated Biological Properties of T. harzianum Isolate T-70D
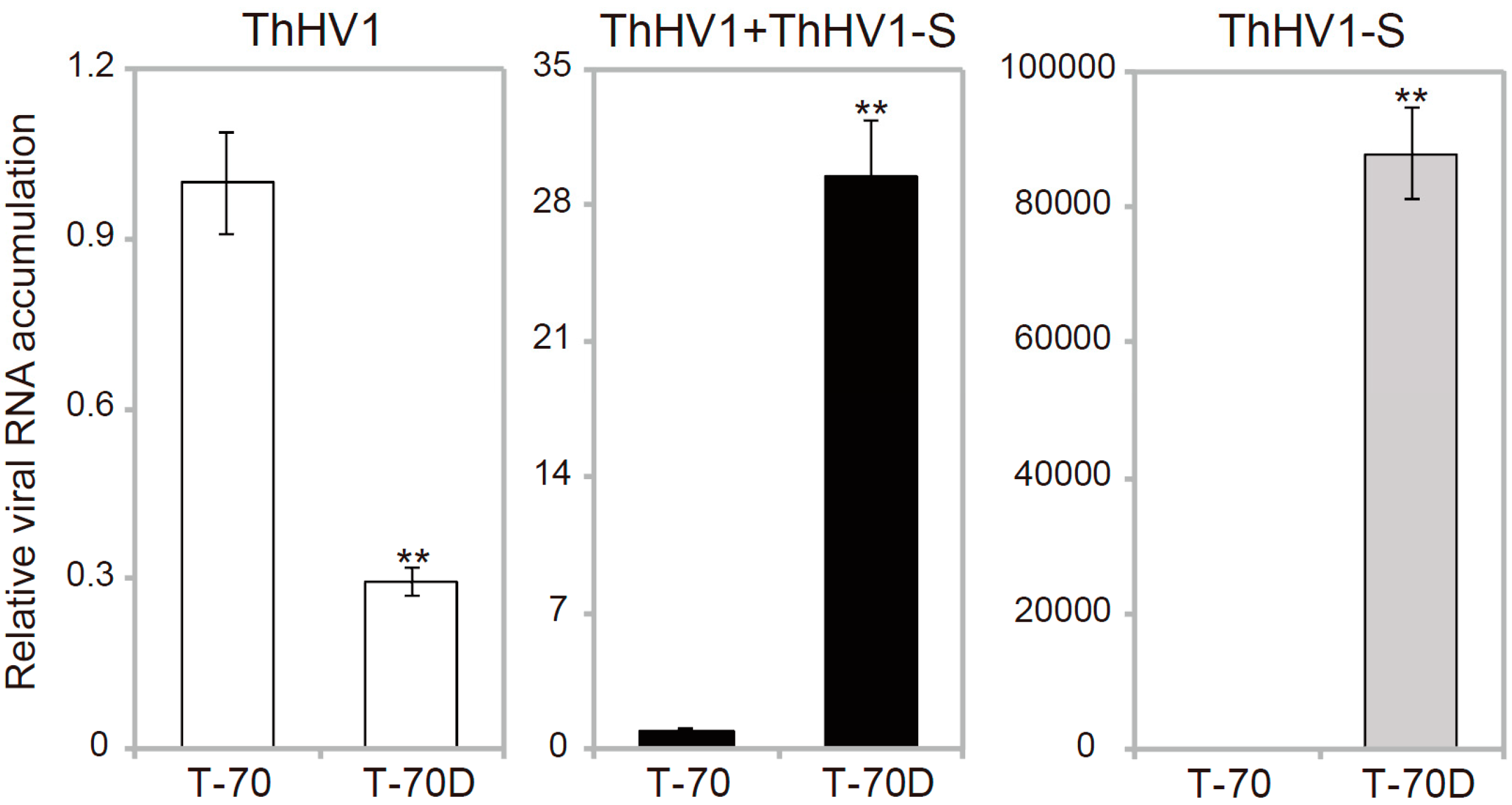
3.4. Accumulation of ThHV1 and ThHV1-S
3.5. Vertical Transmission of ThHV1 and ThHV1-S
3.6. Effects of ThHV1 and ThHV1-S on Bioligical Properties of T. harzianum and T. koningiopsis
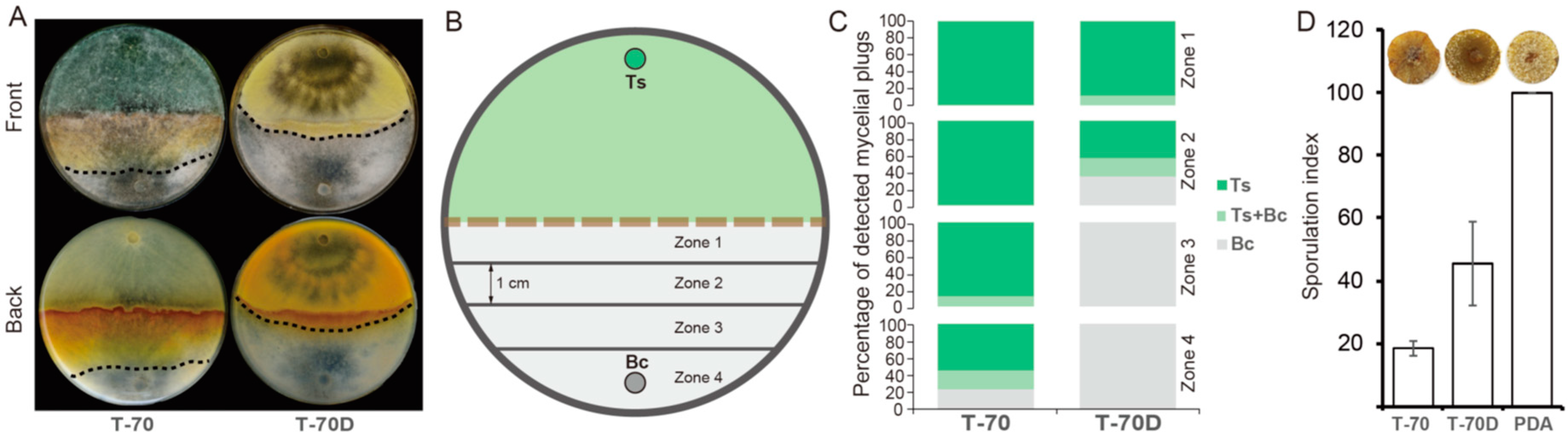
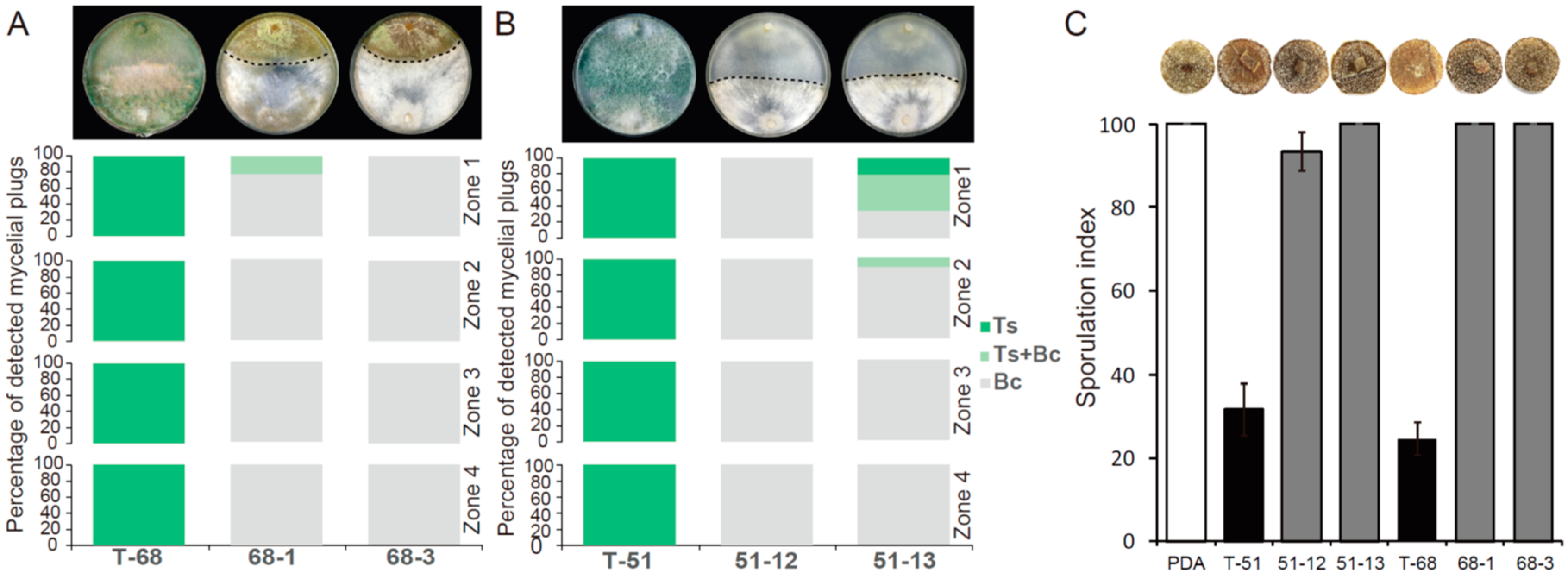
3.7. Effects of ThHV1 and ThHV1-S on Antifungal Activities of T. harzianum and T. koningiopsis
3.8. Incidence and Distribution of ThHV1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Klein, D.; Eveleigh, D.E. Ecology of Trichoderma. In Trichoderma and Gliocladium; Kubicek, C.P., Harman, G.E., Eds.; Taylor & Francis e-Library: London, UK, 1998; Volume 1, pp. 57–74. [Google Scholar]
- Harman, G.E. Myths and dogmas of biocontrol changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis. 2000, 84, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—opportunistic, avirulent plant symbionts. Nat. Rev. microbial. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Affokpon, A.; Coyne, D.L.; Htay, C.C.; Agbèdè, R.D.; Lawouin, L.; Coosemans, J. Biocontrol potential of native Trichoderma isolates against root-knot nematodes in West African vegetable production systems. Soil Biol. Biochem. 2011, 43, 600–608. [Google Scholar] [CrossRef]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
- Schuster, A.; Schmoll, M. Biology and biotechnology of Trichoderma. Appl. Microbiol. Biot. 2010, 87, 787–799. [Google Scholar] [CrossRef]
- Nicot, P.; Bardin, M.; Alabouvette, C.; Köhl, J. Potential of biological control based on published research. 1. Protection against plant pathogens of selected crops. In Classical and Augmentative Biological Control against Diseases and Pests: Critical Status Analysis and Review of Factors Influencing Their Success; IOBC—International Organisation for Biological and Integrated Control of Noxious Animals and Plants: Zurich, Switzerland, 2011; pp. 1–11. [Google Scholar]
- Nicot, P.C.; Stewart, A.; Bardin, M.; Elad, Y. Biological control and biopesticide suppression of Botrytis-incited diseases. In Botrytis–the Fungus, the Pathogen and its Management in Agricultural Systems; Springer: Cham, Switzerland, 2016; pp. 165–187. [Google Scholar]
- Massart, S.; Martinez-Medina, M.; Jijakli, M.H. Biological control in the microbiome era: Challenges and opportunities. Biol. Control. 2015, 89, 98–108. [Google Scholar] [CrossRef]
- Ghabrial, S.A.; Suzuki, N. Viruses of plant pathogenic fungi. Annu. Rev. Phytopathol. 2009, 47, 353–384. [Google Scholar] [CrossRef]
- Ghabrial, S.A.; Castón, J.R.; Jiang, D.; Nibert, M.L.; Suzuki, N. 50-plus years of fungal viruses. Virology 2015, 479–480, 356–368. [Google Scholar] [CrossRef]
- Nuss, D.L. Hypovirulence: Mycoviruses at the fungal–plant interface. Nat. Rev. Microbiol. 2005, 3, 632–642. [Google Scholar] [CrossRef]
- Milgroom, M.G.; Hillman, B.I. The ecology and evolution of fungal viruses. In Studies in viral ecology: Microbial and botanical host systems; Wiley-Blackwell: Oxford, UK, 2011; Volume 1, pp. 217–253. [Google Scholar]
- Dawe, A.L.; Nuss, D.L. Hypoviruses and Chestnut Blight: Exploiting Viruses to Understand and Modulate Fungal Pathogenesis. Genetics 2001, 35, 1–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, R.; Ichinose, S.; Takeshita, K.; Urayama, S.I.; Fukuhara, T.; Komatsu, K.; Arie, T.; Ishihara, A.; Egusa, M.; Kodama, M. Molecular characterization of a novel mycovirus in Alternaria alternata manifesting two-sided effects: Down-regulation of host growth and up-regulation of host plant pathogenicity. Virology 2018, 519, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Márquez, L.M.; Redman, R.S.; Rodriguez, R.J.; Roossinck, M.J. A virus in a fungus in a plant: Three-way symbiosis required for thermal tolerance. Science 2007, 315, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Kotta-Loizou, I.; Coutts, R.H. Studies on the virome of the entomopathogenic fungus Beauveria bassiana reveal novel dsRNA elements and mild hypervirulence. PLoS Pathog. 2017, 13, e1006183. [Google Scholar] [CrossRef] [PubMed]
- Yaegashi, H.; Kanematsu, S.; Ito, T. Molecular characterization of a new hypovirus infecting a phytopathogenic fungus, Valsa ceratosperma. Virus Res. 2012, 165, 143–150. [Google Scholar] [CrossRef]
- Pathak, K.B.; Nagy, P.D. Defective interfering RNAs: Foes of viruses and friends of virologists. Viruses 2009, 1, 895–919. [Google Scholar] [CrossRef]
- Chiba, S.; Lin, Y.H.; Kondo, H.; Kanematsu, S.; Suzuki, N. Effects of defective interfering RNA on symptom induction by, and replication of, a novel partitivirus from a phytopathogenic fungus, Rosellinia necatrix. J. Virol. 2013, 87, 2330–2341. [Google Scholar] [CrossRef]
- Zhang, X.; Segers, G.C.; Sun, Q.; Deng, F.; Nuss, D.L. Characterization of hypovirus-derived small RNAs generated in the chestnut blight fungus by an inducible DCL-2-dependent pathway. J. Virol. 2008, 82, 2613–2619. [Google Scholar] [CrossRef]
- You, J.; Zhang, J.; Wu, M.; Yang, L.; Chen, W.; Li, G. Multiple criteria-based screening of Trichoderma isolates for biological control of Botrytis cinerea on tomato. Biol. Control. 2016, 101, 31–38. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, L.; Li, G.; Jiang, D.; Hou, M.; Huang, H.C. Hypovirulence and double-stranded RNA in Botrytis cinerea. Phytopathology 2007, 97, 1590–1599. [Google Scholar] [CrossRef]
- Möller, E.; Bahnweg, G.; Sandermann, H.; Geiger, H. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 1992, 20, 6115. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, L.; Li, G.; Jiang, D.; Ghabrial, S.A. Genome characterization of a debilitation-associated mitovirus infecting the phytopathogenic fungus Botrytis cinerea. Virology 2010, 406, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Morris, T.; Dodds, J. Isolation and analysis of double-stranded RNA from virus-infected plant and fungal tissue. Phytopathology 1979, 69, 854–858. [Google Scholar] [CrossRef]
- Yu, L.; Sang, W.; Wu, M.D.; Zhang, J.; Yang, L.; Zhou, Y.J.; Chen, W.D.; Li, G.Q. Novel hypovirulence-associated RNA mycovirus in the plant-pathogenic fungus Botrytis cinerea: Molecular and biological characterization. Appl. Environ. Microbiol. 2015, 81, 2299–2310. [Google Scholar] [CrossRef]
- Wu, M.; Jin, F.; Zhang, J.; Yang, L.; Jiang, D.; Li, G. Characterization of a novel bipartite double-stranded RNA mycovirus conferring hypovirulence in the phytopathogenic fungus Botrytis porri. J. Virol. 2012, 86, 6605–6619. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Sperschneider, J.; Datta, A.; Wise, M.J. Heuristic RNA pseudoknot prediction including intramolecular kissing hairpins. RNA 2011, 17, 27–38. [Google Scholar] [CrossRef]
- Hao, F.; Zhou, Z.; Wu, M.; Li, G. Molecular characterization of a novel endornavirus from the phytopathogenic fungus Botrytis cinerea. Arch. Virol. 2017, 162, 313–316. [Google Scholar] [CrossRef]
- Priscila, D.S.D.; Rodrigues, G.N.; Zubieta, M.P.; Ramoni, J.; Codima, C.A.; Lima, D.J.; Farinas, C.S.; da Cruz Pradella, J.G.; Seiboth, B. The relation between xyr1 overexpression in Trichoderma harzianum and sugarcane bagasse saccharification performance. J. Biotechnol. 2017, 246, 24–32. [Google Scholar] [CrossRef]
- Zeng, L.M.; Zhang, J.; Han, Y.C.; Yang, L.; Wu, M.d.; Jiang, D.H.; Chen, W.; Li, G.Q. Degradation of oxalic acid by the mycoparasite Coniothyrium minitans plays an important role in interacting with Sclerotinia sclerotiorum. Environ. Microbiol. 2014, 16, 2591–2610. [Google Scholar] [CrossRef]
- Lou, Y.; Han, Y.; Yang, L.; Wu, M.; Zhang, J.; Cheng, J.; Wang, M.; Jiang, D.; Chen, W.; Li, G. CmpacC regulates mycoparasitism, oxalate degradation and antifungal activity in the mycoparasitic fungus Coniothyrium minitans. Environ. Microbiol. 2015, 17, 4711–4729. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wu, S.; Cheng, J.; Fu, Y.; Jiang, D.; Xie, J. Molecular characterization of two positive-strand RNA viruses co-infecting a hypovirulent strain of Sclerotinia sclerotiorum. Virology 2014, 464, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.E.; Pearson, M.N. Characterisation of a novel hypovirus from Sclerotinia sclerotiorum potentially representing a new genus within the Hypoviridae. Virology 2014, 464, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.E.; Pearson, M.N. Molecular characterisation of an endornavirus infecting the phytopathogen Sclerotinia sclerotiorum. Virus Res. 2014, 189, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Matsufuji, S.; Matsufuji, T.; Miyazaki, Y.; Murakami, Y.; Atkins, J.F.; Gesteland, R.F.; Hayashi, S. Autoregulatory frameshifting in decoding mammalian ornithine decarboxylase antizyme. Cell 1995, 80, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Shapira, R.; Choi, G.H.; Hillman, B.I.; Nuss, D.L. The contribution of defective RNAs to the complexity of viral-encoded double-stranded RNA populations present in hypovirulent strains of the chestnut blight fungus Cryphonectria parasitica. EMBO J. 1991, 10, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Hillman, B.; Foglia, R.; Yuan, W. Satellite and defective RNAs of Cryphonectria hypovirus 3-grand haven 2, a virus species in the family Hypoviridae with a single open reading frame. Virology 2000, 276, 181–189. [Google Scholar] [CrossRef]
- Li, P.; Zhang, H.; Chen, X.; Qiu, D.; Guo, L. Molecular characterization of a novel hypovirus from the plant pathogenic fungus Fusarium graminearum. Virology 2015, 481, 151–160. [Google Scholar] [CrossRef]
- Cheng, J.; Jiang, D.Y.; Li, G.; Peng, Y.; Ghabrial, S.A. Molecular characterization of a dsRNA totivirus infecting the sclerotial parasite Coniothyrium minitans. Virus Res. 2003, 93, 41–50. [Google Scholar] [CrossRef]
- Yun, S.H.; Lee, S.H.; So, K.K.; Kim, J.M.; Kim, D.H. Incidence of diverse dsRNA mycoviruses in Trichoderma spp. causing green mold disease of shiitake Lentinula edodes. FEMS Microbiol. Lett. 2016, fnw220. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, S.H.; Chun, J.; Kim, D.H. Characterization of a novel dsRNA mycovirus of Trichoderma atroviride NFCF028. Arch. Virol. 2017, 162, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Yang, H.E.; Kim, D.H. Identification and molecular characterization of a novel partitivirus from Trichoderma atroviride NFCF394. Viruses 2018, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zeng, X.; Cai, X.; Liu, H.; Zeng, Z. Molecular characterization of a novel double-stranded RNA mycovirus of Trichoderma asperellum strain JLM45-3. Arch. Virol. 2018, 163, 3433–3437. [Google Scholar] [CrossRef] [PubMed]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Yaegashi, H.; Nakamura, H.; Sawahata, T.; Sasaki, A.; Iwanami, Y.; Ito, T.; Kanematsu, S. Appearance of mycovirus-like double-stranded RNAs in the white root rot fungus, Rosellinia necatrix, in an apple orchard. FEMS Microbiol. Ecol. 2013, 83, 49–62. [Google Scholar] [CrossRef]
- Vainio, E.J.; Müller, M.M.; Korhonen, K.; Piri, T.; Hantula, J. Viruses accumulate in aging infection centers of a fungal forest pathogen. ISME J. 2015, 9, 497. [Google Scholar] [CrossRef]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Anagnostakis, S.L. Biological control of chestnut blight. Science 1982, 215, 466–471. [Google Scholar] [CrossRef]
| Strains | Origin (Place and Collected Time) | Presence of Virus | |
|---|---|---|---|
| ThHV1 | ThHV1-S | ||
| Field strains | |||
| T-70 | Ezhou, China, 2012 | + 1 | – |
| T-68 | Ezhou, China, 2012 | + | – |
| T-51 | Wuhan, China, 2012 | – | – |
| Laboratory-Derived Strains | |||
| T-70D | colony edge progeny of T-70, 2013 | + | + |
| 68-1 | T-68 in a pairing-culture of T-68 and T-70D, 2016 | + | + |
| 68-3 | T-68 in a pairing-culture of T-68 and T-70D, 2016 | + | + |
| 51-12 | T-51 in a pairing-culture of T-51 and T-70D, 2016 | + | + |
| 51-13 | T-51 in a pairing-culture of T-51 and T-70D, 2016 | + | + |
| 51-70-2 | T-51 in a pairing-culture of T-51 and T-70, 2016 | + | – |
| 51-70-4 | T-51 in a pairing-culture of T-51 and T-70, 2016 | + | – |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, J.; Zhou, K.; Liu, X.; Wu, M.; Yang, L.; Zhang, J.; Chen, W.; Li, G. Defective RNA of a Novel Mycovirus with High Transmissibility Detrimental to Biocontrol Properties of Trichoderma spp. Microorganisms 2019, 7, 507. https://doi.org/10.3390/microorganisms7110507
You J, Zhou K, Liu X, Wu M, Yang L, Zhang J, Chen W, Li G. Defective RNA of a Novel Mycovirus with High Transmissibility Detrimental to Biocontrol Properties of Trichoderma spp. Microorganisms. 2019; 7(11):507. https://doi.org/10.3390/microorganisms7110507
Chicago/Turabian StyleYou, Jiaqi, Kang Zhou, Xiaolin Liu, Mingde Wu, Long Yang, Jing Zhang, Weidong Chen, and Guoqing Li. 2019. "Defective RNA of a Novel Mycovirus with High Transmissibility Detrimental to Biocontrol Properties of Trichoderma spp." Microorganisms 7, no. 11: 507. https://doi.org/10.3390/microorganisms7110507
APA StyleYou, J., Zhou, K., Liu, X., Wu, M., Yang, L., Zhang, J., Chen, W., & Li, G. (2019). Defective RNA of a Novel Mycovirus with High Transmissibility Detrimental to Biocontrol Properties of Trichoderma spp. Microorganisms, 7(11), 507. https://doi.org/10.3390/microorganisms7110507





