Seroepidemiology and the Molecular Detection of Animal Brucellosis in Punjab, Pakistan
Abstract
1. Introduction
2. Materials and Methods
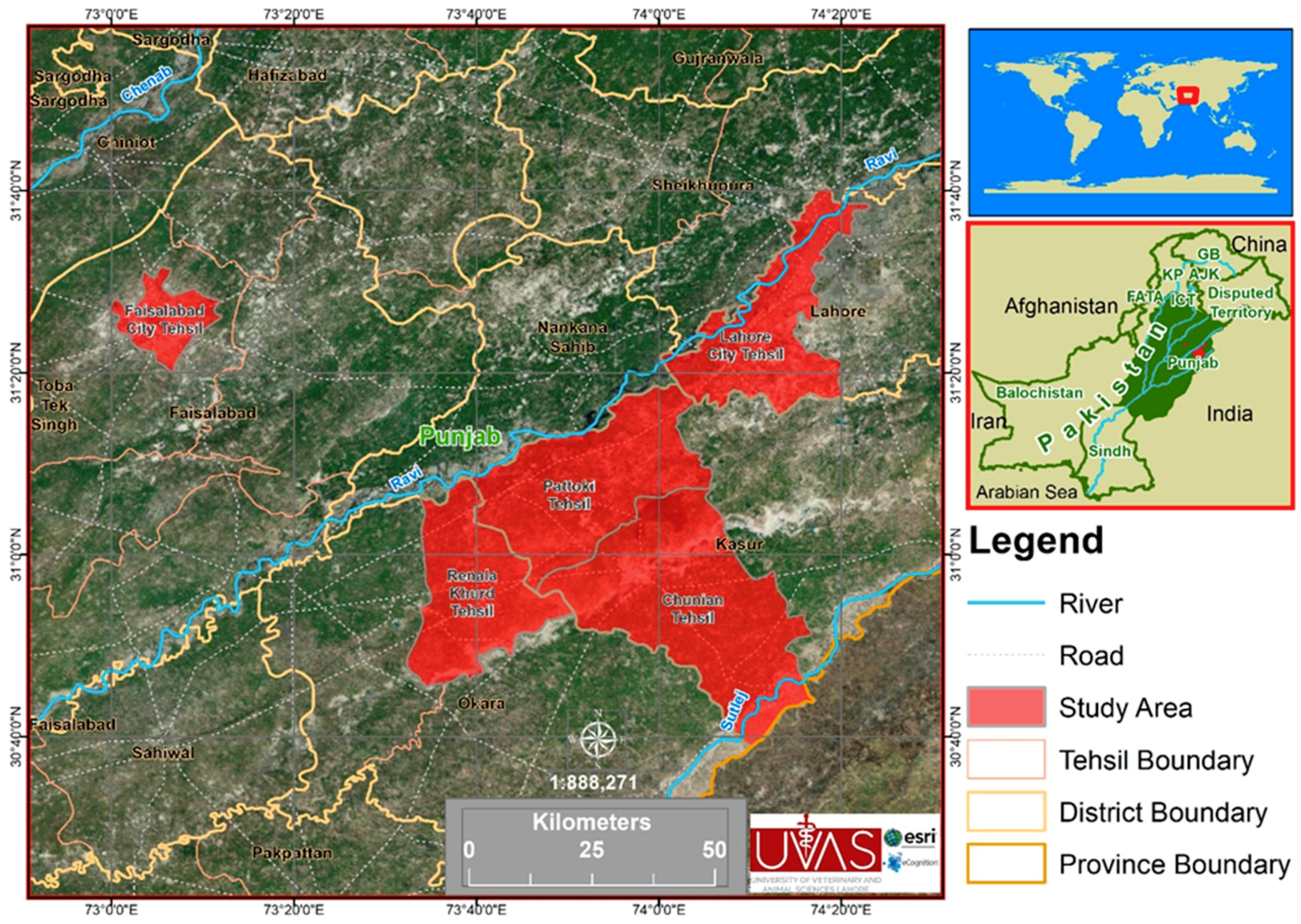
2.1. Sampling Sites
2.2. Epidemiological Data Collection
2.3. Blood Sampling
2.4. Serum Separation
2.5. Rose Bengal Plate Test (RBPT)
2.6. DNA Extraction
2.7. Quantitative Real-Time (RT) PCR
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Islam, M.A.; Khatum, M.M.; Were, S.R.; Sriranganathan, N.; Boyle, S.M. A review of Brucella seroprevalence among humans and animals in Bangladesh with special emphasis on epidemiology risk factors and control opportunities. Vet. Microbiol. 2013, 166, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Schelling, E.; Diguimbave, C.; Daoud, S.; Nicolet, J.; Boerlin, P.; Tanner, M.; Zinsstag, J. Brucellosis and Q-fever seroprevalence of nomadic pastoralists and their livestock in Chad. Prev. Vet. Med. 2003, 61, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ali, Q.; Neubauer, H.; Melzer, F.; Elschner, M.; Khan, I.; Abatih, E.N.; Ullah, N.; Irfan, M.; Akhter, S. Seroprevalence and risk factors associated with brucellosis as a professional hazard in Pakistan. Foodborne Path. Dis. 2013, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Verma, D.K.; Rout, P.K.; Singh, S.V.; Vihan, V.S. Polymerase chain reaction for detection of B. melitensis in goat milk. Small Rumin. Res. 2006, 65, 79–84. [Google Scholar] [CrossRef]
- Tasaime, W.; Emikpe, B.; Folitse, R.; Fofie, C.; Burimuah, V.; Johnson, S. The prevalence of brucellosis in cattle and their handle in North Tongu district, Volta region Ghana. Afr. J. Infect. Dis. 2016, 10, 111–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogugua, A.J.; Akinseye, V.O.; Ayoola, M.C.; Stack, J.; Babalola, C.S.I. Risk factors associated with brucellosis among slaughtered cattle: Epidemiological insight from two metropolitan abattoirs in south western Nigeria. Asian Pacific J. Trop. Dis. 2015, 5, 747–753. [Google Scholar]
- Zeng, J.; Duoji, C.; Yuan, Z.; Yuzhen, S.; Fan, W.; Tian, L. Seroprevalence and risk factors for bovine brucellosis in domestic Yaks (Bos grunniens) in Tibet, China. Trop. Anim. Health Pro. 2017, 49, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Slack, M.P.; Cohen, J.; Powderly, W.G.; Opal, S.M. Gram-negative coccobacilli. Brucella species. In Infectious Diseases, 2nd ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Mosby Elsevier: Edinburg, UK, 2004; pp. 2–8. [Google Scholar]
- Winn, W.; Allen, S.; Janda, W.; Koneman, W. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology, 6th ed.; Lippincott Williams and Wilkins: New York, NY, USA, 2006; pp. 482–491. [Google Scholar]
- Maadi, H.; Moharamnejad, M.; Haghi, M. Prevalence of brucellosis in cattle in Urmia, Iran. Pak. Vet. J. 2011, 31, 81–82. [Google Scholar]
- Hussain, I.; Arshad, M.I.; Mahmood, M.S. Seroprevalence of brucellosis in human, cattle and buffalo population in Pakistan. Turk. J. Vet. Anim. Sci. 2008, 32, 315–318. [Google Scholar]
- Amen, K.M.R.; Rahman, M.B.; Rahman, M.S.; Han, J.C.; Park, J.H.; Chae, J.S. Prevalence of Brucella antibodies in sera of cows in Bangladesh. J. Vet. Sci. 2005, 6, 223–226. [Google Scholar] [CrossRef][Green Version]
- Kara, R.; Akkaya, L. Investigation of B. abortus and B. melitensis at cheeses in Afyonkarahisar, Turkey. Br. J. Dairy Sci. 2013, 3, 5–8. [Google Scholar]
- Godfroid, J.; Scholz, H.C.; BArbier, T.; Nicolas, C.; Wattiau, P.; Fretin, D. Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Prev. Vet. Med. 2011, 102, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.A.M.; Shah, M.A.; Rashid, M. Prevalence of antibody to Brucella in sheep and goats of Punjab region. Pak. J. Biol. Sci. 2000, 3, 196–198. [Google Scholar]
- 9211 Virtual Governance System. Available online: http://www.livestockpunjab.gov.pk/page/pages/9211_virtual_governance (accessed on 20 June 2019).
- Alton, G.G.; Jones, L.M.; Angus, R.D.; Verges, J.M. Techniques for the brucellosis laboratory Paris Institute National de la Recherche Agronomique. J. Clin. Microbiol. 1988, 33, 3198–3200. [Google Scholar]
- Probert, W.S.; Schrader, K.N.; Khuong, N.Y. Real time multiplies PCR assay for detection of Brucella spp, B. abortus and B. melitensis. J. Clin. Microbiol. 2004, 42, 1290–1293. [Google Scholar] [CrossRef]
- Fatima, S.; Khan, I.; Nasir, A.; Younas, M.; Saqib, M.; Melzer, F.; Neubauer, H.; El-Adawy, H. Serological, molecular detection and potential risk factors associated with camel brucellosis in Pakistan. Trop. Anim. Health Pro. 2016, 48, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Gwida, M.M.; Abdel, H.E.G.; Falk, M. Comparison of diagnostic tests for the detection of Brucella spp, in camel sera. BMC Res. Note 2011, 4, 525. [Google Scholar] [CrossRef]
- Charachon, M.S.; Foulongne, V.; Callaghan, O.D.; Ramuz, M. Brucella at the dawn of the third millennium: Genomic organization and pathogenesis. Thol. Biol. 2002, 50, 401–412. [Google Scholar]
- Thakur, S.D.; Kumar, R.; Thapliyal, D.C. Human brucellosis: A review of an under-diagnosed animal transmitted disease. J. Com. Dis. 2002, 34, 287–301. [Google Scholar]
- Ali, S.; Akther, S.; Neubauer, H.; Melzer, F.; Khan, I.; Qurban, A.; Irfan, M. Serological, cultural, and molecular evidences of Brucella infection in small ruminants in Pakistan. J. Infect. Dev. Countr. 2015, 9, 470–475. [Google Scholar] [CrossRef]
- Shahzad, A.; Khan, A.; Khan, M.Z.; Saqib, M. Seroprevalence and molecular investigation of brucellosis in camels of selected districts of Punjab, Pakistan. Thai J. Vet. Med. 2017, 47, 207–215. [Google Scholar]
- Makita, K.; Fever, E.; Waiswa, C.; Eisler, M.; Thrusfield, M.; Welburn, S. Herd prevalence of bovine brucellosis and analysis of risk factor in cattle in urban and peri-urban areas of the Kampala economic zone, Uganda. BMC Vet. Res. 2011, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yibeltal, M. A Seroprevalence Study of Small Ruminant’s Brucellosis in Selected Sites of the Afar and Somali Region, Ethiopia. DVM Thesis, Faculty of Veterinary Medicine, Addis Ababa University, Debre Zeit, Ethiopia, 2005. [Google Scholar]
- Shafee, M.; Rabbani, M.; Sheik, A.; Ahmad, M.; Razzaq, A. Prevalence of bovine brucellosis in organized dairy farms, using milk ELISA, in Quetta city, Baluchistan, Pakistan. Vet. Med. Int. 2011, 2011, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ogugua, A.J.; Akinseye, V.O.; Ayoola, M.C.; Oyesola, O.O.; Shima, F.K.; Tijjani, A.O.; Aderemi, N.A.; Adesokan, H.K.; Lorraine, P.; Andrew, T.; et al. Seroprevalence and risk factors for brucellosis in goats in selected in Nigeria and the public health implication. Afr. J. Med. Med. Sci. 2014, 43, 121–129. [Google Scholar] [PubMed]
- Hegazy, Y.; Moqwad, A.; Osman, S.; Ridler, A.; Guitian, J. Ruminant Brucellosis in the Kafr El Sheikh Governorate of the Nile Delta, Egypt: Prevalence of a neglected Zoonosis. PLoS Negl. Trop. Dis. 2011, 5, e944. [Google Scholar] [CrossRef] [PubMed]
- Akthar, L.; Islam, M.A.; Das, S.; Khatun, M.; Islam, A. Seroprevalence of Brucellosis and its associated risk factors in sheep and goat in the farms and slaughter house in Mymensingh, Bangladesh. Micro. Health 2014, 3, 25–28. [Google Scholar]
- Rivera, J.L.S.; Correa, J.C.S.; Gil, L.G.S. Seroprevalence of and risk factors for brucellosis of goats in herds of Michoacan, Mexico. Prev. Vet. Med. 2007, 82, 282–290. [Google Scholar] [CrossRef]
- Jain, N.; Boyle, S.M.; Sriranganathan, N. Effect of exogenous erythritol on growth and survival of Brucella. Vet. Microbiol. 2012, 160, 513–516. [Google Scholar] [CrossRef]
- Ali, S.; Akthar, S.; Neubauer, H.; Melzer, F.; Khan, I.; Abatih, E.N.; Adawy, H.E.; Irfan, M.; Muhammad, A.; Akbar, M.W.; et al. Seroprevalence and risk factors associated with bovine brucellosis in the Potohar Plateau, Pakistan. BMC Res. Notes 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Radostits, O.M.; Gay, C.C.; Blood, D.C.; Hinchclif, K.W. Veterinary Medicine, Textbook of the Disease of Cattle, Sheep, Pigs, Goats and Horses, 10th ed.; ELBS Baillière-Tindall: London, UK, 2007; pp. 963–994. [Google Scholar]
- Radostits, O.M.; Gay, C.C.; Blood, D.C.; Hinchcliff, K.W. Veterinary Medicine, A Textbook of Diseases of Cattle, Sheep, Goats, Pigs and Horses, 9th ed.; ELBS Baillière-Tindall: London, UK, 2000; pp. 870–871. [Google Scholar]
- Harbord, R.; Whiting, P.M. Metandi: Meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata J. 2009, 9, 211–229. [Google Scholar] [CrossRef]
- Muendo, E.; Mbatha, P.; Macharia, J.; Abdoel, T.; Janszen, P.; Pastoor, R.; Smits, H. Infection of cattle in Kenya with Brucella abortus biovar 3 and Brucella melitensis biovar 1 genotypes. Trop. Anim. Health Prod. 2011, 44, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Tsegay, A.; Tuli, G.; Kassa, T.; Kebede, N. Seroprevalence and risk factors of brucellosis in small ruminants slaughtered at Debra Ziet and Mojdjo export abattoirs, Ethiopia. J. Infect. Dev. Countr. 2014, 9, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, R.; Waheed, U.; Ali, T.; Gopaul, K.K.; Dainty, A.C.; Muchowski, J.K.; Koylass, M.S.; Brew, S.D.; Perrett, L.L.; Whatmore, A.M.; et al. Serological and nucleic acid based detection of brucellosis in livestock species and molecular characterization of Brucella melitensis strains isolated from Pakistan. Int. J. Agric. Biol. 2016, 18, 311–318. [Google Scholar] [CrossRef]
- Khan, M.Z.; Usman, T.; Sadique, U.; Qureshi, M.S.; Hassan, M.F.; Shahid, M.; Khan, A. Molecular characterization of Brucella abortus and Brucella melitensis in cattle and humans at the North West of Pakistan. Pak. Vet. J. 2017, 37, 360–363. [Google Scholar]
- Cao, X.; Shang, Y.; Liu, Y.; Li, Z.; Jing, Z. Genetic Characterization of Animal Brucella Isolates from Northwest Region in China. BioMed Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, M.; Shome, R.; Balamurugan, V.; Shome, B.R.; NarayanaRao, K.; Vivekananda; Isloor, S.; Prabhudas, K. Molecular typing of Brucella species isolates from livestock and human. Trop. Anim. Health Prod. 2012, 44, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Pishva, E.; Salehi, R.; Hoseini, A.; Kargar, A.; Taba, F.E.; Hajiyan, M.; Fadaei, R.; Ramezanpour, J. Molecular typing of Brucella species isolates from Human and livestock bloods in Isfahan province. Adv. Biomed Res. 2015, 4, 104. [Google Scholar] [PubMed]
- Matope, G.; Bhebhe, E.; Muma, J.; Oloya, J.; Madekurozwa, R.; Lund, A.; Skjerve, E. Seroprevalence of brucellosis and its associated risk factors in cattle from small holder’s dairy farm in Zimbabwe. Trop. Anim. Health Pro. 2011, 43, 975–982. [Google Scholar] [CrossRef]
- Dehkordi, F.S.; Saberian, S.; Momtaz, H. Detection and segregation of Brucella abortus and Brucella melitensis in aborted bovine, ovine, caprine, Buffalo and camelid Fetuses by application of conventional and real time PCR. Thai J. Vet. Med. 2013, 42, 13–20. [Google Scholar]
| Primers and Probes | Brucella genus | Brucella abortus | Brucella melitensis |
|---|---|---|---|
| Forward | 5′GCTCGGTTGCCAATATCAATGC3′ | 5′GCGGCTTTTCTATCACGGTATTC3′ | 5′AACAAGCGGCACCCCTAAAA3′ |
| Reverse | 5′GGGTAAAGCGTCGCCAGAAG3′ | 5′CATGCGCTATGATCTGGTTACG3′ | 5′CATGCGCTATGATCTGGTTACG3′ |
| Probe | 5′AAATCTTCCACCTTGCCCCTTGCCATCA3′ | 5′CGCTCATGCTCGCCAGACTTCAATG3′ | 5′CAGGAGTGTTTCGGCTCAGAATAATCCACA3′ |
| 5′Flourophore/3′ Quencher | 6-Fam/BHQ_1 | HEX/BHQ_1 | Cy5/BHQ_2 |
| Target Gene | bcsp31 | IS711 element downstream of the alkB | IS711 element downstream of BMEI1162 |
| Variables | Categories | Sample Examined | Sample Positive (%) | Statistical Analysis | |
|---|---|---|---|---|---|
| Chi Square Value | P-Value | ||||
| District | Faisalabad | 150 | 17 (11.3) | 39.947 | 0.000 |
| Okara | 169 | 7 (4.1) | |||
| Lahore | 152 | 3 (2.0) | |||
| Kasur | 612 | 8 (1.3) | |||
| Tehsil | Chunian | 12 | 2 (16.7) | 49.181 | 0.000 |
| Faisalabad | 150 | 17 (11.3) | |||
| Renala Khurd | 169 | 7 (4.1) | |||
| Lahore | 152 | 3 (2.0) | |||
| Pattoki | 600 | 6(1.0) | |||
| Variables | Factors | Sample Examined | Positive for RBPT (%) | Statistical Analysis | |
|---|---|---|---|---|---|
| Chi Square Value | P-Value | ||||
| Species | Buffalo | 234 | 12 (5.1) | 5.813 | 0.121 |
| Cow | 206 | 8 (3.8) | |||
| Sheep | 203 | 7 (3.4) | |||
| Goat | 440 | 8 (1.8) | |||
| Gender | Male | 148 | 11 (7.4) | 9.673 | 0.000 |
| Female | 935 | 24 (2.5) | |||
| Breed | Nili Ravi | 234 | 12 (5.1) | 5.815 | 0.213 |
| Rojhan | 206 | 8 (3.8) | |||
| Kajli | 203 | 7 (3.4) | |||
| Teddy | 52 | 1 (1.9) | |||
| Beetle | 388 | 7 (1.8) | |||
| Age | Adult | 1065 | 35 (3.3) | 0.611 | 0.434 |
| Young | 18 | 0 (0) | |||
| Herd size (n = 149) | ≤10 | 96 | 5 (5.2) | 13.803 | 0.000 |
| >10 | 53 | 14 (26.4) | |||
| Abortion history | No | 950 | 23 (2.4) | 16.258 | 0.000 |
| Yes | 133 | 12 (9.0) | |||
| Have their own Sire | No | 368 | 20 (5.4) | 8.650 | 0.000 |
| Yes | 715 | 15 (2.1) | |||
| Cleaning up the coral in herd (n = 149) | No | 37 | 3 (8.1) | 0.954 | 0.329 |
| Yes | 112 | 16 (14.2) | |||
| Body condition | Weak | 20 | 0 (0) | 14.304 | 0.001 |
| Medium | 861 | 20 (2.3) | |||
| Healthy | 202 | 15 (7.4) | |||
| Stock replacement in herd (n = 149) | Self-reared | 130 | 13 (10.0) | 6.938 | 0.008 |
| Purchased | 19 | 6 (31.5) | |||
| Variables | Binary Logistic Regression | P-Value | ||
|---|---|---|---|---|
| OR | (95% CI) | |||
| Lower | Upper | |||
| District | 1.484 | 0.987 | 2.231 | 0.058 |
| Tehsil | 1.407 | 0.976 | 2.029 | 0.068 |
| Herd Size | 0.639 | 0.147 | 2.771 | 0.550 |
| Species | 0.871 | 0.518 | 1.465 | 0.604 |
| Gender | 0.918 | 0.286 | 2.954 | 0.886 |
| Breed | 1.113 | 0.788 | 1.574 | 0.543 |
| Age | 21763518.14 | 0.000 | 0.999 | |
| Abortion history | 4.603 | 1.853 | 11.430 | 0.001 |
| Have their own sire | 0.230 | 0.086 | 0.612 | 0.003 |
| Cleaning up the coral | 0.469 | 0.094 | 2.338 | 0.356 |
| Body condition | 1.440 | 0.950 | 2.181 | 0.085 |
| Stock replacement | 1.542 | 0.309 | 7.685 | 0.597 |
| Case No. | Host | Serological Assay | PCR | ||
|---|---|---|---|---|---|
| RBPT | Brucella Genus | B. abortus | B. melitensis | ||
| 94 | Goat | + | + | − | + |
| 160 | Goat | + | + | − | + |
| 167 | Goat | + | + | − | + |
| 192 | Goat | + | + | − | + |
| 198 | Sheep | + | + | − | + |
| 278 | Goat | + | + | − | + |
| 309 | Goat | + | + | − | + |
| 315 | Goat | + | + | − | + |
| 316 | Goat | + | + | − | + |
| 319 | Sheep | + | + | − | + |
| 320 | Sheep | + | + | − | + |
| 322 | Sheep | + | + | − | + |
| 324 | Buffalo | + | + | − | + |
| 334 | Buffalo | + | + | − | + |
| 342 | Cow | + | + | − | + |
| 348 | Cow | + | + | − | + |
| 350 | Cow | + | + | − | + |
| 440 | Sheep | + | + | − | + |
| 456 | Buffalo | + | + | − | + |
| 463 | Buffalo | + | + | − | + |
| 470 | Buffalo | + | + | − | + |
| 473 | Buffalo | + | + | − | + |
| 479 | Cow | + | + | − | + |
| 489 | Cow | + | + | − | + |
| 491 | Cow | + | + | − | + |
| 499 | Cow | + | + | − | + |
| 503 | Buffalo | + | + | − | + |
| 504 | Buffalo | + | + | − | + |
| 506 | Buffalo | + | + | − | + |
| 513 | Buffalo | + | + | − | + |
| 517 | Buffalo | + | + | − | + |
| 553 | Buffalo | + | + | − | + |
| 569 | Cow | + | + | − | + |
| 578 | Sheep | + | + | − | + |
| 580 | Sheep | + | + | − | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, U.; Ali, S.; Khan, T.M.; El-Adawy, H.; Melzer, F.; Khan, A.U.; Iftikhar, A.; Neubauer, H. Seroepidemiology and the Molecular Detection of Animal Brucellosis in Punjab, Pakistan. Microorganisms 2019, 7, 449. https://doi.org/10.3390/microorganisms7100449
Saeed U, Ali S, Khan TM, El-Adawy H, Melzer F, Khan AU, Iftikhar A, Neubauer H. Seroepidemiology and the Molecular Detection of Animal Brucellosis in Punjab, Pakistan. Microorganisms. 2019; 7(10):449. https://doi.org/10.3390/microorganisms7100449
Chicago/Turabian StyleSaeed, Usama, Shahzad Ali, Tahir Mahmood Khan, Hosny El-Adawy, Falk Melzer, Aman Ullah Khan, Anam Iftikhar, and Heinrich Neubauer. 2019. "Seroepidemiology and the Molecular Detection of Animal Brucellosis in Punjab, Pakistan" Microorganisms 7, no. 10: 449. https://doi.org/10.3390/microorganisms7100449
APA StyleSaeed, U., Ali, S., Khan, T. M., El-Adawy, H., Melzer, F., Khan, A. U., Iftikhar, A., & Neubauer, H. (2019). Seroepidemiology and the Molecular Detection of Animal Brucellosis in Punjab, Pakistan. Microorganisms, 7(10), 449. https://doi.org/10.3390/microorganisms7100449








