Quantitative Characterization of the Growth of Deinococcus geothermalis DSM-11302: Effect of Inoculum Size, Growth Medium and Culture Conditions
Abstract
:1. Introduction
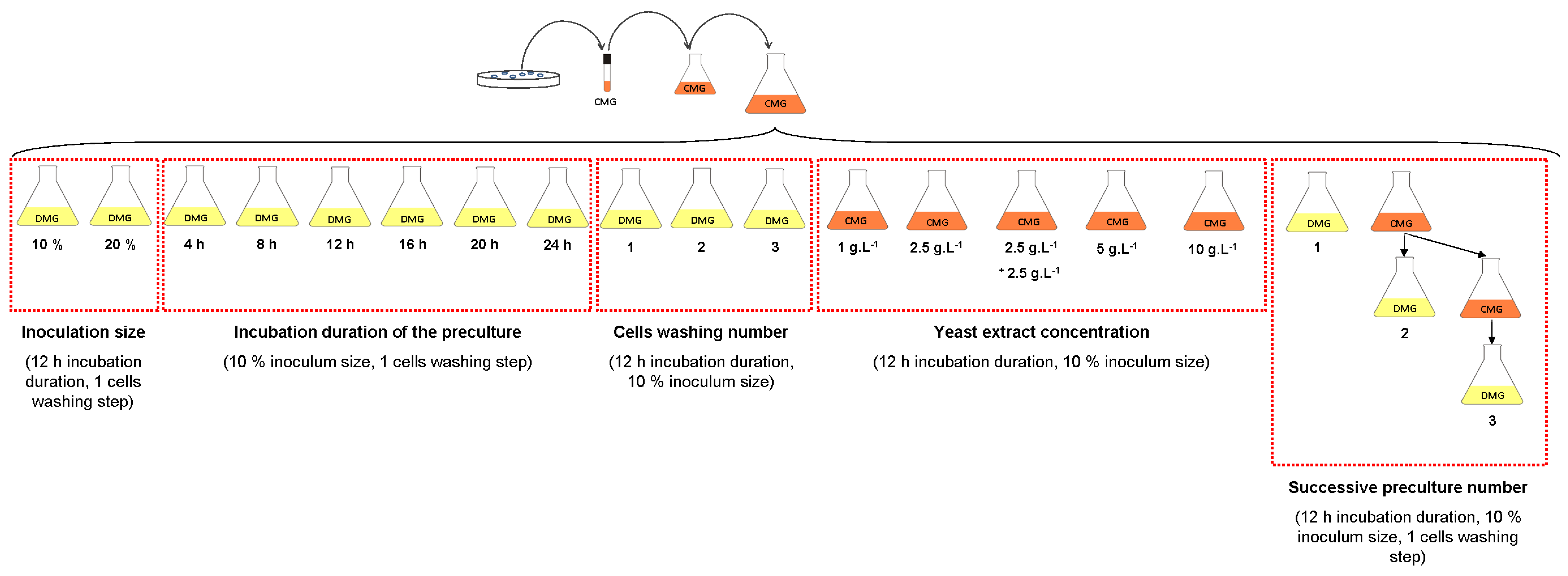
2. Materials and Methods
2.1. Microorganisms, Media and Growth Conditions
2.1.1. Bacterial Strains
2.1.2. Media and Growth Conditions
2.1.3. Erlenmeyer Flask Experiments
2.1.3.1. Culture of the Three Strains of Deinococcus geothermalis
2.1.3.2. Effect of Yeast Extract Concentration
2.1.3.3. Effect of Inoculation Size and Incubation Duration of the Preculture
2.1.3.4. Effect of One- to Three-Stage Inoculum Cultures in Complex Medium
2.1.3.5. Effect of Dilution of the Residual Yeast Extract in the Medium with Cell Washing Before Inoculation
2.1.3.6. Growth of Deinococcus geothermalis DSM-11302 in Defined Media on Carbon Sources and Complex Nutrients
| Vitamins | Concentration (mg·L−1) |
|---|---|
| Thiamine | 0.105 |
| Riboflavine | 0.625 |
| Pyridoxine | 0.12 |
| Nicotinic acid | 3 |
| Panthotenic acid | 0.525 |
| Folic acid | 0.03 |
| Choline | 7.5 |
| Biotine | 0.02 |
2.1.4. Determination of the Respiratory Type of Deinococcus geothermalis DSM-11302
2.1.5. Culture in Bioreactor
2.1.6. Chemical Products
2.2. Analytical Methods
2.2.1. Biomass Analysis
2.2.2. Supernatant Analysis
3. Results and Discussion
3.1. Comparison of the Growth of Deinococcus geothermalis Strains DSM-11300, DSM-11301 and DSM-11302
| Strains | Deinococcus geothermalis DSM 11300 | Deinococcus geothermalis DSM 11301 | Deinococcus geothermalis DSM 11302 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Media | DMG | CMG | TH162 | Holland | DMG | CMG | TH162 | Holland | DMG | CMG | TH162 | Holland | |
| 24 h | Number of generations | 1.3 | 3.7 | 1.4 | 0.1 | 1.6 | 3.7 | 1.6 | 0.3 | 2.8 | 3.7 | 1.6 | 0.4 |
| Glucose uptake (%) | 16 | 75 | / | / | 41 | 86 | / | / | 55 | 94 | / | / | |
| 48 h | Number of generations | 1.6 | 3.7 | 1.4 | 0.1 | 1.6 | 3.7 | 1.6 | 0.3 | 3.2 | 4.1 | 1.6 | 0.4 |
| Glucose uptake (%) | 24 | 100 | / | / | 81 | 100 | / | / | 89 | 100 | / | / | |
3.2. Effect of the Temperature on the Growth of Deinococcus geothermalis DSM-11302 and Respiratory Type
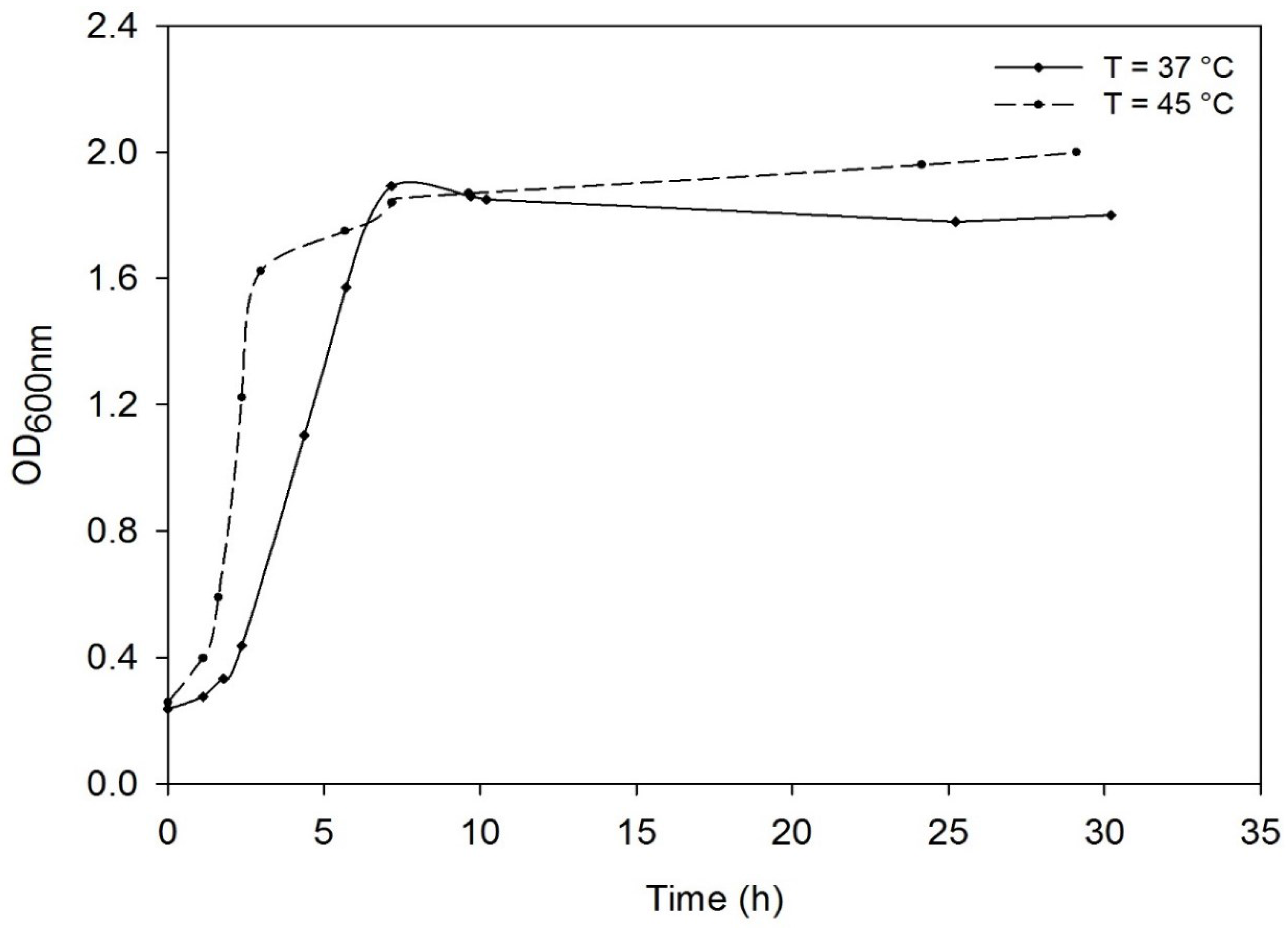
3.3. Effect of Yeast Extract Concentration
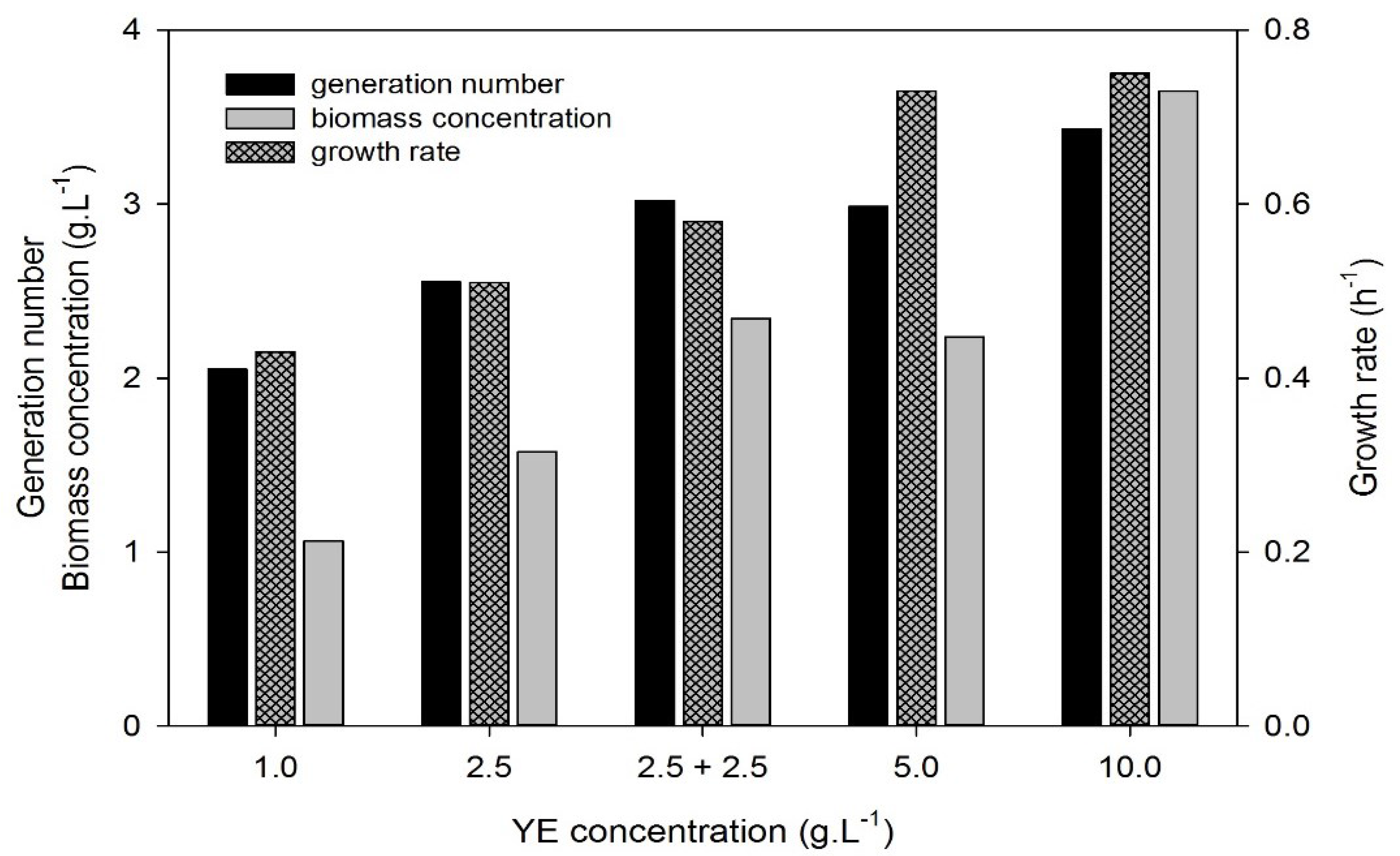
3.4. Effect of Method of Strain Preservation, Frozen Storage Period, Type of Strain Revivification and Variability between CFU on Petri Dishes
3.5. Influence of Inoculation Size and Duration of Incubation Phase of Pre-Culture
| Medium | CMG | ||||||
|---|---|---|---|---|---|---|---|
| Preservation method | Freeze dried cells | Glycerol stocks (−80 °C) | |||||
| Storage period (months) | – | 20 | 12 | ||||
| Revivification | Petri dishes culture | Direct tube inoculation | Petri dishes culture | Petri dishes culture | Petri dishes culture | Petri dishes culture | Direct tube inoculation |
| Initial OD600 nm | 0.282 | 0.334 | 0.286 | 0.270 | 0.270 | 0.338 | 0.324 |
| Final OD600 nm | 1.58 | 1.77 | 1.63 | 1.72 | 1.72 | 1.62 | 1.72 |
| Number of generations | 2.5 | 2.4 | 2.5 | 2.7 | 2.7 | 2.3 | 2.4 |
| µ (h−1) | 0.76 | 0.75 | 0.75 | 0.76 | 0.76 | 0.76 | 0.71 |
| Glucose uptake (%) | 44 | 42 | 41 | 50 | 60 | 40 | 42 |
| Medium | DMG | ||||||
|---|---|---|---|---|---|---|---|
| Preservation method | Freeze dried cells | Glycerol stocks (–80 °C) | |||||
| Storage period (months) | – | 20 | 12 | ||||
| Revivification | Petri dishes culture | Direct tube inoculation | Petri dishes culture | Petri dishes culture | Petri dishes culture | Petri dishes culture | Direct tube inoculation |
| Initial OD600 nm | 0.232 | 0.224 | 0.216 | 0.233 | 0.246 | 0.22 | 0.218 |
| Final OD600 nm | 1.37 | 1.37 | 1.34 | 1.35 | 1.36 | 1.36 | 1.52 |
| Number of generations | 2.6 | 2.6 | 2.6 | 2.5 | 2.5 | 2.6 | 2.8 |
| µ (h−1) | 0.17 | 0.17 | 0.16 | 0.15 | 0.16 | 0.15 | 0.15 |
| Glucose uptake (%) | 38 | 36 | 42 | 50 | 26 | 40 | 51 |
| YS,X (gx·gGlc−1) | 0.40 | 0.44 | 0.36 | 0.31 | 0.59 | 0.41 | 0.35 |
| Age of Pre-Culture (h) | 4 | 8 | 12 | 16 | 20 | 24 | |
|---|---|---|---|---|---|---|---|
| Inoculation size (%) | 10 | 10 | 10 | 20 | 10 | 10 | 10 |
| Initial OD600 nm | 0.201 | 0.270 | 0.213 | 0.439 | 0.203 | 0.231 | 0.260 |
| Final OD600 nm | 1.72 | 1.79 | 1.61 | 2.00 | 1.41 | 1.51 | 1.57 |
| Number of generations | 3.0 | 2.7 | 2.9 | 2.2 | 2.7 | 2.7 | 2.6 |
| µ (h−1) | 0.17 | 0.11 | 0.14 | 0.14 | 0.09 | 0.09 | 0.08 |
| Glucose uptake (%) | 45 | 42 | 40 | 55 | 37 | 28 | 40 |
| YS,X (gx·gGlc−1) | 0.26 | 0.31 | 0.19 | 0.21 | 0.22 | 0.29 | 0.23 |
3.6. Influence of Successive Subcultures, Dilution of The Residual Yeast Extract in the Medium and Cell Washing before Inoculation on the Growth of Deinococcus geothermalis DSM-11302 in DMG
3.7. Growth of Deinococcus geothermalis DSM-11302 in Defined Media on Carbon Sources and Complex Nutrients
| Precultures nb | 1 | 2 | 3 | 1 | 1 | 1 |
|---|---|---|---|---|---|---|
| Cell washing nb | 1 | 1 | 1 | 1 | 2 | 3 |
| Dilution of residual yeast extract | 2.10−4 | 2.10−4 | 2.10−4 | 2.10−4 | 4.10−7 | 8.10−10 |
| Initial OD600 nm | 0.192 | 0.245 | 0.233 | 0.292 | 0.285 | 0.239 |
| Final OD600 nm | 1.76 | 1.84 | 1.90 | 1.70 | 1.66 | 1.62 |
| Number of generations | 3.2 | 2.9 | 3.0 | 2.5 | 2.5 | 2.8 |
| µ (h−1) | 0.13 | 0.10 | 0.06 | 0.13 | 0.13 | 0.13 |
| Glucose uptake (%) | 63 | 67 | 45 | 50 | 53 | 44 |
| YS,X (gx·gGlc−1) | 0.14 | 0.15 | 0.25 | 0.20 | 0.18 | 0.25 |
| Carbon Sources | CMC (Carboxy Methyl Cellulose) | Mannose | Trehalose | Galactose | Fructose | Maltose | Lactose | Sucrose | Glucose |
|---|---|---|---|---|---|---|---|---|---|
| Initial OD600 nm | 0.269 | 0.257 | 0.245 | 0.296 | 0.272 | 0.261 | 0.248 | 0.240 | 0.242 |
| Final OD600 nm | 0.222 | 1.880 | 2.120 | 0.690 | 1.540 | 1.990 | 0.218 | 1.990 | 2.000 |
| Number of generations | 0 | 2.9 | 3.1 | 1.2 | 2.5 | 2.9 | 0 | 3.1 | 3.1 |
| Complex Sources | YE (Yeast Extract) Vitamins | Casamino Acids | D. geothermalis Extract | S. cerevisiae Extract | E. coli extract | None |
|---|---|---|---|---|---|---|
| Initial OD600 nm | 0.242 | 0.248 | 0.288 | 0.301 | 0.230 | 0.246 |
| Final OD600 nm | 1.840 | 1.650 | 1.850 | 2.000 | 1.760 | 1.860 |
| Number of generations | 2.9 | 2.7 | 2.7 | 2.7 | 2.9 | 2.9 |
| Glucose uptake (%) | 100 | 90 | 100 | 100 | 100 | 94 |
| Y S,X (gx·gGlc−1) | 0.23 | 0.21 | 0.23 | 0.23 | 0.23 | 0.23 |
3.8. Quantification of Deinococcus geothermalis DSM-11302 Growth on a Mineral Medium
| Physiological Parameters | Phase I | Phase II | Phase III |
|---|---|---|---|
| Growth rate (h−1) | 0.28 | 0.16 | 0.04 |
| Number of generations | 1.2 | 1.5 | – |
| qO2 (mmolO2·CmolX−1·h−1) | 216 | 186 105 | 67 |
| qCO2 (mmolCO2·CmolX−1·h−1) | 226 | 206 121 | 73 |
| qGlc (CmolGlc·CmolX−1·h−1) | 2.2 | 0.22 | 0.22 |
| QR | 1.7 | 1.12 | 1.12 |

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix

References
- Battista, J.R.; Earl, A.M.; White, O. The stress responses of Deinococcus radiodurans. In Bacterial Stress Responses; Storz, G., Hengge-Aronis, R., Eds.; ASM Press: Washington, DC, USA, 2000; pp. 383–391. [Google Scholar]
- Ferreira, A.C.; Nobre, M.F.; Rainey, F.A.; Silva, M.T.; Wait, R.; Burghardt, J.; DaCosta, M.S. Deinococcus geothermalis sp. nov. and Deinococcus murrayi sp. nov. two extremely radiation-resistant and slightly thermophilic species from hot springs. Int. J. Syst. Evol. Microbiol. 1997, 47, 939–947. [Google Scholar]
- Blasius, M.; Sommer, S.; Hübscher, U. Deinococcus radiodurans: What belongs to the survival kit? Crit. Rev. Biochem. Mol. Biol. 2008, 43, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Mattimore, V.; Battista, J.R. Radioresistance of Deinococcus radiodurans: Functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 1996, 178, 633–637. [Google Scholar] [PubMed]
- Slade, D.; Radman, M. Oxidative stress resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. Rev. 2011, 75, 133–191. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, J.-P.; Matic, I.; Biton, J.; Pouletty, P. Use of Bacteria for the Production of Bioenergy. U.S. Patent 0,104,766, 5 May 2011. [Google Scholar]
- Brim, H.; McFarlan, S.C.; Fredrickson, J.K.; Minton, K.W.; Zhai, M.; Wackett, L.P.; Daly, M.J. Engineering Deinococcus radiodurans for metal remediation in radioactive mixed waste environments. Nat. Biotechnol. 2000, 18, 85–90. [Google Scholar] [PubMed]
- Daly, M.J. Engineering radiation-resistant bacteria for environmental biotechnology. Curr. Opin. Biotech. 2000, 11, 280–285. [Google Scholar] [CrossRef]
- Lange, C.C.; Wackett, L.P.; Minton, K.W.; Daly, M.J. Engineering a recombinant Deinococcus radiodurans for organopollutant degradation in radioactive mixed waste environments. Nat. Biotechnol. 1998, 16, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Kolari, M.; Schmidt, U.; Kuismanen, E.; Salkinoja-Salonen, M.S. Firm but slippery attachment of Deinococcus geothermalis. J. Bacteriol. 2002, 184, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Adhesion, Presence and Antifouling of Deinococcus geothermalis in Paper Machine Environment. Available online: https://helda.helsinki.fi/handle/10138/24770 (accessed on 9 June 2015).
- Väisänen, O.M.; Weber, A.; Bennasar, A.; Rainey, F.A.; Busse, H.-J.; Salkinoja-Salonen, M.S. Microbial communities of printing paper machines. J. Appl. Microbiol. 1998, 84, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Kongpol, A.; Kato, J.; Vangnai, A.S. Isolation and characterization of Deinococcus geothermalis T27, a slightly thermophilic and organic solvent-tolerant bacterium able to survive in the presence of high concentrations of ethyl acetate. FEMS Microbiol. Lett. 2008, 286, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Brim, H.; Venkateswaran, A.; Kostandarithes, H.M.; Fredrickson, J.K.; Daly, M.J. Engineering Deinococcus geothermalis for bioremediation of high-temperature radioactive waste environments. Appl. Environ. Microb. 2003, 69, 4575–4582. [Google Scholar] [CrossRef]
- Bornot, J.; Molina-Jouve, C.; Uribelarrea, J.-L.; Gorret, N. Growth of the extremophilic Deinococcus geothermalis DSM 11302 using co-substrate fed-batch culture. Appl. Microbiol. Biot. 2014, 98, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- He, Y. High cell density production of Deinococcus radiodurans under optimized conditions. J. Ind. Microbiol. Biot. 2009, 36, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.; Rothfuss, H.; Lidstrom, M. Development of a defined medium supporting rapid growth for Deinococcus radiodurans and analysis of metabolic capacities. Appl. Microbiol. Biot. 2006, 72, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Bornot, J.; Aceves-Lara, C.-A.; Molina-Jouve, C.; Uribelarrea, J.-L.; Gorret, N. Experimental and statistical analysis of nutritional requirements for the growth of the extremophile Deinococcus geothermalis DSM 11300. Extremophiles 2014, 18, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, J.; Barford, J. The effect of inoculum preparation on the fully aerated growth of Saccharomyces cerevisiae with a glucose substrate. J. Gen. Appl. Microbiol. 1987, 33, 113–121. [Google Scholar] [CrossRef]
- Parton, C.; Willis, P. Strain preservation, inoculum preparation and development. In Fermentation: A Practical Approach; McNeil, B., Harvey, L.M., Eds.; Oxford University Press: New York, NY, USA, 1990; pp. 39–64. [Google Scholar]
- Hunt, G.R.; Stieber, R.W. Inoculum development. In Manual of Industrial Microbiology and Biotechnology; Demain, A.L., Solomon, N.A., Eds.; American Society for Microbiology: Washington, DC, USA, 1986; pp. 32–40. [Google Scholar]
- Vosti, D.; Joslyn, M. Autolysis of bakers’ yeast. Appl. Microb. 1954, 2, 70–78. [Google Scholar]
- Leduc, M.; van Heijenoort, J. Autolysis of Escherichia coli. J. Bacteriol. 1980, 142, 52–59. [Google Scholar] [PubMed]
- Liedert, C.; Peltola, M.; Bernhardt, J.; Neubauer, P.; Salkinoja-Salonen, M. Physiology of resistant Deinococcus geothermalis bacterium aerobically cultivated in low-manganese medium. J. Bacteriol. 2012, 194, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Lemee, L.; Peuchant, E.; Clerc, M.; Brunner, M.; Pfander, H. Deinoxanthin: A new carotenoid isolated from Deinococcus radiodurans. Tetrahedron 1997, 53, 919–926. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wong, T.Y.; Chen, L.Y.; Lin, C.S.; Liu, J.K. Induction of a futile Embden-Meyerhof-Parnas pathway in Deinococcus radiodurans by Mn: Possible role of the pentose phosphate pathway in cell survival. Appl. Environ. Microb. 2000, 66, 105–112. [Google Scholar] [CrossRef]
- Makarova, K.S.; Omelchenko, M.V.; Gaidamakova, E.K.; Matrosova, V.Y.; Vasilenko, A.; Zhai, M.; Daly, M.J. Deinococcus geothermalis: The pool of extreme radiation resistance genes shrinks. PLoS ONE 2007, 2. [Google Scholar] [CrossRef]
- Venkateswaran, A.; McFarlan, S.C.; Ghosal, D.; Minton, K.W.; Vasilenko, A.; Makarova, K.; Daly, M.J. Physiologic determinants of radiation resistance in Deinococcus radiodurans. Appl. Environ. Microb. 2000, 66, 2620–2626. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bornot, J.; Molina-Jouve, C.; Uribelarrea, J.-L.; Gorret, N. Quantitative Characterization of the Growth of Deinococcus geothermalis DSM-11302: Effect of Inoculum Size, Growth Medium and Culture Conditions. Microorganisms 2015, 3, 441-463. https://doi.org/10.3390/microorganisms3030441
Bornot J, Molina-Jouve C, Uribelarrea J-L, Gorret N. Quantitative Characterization of the Growth of Deinococcus geothermalis DSM-11302: Effect of Inoculum Size, Growth Medium and Culture Conditions. Microorganisms. 2015; 3(3):441-463. https://doi.org/10.3390/microorganisms3030441
Chicago/Turabian StyleBornot, Julie, Carole Molina-Jouve, Jean-Louis Uribelarrea, and Nathalie Gorret. 2015. "Quantitative Characterization of the Growth of Deinococcus geothermalis DSM-11302: Effect of Inoculum Size, Growth Medium and Culture Conditions" Microorganisms 3, no. 3: 441-463. https://doi.org/10.3390/microorganisms3030441
APA StyleBornot, J., Molina-Jouve, C., Uribelarrea, J.-L., & Gorret, N. (2015). Quantitative Characterization of the Growth of Deinococcus geothermalis DSM-11302: Effect of Inoculum Size, Growth Medium and Culture Conditions. Microorganisms, 3(3), 441-463. https://doi.org/10.3390/microorganisms3030441





