Role of Bacterial Exopolysaccharides as Agents in Counteracting Immune Disorders Induced by Herpes Virus
Abstract
:1. Introduction
2. Herpes Simplex Virus
3. Marine Microbial Exopolysaccharides
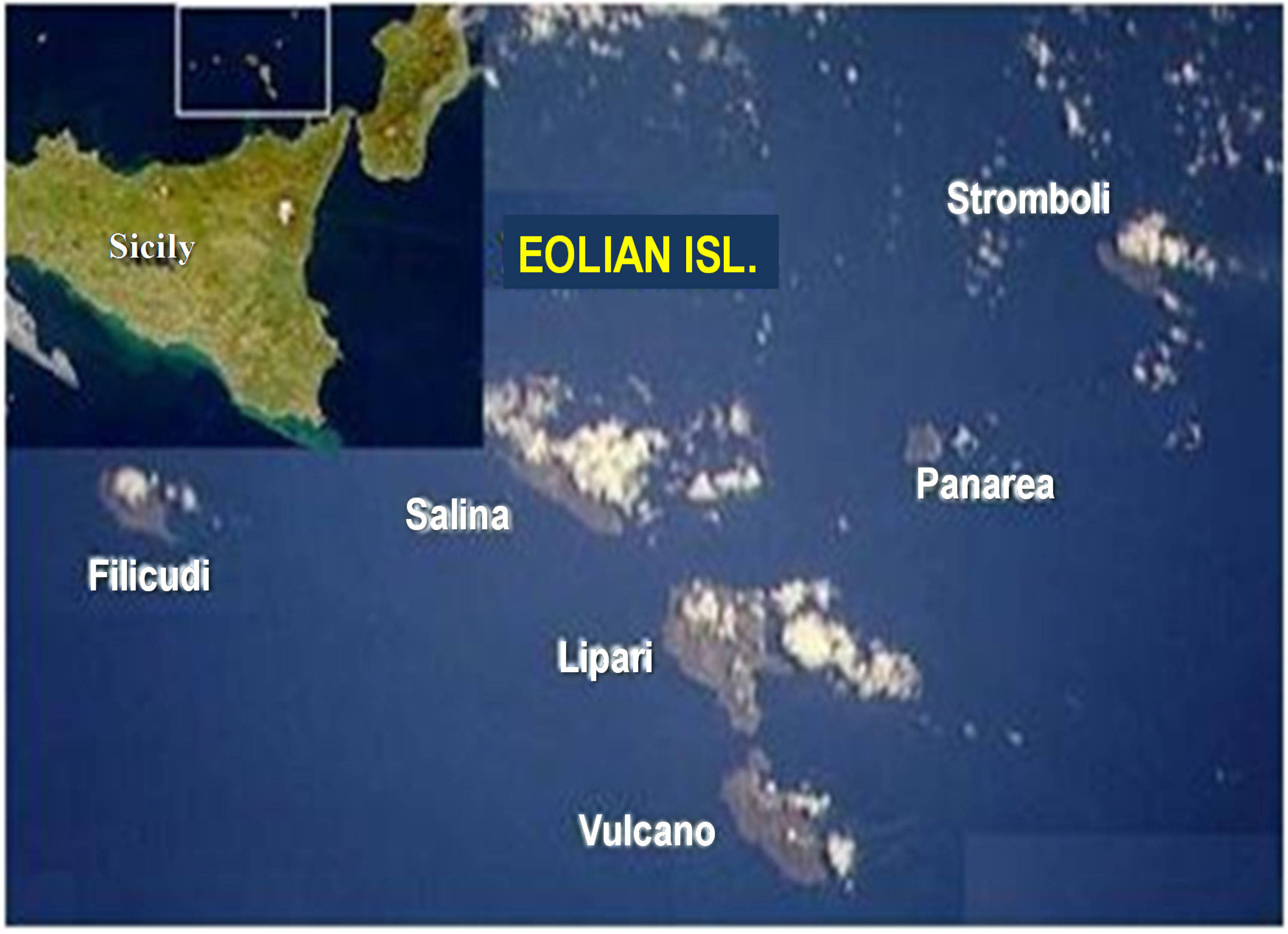
4. Eolian Shallow Vents and Bacterial EPS Producers
| Site | Depth (m) | T (°C) | pH | Conductivity (mS/cm) | Strain |
|---|---|---|---|---|---|
| Bottaro, Panarea Island | 8.0 | 55 | 5.4 | 42.90 | T14 |
| Porto di Levante, Vulcano Island | 0.7 | 70 | 5.2 | - | B3-15, B3-72 |
| Phenotypic Characteristics | Bacillus licheniformis | Geobacillus thermodenitrificans | |
|---|---|---|---|
| Strain B3-15 | Strain T14 | Strain B3-72 | |
| Growth temperature (°C) | 25–60 | 25–60 | 45–70 |
| Optimum temperature (°C) | 45 | 50 | 65 |
| Growth pH | 5.5–9 | 4–10 | 6–9 |
| Optimum pH | 7 | 8 | 7 |
| Growth NaCl | 0–7 | 2–10 | 0–2 |
| Optimum NaCl | 2 | 5 | 0 |
| Reduction of nitrate to nitrite | − | + | + |
| Catalase | + | + | − |
| Oxidase | + | + | − |
| Hydrolysis of: | |||
| Starch | − | + | − |
| Tween 20 | + | − | + |
| Tween 80 | + | − | − |
| Acid production from: | |||
| l-arabinose | − | + | − |
| d-galactose | − | + | − |
| d-glucose | + | + | − |
| Inositol | − | + | − |
| d-mannitol | + | + | − |
| d-sorbitol | − | + | − |
| Methyl-α-d-glucopyranoside | − | + | − |
| Amygdalin | − | + | − |
| Arbutin | − | + | + |
| Salicin | − | + | + |
| d-cellobiose | + | + | + |
| d-maltose | − | + | + |
| d-melibiose | − | + | |
| d-saccharose | − | + | + |
| d-melezitose | + | − | − |
| d-raffinose | − | − | + |
| Potassium 2-ketogluconate | + | − | + |
| Acid production from: | |||
| Potassium 5-ketogluconate | + | − | + |
| Antibiotic resistence to: | |||
| Bacitracin | + | − | − |
| Polymyxin B | + | + | − |
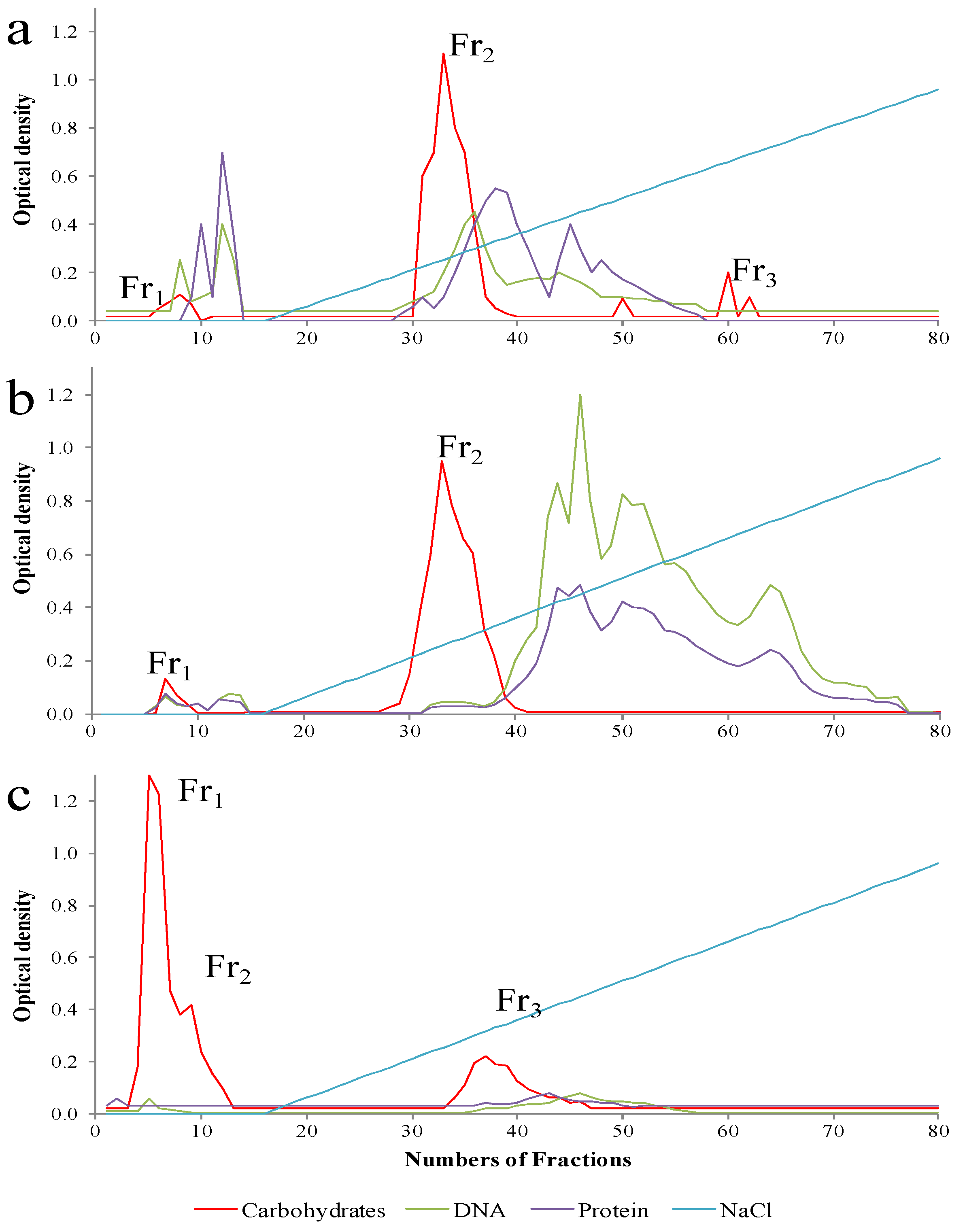
5. EPSs Production and Characterization
| Properties | EPS2-B3-15 | EPS2-B3-72 | EPS1-T14 |
|---|---|---|---|
| EPS production (mg·L−1) | 165 | 70 | 366 |
| Sugar-media | Glucose | Glucose | Sucrose |
| Carbohydrate content (%) | 66 | 80 | 99 |
| Protein content (%) | 5 | 3 | 1.2 |
| Molecular weight (KDa) | 600 | 400 | 1000 |
| Monosaccharide composition (ratio of relative portion) | Man | Man/Glu (1:0.2) | Fru/Fuc/Glu/GalN/Man (1.0:0.75:0.28:trace:trace) |
| Saccaride repeating unit | Tetrasaccharide | Trisaccharide | Trisaccharide |
| Anomeric configuration | Manno-pyranosidic | Manno-pyranosidic | Manno-pyranosidic |


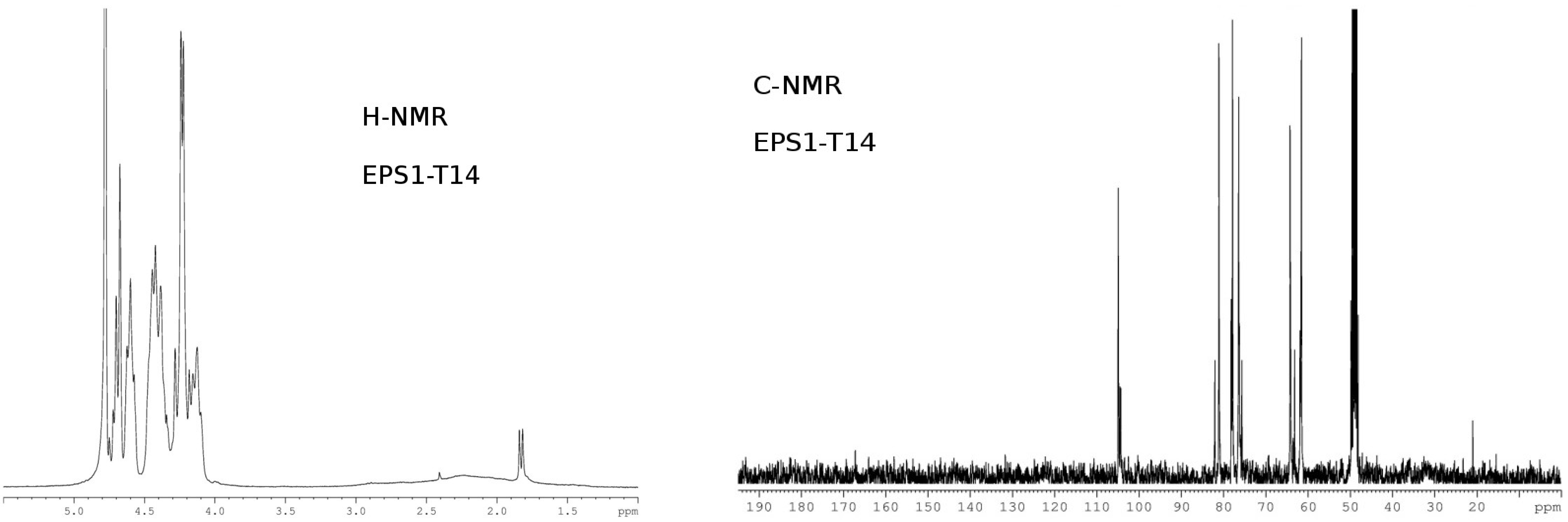
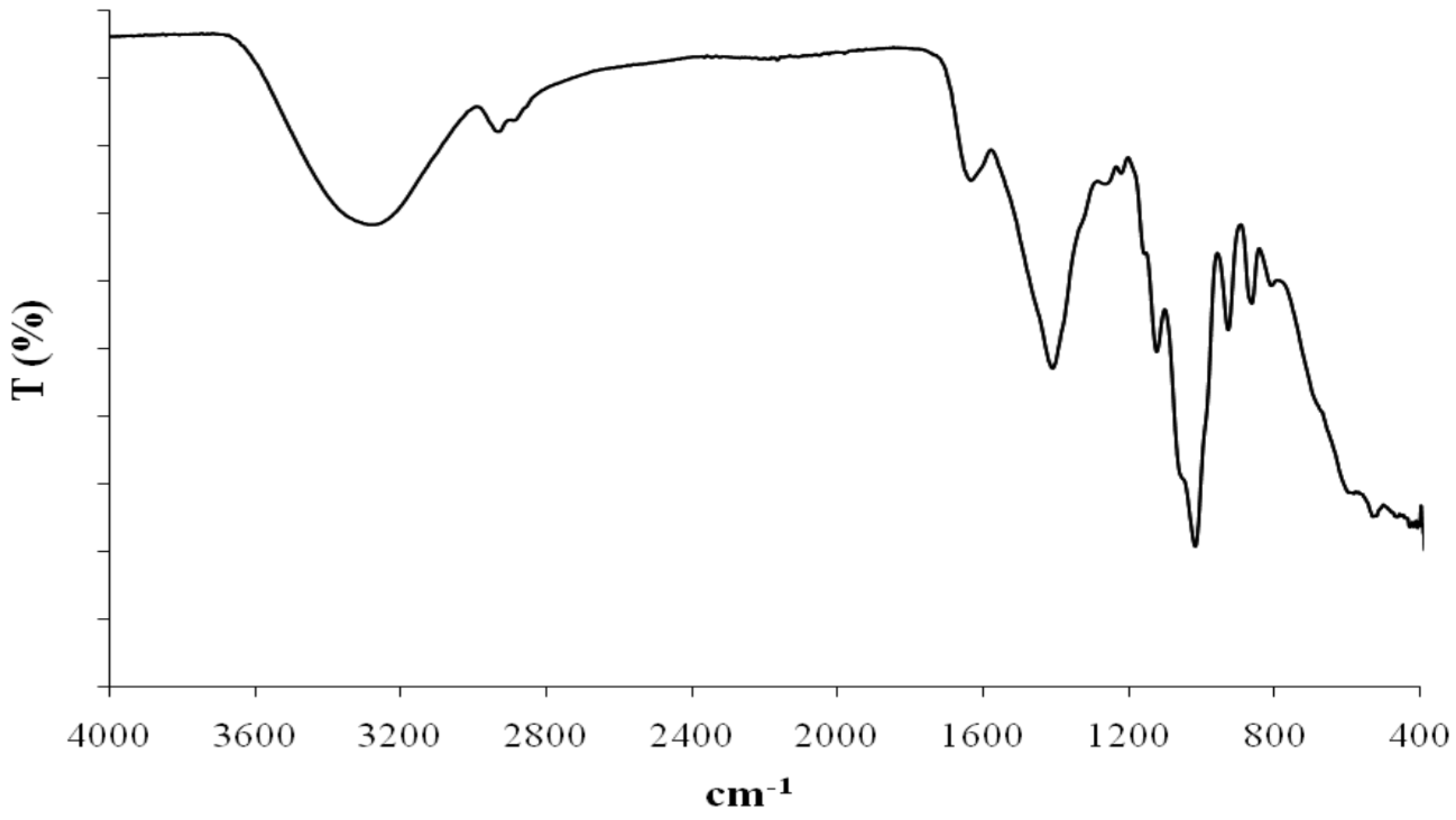
6. EPSs Anti-Herpes Virus Activity
| EPS | Cytotoxicity Percentage on PBMC Cells | |||||
|---|---|---|---|---|---|---|
| 200 μg·mL−1 * | 300 μg·mL−1 * | 400 μg·mL−1 * | 500 μg·mL−1 * | 600 μg·mL−1 * | 700 μg·mL−1 * | |
| EPS2-B3-15 | 0 | 0 | 12 ± 2.0 | 29 ± 5.0 | 48 ± 2.9 | 57 ± 4.5 |
| EPS2-B3-72 | 0 | 0 | 4 ± 0.6 | 12 ± 2.2 | 29 ± 6.8 | 40 ± 7.3 |
| EPS1-T14 | 0 | 0 | 0 | 12 ± 1.1 | 31 ± 4.9 | 60 ± 1.9 |
7. Immunomodulator and Immunostimulant Effects
| Inducer | IFN-γ | IFN-α | TNF-α | IL-12 | IL-18 |
|---|---|---|---|---|---|
| None | ˂0.06 | ˂3.1 | ˂0.13 | ˂2.1 | ˂9.2 |
| HSV-2 | ˂0.08 | ˂3.6 | ˂0.12 | ˂2.2 | ˂8.9 |
| EPS2-B3-15 (300 μg·mL−1) | 165 ± 19 *,† | 480 ± 76 *,† | 2151 ± 328 *,† | 420 ± 78 *,† | 140 ± 35 *,† |
| EPS2-B3-72 (300 μg·mL−1) | 115 ± 18 *,† | 116 ± 13 *,† | 1980 ± 101 *,† | 320 ± 49 *,† | 183 ± 29 *,† |
| EPS1-T14 (300 μg·mL−1) | 58 ± 13 *,† | 45 ± 3 *,† | 610 ± 43 *,† | 128 ± 19 *,† | 49 ± 3 *,† |
| EPS1-T14 (400 μg·mL−1) | 105 ± 28 *,† | 108 ± 25 *,† | 1310 ± 73 *,† | 358 ± 69 *,† | 86 ± 3 *,† |
| EPS2-B3-15 (300 μg·mL−1) + HSV-2 | 79 ± 24 | 295 ± 93 | 780 ± 98 | 115 ± 28 | 84 ± 22 |
| EPS2-B3-72 (300 μg·mL−1) + HSV-2 | 61 ± 9 | 42 ± 5 | 680 ± 71 | 122 ± 17 | 95 ± 13 |
| EPS1-T14 (300 μg·mL−1) + HSV-2 | 27 ± 2 | 29 ± 2 | 301 ± 28 | 57 ± 11 | 23 ± 8 |
| EPS1-T14 (400 μg·mL−1) + HSV-2 | 37 ± 2 | 26 ± 2 | 317 ± 88 | 166 ± 20 | 28 ± 10 |
| Inducer | IL-4 | IL-10 |
|---|---|---|
| None | ˂0.1 | ˂0.5 |
| HSV-2 | 41 ± 6 | 35 ± 5 |
| EPS2-B3-15 (300 μg·mL−1) | ˂0.1 | ˂0.5 |
| EPS2-B3-72 (300 μg·mL−1) | ˂0.1 | ˂0.5 |
| EPS1-T14 (400 μg·mL−1) | ˂0.1 | ˂0.5 |
| EPS2-B3-15 (300 μg·mL−1) + HSV-2 | ˂0.1 * | ˂0.5 * |
| EPS2-B3-72 (300 μg·mL−1) + HSV-2 | ˂0.1 * | ˂0.5 * |
| EPS1-T14 (400 μg·mL−1) + HSV-2 | 23 ± 6 | 37 ± 2 |
8. Conclusions
9. Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interests
References
- Maugeri, T.L.; Bianconi, G.; Canganella, F.; Danovaro, R.; Gugliandolo, C.; Italiano, F.; Lentini, V.; Manini, E.; Nicolaus, B. Shallow hydrothermal vents in the southern Tyrrhenian Sea. Chem. Ecol. 2010, 26, 285–298. [Google Scholar] [CrossRef]
- Maugeri, T.L.; Gugliandolo, C.; Caccamo, D.; Stackebrandt, E. A polyphasic taxonomic study of thermophilic bacilli from shallow, marine vents. Syst. Appl. Microbiol. 2001, 24, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, T.L.; Lentini, V.; Gugliandolo, C.; Italiano, F.; Cousin, S.; Stackebrandt, E. Bacterial and archaeal populations at two shallow hydrothermal vents of Panarea Island (Eolian Islands, Italy). Extremophiles 2009, 13, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, C.; Lentini, V.; Spanò, A.; Maugeri, T.L. New bacilli from shallow hydrothermal vents of Panarea Island (Italy) and their biotechnological potential. J. Appl. Microbiol. 2012, 112, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Finore, I.; di Donato, P.; Mastascusa, V.; Nicolaus, B.; Poli, A. Fermentation technologies for the optimization of marine microbial exopolysaccharide production. Mar. Drugs 2014, 12, 3005–3024. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, T.L.; Gugliandolo, C.; Caccamo, D.; Panico, A.; Lama, L.; Gambacorta, A.; Nicolaus, B. A halophilic thermotolerant Bacillus isolated from a marine hot spring able to produce a new exopolysaccharide. Biotechnol. Lett. 2002, 24, 515–519. [Google Scholar] [CrossRef]
- Nicolaus, B.; Panico, A.; Manca, M.C.; Lama, L.; Gambacorta, A.; Maugeri, T.L. A thermophilic Bacillus isolated from an Eolian shallow hydrothermal vent, able to produce exopolysaccharides. Syst. Appl. Microbiol. 2000, 23, 426–432. [Google Scholar] [CrossRef]
- Spanò, A.; Gugliandolo, C.; Lentini, V.; Maugeri, T.L.; Anzelmo, G.; Poli, A.; Nicolaus, B. A novel EPS-producing strain of Bacillus licheniformis isolated from a shallow vent off Panarea Island (Italy). Curr. Microbiol. 2013, 67, 21–29. [Google Scholar]
- Arena, A.; Maugeri, T.L.; Pavone, B.; Iannello, D.; Gugliandolo, C.; Bisignano, G. Antiviral and immunomodulatory effect of a novel exopolysaccharide from a marine thermotolerant Bacillus licheniformis. Int. Immunopharmacol. 2006, 6, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Gugliandolo, C.; Stassi, G.; Pavone, B.; Iannello, D.; Bisignano, G.; Maugeri, T.L. An exopolysaccharide produced by Geobacillus thermodenitrificans strain B3-72: Antiviral activity on immunocompetent cells. Immunol. Lett. 2009, 123, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.R.; Gustafson, K.; McMahon, J.; Shoemaker, R. Discovery of cyanovirin-N, a novel HIV-inactivating protein from Nostoc ellipsosporum that targets viral gp120. Int. Conf. AIDS 1996, 11, 71. [Google Scholar]
- Gugliandolo, C.; Spanò, A.; Lentini, V.; Arena, A.; Maugeri, T.L. Antiviral and immunomodulatory effects of a novel bacterial exopolysacchatide of shallow marine vent origin. J. Appl. Microbiol. 2014, 116, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara-Bell, J.; Lu, Y. Marine compounds and their antiviral activities. Antivir. Res. 2010, 86, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.S.; Ngo, D.H.; Ta, Q.V.; Kim, S.K. Marine organisms as a therapeutic source against herpes simplex virus infection. Eur. J. Pharm. Sci. 2011, 44, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Roizmann, B.; Desrosiers, R.C.; Fleckenstein, B.; Lopez, C.; Minson, A.C.; Studdert, M.J. The family herpesviridae: An update. The herpesvirus study group of the international committee on taxonomy of viruses. Arch. Virol. 1992, 123, 425–449. [Google Scholar] [CrossRef] [PubMed]
- White, M.K.; Gorrill, T.S.; Khalili, K. Reciprocal transactivation between HIV-1 and other human viruses. Virology 2006, 352, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Bisignano, C.; Stassi, G.; Mandalari, G.; Wickham, M.S.J.; Bisignano, G. Immunomodulatory and antiviral activity of almond skins. Immunol. Lett. 2010, 132, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.D.; Secrist, H.; DeKruyoff, R.H.; Wolf, S.F.; Umetsu, D.T. IL-12 inhibits the production of IL-4 and IL-10 in allergen specific human CD4 T lymphocytes. J. Immunol. 1995, 155, 111–117. [Google Scholar] [PubMed]
- García-Satre, A.; Biron, C.A. Type 1 interferons and the virus-host relationship: A lesson in Détente. Science 2006, 312, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Tsutsui, H.; Yoshimoto, T.; Adachi, O.; Yoshida, N.; Kishimoto, T.; Okamura, H.; Nakanishi, K.; Akira, S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 1998, 8, 383–390. [Google Scholar] [CrossRef]
- Fujioka, N.; Akazawa, R.; Ohashi, K.; Fujii, M.; Ikeda, M.; Kurimoto, M. Interleukin-18 protects mice against acute herpes simplex virus type 1 infection. J. Virol. 1999, 73, 2401–2409. [Google Scholar] [PubMed]
- Boulanger, M.J.; Chow, D.C.; Breynova, E.; Martick, M.; Stanford, G.; Nocholas, J. Molecular mechanisms for viral mimicry of a human cytokine: Activation of gp130 by HHV-8 interleukin-6. J. Mol. Biol. 2004, 335, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.B.; Sørensen, L.N.; Malmgaard, L.; Ank, N.; Baines, J.D.; Chen, Z.J. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J. Virol. 2007, 81, 13315–13324. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H.; Melchjorsen, J.; Malmgaard, L.; Casola, A.; Paludan, S.R. Suppression of proinflammatory cytokine expression by herpes simplex virus type 1. J. Virol. 2004, 78, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- Chew, T.; Taylor, K.E.; Mossman, K.L. Innate and adaptive immune responses to herpes simplex virus. Viruses 2009, 1, 979–1002. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W. Chemical studies of marine bacteria: Developing a new resource. Chem. Rev. 1993, 93, 1673–1683. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv. 2010, 28, 436–450. [Google Scholar]
- Donot, F.; Fontana, A.; Baccou, J.C.; Schorr-Galindo, S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012, 87, 951–962. [Google Scholar] [CrossRef]
- Decho, A.W. Microbial exopolymer secretions in ocean environments: Their role(s) in food webs and marine processes. In Oceanography Marine Biology; Barnes, M., Ed.; Aberdeen University Press: Aberdeen, UK, 1990; pp. 73–153. [Google Scholar]
- Nicolaus, B.; Kambourova, M.; Oner, E.T. Exopolysaccharides from extremophiles: From fundamentals to biotechnology. Environ. Technol. 2010, 31, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial exopolysaccharides from extreme habitat: Production, characterization and biological activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef] [PubMed]
- Guezennec, J.G. From extreme environments to biologically active exopolysaccharides. Commun. Agric. Appl. Biol. Sci. 2003, 68, 227–234. [Google Scholar] [PubMed]
- Nichols, C.M.; Lardière, S.G.; Bowman, J.P.; Nichols, P.D.; Gibson, J.A.E.; Guézennec, J. Chemical characterization of exopolysaccharides from Antarctic marine bacteria. Microb. Ecol. 2005, 49, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Laurenzio, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.; Alves, V.D.; Reis, M.A.M. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Delbarre-Ladrat, C.; Sinquin, C.; Lebellenger, L.; Zykwinska, A.; Colliec-Jouault, S. Exopolysaccharides produced by marine bacteria and their applications as glycosaminoglycan-like molecules. Front. Chem. 2014, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rechter, S.; Konig, T.; Auerochs, S.; Thulke, S.; Walter, H.; Dornenburg, H.; Walter, C.; Marschall, M. Antiviral activity of Arthrospira-derived spirulan-like substances. Antivir. Res. 2006, 72, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.A.; Mody, K.; Jha, B. Evaluation of biosurfactant/bioemulsifier production by a marine bacterium. Bull. Environ. Contam. Toxicol. 2007, 79, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Mody, K.; Jha, B. Characterization of an exopolysaccharide produced by a marine Enterobacter Cloacae. Indian J. Exp. Biol. 2005, 43, 467–471. [Google Scholar] [PubMed]
- Al-Nahas, M.O.; Darwish, M.M.; Ali, A.E.; Amin, M.A. Characterization of an exopolysaccharide-producing marine bacterium, isolate Pseudoalteromonas sp. AM. Afr. J. Microbiol. Res. 2011, 5, 3823–3831. [Google Scholar] [CrossRef]
- Matsuda, M.; Shigeta, S.; Okutani, K. Antiviral activities of marine Pseudomonas polysaccharides and their oversulfated derivatives. Mar. Biotechnol. 1999, 1, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Rinker, K.D.; Kelly, R.M. Effect of carbon and nitrogen sources on growth dynamics and exopolysaccharide production for the hyper thermophilic archaeon Thermococcus litoralis and bacterium Thermotoga maritima. Biotechnol. Bioeng. 2000, 69, 537–547. [Google Scholar] [CrossRef]
- Ito, M.; Baba, M.; Hirabayashi, K.; Matsumoto, T.; Suzuki, M.; Suzuki, S.; Shigeta, S.; de Clercq, E. In vitro activity of mannan sulfate, a novel sulfated polysaccharide, against human immunodeficiency virus type I and other enveloped viruses. Eur. J. Clin. Microbiol. 1989, 8, 171–173. [Google Scholar] [CrossRef]
- Lin, M.H.; Yang, Y.L.; Chen, Y.P.; Hua, K.F.; Lu, C.P.; Sheu, F.; Lin, G.H.; Tsay, S.S.; Liang, S.M.; Wu, S.H. A novel exopolysaccharide from the biofilm of Thermus aquaticus YT-1 induces the immune response through Toll-like receptor 2. J. Biol. Chem. 2011, 286, 17736–17745. [Google Scholar] [CrossRef] [PubMed]
- Bejar, V.; Llamas, I.; Calvo, C.; Quesada, E. Characterization of exopolysaccharides produced by 19 halophilic strains of the species Halomonas eurihalina. J. Biotechnol. 1998, 61, 135–141. [Google Scholar] [CrossRef]
- Bouchotroch, S.; Quesada, E.; del Moral, A.; Llamas, I.; Bejar, V. Halomonas maura sp. nov., a novel moderately halophilic, exopolysaccharide-producing bacterium. Int. J. Syst. Evol. Microbiol. 2001, 51, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Arias, S.; Ruiz-Garcìa, C.; Martinez-Cánovas, J.; Páez, R. Moderately halophilic exopolysaccharide-producing bacteria. In Halophilic Microorganisms; Ventosa, A., Ed.; Springer-Verlag: Heidelberg, Germany, 2004; pp. 297–314. [Google Scholar]
- Martínez-Cánovas, M.J.; Béjar, V.; Martínez-Checa, F.; Quesada, E. Halomonas anticariensis sp. nov., from Fuente de Piedra, a saline-wetland wildfowl reserve in Malaga, Southern Spain. Int. J. Syst. Evol. Microbiol. 2004, 54, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cánovas, M.J.; Quesada, E.; Llamas, I.; Béjar, V. Halomonas ventosae sp. nov., a moderately halophilic, denitrifying, exopolysaccharide-producing bacterium. Int. J. Syst. Evol. Microbil. 2004, 54, 733–737. [Google Scholar] [CrossRef]
- Llamas, I.; del Moral, A.; Martınez-Checa, F.; Arco, Y.; Arias, S.; Quesada, E. Halomonas maura is a physiologically versatile bacterium of both ecological and biotechnological interest. Antonie van Leeuwenhoek 2006, 89, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Snoeck, R.; Pauwels, R.; de Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988, 32, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.A.; Carlucci, M.J.; Matulewicz, M.C.; Damonte, E.B. Natural sulfated polysaccharides for the prevention and control of viral infections. Top Heterocycl. Chem. 2007, 11, 259–281. [Google Scholar]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiol. 2009, 19, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Péterszegi, G.; Fodil-Bourahla, I.; Robert, A.M.; Robert, L. Pharmacological properties of fucose. Applications in age-related modifications of connective tissues. Biomed. Pharmacother. 2003, 57, 240–245. [Google Scholar] [CrossRef]
- Uyangaa, E.; Patil, A.M.; Eo, S.K. Prophylactic and therapeutic modulation of innate and adaptive immunity against mucosal infection of herpes simplex virus. Immune Netw. 2014, 14, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Tommonaro, G.; Poli, A.; di Donato, P.; Abbamondi, G.R.; Finore, I.; Nicolaus, B. Bioactive polysaccharides of vegetable and microbial origins: An overview. In Handbook of Polymers for Pharmaceutical Technologies; Vijay, K.T., Manju, K.T., Eds.; Wiley-Blackwell: Washington, DC, USA, 2015; Chapter 1; pp. 1–32. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugliandolo, C.; Spanò, A.; Maugeri, T.L.; Poli, A.; Arena, A.; Nicolaus, B. Role of Bacterial Exopolysaccharides as Agents in Counteracting Immune Disorders Induced by Herpes Virus. Microorganisms 2015, 3, 464-483. https://doi.org/10.3390/microorganisms3030464
Gugliandolo C, Spanò A, Maugeri TL, Poli A, Arena A, Nicolaus B. Role of Bacterial Exopolysaccharides as Agents in Counteracting Immune Disorders Induced by Herpes Virus. Microorganisms. 2015; 3(3):464-483. https://doi.org/10.3390/microorganisms3030464
Chicago/Turabian StyleGugliandolo, Concetta, Antonio Spanò, Teresa L. Maugeri, Annarita Poli, Adriana Arena, and Barbara Nicolaus. 2015. "Role of Bacterial Exopolysaccharides as Agents in Counteracting Immune Disorders Induced by Herpes Virus" Microorganisms 3, no. 3: 464-483. https://doi.org/10.3390/microorganisms3030464
APA StyleGugliandolo, C., Spanò, A., Maugeri, T. L., Poli, A., Arena, A., & Nicolaus, B. (2015). Role of Bacterial Exopolysaccharides as Agents in Counteracting Immune Disorders Induced by Herpes Virus. Microorganisms, 3(3), 464-483. https://doi.org/10.3390/microorganisms3030464









