Biotechnological Applications of Microbial (Per)chlorate Reduction
Abstract
:1. Introduction
2. (Per)chlorate Bioremediation
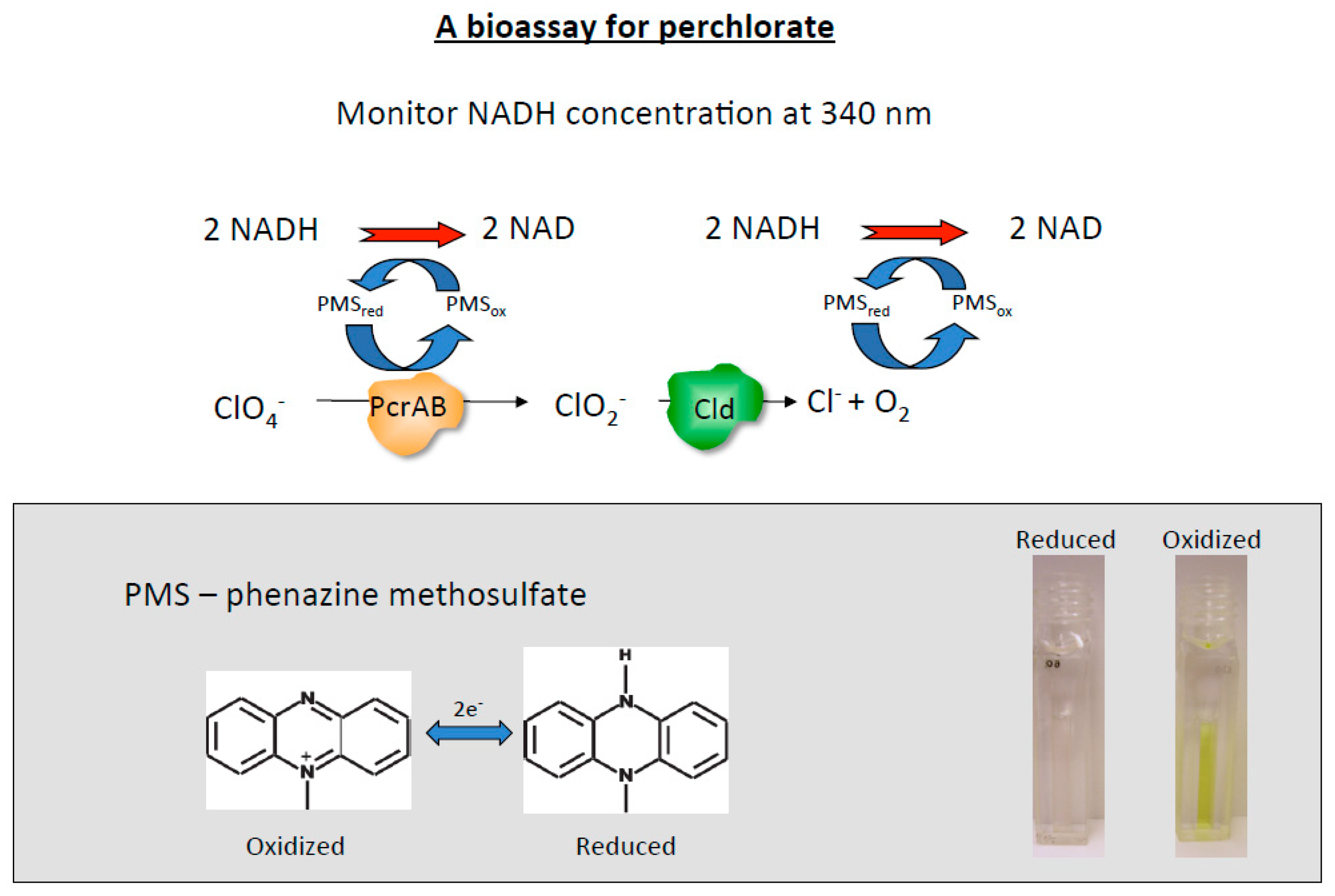
3. Enzymatic Bioassay for the Detection of Perchlorate
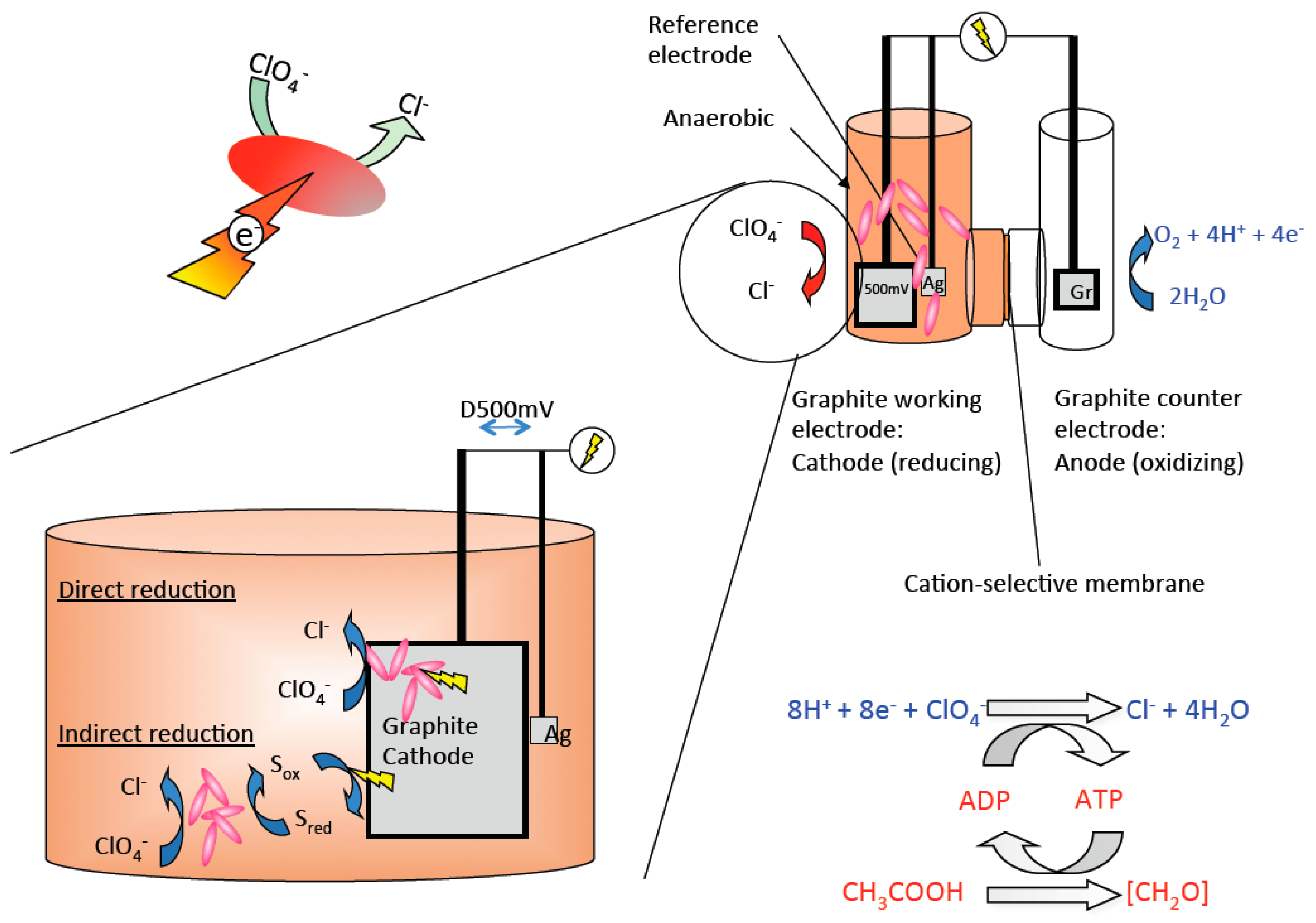
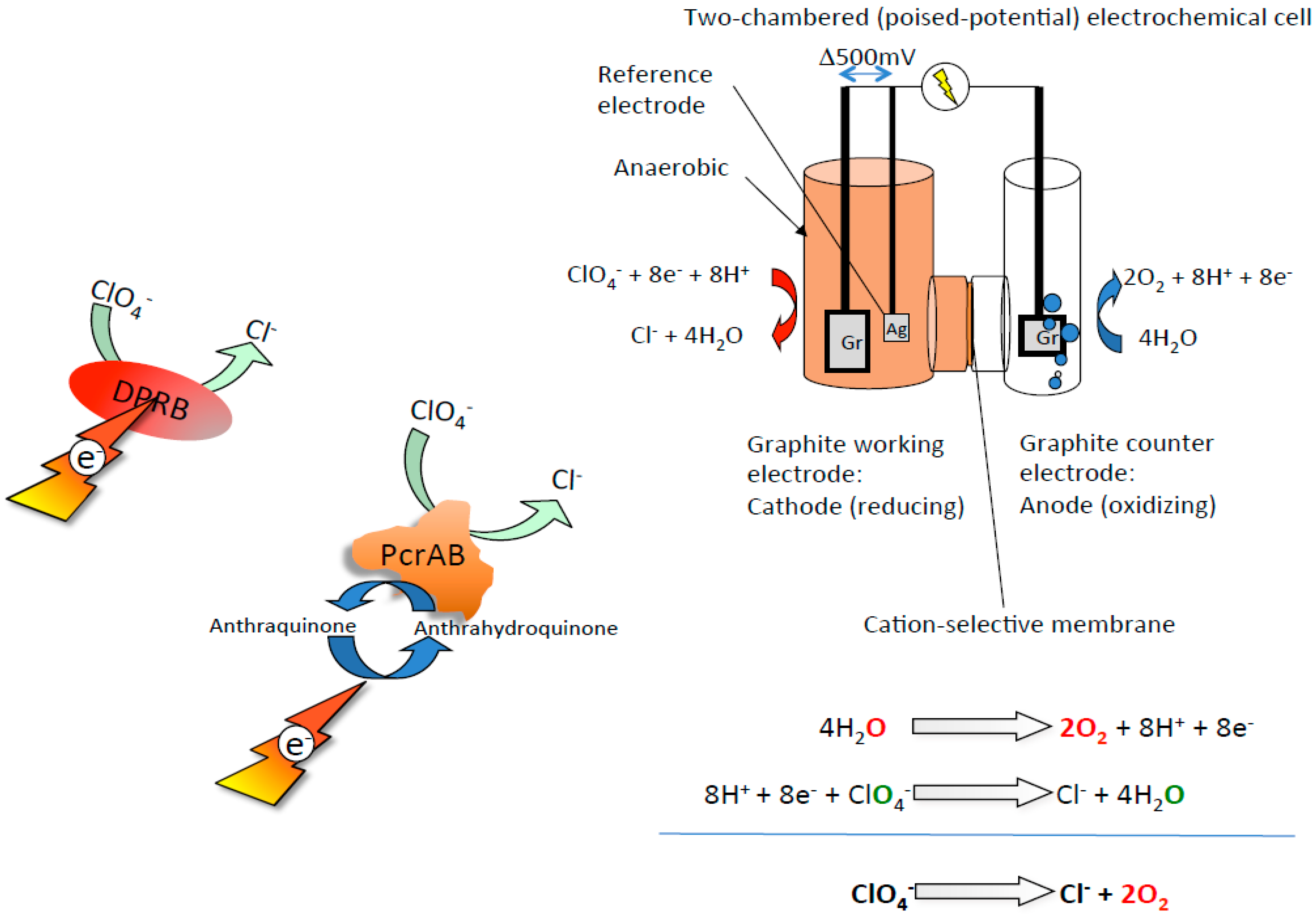
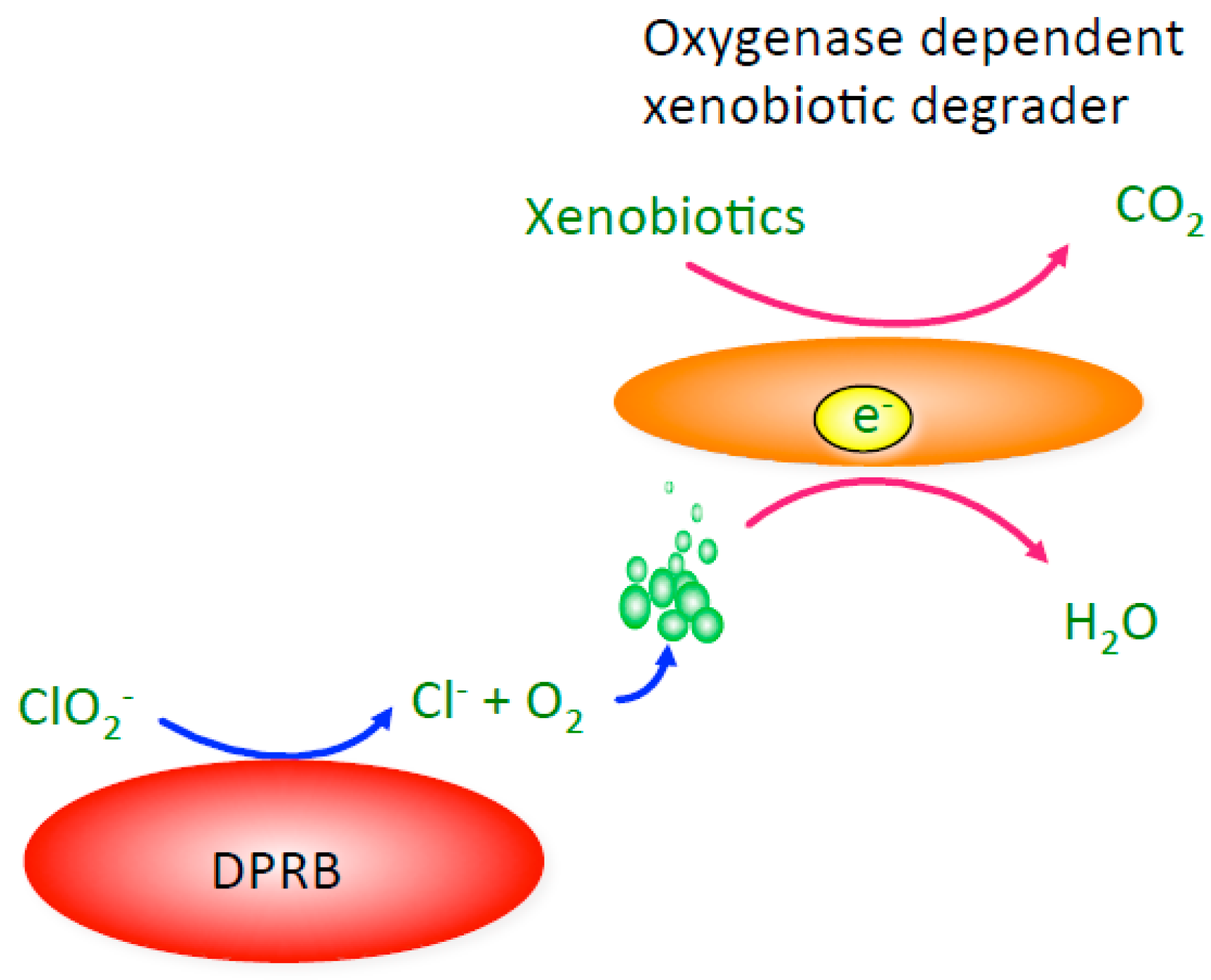
4. Bioeletrochemical O2 Production from Perchlorate
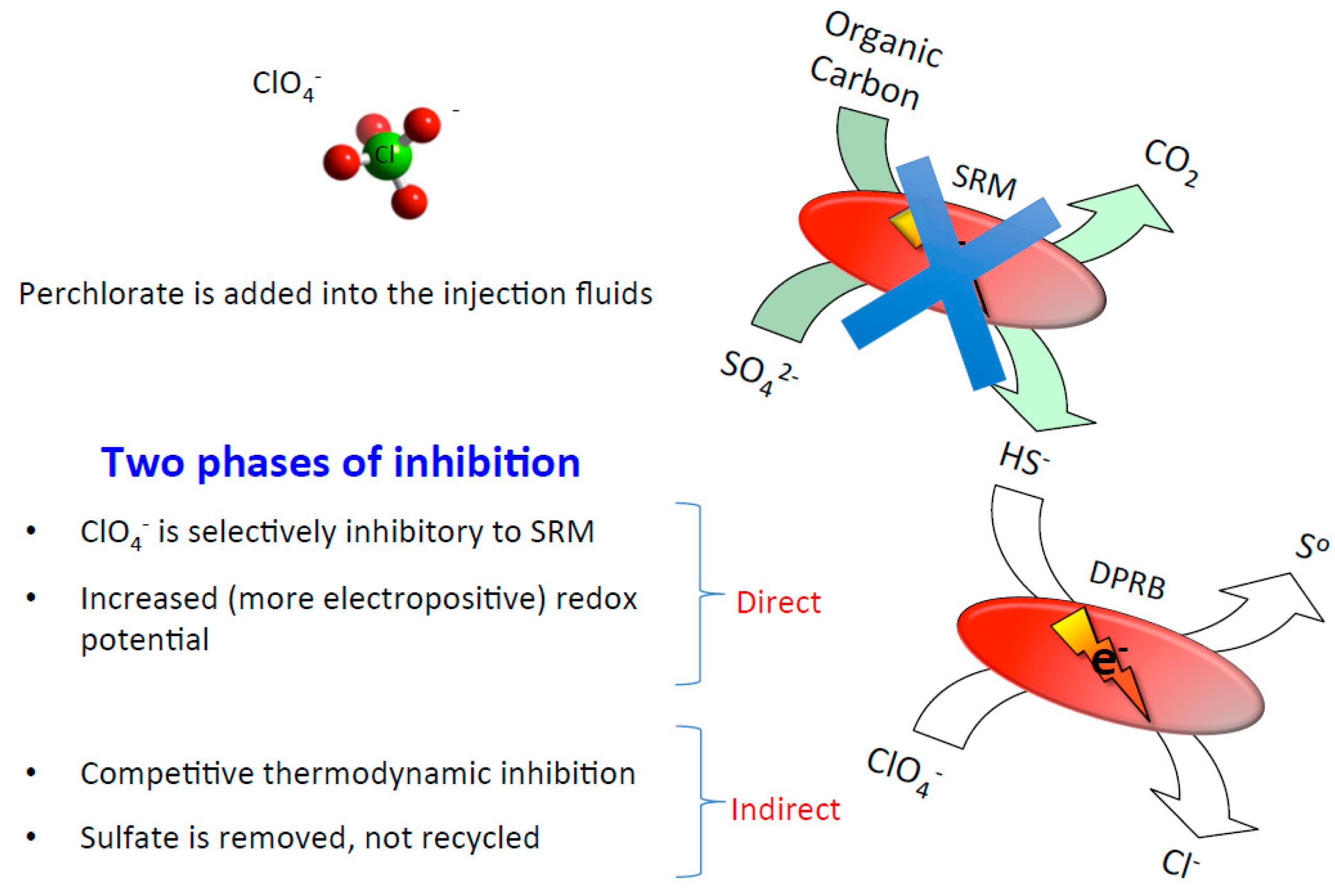
5. Oil Reservoir Bio-Souring Control
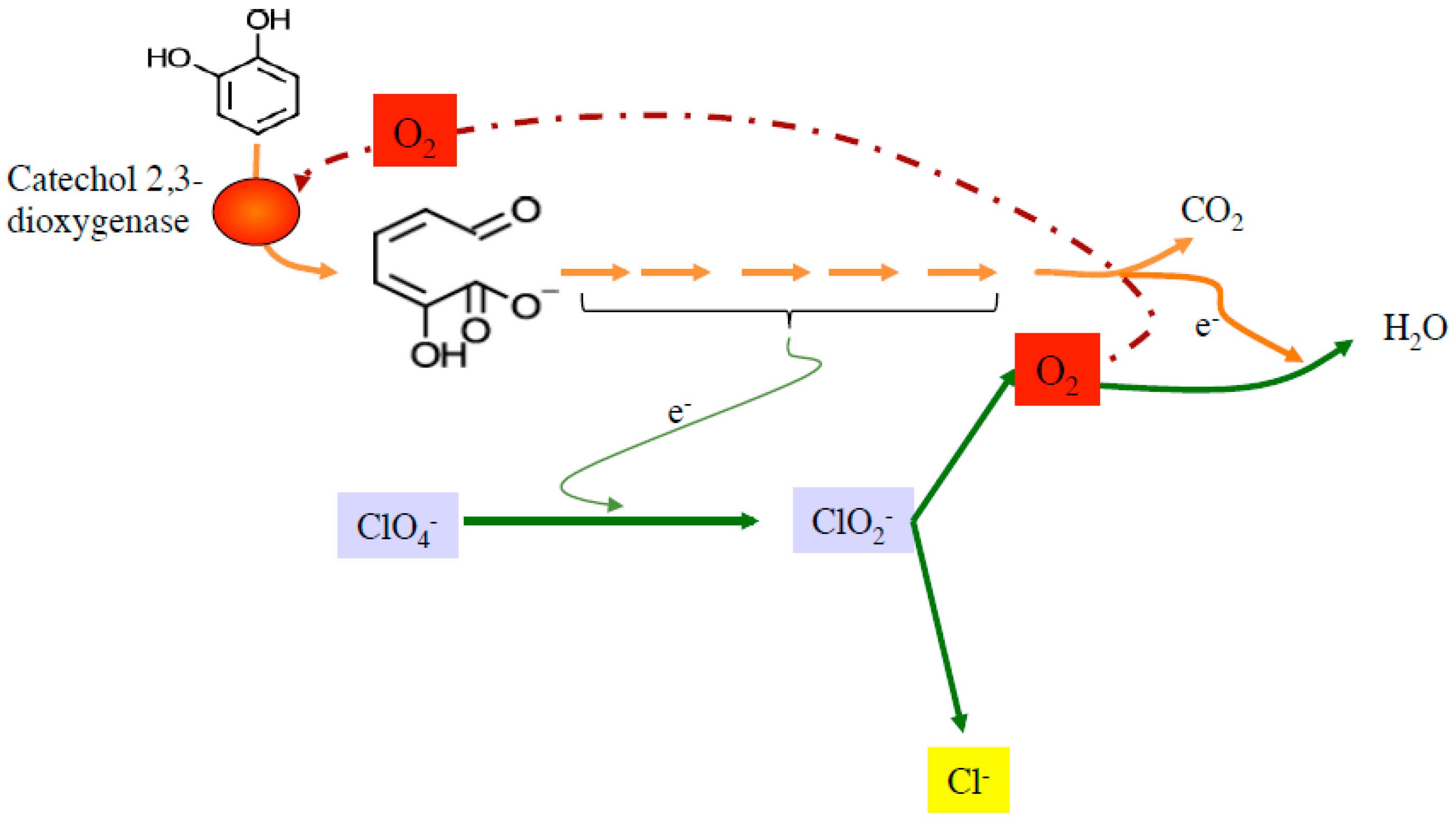
6. Xenobiotic Bioremediation by DPRB and DCRB
7. Genetic Optimization of DPRB and DCRB
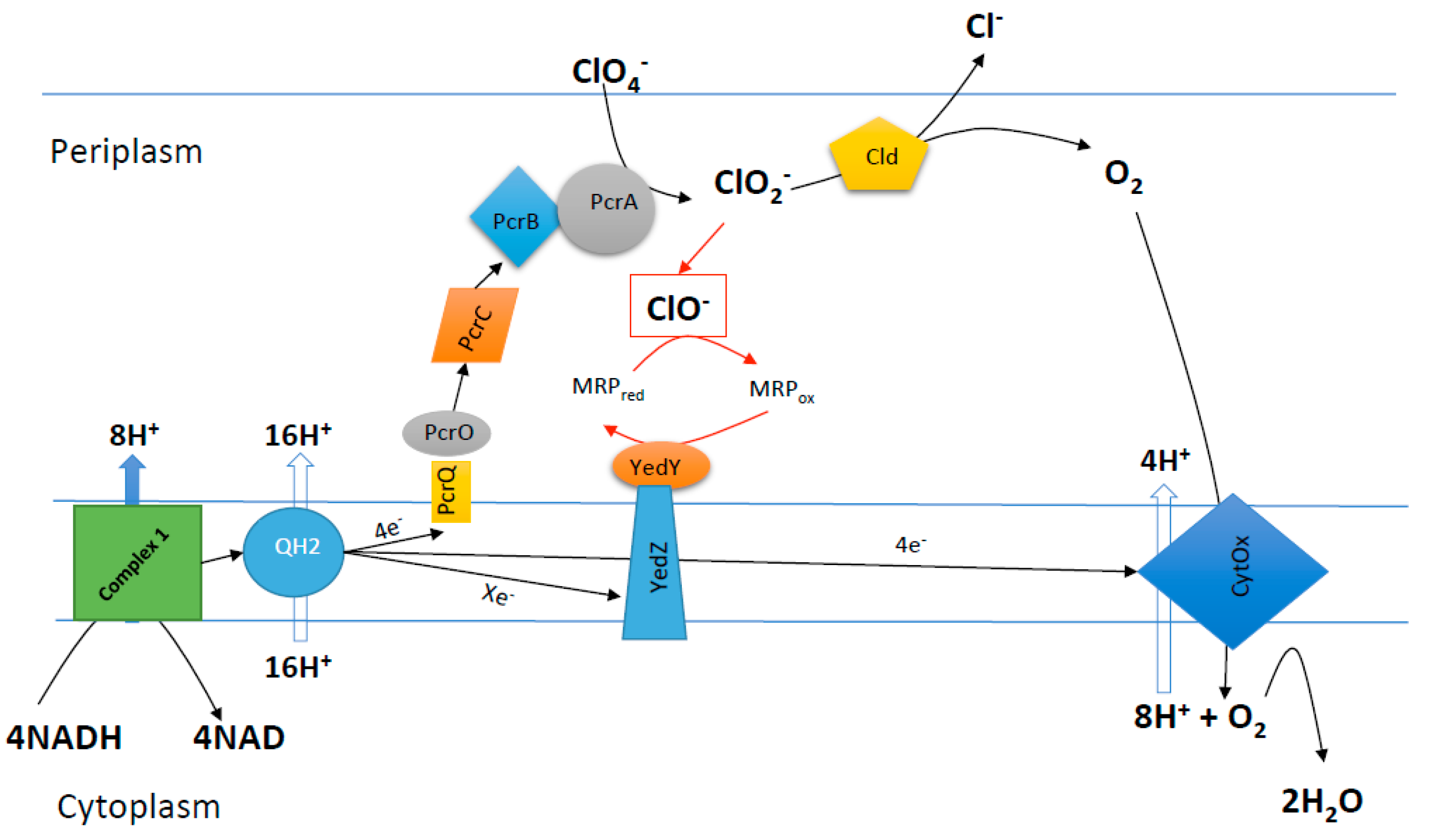
8. Chlorite Mediated Bioreactor Hygiene Control
9. Chlorite Mediated Aeration Enhancement
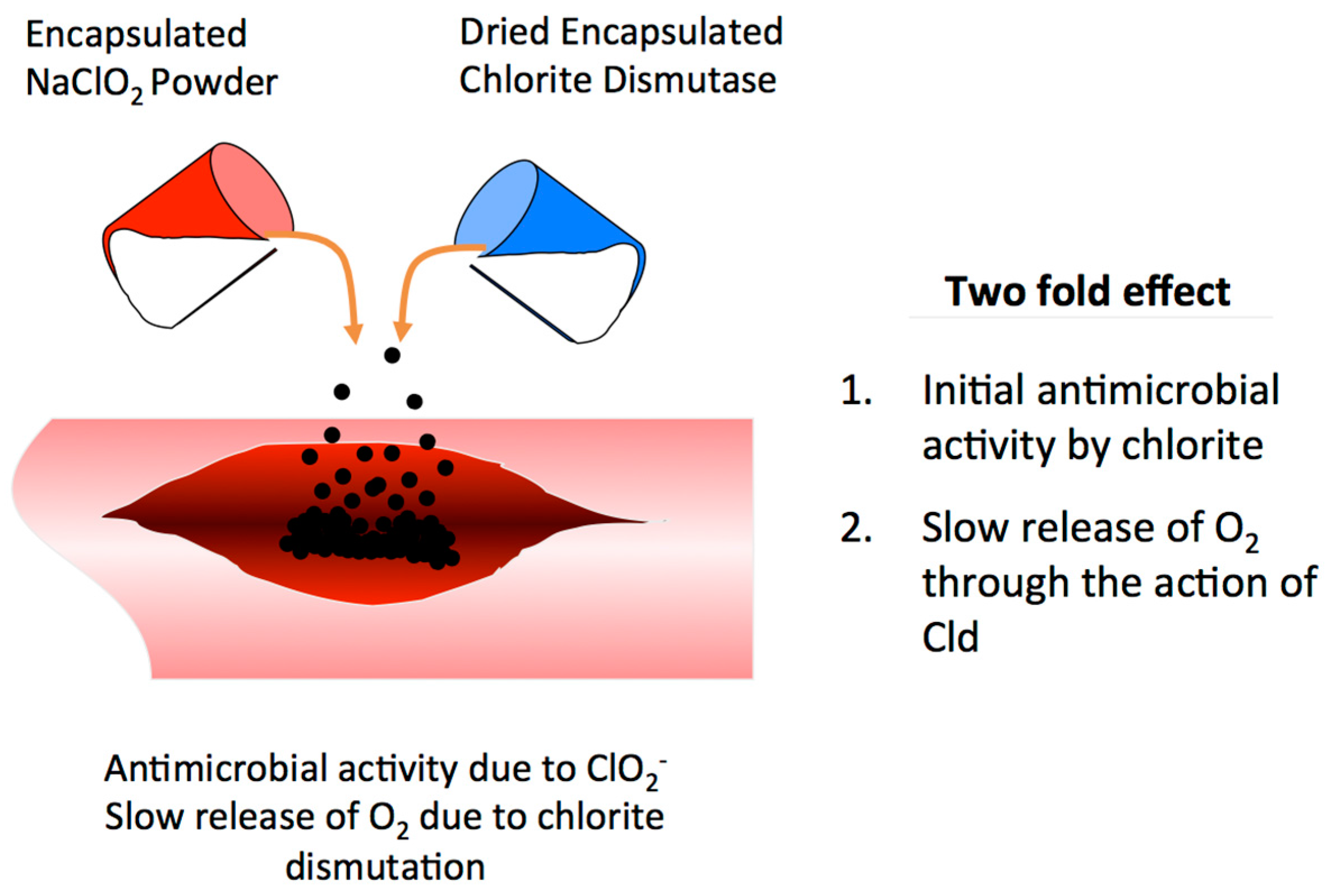
10. Gas Gangrene Treatment
11. Conclusions
Acknowledgments
Conflicts of Interest
References
- Urbansky, E.T. Perchlorate as an environmental contaminant. Environ. Sci. Pollut. Res. 2002, 9, 187–192. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Anderson, T.; Cox, S.; Harvey, G.; Cheng, Q.; Jackson, W.A. Perchlorate in wet deposition across north america. Environ. Sci. Technol. 2009, 43, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, T.; Rova, M.; Smedja Bäcklund, A. Microbial metabolism of oxochlorates: A bioenergetic perspective. Biochim. Biophys. Acta 2013, 1827, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Motzer, W.E. Perchlorate: Problems, detection, and solutions. Environ. Forensics 2001, 2, 301–311. [Google Scholar] [CrossRef]
- Dasgupta, P.K.; Martinelango, P.K.; Jackson, W.A.; Anderson, T.A.; Tian, K.; Tock, R.W.; Rajagopalan, S. The origin of naturally occurring perchlorate: The role of atmospheric processes. Environ. Sci. Technol. 2005, 39, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Catling, D.; Claire, M.; Zahnle, K.; Quinn, R.; Clark, B.; Hecht, M.; Kounaves, S. Atmospheric origins of perchlorate on mars and in the atacama. J. Geophys. Res. Planets (1991–2012) 2010, 115. [Google Scholar] [CrossRef]
- Mastrocicco, M.; Di Giuseppe, D.; Vincenzi, F.; Colombani, N.; Castaldelli, G. Chlorate origin and fate in shallow groundwater below agricultural landscapes. Environ. Pollut. 2017, 231, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Kounaves, S.P.; Stroble, S.T.; Anderson, R.M.; Moore, Q.; Catling, D.C.; Douglas, S.; McKay, C.P.; Ming, D.W.; Smith, P.H.; Tamppari, L.K.; et al. Discovery of natural perchlorate in the antarctic dry valleys and its global implications. Environ. Sci. Technol. 2010, 44, 2360–2364. [Google Scholar] [CrossRef] [PubMed]
- Urbansky, E.T. Perchlorate chemistry: Implications for analysis and remediation. Bioremediat. J. 1998, 2, 81–95. [Google Scholar] [CrossRef]
- Urbanski, T. Chemistry and Technology of Explosives; Pergamon Press: New York, NY, USA, 1988; Volume 4, pp. 602–620. [Google Scholar]
- Roote, D. Technology Status Report—Perchlorate Treatment Technologies; Ground-Water Remediation Technologies Analysis Center: Pittsburgh, PA, USA, 15 May 2001.
- Stanbury, J.B.; Wyngaarden, J.B. Effect of perchlorate on the human thyroid gland. Metab. Clin. Exp. 1952, 1, 533–539. [Google Scholar] [PubMed]
- Wolff, J. Perchlorate and the thyroid gland. Pharmacol. Rev. 1998, 50, 89–106. [Google Scholar] [PubMed]
- Clark, J.J.J. Toxicology of perchlorate. In Perchlorate in the Environment; Urbansky, E.T., Ed.; Springer: Boston, MA, USA, 2000; pp. 15–29. [Google Scholar]
- Abt, E.; Spungen, J.; Pouillot, R.; Gamalo-Siebers, M.; Wirtz, M. Update on dietary intake of perchlorate and iodine from U.S. Food and drug administration’s total diet study: 2008–2012. J. Expo. Sci. Environ. Epidemiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Engelbrektson, A.; Hubbard, C.G.; Tom, L.M.; Boussina, A.; Jin, Y.T.; Wong, H.; Piceno, Y.M.; Carlson, H.K.; Conrad, M.E.; Anderson, G. Inhibition of microbial sulfate reduction in a flow-through column system by (per)chlorate treatment. Front. Microbiol. 2014, 5, 315. [Google Scholar] [CrossRef] [PubMed]
- Deshwal, B.; Lee, H.-K. Kinetics and mechanism of chloride based chlorine dioxide generation process from acidic sodium chlorate. J. Hazard. Mater. 2004, 108, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.D.; Achenbach, L.A. Microbial perchlorate reduction: Rocket-fuelled metabolism. Nat. Rev. Microbiol. 2004, 2, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Youngblut, M.D.; Wang, O.; Barnum, T.P.; Coates, J.D. (Per)chlorate in biology on earth and beyond. Annu. Rev. Microbiol. 2016, 70, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Condie, L.W. Toxicological problems associated with chlorine dioxide. J. (Am. Water Works Assoc.) 1986, 78, 73–78. [Google Scholar]
- Daniel, F.B.; Condie, L.W.; Robinson, M.; Stober, J.A.; York, R.G.; Olson, G.R.; Wang, S.-R. Comparative subchronic toxicity studies of three disinfectants (pdf). J. (Am. Water Works Assoc.) 1990, 82, 61–69. [Google Scholar]
- Vanwijk, D.J.; Hutchinson, T.H. The ecotoxicity of chlorate to aquatic organisms: A critical review. Ecotoxicol. Environ. Saf. 1995, 32, 244–253. [Google Scholar] [CrossRef]
- Ingram, P.R.; Pitt, A.R.; Wilson, C.G.; Olejnik, O.; Spickett, C.M. A comparison of the effects of ocular preservatives on mammalian and microbial atp and glutathione levels. Free Radic. Res. 2004, 38, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Ingram, P.R.; Homer, N.Z.; Smith, R.A.; Pitt, A.R.; Wilson, C.G.; Olejnik, O.; Spickett, C.M. The interaction of sodium chlorite with phospholipids and glutathione: A comparison of effects in vitro, in mammalian and in microbial cells. Arch. Biochem. Biophys. 2003, 410, 121–133. [Google Scholar] [CrossRef]
- Van Wijk, D.J.; Kroon, S.G.; Garttener-Arends, I.C. Toxicity of chlorate and chlorite to selected species of algae, bacteria, and fungi. Ecotoxicol. Environ. Saf. 1998, 40, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, G.; Rand, J.; O’leary, K.; Rygel, A.; Chauret, C.; Andrews, R. Disinfectant efficacy of chlorite and chlorine dioxide in drinking water biofilms. Water Res. 2005, 39, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Allende, A.; McEvoy, J.; Tao, Y.; Luo, Y. Antimicrobial effect of acidified sodium chlorite, sodium chlorite, sodium hypochlorite, and citric acid on Escherichia coli O157:H7 and natural microflora of fresh-cut cilantro. Food Control 2009, 20, 230–234. [Google Scholar] [CrossRef]
- Luo, Y.; Lu, S.; Zhou, B.; Feng, H. Dual effectiveness of sodium chlorite for enzymatic browning inhibition and microbial inactivation on fresh-cut apples. LWT Food Sci. Technol. 2011, 44, 1621–1625. [Google Scholar] [CrossRef]
- Bichai, F.; Barbeau, B. Assessing the disinfecting power of chlorite in drinking water. Water Qual. Res. J. Can. 2006, 41, 375–382. [Google Scholar]
- Dempster, R.P.; Morales, P.; Glennon, F.X. Use of sodium chlorite to combat anchorworm infestations of fish. Progress. Fish-Cult. 1988, 50, 51–55. [Google Scholar] [CrossRef]
- Åslander, A. Experiments on the Eradication of Canada Thistle: Cirsium Arvense, with Chlorates and Other Herbicides; US Government Printing Office: Washington, DC, USA, 1928.
- Youngblut, M.D.; Tsai, C.-L.; Clark, I.C.; Carlson, H.K.; Maglaqui, A.P.; Gau-pan, P.S.; Redford, S.A.; Wong, A.; Tainer, J.A.; Coates, J.D. Perchlorate reductase is distinguished by active site aromatic gate residues. J. Biol. Chem. 2016, 291, 9190–9202. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.C.; Melnyk, R.A.; Engelbrektson, A.; Coates, J.D. Structure and evolution of chlorate reduction composite transposons. mBio 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.C.; Melnyk, R.A.; Iavarone, A.T.; Novichkov, P.S.; Coates, J.D. Chlorate reduction in shewanella algae acdc is a recently acquired metabolism characterized by gene loss, suboptimal regulation and oxidative stress. Mol. Microbiol. 2014, 94, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.C.; Melnyk, R.A.; Youngblut, M.D.; Carlson, H.K.; Iavarone, A.T.; Coates, J.D. Synthetic and evolutionary construction of a chlorate-reducing shewanella oneidensis MR-1. mBio 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.C.; Youngblut, M.; Jacobsen, G.; Wetmore, K.M.; Deutschbauer, A.; Lucas, L.; Coates, J.D. Genetic dissection of chlorate respiration in pseudomonas stutzeri pda reveals syntrophic (per)chlorate reduction. Environ. Microbiol. 2016, 18, 3342–3354. [Google Scholar] [CrossRef] [PubMed]
- Thorell, H.D.; Stenklo, K.; Karlsson, J.; Nilsson, T. A gene cluster for chlorate metabolism in ideonella dechloratans. Appl. Environ. Microbiol. 2003, 69, 5585–5592. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.; Jackson, A. Principles of perchlorate treatment. In In Situ Bioremediation of Perchlorate in Groundwater; Springer: Norwell, MA, USA, 2008. [Google Scholar]
- Stepanov, V.G.; Xiao, Y.; Tran, Q.; Rojas, M.; Willson, R.C.; Fofanov, Y.; Fox, G.E.; Roberts, D.J. The presence of nitrate dramatically changed the predominant microbial community in perchlorate degrading cultures under saline conditions. BMC Microbiol. 2014, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Viraraghavan, T. Perchlorate: Health effects and technologies for its removal from water resources. Int. J. Environ. Res. Public Health 2009, 6, 1418–1442. [Google Scholar] [CrossRef] [PubMed]
- Carlstrom, C.I.; Loutey, D.E.; Wang, O.; Engelbrektson, A.; Clark, I.; Lucas, L.N.; Somasekhar, P.Y.; Coates, J.D. Phenotypic and genotypic description of sedimenticola selenatireducens strain CUZ, a marine (per)chlorate-respiring gammaproteobacterium, and its close relative the chlorate-respiring sedimenticola strain NSS. Appl. Environ. Microbiol. 2015, 81, 2717–2726. [Google Scholar] [CrossRef] [PubMed]
- Carlström, C.I.; Wang, O.; Melnyk, R.A.; Bauer, S.; Lee, J.; Engelbrektson, A.; Coates, J.D. Physiological and genetic description of dissimilatory perchlorate reduction by the novel marine bacterium Arcobacter sp. Strain CAB. mBio 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Okeke, B.C.; Giblin, T.; Frankenberger, W.T., Jr. Reduction of perchlorate and nitrate by salt tolerant bacteria. Environ. Pollut. 2002, 118, 357–363. [Google Scholar] [CrossRef]
- Xiao, Y.; Roberts, D.J. Kinetics analysis of a salt-tolerant perchlorate-reducing bacterium: Effects of sodium, magnesium, and nitrate. Environ. Sci. Technol. 2013, 47, 8666–8673. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.H.; Oh, H.; Ki, D.; Van Ginkel, S.W.; Rittmann, B.E.; Park, J. Bacterial biofilm-community selection during autohydrogenotrophic reduction of nitrate and perchlorate in ion-exchange brine. Appl. Microbiol. Biotechnol. 2009, 81, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Shin, S.; Oh, J. Characterization of a microbial community capable of reducing perchlorate and nitrate in high salinity. Biotechnol. Lett. 2009, 31, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Roberts, D.; Lehman, S.; Jackson, G.; Fox, G.; Willson, R. Molecular assessment of salt-tolerant, perchlorate-and nitrate-reducing microbial cultures. Water Sci. Technol. 2009, 60, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Giblin, T.L.; Herman, D.C.; Frankenberger, W. Removal of perchlorate from ground water by hydrogen-utilizing bacteria. J. Environ. Qual. 2000, 29, 1057–1062. [Google Scholar] [CrossRef]
- Logan, B.E.; LaPoint, D. Treatment of perchlorate-and nitrate-contaminated groundwater in an autotrophic, gas phase, packed-bed bioreactor. Water Res. 2002, 36, 3647–3653. [Google Scholar] [CrossRef]
- Miller, J.P.; Logan, B.E. Sustained perchlorate degradation in an autotrophic, gas-phase, packed-bed bioreactor. Environ. Sci. Technol. 2000, 34, 3018–3022. [Google Scholar] [CrossRef]
- Nerenberg, R.; Rittmann, B. Hydrogen-based, hollow-fiber membrane biofilm reactor for reduction of perchlorate and other oxidized contaminants. Water Sci. Technol. 2004, 49, 223–230. [Google Scholar] [PubMed]
- Son, A.; Lee, J.; Chiu, P.C.; Kim, B.J.; Cha, D.K. Microbial reduction of perchlorate with zero-valent iron. Water Res. 2006, 40, 2027–2032. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.K.; Conneely, T.; Nüsslein, K.R.; Ergas, S.J. Biological perchlorate reduction in packed bed reactors using elemental sulfur. Environ. Sci. Technol. 2009, 43, 4466–4471. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Song, Y.; Min, B.; Steinberg, L.; Logan, B.E. Microbial degradation of perchlorate: Principles and applications. Environ. Eng. Sci. 2003, 20, 405–422. [Google Scholar] [CrossRef]
- Hatzinger, P.B. Perchlorate biodegradation for water treatment. Environ. Sci. Technol. 2005, 39, 239A–247A. [Google Scholar] [CrossRef] [PubMed]
- Thrash, J.C.; Van Trump, J.I.; Weber, K.A.; Miller, E.; Achenbach, L.A.; Coates, J.D. Electrochemical stimulation of microbial perchlorate reduction. Environ. Sci. Technol. 2007, 41, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Anderson, R.D.; Min, J.H.; Boulos, L.; Prasifka, D.; Juby, G.J. Fixed-bed biological treatment of perchlorate-contaminated drinking water. J. (Am. Water Works Assoc.) 2005, 97, 70–81. [Google Scholar]
- Thrash, J.C.; Coates, J.D. Review: Direct and indirect electrical stimulation of microbial metabolism. Environ. Sci. Technol. 2008, 42, 3921–3931. [Google Scholar] [CrossRef] [PubMed]
- Rook, J.J. Haloforms in drinking water. J. (Am. Water Works Assoc.) 1976, 68, 168–172. [Google Scholar]
- Coates, J.D.; Thrash, C.J. Bioelectrical Treatment of Xenobiotics. WO2,008,140,590 A2, 20 November 2008. [Google Scholar]
- Shea, C.; Clauwaert, P.; Verstraete, W.; Nerenberg, R. Adapting a denitrifying biocathode for perchlorate reduction. Water Sci. Technol. 2008, 58, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.C.; Carlson, H.K.; Iavarone, A.T.; Coates, J.D. Bioelectrical redox cycling of anthraquinone-2, 6-disulfonate coupled to perchlorate reduction. Energy Environ. Sci. 2012, 5, 7970–7978. [Google Scholar] [CrossRef]
- Lovley, D.R. Electromicrobiology. Ann. Rev. Microbiol. 2012, 66, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Holmes, D.E.; Zhao, Z.; Woodard, T.L.; Zhang, Y.; Sun, D.; Wang, L.; Nevin, K.P.; Lovley, D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar] [CrossRef] [PubMed]
- El Aribi, H.; Le Blanc, Y.J.; Antonsen, S.; Sakuma, T. Analysis of perchlorate in foods and beverages by ion chromatography coupled with tandem mass spectrometry (IC-ESI-MS/MS). Anal. Chim. Acta 2006, 567, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Gokhale, S.; Streib, T.; Rohrer, J.; Pohl, C. Improved method for the determination of trace perchlorate in ground and drinking waters by ion chromatography. J. Chromatogr. A 2000, 888, 151–158. [Google Scholar] [CrossRef]
- Valentín-Blasini, L.; Blount, B.C.; Delinsky, A. Quantification of iodide and sodium-iodide symporter inhibitors in human urine using ion chromatography tandem mass spectrometry. J. Chromatogr. A 2007, 1155, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Heinnickel, M.; Smith, S.C.; Koo, J.; O’Connor, S.M.; Coates, J.D. A bioassay for the detection of perchlorate in the ppb range. Environ. Sci. Technol. 2011, 45, 2958–2964. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.D.; Heinnickel, M.; Achenbach, L.A. An Enzymatic Bioassay for Perchlorate. Available online: https://clu-in.org/download/contaminantfocus/perchlorate/Perchlorate-ER-1530-FR.pdf (accessed on 23 November 2017).
- Glavin, D.P.; Freissinet, C.; Miller, K.E.; Eigenbrode, J.L.; Brunner, A.E.; Buch, A.; Sutter, B.; Archer, P.D., Jr.; Atreya, S.K.; Brinckerhoff, W.B.; et al. Evidence for perchlorates and the origin of chlorinated hydrocarbons detected by sam at the rocknest aeolian deposit in gale crater. J. Geophys. Res. Planets 2013, 118, 1955–1973. [Google Scholar] [CrossRef]
- Navarro-González, R.; Vargas, E.; de la Rosa, J.; Raga, A.C.; McKay, C.P. Reanalysis of the viking results suggests perchlorate and organics at midlatitudes on Mars. J. Geophys. Res. 2010, 115, E12010. [Google Scholar] [CrossRef]
- Jackson, W.A.; Davila, A.F.; Sears, D.W.G.; Coates, J.D.; McKay, C.P.; Brundrett, M.; Estrada, N.; Böhlke, J.K. Widespread occurrence of (per)chlorate in the solar system. Earth Planet. Sci. Lett. 2015, 430, 470–476. [Google Scholar] [CrossRef]
- Davila, A.F.; Willson, D.; Coates, J.D.; McKay, C.P. Perchlorate on mars: A chemical hazard and a resource for humans. Int. J. Astrobiol. 2013, 12, 321–325. [Google Scholar] [CrossRef]
- Urbansky, E.T.; Brown, S.K. Perchlorate retention and mobility in soils. J. Environ. Monit. 2003, 5, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, P.; Engelbrektson, A.; Hubbard, C.G.; Metlagel, Z.; Csencsits, R.; Auer, M.; Conrad, M.E.; Thieme, J.; Northrup, P.; Coates, J.D. Control of sulfidogenesis through bio-oxidation of H2S coupled to (per)chlorate reduction. Environ. Microbiol. Rep. 2014, 6, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Gieg, L.M.; Jack, T.R.; Foght, J.M. Biological souring and mitigation in oil reservoirs. Appl. Microbiol. Biotechnol. 2011, 92, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.; Elshahed, M.S.; McInerney, M.J. Microbial processes in oil fields: Culprits, problems, and opportunities. Adv. Appl. Microbiol. 2009, 66, 141–251. [Google Scholar] [PubMed]
- Carlson, H.K.; Kuehl, J.V.; Hazra, A.B.; Justice, N.B.; Stoeva, M.K.; Sczesnak, A.; Mullan, M.R.; Iavarone, A.T.; Engelbrektson, A.; Price, M.N. Mechanisms of direct inhibition of the respiratory sulfate-reduction pathway by (per)chlorate and nitrate. ISME J. 2015, 9, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Voordouw, G.; Grigoryan, A.A.; Lambo, A.; Lin, S.; Park, H.S.; Jack, T.R.; Coombe, D.; Clay, B.; Zhang, F.; Ertmoed, R. Sulfide remediation by pulsed injection of nitrate into a low temperature canadian heavy oil reservoir. Environ. Sci. Technol. 2009, 43, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- Hubert, C. Microbial ecology of oil reservoir souring and its control by nitrate injection. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 2753–2766. [Google Scholar]
- Lovley, D.R.; Goodwin, S. Hydrogen concentrations as an indicator of the predominant terminal electron accepting reactions in aquatic sediments. Geochim. Cosmochim. Acta 1988, 52, 2993–3003. [Google Scholar] [CrossRef]
- Callbeck, C.M.; Agrawal, A.; Voordouw, G. Acetate production from oil under sulfate-reducing conditions in bioreactors injected with sulfate and nitrate. Appl. Environ. Microbiol. 2013, 79, 5059–5068. [Google Scholar] [CrossRef] [PubMed]
- Moura, I.; Bursakov, S.; Costa, C.; Moura, J.J. Nitrate and nitrite utilization in sulfate-reducing bacteria. Anaerobe 1997, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Mehta-Kolte, M.G.; Loutey, D.; Wang, O.; Youngblut, M.D.; Hubbard, C.G.; Wetmore, K.M.; Conrad, M.E.; Coates, J.D. Mechanism of H2S oxidation by the dissimilatory perchlorate-reducing microorganism Azospira suillum PS. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Williamson, A.D.; Liu, Y.; Nowak, J.; Loutey, D.; Louie, W.; Kim, H.; Goldstein, A.; Ajo-Franklin, J.; Coates, J.D. University of California: Berkeley, CA, USA, Unpublished work. 2017.
- Díaz, E.; Jiménez, J.I.; Nogales, J. Aerobic degradation of aromatic compounds. Curr. Opin. Biotechnol. 2013, 24, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Carmona, M.; Zamarro, M.T.; Blázquez, B.; Durante-Rodríguez, G.; Juárez, J.F.; Valderrama, J.A.; Barragán, M.J.; García, J.L.; Díaz, E. Anaerobic catabolism of aromatic compounds: A genetic and genomic view. Microbiol. Mol. Biol. Rev. 2009, 73, 71–133. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.K.; Singh, O.V.; Jain, R.K. Polycyclic aromatic hydrocarbons: Environmental pollution and bioremediation. Trends Biotechnol. 2002, 20, 243–248. [Google Scholar] [CrossRef]
- Jiménez, J.I.; Miñambres, B.; García, J.L.; Díaz, E. Genomic analysis of the aromatic catabolic pathways from Pseudomonas putida KT2440. Environ. Microbiol. 2002, 4, 824–841. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Apte, S.K.; Phale, P.S. Preferential utilization of aromatic compounds over glucose by pseudomonas putida CSV86. Appl. Environ. Microbiol. 2006, 72, 2226–2230. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D. Potential for anaerobic bioremediation of btex in petroleum-contaminated aquifers. J. Ind. Microbiol. Biotechnol. 1997, 18, 75–81. [Google Scholar] [CrossRef]
- Fuchs, G.; Boll, M.; Heider, J. Microbial degradation of aromatic compounds—From one strategy to four. Nat. Rev. Microbiol. 2011, 9, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Ismail, W.; Gescher, J. Epoxy coenzyme a thioester pathways for degradation of aromatic compounds. Appl. Environ. Microbiol. 2012, 78, 5043–5051. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.D.; Chakraborty, R.; Lack, J.G.; O’connor, S.M.; Cole, K.A.; Bender, K.S.; Achenbach, L.A. Anaerobic benzene oxidation coupled to nitrate reduction in pure culture by two strains of dechloromonas. Nature 2001, 411, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.D.; Bruce, R.A.; Patrick, J.A.; Achenbach, L.A. Hydrocarbon bioremediative potential of (per)chlorate-reducing bacteria. Bioremediat. J. 1999, 3, 323–334. [Google Scholar] [CrossRef]
- Coates, J.D.; Bruce, R.A.; Haddock, J.D. Anoxic bioremediation of hydrocarbons. Nature 1998, 396, 730. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.; Horita, J.; Anderson, T.; Pardue, J.; Reible, D.; Jackson, W.A. The use of chlorate, nitrate, and perchlorate to promote crude oil mineralization in salt marsh sediments. Environ. Sci. Pollut. Res. 2015, 22, 15377–15385. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.G.; Baesman, S.M.; Carlstrom, C.I.; Coates, J.D.; Oremland, R.S. Methane oxidation linked to chlorite dismutation. Front. Microbiol. 2014, 5, 275. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, R.A.; Clark, I.C.; Liao, A.; Coates, J.D. Transposon and deletion mutagenesis of genes involved in perchlorate reduction in azospira suillum PS. mBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, R.A.; Youngblut, M.D.; Clark, I.C.; Carlson, H.K.; Wetmore, K.M.; Price, M.N.; Iavarone, A.T.; Deutschbauer, A.M.; Arkin, A.P.; Coates, J.D. Novel mechanism for scavenging of hypochlorite involving a periplasmic methionine-rich peptide and methionine sulfoxide reductase. mBio 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Verreault, D.; Gendron, L.; Rousseau, G.M.; Veillette, M.; Massé, D.; Lindsley, W.G.; Moineau, S.; Duchaine, C. Detection of airborne lactococcal bacteriophages in cheese manufacturing plants. Appl. Environ. Microbiol. 2011, 77, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Shirley, M.; Wu, X.; Keis, S. Bacteriophage infections in the industrial acetone butanol (ab) fermentation process. J. Mol. Microbiol. Biotechnol. 2000, 2, 21–26. [Google Scholar] [PubMed]
- Marcó, M.B.; Moineau, S.; Quiberoni, A. Bacteriophages and dairy fermentations. Bacteriophage 2012, 2, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Callanan, M.J.; Klaenhammer, T.R. Bacteriophages in industry. eLS 2008. [Google Scholar] [CrossRef]
- Martín, M.; Montes, F.J.; Galán, M.A. Bubbling process in stirred tank reactors II: Agitator effect on the mass transfer rates. Chem. Eng. Sci. 2008, 63, 3223–3234. [Google Scholar] [CrossRef]
- Karimi, A.; Golbabaei, F.; Mehrnia, M.R.; Neghab, M.; Mohammad, K.; Nikpey, A.; Pourmand, M.R. Oxygen mass transfer in a stirred tank bioreactor using different impeller configurations for environmental purposes. Iran. J. Environ. Health Sci. Eng. 2013, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Krebs, C.; Dassama, L.M.; Matthews, M.L.; Jiang, W.; Price, J.C.; Korboukh, V.; Li, N.; Bollinger, J.M. Novel approaches for the accumulation of oxygenated intermediates to multi-millimolar concentrations. Coord. Chem. Rev. 2013, 257, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Dassama, L.M.; Yosca, T.H.; Conner, D.A.; Lee, M.H.; Blanc, B.; Streit, B.R.; Green, M.T.; DuBois, J.L.; Krebs, C.; Bollinger, J.M., Jr. O2-evolving chlorite dismutase as a tool for studying O2-utilizing enzymes. Biochemistry 2012, 51, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Mölder, E.; Mashirin, A.; Tenno, T. Measurement of the oxygen mass transfer through the air-water interface (5 pp). Environ. Sci. Pollut. Res. 2005, 12, 66–70. [Google Scholar] [CrossRef]
- Rui, Z.; Li, X.; Zhu, X.; Liu, J.; Domigan, B.; Barr, I.; Cate, J.H.; Zhang, W. Microbial biosynthesis of medium-chain 1-alkenes by a nonheme iron oxidase. Proc. Natl. Acad. Sci. USA 2014, 111, 18237–18242. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chang, W.-C.; Guo, Y.; Huang, H.; Peck, S.C.; Pandelia, M.E.; Lin, G.-M.; Liu, H.-W.; Krebs, C.; Bollinger, J.M. Evidence that the fosfomycin-producing epoxidase, HppE, is a non–heme-iron peroxidase. Science 2013, 342, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.D.; Michaelidou, U.; Bruce, R.A.; O’Connor, S.M.; Crespi, J.N.; Achenbach, L.A. The ubiquity and diversity of dissimilatory (per)chlorate-reducing bacteria. Appl. Environ. Microbiol. 1999, 65, 5234–5241. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, O.; Coates, J.D. Biotechnological Applications of Microbial (Per)chlorate Reduction. Microorganisms 2017, 5, 76. https://doi.org/10.3390/microorganisms5040076
Wang O, Coates JD. Biotechnological Applications of Microbial (Per)chlorate Reduction. Microorganisms. 2017; 5(4):76. https://doi.org/10.3390/microorganisms5040076
Chicago/Turabian StyleWang, Ouwei, and John D. Coates. 2017. "Biotechnological Applications of Microbial (Per)chlorate Reduction" Microorganisms 5, no. 4: 76. https://doi.org/10.3390/microorganisms5040076
APA StyleWang, O., & Coates, J. D. (2017). Biotechnological Applications of Microbial (Per)chlorate Reduction. Microorganisms, 5(4), 76. https://doi.org/10.3390/microorganisms5040076



