Performance of Winter Wheat Cultivars Grown Organically and Conventionally with Focus on Fusarium Head Blight and Fusarium Trichothecene Toxins
Abstract
1. Introduction
2. Material and Methods
2.1. Field Experiments
2.2. Analysis of Mineral Elements in Soil
2.3. Seed Quality
2.4. Fusarium DNA Quantification with Real-Time PCR
2.4.1. Isolation of Total DNA from Grain
2.4.2. Preparation of Standard Curve
2.4.3. Preparation of DNA Samples for Real-Time PCR
2.4.4. Real-Time PCR Reaction Conditions
2.5. Analysis of Trichothecenes
2.6. Chemical Analysis of Ergosterol
2.7. Statistics
3. Results
3.1. Concentration of Mineral Elements in Soil
3.2. Phenotypic Data and Fungal Diseases
3.3. Characteristic of Seed Germination
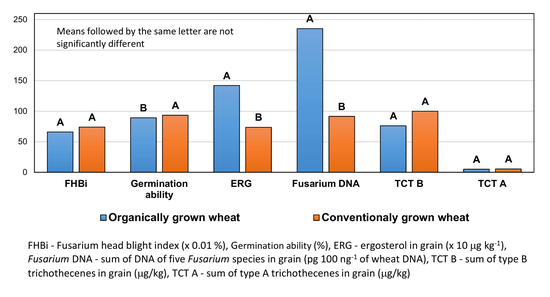
3.4. Concentration of Ergosterol and Trichothecenes
3.5. Fusarium Species
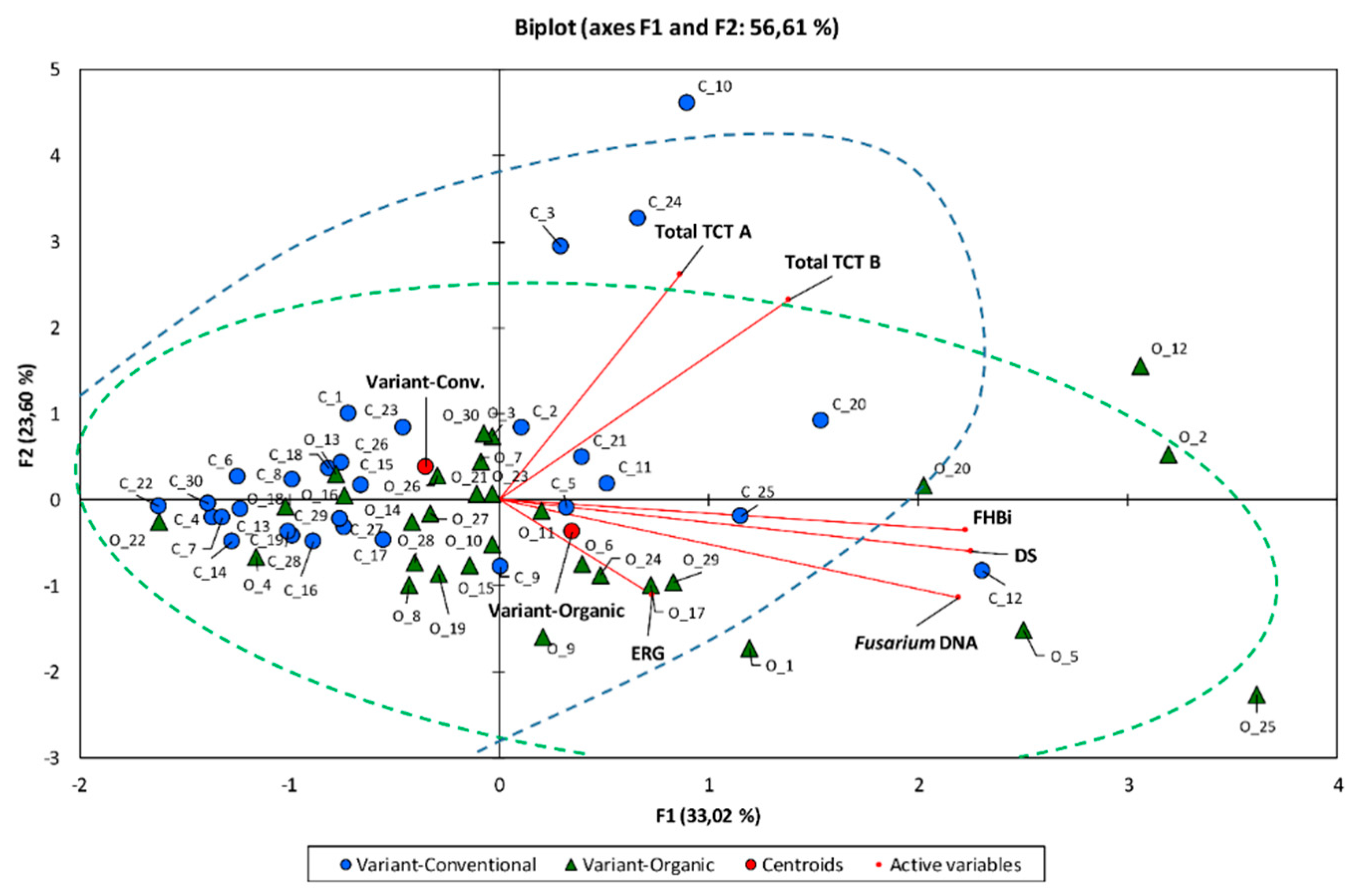
3.6. Correlation Between Experimental Components
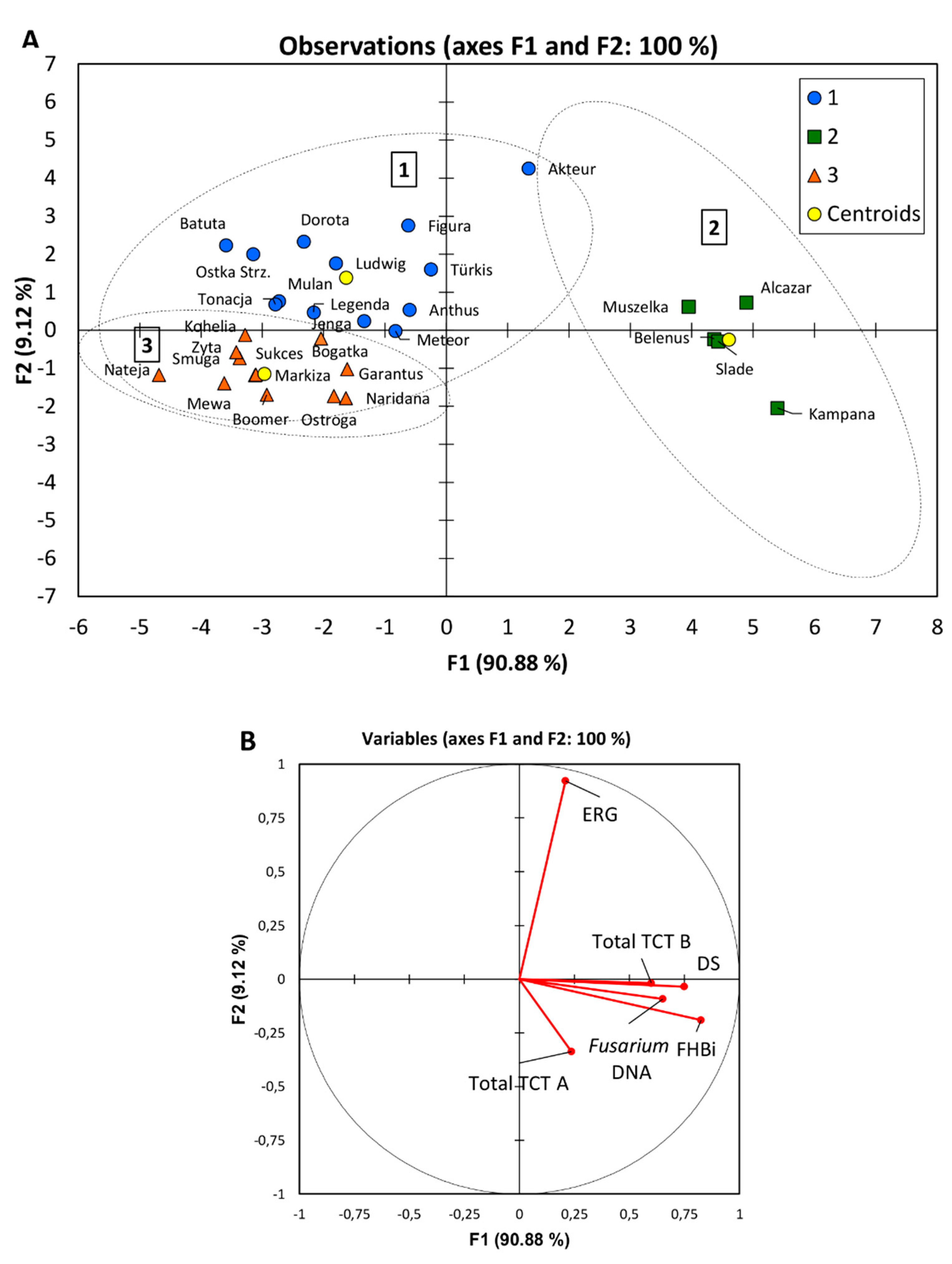
3.7. Multivariate Principal Component Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Anonym Raport o Stanie Rolnictwa Ekologicznego w Polsce w Latach 2015–2016 [The Report on Organic Farming in Poland in 2015–2016]; Agricultural and Food Quality Inspection (GIJHARS): Warszawa, Poland, 2017.
- Borgen, A. Strategies for regulation of seed borne diseases in organic farming. Seed Test. Int. ISTA News Bull. 2004, 127, 19–21. [Google Scholar]
- Wolfe, M.S.; Baresel, J.P.; Desclaux, D.; Goldringer, I.; Hoad, S.; Kovacs, G.; Löschenberger, F.; Miedaner, T.; Østergård, H.; Lammerts Van Bueren, E.T. Developments in breeding cereals for organic agriculture. Euphytica 2008, 163, 323–346. [Google Scholar] [CrossRef]
- Letourneau, D.; van Bruggen, A.H.C. Crop protection in organic agriculture. In Organic Agriculture: A Global Perspective; Kristiansen, P., Acram, T., Reganold, J., Eds.; CABI Publishing: Wallingford, UK, 2006; pp. 93–121. ISBN 0 643 09090 8. [Google Scholar]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Bottalico, A. Fusarium diseases of cereals: Species complex and related mycotoxin profiles, in Europe. J. Plant Pathol. 1998, 80, 85–103. [Google Scholar]
- Wagacha, J.M.; Muthomi, J.W. Fusarium culmorum: Infection process, mechanisms of mycotoxin production and their role in pathogenesis in wheat. Crop. Prot. 2007, 26, 877–885. [Google Scholar] [CrossRef]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Dweba, C.C.; Figlan, S.; Shimelis, H.A.; Motaung, T.E.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T.J. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop. Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Juan, C.; Ritieni, A.; Mañes, J. Occurrence of Fusarium mycotoxins in Italian cereal and cereal products from organic farming. Food Chem. 2013, 141, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Vrček, I.V.; Čepo, D.V.; Rašić, D.; Peraica, M.; Žuntar, I.; Bojić, M.; Mendaš, G.; Medić-Šarić, M. A comparison of the nutritional value and food safety of organically and conventionally produced wheat flours. Food Chem. 2014, 143, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Malmauret, L.; Parent-Massin, D.; Hardy, J.-L.; Vergey, P. Contaminants in organic and conventional foodstuffs s in France. Food Addit. Contam. 2002, 19, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Marx, H.; Gedek, B.; Kollarczik, B. Vergleichende Untersuchungen zum mykotoxikologischen Status von ökologisch und konventionell angebautem Getreide. Z. Lebensm. Unters. Forsch. 1995, 201, 83–86. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, A.J.; Whittingham, M.J. Birds select conventional over organic wheat when given free choice. J. Sci. Food Agric. 2010, 90, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Champeil, A.; Fourbet, J.F.; Doré, T.; Rossignol, L. Influence of cropping system on Fusarium head blight and mycotoxin levels in winter wheat. Crop. Prot. 2004, 23, 531–537. [Google Scholar] [CrossRef]
- Harcz, P.; De Temmerman, L.; De Voghel, S.; Waegeneers, N.; Wilmart, O.; Vromman, V.; Schmit, J.F.; Moons, E.; Van Peteghem, C.; De Saeger, S.; et al. Contaminants in organically and conventionally produced winter wheat (Triticum aestivum) in Belgium. Food Addit. Contam. 2007, 24, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Mäder, P.; Hahn, D.; Dubois, D.; Gunst, L.; Alföldi, T.; Bergmann, H.; Oehme, M.; Amadò, R.; Schneider, H.; Graf, U.; et al. Wheat quality in organic and conventional farming: Results of a 21 year field experiment. J. Sci. Food Agric. 2007, 87, 1826–1835. [Google Scholar] [CrossRef]
- Magkos, F.; Arvaniti, F.; Zampelas, A. Organic Food: Buying More Safety or Just Peace of Mind? A Critical Review of the Literature. Crit. Rev. Food Sci. Nutr. 2006, 46, 23–56. [Google Scholar] [CrossRef] [PubMed]
- Góral, T.; Stuper-Szablewska, K.; Buśko, M.; Boczkowska, M.; Walentyn-Góral, D.; Wiśniewska, H.; Perkowski, J. Relationships between genetic diversity and Fusarium toxin profiles of winter wheat cultivars. Plant Pathol. J. 2015, 31, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska-Kolodziejczak, A.; Stuper-Szablewska, K.; Kulik, T.; Busko, M.; Rissmann, I.; Wiwart, M.; Perkowski, J. Concentration of fungal metabolites, phenolic acids and metals in mixtures of cereals grown in organic and conventional farms. J. Anim. Feed Sci. 2016, 25, 74–81. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing 2019; International Seed Testing Association—ISTA: Bassersdorf, Switzerland, 2019; ISSN 2310-3655. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus (Madison) 1990, 12, 13–15. [Google Scholar]
- Turner, A.S.; Lees, A.K.; Rezanoor, H.N.; Nicholson, P. Refinement of PCR-detection of Fusarium avenaceum and evidence from DNA marker studies for phenetic relatedness to Fusarium tricinctum. Plant Pathol. 1998, 47, 278–288. [Google Scholar] [CrossRef]
- Nicholson, P.; Simpson, D.R.; Weston, G.; Rezanoor, H.N.; Lees, A.K.; Parry, D.W.; Joyce, D.; Centre, J.I.; Lane, C. Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiol. Mol. Plant Pathol. 1998, 53, 17–37. [Google Scholar] [CrossRef]
- Wilson, A.; Simpson, D.; Chandler, E.; Jennings, P.; Nicholson, P. Development of PCR assays for the detection and differentiation of Fusarium sporotrichoides and Fusarium langsethiae. FEMS Microbiol. Lett. 2004, 233, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.W.; Nicholson, P. Development of a PCR assay to detect Fusarium poae in wheat. Plant Pathol. 1996, 45, 383–391. [Google Scholar] [CrossRef]
- Perkowski, J.; Kiecana, I.; Kaczmarek, Z. Natural occurrence and distribution of Fusarium toxins in contaminated barley cultivars. Eur. J. Plant Pathol. 2003, 109, 331–339. [Google Scholar] [CrossRef]
- Young, J.C. Microwave-assisted extraction of the fungal metabolite ergosterol and total fatty acids. J. Agric. Food Chem. 1995, 43, 2904–2910. [Google Scholar] [CrossRef]
- Perkowski, J.; Wiwart, M.; Busko, M.; Laskowska, M.; Berthiller, F.; Kandler, W.; Krska, R. Fusarium toxins and total fungal biomass indicators in naturally contaminated wheat samples from north-eastern Poland in 2003. Food Addit. Contam. 2007, 24, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Perkowski, J.; Buśko, M.; Stuper, K.; Kostecki, M.; Matysiak, A.; Szwajkowska-Michałek, L. Concentration of ergosterol in small-grained naturally contaminated and inoculated cereals. Biologia 2008, 63, 542–547. [Google Scholar] [CrossRef]
- Romero, A.; Munévar, F.; Cayón, G. Silicon and plant diseases. A review. Agron. Colomb. 2011, 29, 473–480. [Google Scholar]
- Dorneles, K.R.; Dallagnol, L.J.; Pazdiora, P.C.; Rodrigues, F.A.; Deuner, S. Silicon potentiates biochemical defense responses of wheat against tan spot. Physiol. Mol. Plant Pathol. 2017, 97, 69–78. [Google Scholar] [CrossRef]
- Wasilkowski, D.; Mrozik, A.; Piotrowska-Seget, Z.; Krzyzak, J.; Pogrzeba, M.; Plaza, G. Changes in enzyme activities and microbial community structure in heavy metal-contaminated soil under in situ aided phytostabilization. Clean Soil Air Water 2014, 42, 1618–1625. [Google Scholar] [CrossRef]
- Ahmad, I.; Hayat, S.; Ahmad, A.; Inam, A.; Samiullah, I. Effect of heavy metal on survival of certain groups of indigenous soil microbial population. J. Appl. Sci. Environ. Manag. 2005, 9, 115–121. [Google Scholar]
- Martyniuk, S.; Martyniuk, M. Occurrence of Azotobacter spp. in some Polish soils. Polish J. Environ. Stud. 2003, 12, 371–374. [Google Scholar]
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Mason, K.E.; van Agtmaal, M.; et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Góral, T.; Walentyn-Góral, D. Variation for resistance to Fusarium head blight in winter and spring wheat cultivars studied in 2009–2016. Short communication. Biul. IHAR 2018, 284, 3–12. [Google Scholar]
- Małuszyńska, E.; Mańkowski, D.R. Seed sowing value and response to drought stress of organic and conventional oat (Avena sativa L.) seeds during 5 years of storage. Acta Sci. Pol. Agric. 2016, 15, 27–36. [Google Scholar]
- Wiwart, M.; Perkowski, J.; Budzyński, M.; Suchowilska, E.; Buśko, M.; Matysiak, A. Concentrations of ergosterol and trichothecenes in the grains of three Triticum species. Czech J. Food Sci. 2011, 29, 430–440. [Google Scholar] [CrossRef]
- Buśko, M.; Stuper, K.; Jeleń, H.; Góral, T.; Chmielewski, J.; Tyrakowska, B.; Perkowski, J. Comparison of volatiles profile and contents of trichothecenes group B, ergosterol, and ATP of bread wheat, durum wheat, and triticale grain naturally contaminated by mycobiota. Front. Plant Sci. 2016, 7, 1243. [Google Scholar] [CrossRef]
- Karlsson, I.; Friberg, H.; Kolseth, A.K.; Steinberg, C.; Persson, P. Organic farming increases richness of fungal taxa in the wheat phyllosphere. Mol. Ecol. 2017, 26, 3424–3436. [Google Scholar] [CrossRef]
- Lori, M.; Symnaczik, S.; Mäder, P.; De Deyn, G.; Gattinger, A. Organic farming enhances soil microbial abundance and activity—A meta-analysis and meta-Regression. PLoS ONE 2017, 12, 1–25. [Google Scholar] [CrossRef]
- Sapkota, R.; Knorr, K.; Jørgensen, L.N.; O’Hanlon, K.A.; Nicolaisen, M. Host genotype is an important determinant of the cereal phyllosphere mycobiome. New Phytol. 2015, 207, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.; van der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 1–14. [Google Scholar]
- Lukanowski, A.; Sadowski, C. Fusarium langsethiae on kernels of winter wheat in Poland—Occurrence and mycotoxigenic abilities. Cereal Res. Commun. 2008, 36, 453–457. [Google Scholar] [CrossRef]
- Stenglein, S.A. Fusarium poae: A pathogen that needs more attention. J. Plant Pathol. 2009, 91, 25–36. [Google Scholar]
- Xu, X.M.; Parry, D.W.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Edwards, S.G.; Cooke, B.M.; Doohan, F.M.; Brennan, J.M.; Moretti, A.; et al. Predominance and association of pathogenic fungi causing Fusarium ear blightin wheat in four European countries. Eur. J. Plant Pathol. 2005, 112, 143–154. [Google Scholar] [CrossRef]
- Reischer, G.H.; Lemmens, M.; Farnleitner, A.; Adler, A.; Mach, R.L. Quantification of Fusarium graminearum in infected wheat by species specific real-time PCR applying a TaqMan probe. J. Microbiol. Methods 2004, 59, 141–146. [Google Scholar] [CrossRef]
- Nicholson, P.; Turner, A.S.; Edwards, S.G.; Bateman, G.L.; Morgan, L.W.; Parry, D.W.; Marshall, J.; Nuttall, M. Development of stem-base pathogens on different cultivars of winter wheat determined by quantitative PCR. Eur. J. Plant Pathol. 2002, 108, 163–177. [Google Scholar] [CrossRef]
- Justesen, A.F.; Hansen, H.J.; Pinnschmidt, H.O. Quantification of Pyrenophora graminea in barley seed using real-time PCR. Eur. J. Plant Pathol. 2008, 122, 253–263. [Google Scholar] [CrossRef]
- Váňová, M.; Klem, K.; Míša, P.; Matušinsky, P.; Hajšlová, J.; Lancová, K. The content of Fusarium mycotoxins, grain yield and quality of winter wheat cultivars under organic and conventional cropping systems. Plant Soil Environ. 2008, 54, 395–402. [Google Scholar] [CrossRef]
- Ibáñez-Vea, M.; González-Peñas, E.; Lizarraga, E.; López De Cerain, A. Co-occurrence of aflatoxins, ochratoxin A and zearalenone in barley from a northern region of Spain. Food Chem. 2012, 132, 35–42. [Google Scholar] [CrossRef]
- Edwards, S.G. Fusarium mycotoxin content of UK organic and conventional barley. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2009, 26, 1185–1190. [Google Scholar] [CrossRef]
- Edwards, S.G. Fusarium mycotoxin content of UK organic and conventional oats. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2009, 26, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Remža, J.; Lacko-Bartošová, M.; Kosík, T. Fusarium mycotoxin content of Slovakian organic and conventional cereals. J. Cent. Eur. Agric. 2016, 17, 164–175. [Google Scholar] [CrossRef]
- Brodal, G.; Hofgaard, I.S.; Eriksen, G.S.; Bernhoft, A.; Sundheim, L. Mycotoxins in organically versus conventionally produced cereal grains and some other crops in temperate regions. World Mycotoxin J. 2016, 9, 755–770. [Google Scholar] [CrossRef]
- Hoogenboom, L.A.P.; Bokhorst, J.G.; Northolt, M.D.; van de Vijver, L.P.L.; Broex, N.J.G.; Mevius, D.J.; Meijs, J.A.C.; Van der Roest, J. Contaminants and microorganisms in Dutch organic food products: A comparison with conventional products. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, A.; Clasen, P.-E.; Kristoffersen, A.B.; Torp, M. Less Fusarium infestation and mycotoxin contamination in organic than in conventional cereals. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2010, 27, 842–852. [Google Scholar] [CrossRef]
- Meister, U. Fusarium toxins in cereals of integrated and organic cultivation from the Federal State of Brandenburg (Germany) harvested in the years 2000–2007. Mycotoxin Res. 2009, 25, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F.; Moser, G.; Müller, H.; Berg, G. Functional and structural microbial diversity in organic and conventional viticulture: organic farming benefits natural biocontrol agents. Appl. Environ. Microbiol. 2011, 77, 2188–2191. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, A.; Torp, M.; Clasen, P.-E.-E.; Løes, A.-K.; Kristoffersen, A.B. Influence of agronomic and climatic factors on Fusarium infestation and mycotoxin contamination of cereals in Norway. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2012, 29, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, I.; Moretti, A.; Giorni, P.; Brera, C.; Battilani, P. Organic vs conventional farming: differences in infection by mycotoxin-producing fungi on maize and wheat in Northern and Central Italy. Crop. Prot. 2015, 72, 22–30. [Google Scholar] [CrossRef]
- Newton, A.C.; Guy, D.C.; Preedy, K. Wheat cultivar yield response to some organic and conventional farming conditions and the yield potential of mixtures. J. Agric. Sci. 2017, 155, 1045–1060. [Google Scholar] [CrossRef]
- Mason, H.E.; Spaner, D. Competitive ability of wheat in conventional and organic management systems: a review of the literature. Can. J. Plant Sci. 2006, 86, 333–343. [Google Scholar] [CrossRef]
- Osman, A.M.; Almekinders, C.J.M.; Struik, P.C.; Lammerts van Bueren, E.T. Adapting spring wheat breeding to the needs of the organic sector. NJAS Wageningen J. Life Sci. 2016, 76, 55–63. [Google Scholar] [CrossRef]
- Scholten, O.E.; Steenhuis-Broers, G.; Timmermsns, B.; Osman, A. Screening for resistance to Fusarium head blight in organic wheat production. In Proceedings of the COST SUSVAR Fusarium Workshop: Fusarium Diseases in Cereals—Potential Impact from Sustainable Cropping Systems, Velence, Hungary, 1–2 June 2007; Vogelgsang, S., Jalli, M., Kovacs, G., Vida, G., Eds.; Risø National Laboratory: Roskilde, Denmark, 2007; pp. 20–23. [Google Scholar]
| No. | Cultivar | No. | Cultivar | No. | Cultivar | |||
|---|---|---|---|---|---|---|---|---|
| 1 | Akteur | MS * | 11 | Jenga | MS | 21 | Naridana | MS |
| 2 | Alcazar | S | 12 | Kampana | S | 22 | Nateja | R |
| 3 | Anthus | MS | 13 | Kohelia | MR | 23 | Ostka Strzelecka | MS |
| 4 | Batuta | MS | 14 | Legenda | MR | 24 | Ostroga | MR |
| 5 | Belenus | MS | 15 | Ludwig | MS | 25 | Slade | MS |
| 6 | Bogatka | MR | 16 | Markiza | MS | 26 | Smuga | S |
| 7 | Boomer | MR | 17 | Meteor | MS | 27 | Sukces | MR |
| 8 | Dorota | MR | 18 | Mewa | MS | 28 | Tonacja | MR |
| 9 | Figura | MS | 19 | Mulan | MS | 29 | Türkis | MS |
| 10 | Garantus | MS | 20 | Muszelka | S | 30 | Zyta | MR |
| Fusarium Species | Primer Name | Sequence (5′–3′) | Source |
|---|---|---|---|
| F. avenaceum | JIAf | GCTAATTCTTAACTTACTAGGGGCC | [24] |
| JIAr | CTGTAATAGGTTATTTACATGGGCG | ||
| F. culmorum | Fc01F | ATGGTGAACTCGTCGTGGC | [25] |
| Fc01R | CCCTTCTTACGCCAATCTCG | ||
| F. graminearum | Fg16F | CTCCGGATATGTTGCGTCAA | [25] |
| Fg16R | GGTAGGTATCCGACATGGCAA | ||
| F. langsethiae | FlangF3 | CAAAGTTCAGGGCGAAAACT | [26] |
| LanspoR1 | TACAAGAAGACGTGGCGATAT | ||
| F. poae: | Fp82F | CAAGCAAACAGGCTCTTCACC | [27] |
| Fp82R | TGTTCCACCTCAGTGACAGGTT | ||
| F. sporotrichioides | FsporF1 | CGCACAACGCAAACTCATC | [26] |
| LanspoR1 | TACAAGAAGACGTGGCGATAT |
| Variant | Heading (Days from 1st May) | Flowering (Days from 1st May) | Plant Height (cm) | Grain Yield Per Plot (kg) | FHBi (%) |
|---|---|---|---|---|---|
| Conventional | |||||
| Mean | 29.7 b | 31.6 b | 97.8 a | 5.0 a | 0.74 a |
| Std. deviation | 2.53 | 2.39 | 12.22 | 0.89 | 1.00 |
| Organic | |||||
| Mean | 28.0 a | 29.8 a | 99.0 a | 5.1 a | 0.66 a |
| Std. deviation | 2.39 | 2.55 | 10.39 | 0.76 | 0.77 |
| Variant | Germination Energy (%) | Germination Capacity (%) | Abnormal Seedlings (%) | Dead Seeds (%) | Fresh, Ungerminated Seeds (%) |
|---|---|---|---|---|---|
| Conventional | |||||
| Mean | 87.0 b *** | 93.4 b *** | 3.6 a ** | 2.5 a ** | 0.6 a |
| Std. deviation | 10.86 | 3.81 | 2.26 | 1.79 | 0.63 |
| Organic | |||||
| Mean | 63.2 a *** | 89.3 a *** | 5.3 b ** | 4.2 b ** | 1.2 a |
| Std. deviation | 24.00 | 4.81 | 2.10 | 3.01 | 1.91 |
| Variant | ERG | DON | FUS-X | 3-AcDON | 15-AcDON | NIV | Total TCT B |
|---|---|---|---|---|---|---|---|
| Conventional | |||||||
| Mean | 0.74 a ** | 84.8 a | 0.9 a | 7.3 b *** | 1.5 a | 5.6 a | 100.0 a |
| Range | 0.26–1.85 | 5.8–444.4 | 0–11.6 | 2.3–30.3 | 0–14.3 | 0–19.0 | 8.9–460.2 |
| Std. deviation | 0.39 | 97.3 | 2.4 | 5.9 | 2.6 | 5.0 | 101.4 |
| Organic | |||||||
| Mean | 1.42 b ** | 63.7 a | 0.9 a | 3.1 a *** | 1.1 a | 7.4 a | 76.2 a |
| Range | 0.26–3.46 | 2.2–348.4 | 0–2.9 | 0–6.2 | 0–3.3 | 0–29.5 | 10.1–384.5 |
| Std. deviation | 0.87 | 86.2 | 1.0 | 1.5 | 1.2 | 7.3 | 93.6 |
| Variant | F. a. DNA | F. c. DNA | F. g. DNA | F. p. DNA | F. sp. DNA | Fusarium DNA |
|---|---|---|---|---|---|---|
| Conventional | ||||||
| Mean | 10.8 a *** | 23.3 a | 22.7 a *** | 34.7 a *** | 15,1 a * | 106.6 a *** |
| Range | 0–106.7 | 0–346.2 | 0.7–79.5 | 9.4–132.4 | 0–113.3 | 15.4–405.2 |
| Std. deviation | 19.61 | 63.14 | 21.58 | 24.82 | 29.10 | 90.91 |
| Organic | ||||||
| Mean | 30.2 b *** | 41.1 a | 67.0 b *** | 98.2 b *** | 50.5 b * | 285.7 b *** |
| Range | 0.2–184.6 | 0–415.5 | 0.5–280.0 | 14.1–222.0 | 0–350.0 | 15.3–1205.8 |
| Std. deviation | 44.88 | 85.73 | 62.92 | 56.44 | 94.3 | 244.83 |
| Variables | GE | GC | Dead Seeds | AS | FUS | FHBi | ERG | Total TCT B | Total TCT A | F. a. DNA | F. c. DNA | F. g. DNA | F. p. DNA | F. sp. DNA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Final count | 0.584 *** | |||||||||||||
| Dead seeds | −0.419 * | −0.803 *** | ||||||||||||
| AS | −0.553 ** | −0.777 *** | 0.436 * | |||||||||||
| FUS | −0.112 | −0.320 | 0.048 | 0.012 | ||||||||||
| FHBi | −0.125 | −0.476 ** | 0.545 ** | 0.219 | 0.058 | |||||||||
| ERG | −0.022 | 0.222 | −0.111 | −0.190 | −0.162 | −0.064 | ||||||||
| Total TCT B | −0.019 | −0.431 * | 0.369 * | 0.312 | 0.160 | 0.290 | −0.163 | |||||||
| Total TCT A | −0.216 | −0.252 | 0.185 | 0.373 * | 0.044 | 0.096 | 0.011 | 0.573 *** | ||||||
| F. a DNA | 0.258 | −0.221 | 0.412 * | 0.095 | −0.170 | 0.430 * | 0.016 | − | ||||||
| F. c DNA | −0.052 | −0.215 | 0.168 | 0.002 | 0.289 | 0.011 | −0.325 | 0.174 | − | −0.013 | ||||
| F. g DNA | −0.204 | −0.502 ** | 0.661 *** | 0.288 | 0.010 | 0.586 *** | −0.104 | 0.501 ** | − | 0.583 *** | 0.347 | |||
| F. p DNA | 0.259 | −0.169 | 0.393 * | −0.020 | 0.010 | 0.388 * | −0.285 | 0.202 | 0.108 | 0.531 ** | 0.053 | 0.497 ** | ||
| F. sp DNA | 0.181 | 0.016 | 0.197 | 0.125 | −0.564 *** | 0.193 | −0.270 | − | 0.162 | 0.451 * | −0.241 | 0.215 | 0.447 * | |
| Total DNA | 0.124 | −0.297 | 0.565 | 0.040 | −0.107 | 0.481 ** | −0.267 | 0.201 | 0.063 | 0.657 *** | 0.343 | 0.712 *** | 0.762 *** | 0.515 ** |
| Variables | GE | GC | Dead Seeds | AS | FUS | FHBi | ERG | Total TCT B | Total TCT B | F. a. DNA | F. c. DNA | F. g. DNA | F. p. DNA | F. sp. DNA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Final count | 0.639 *** | |||||||||||||
| Dead seeds | −0.511 ** | −0.874 *** | ||||||||||||
| AS | −0.059 | −0.327 | 0.203 | |||||||||||
| FUS | −0.538 ** | −0.579 *** | 0.470 ** | −0.346 | ||||||||||
| FHBi | −0.274 | −0.533 ** | 0.456 * | 0.360 | 0.002 | |||||||||
| ERG | −0.083 | −0.056 | 0.080 | 0.257 | −0.152 | 0.036 | ||||||||
| Total TCT B | −0.104 | −0.219 | 0.207 | 0.309 | −0.158 | 0.173 | 0.182 | |||||||
| Total TCT A | 0.097 | 0.027 | 0.158 | 0.056 | −0.225 | 0.315 | −0.072 | 0.162 | ||||||
| F. a DNA | −0.023 | −0.479 ** | 0.557 ** | 0.357 | 0.117 | 0.511 ** | 0.192 | − | − | |||||
| F. c DNA | −0.052 | 0.068 | 0.006 | −0.202 | −0.111 | 0.077 | 0.165 | 0.235 | − | −0.210 | ||||
| F. g DNA | −0.127 | −0.379 * | 0.459 * | 0.370 * | −0.003 | 0.461 ** | −0.031 | −0.006 | − | 0.506 ** | −0.039 | |||
| F. p DNA | −0.382 * | −0.577 *** | 0.653 *** | 0.220 | 0.282 | 0.508 ** | −0.055 | 0.208 | 0.163 | 0.550 ** | 0.006 | 0.546 ** | ||
| F. sp DNA | −0.338 | −0.590 *** | 0.593 *** | 0.302 | 0.264 | 0.584 *** | 0.098 | − | 0.106 | 0.607 *** | −0.085 | 0.323 | 0.530 ** | |
| Total DNA | −0.201 | −0.475 ** | 0.557 *** | 0.323 | 0.089 | 0.636 *** | 0.004 | 0.216 | 0.243 | 0.640 *** | 0.214 | 0.760 *** | 0.838 *** | 0.623 *** |
| Group | Number of Cultivars | FHBi (%) | DS (%) | ERG (mg kg−1) | Total TCT B (μg kg−1) | Total TCT A (μg kg−1) | Fusarium DNA (pg 100 ng−1) |
|---|---|---|---|---|---|---|---|
| 1 | 13 | 0.4 | 3.8 | 2.10 | 63 | 4 | 206 |
| 2 | 5 | 2.0 | 8.7 | 1.48 | 191 | 7 | 610 |
| 3 | 12 | 0.4 | 2.8 | 0.67 | 43 | 6 | 237 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Góral, T.; Łukanowski, A.; Małuszyńska, E.; Stuper-Szablewska, K.; Buśko, M.; Perkowski, J. Performance of Winter Wheat Cultivars Grown Organically and Conventionally with Focus on Fusarium Head Blight and Fusarium Trichothecene Toxins. Microorganisms 2019, 7, 439. https://doi.org/10.3390/microorganisms7100439
Góral T, Łukanowski A, Małuszyńska E, Stuper-Szablewska K, Buśko M, Perkowski J. Performance of Winter Wheat Cultivars Grown Organically and Conventionally with Focus on Fusarium Head Blight and Fusarium Trichothecene Toxins. Microorganisms. 2019; 7(10):439. https://doi.org/10.3390/microorganisms7100439
Chicago/Turabian StyleGóral, Tomasz, Aleksander Łukanowski, Elżbieta Małuszyńska, Kinga Stuper-Szablewska, Maciej Buśko, and Juliusz Perkowski. 2019. "Performance of Winter Wheat Cultivars Grown Organically and Conventionally with Focus on Fusarium Head Blight and Fusarium Trichothecene Toxins" Microorganisms 7, no. 10: 439. https://doi.org/10.3390/microorganisms7100439
APA StyleGóral, T., Łukanowski, A., Małuszyńska, E., Stuper-Szablewska, K., Buśko, M., & Perkowski, J. (2019). Performance of Winter Wheat Cultivars Grown Organically and Conventionally with Focus on Fusarium Head Blight and Fusarium Trichothecene Toxins. Microorganisms, 7(10), 439. https://doi.org/10.3390/microorganisms7100439