Harnessing Microbial Power for a Sustainable Future Food System
Abstract
1. Introduction
- Section 2 describes the PRISMA-guided methodology and the process of keyword mapping across two decades of studies.
- Section 3 presents the results for each of the five domains, including bioremediation, biofertilization, biofuels, biochemical synthesis, and next-generation food systems, highlighting their mechanisms, benefits, and limitations.
- Section 4 discusses the potential of microbial innovations to drive the transition toward sustainable food systems, addressing regulatory and societal challenges for large-scale implementation.
- Section 5 summarizes the key conclusions and provides insights into future research directions.
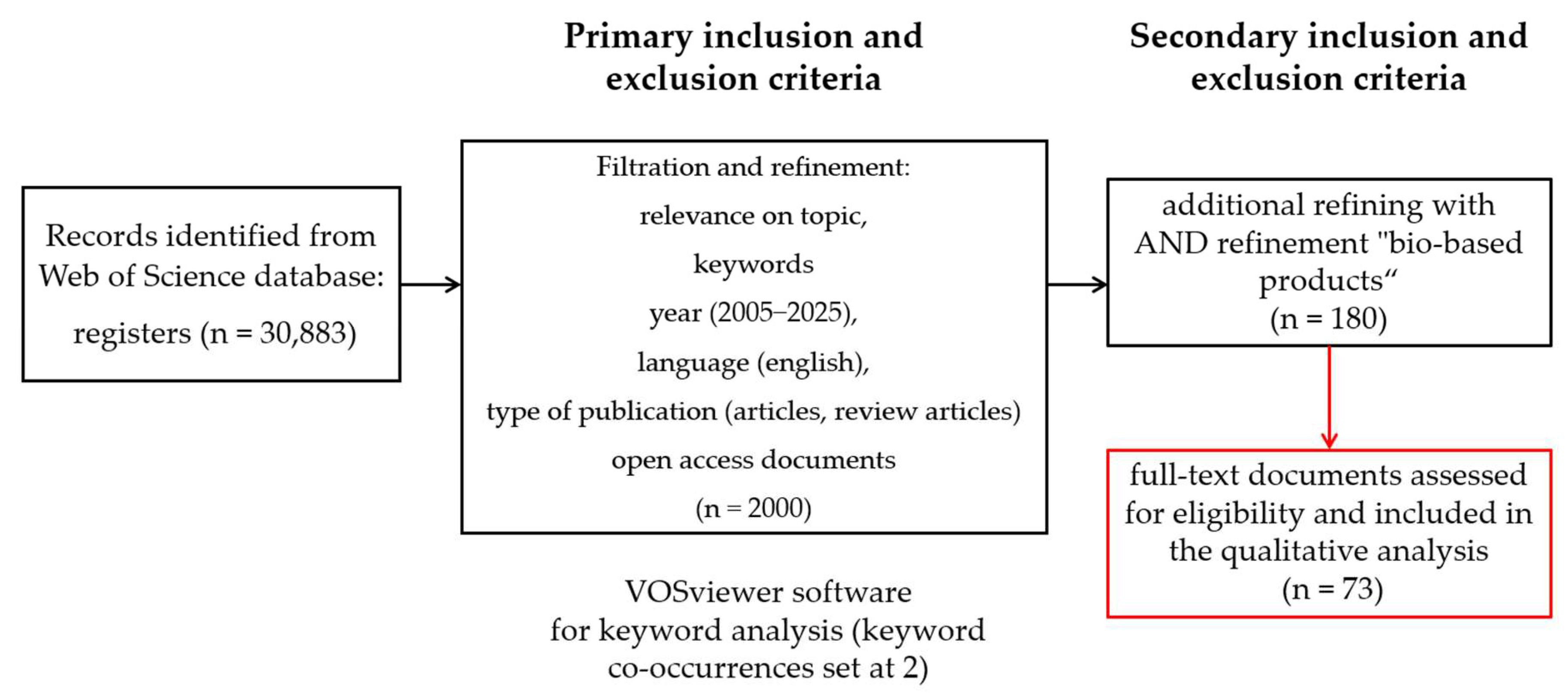
2. Materials and Methods
3. Results
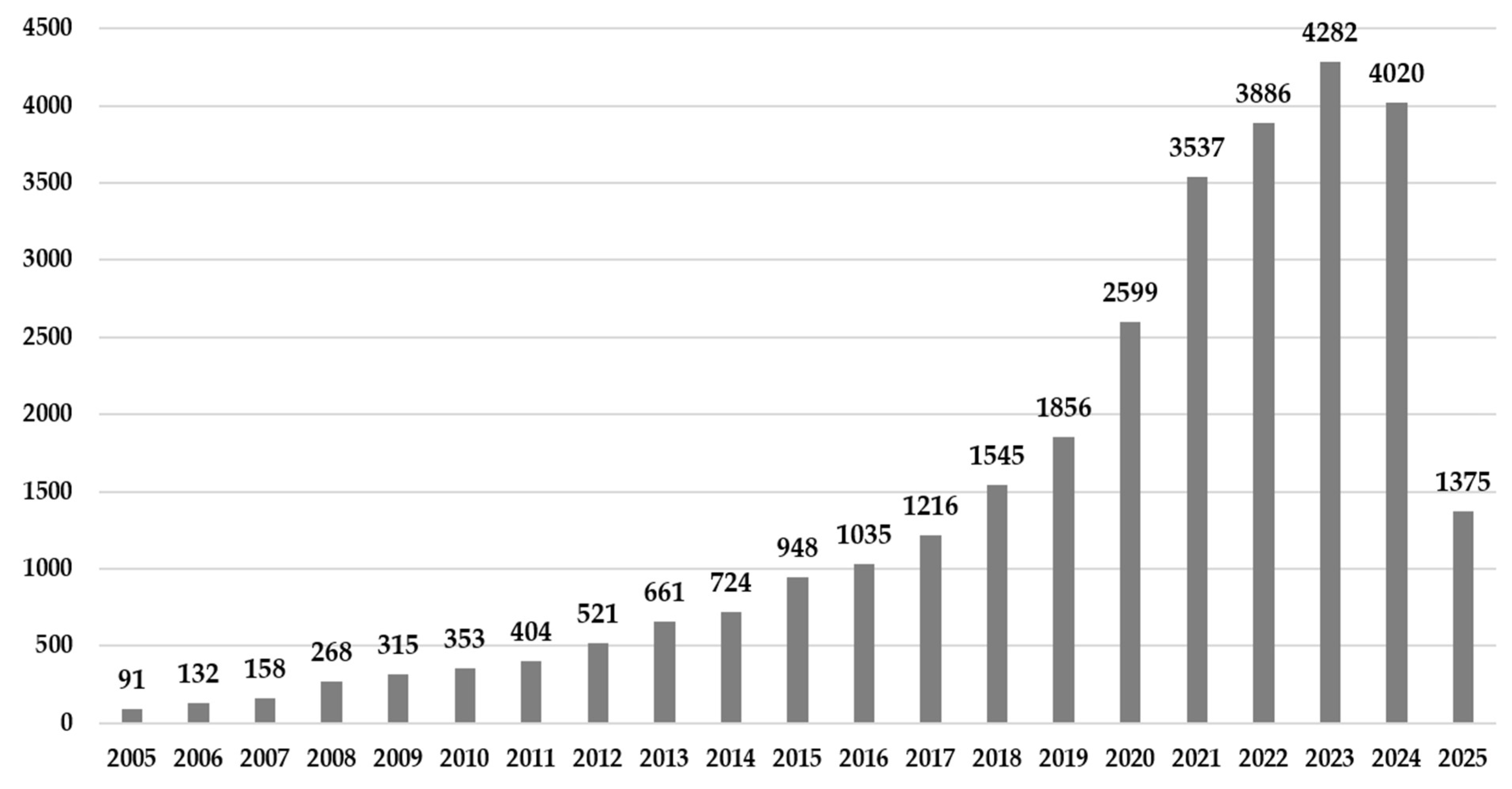
3.1. Context Analysis
3.2. Content Analysis
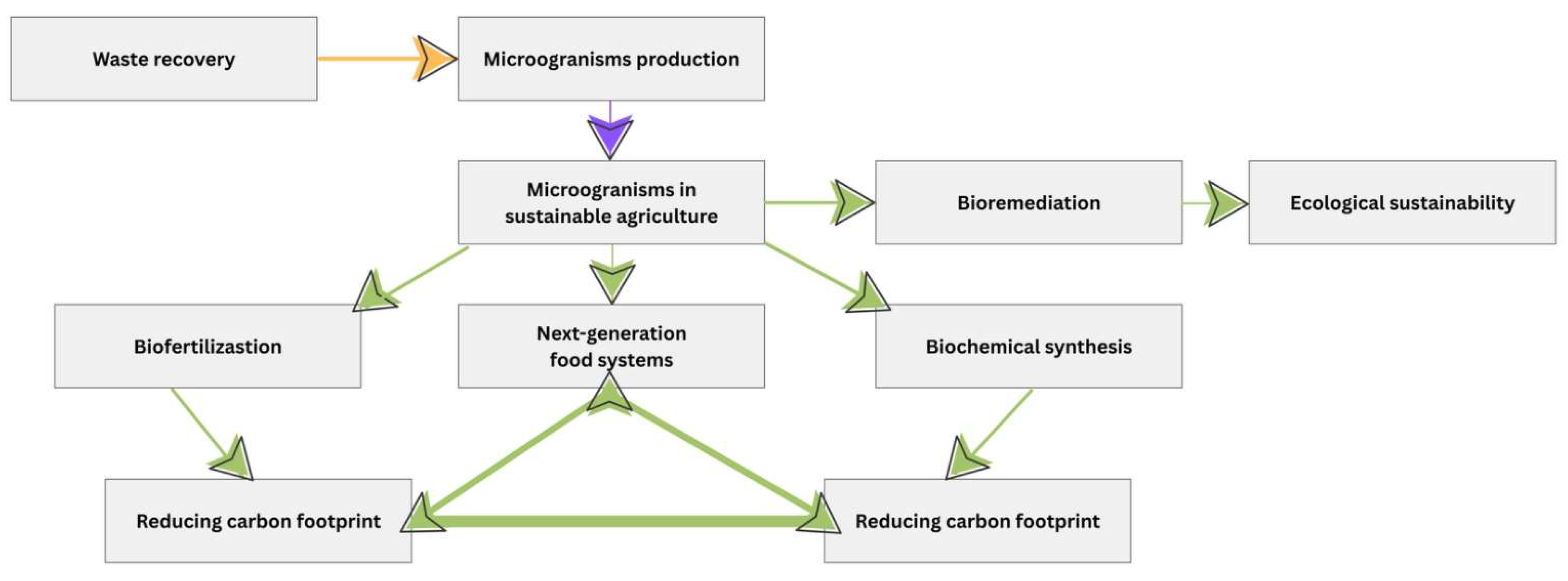
3.2.1. Bioremediation
Microorganisms in the Bioremediation Process
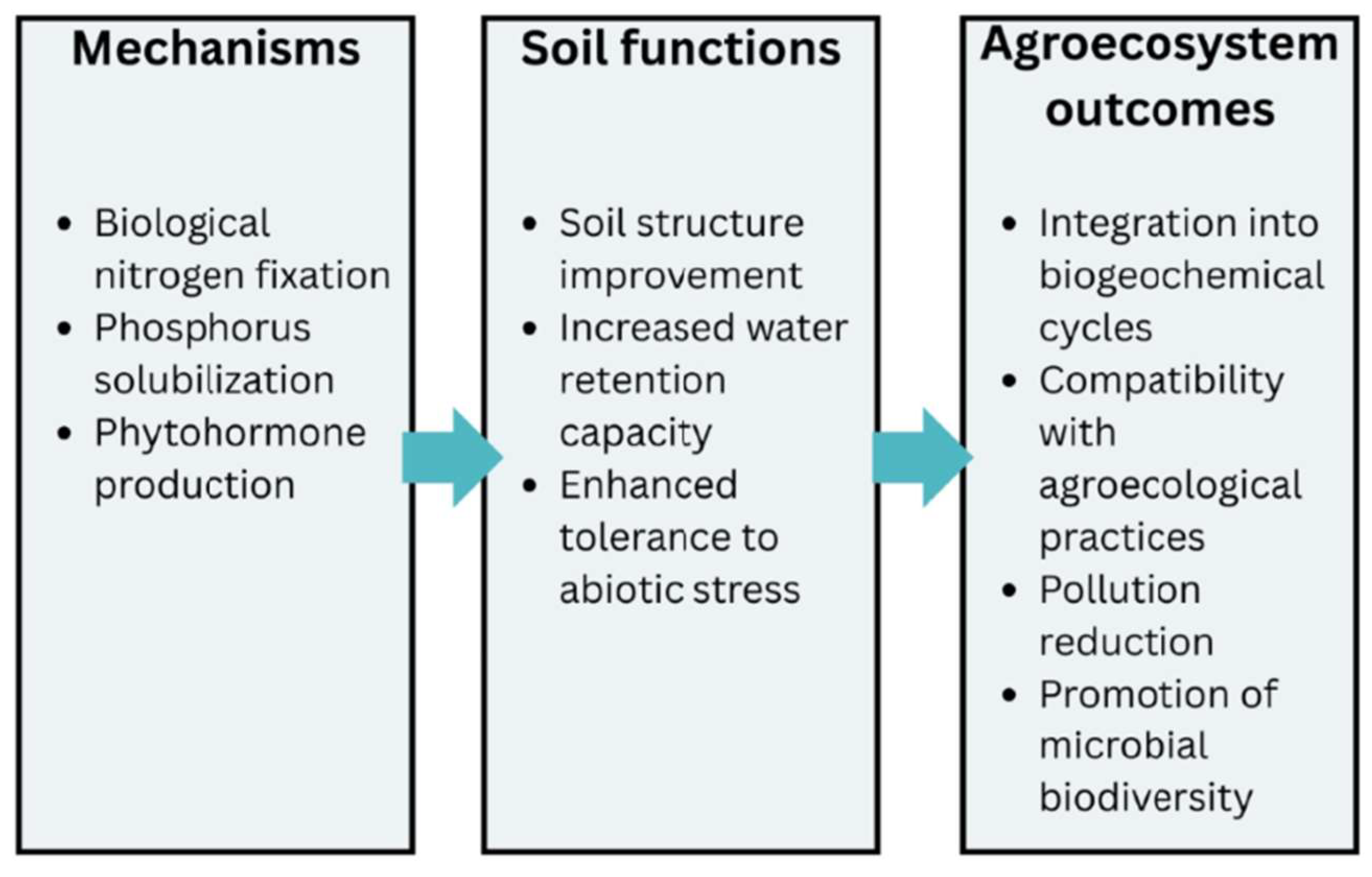
3.2.2. Biofertilization
Microorganisms Involved in the Biofertilization Process
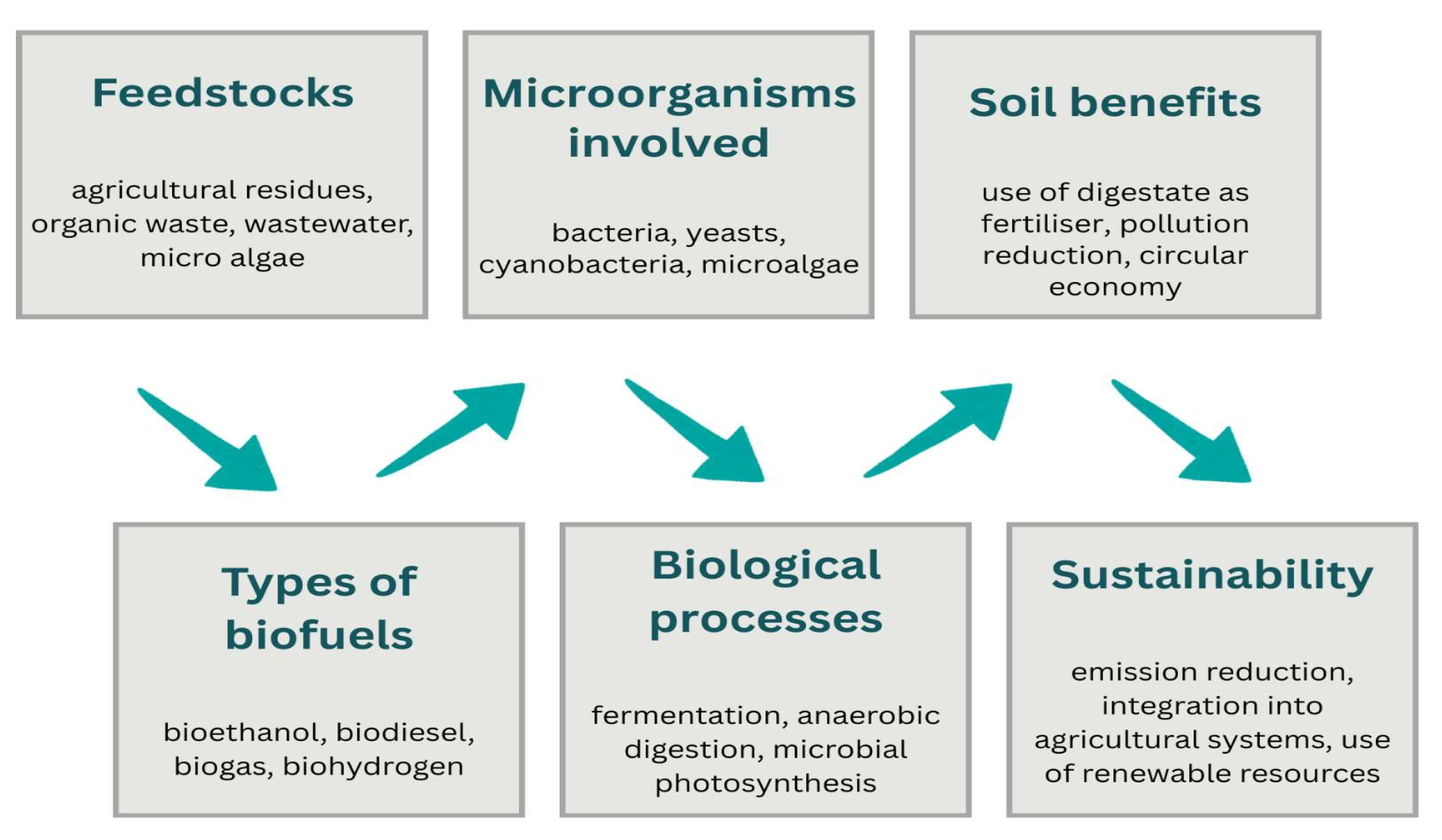
3.2.3. Biofuel Production
Microorganisms in the Biofuel Production Process
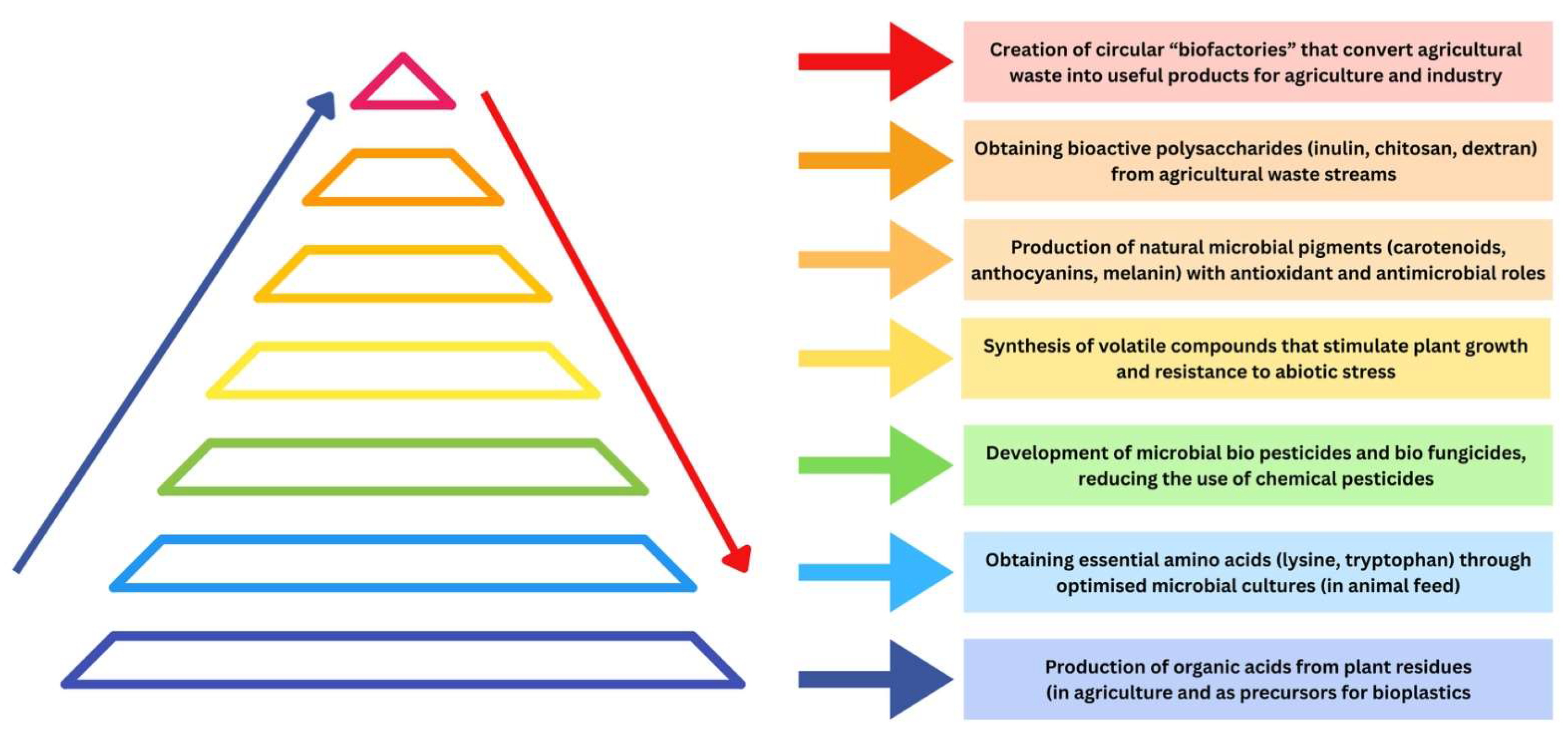
3.2.4. Biochemical Synthesis
Microorganisms in Biochemical Synthesis for Sustainable Agriculture
3.2.5. Next-Generation Food Systems
Key Innovations for Next Generation Food Systems
Challenges in Implementing Next Generation Food Systems
Recommendations for Overcoming the Challenges Associated with Next Generation Food Systems
4. Discussion
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schneider, K.R.; Fanzo, J.; Haddad, L.; Herrero, M.; Moncayo, J.R.; Herforth, A.; Remans, R.; Guarin, A.; Resnick, D.; Covic, N.; et al. The State of Food Systems Worldwide in the Countdown to 2030. Nat. Food 2023, 4, 1090–1110. [Google Scholar] [CrossRef]
- Puma, M.J.; Bose, S.; Chon, S.Y.; Cook, B.I. Assessing the Evolving Fragility of the Global Food System. Environ. Res. Lett. 2015, 10, 024007. [Google Scholar] [CrossRef]
- Calo, A.; McKee, A.; Perrin, C.; Gasselin, P.; McGreevy, S.; Sippel, S.R.; Desmarais, A.A.; Shields, K.; Baysse-Lainé, A.; Magnan, A.; et al. Achieving Food System Resilience Requires Challenging Dominant Land Property Regimes. Front. Sustain. Food Syst. 2021, 5, 683544. [Google Scholar] [CrossRef]
- Pereira, L.M.; Drimie, S.; Maciejewski, K.; Tonissen, P.B.; Biggs, R. Food System Transformation: Integrating a Political–Economy and Social–Ecological Approach to Regime Shifts. Int. J. Environ. Res. Public Health 2020, 17, 1313. [Google Scholar] [CrossRef] [PubMed]
- Linder, T. Making the Case for Edible Microorganisms as an Integral Part of a More Sustainable and Resilient Food Production System. Food Secur. 2019, 11, 265–278. [Google Scholar] [CrossRef]
- Linder, T. Beyond Agriculture─How Microorganisms Can Revolutionize Global Food Production. ACS Food Sci. Technol. 2023, 3, 1144–1152. [Google Scholar] [CrossRef]
- Ferrocino, I.; Rantsiou, K.; McClure, R.; Kostic, T.; de Souza, R.S.C.; Lange, L.; FitzGerald, J.; Kriaa, A.; Cotter, P.; Maguin, E.; et al. The Need for an Integrated Multi-OMICs Approach in Microbiome Science in the Food System. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1082–1103. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.M.; Crispie, F.; Claesson, M.J.; Cotter, P.D. Translating Omics to Food Microbiology. Annu. Rev. Food Sci. Technol. 2017, 8, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, C.; Chen, T.; Chen, X.; Zhao, X. Integrating Metabolomics into a Systems Biology Framework to Exploit Metabolic Complexity: Strategies and Applications in Microorganisms. Appl. Microbiol. Biotechnol. 2006, 70, 151–161. [Google Scholar] [CrossRef]
- Augustin, M.A.; Hartley, C.J.; Maloney, G.; Tyndall, S. Innovation in Precision Fermentation for Food Ingredients. Crit. Rev. Food Sci. Nutr. 2024, 64, 6218–6238. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Ganeshan, S.; Wang, Y.; Tülbek, M.Ç.; Nickerson, M.T. Bioengineered Enzymes and Precision Fermentation in the Food Industry. Int. J. Mol. Sci. 2023, 24, 10156. [Google Scholar] [CrossRef] [PubMed]
- Niyigaba, T.; Küçükgöz, K.; Kołożyn-Krajewska, D.; Królikowski, T.; Trząskowska, M. Advances in Fermentation Technology: A Focus on Health and Safety. Appl. Sci. 2025, 15, 3001. [Google Scholar] [CrossRef]
- Tsegaye, B.; Jaiswal, S.; Jaiswal, A.K. Food Waste Biorefinery: Pathway towards Circular Bioeconomy. Foods 2021, 10, 1174. [Google Scholar] [CrossRef]
- Hassoun, A.; Bekhit, A.E.-D.; Jambrak, A.R.; Regenstein, J.M.; Chemat, F.; Morton, J.D.; Gudjónsdóttir, M.; Carpena, M.; Prieto, M.A.; Varela, P.; et al. The Fourth Industrial Revolution in the Food Industry—Part II: Emerging Food Trends. Crit. Rev. Food Sci. Nutr. 2024, 64, 407–437. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste Biorefinery Models towards Sustainable Circular Bioeconomy: Critical Review and Future Perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhang, X.; Yin, H.; Zhang, X. Current Knowledge on Molecular Mechanisms of Microorganism-Mediated Bioremediation for Arsenic Contamination: A Review. Microbiol. Res. 2022, 258, 126990. [Google Scholar] [CrossRef] [PubMed]
- Masciandaro, G.; Macci, C.; Peruzzi, E.; Ceccanti, B.; Doni, S. Organic Matter–Microorganism–Plant in Soil Bioremediation: A Synergic Approach. Rev. Environ. Sci. Biotechnol. 2013, 12, 399–419. [Google Scholar] [CrossRef]
- Milić, J.; Avdalović, J.; Knudsen, T.Š. Microbial Bioremediation of the Oil Polluted Environment and the Sustainable Development Goals of Pillar Planet of the Agenda 2030. Environ. Dev. Sustain. 2024, 26, 30355–30377. [Google Scholar] [CrossRef]
- Dhankhar, N.; Kumar, J. Impact of Increasing Pesticides and Fertilizers on Human Health: A Review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Kowalska, A.; Biczak, R. Phytoremediation and Environmental Law: Harnessing Biomass and Microbes to Restore Soils and Advance Biofuel Innovation. Energies 2025, 18, 1860. [Google Scholar] [CrossRef]
- Megharaj, M.; Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Bioremediation Approaches for Organic Pollutants: A Critical Perspective. Environ. Int. 2011, 37, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K.; Dash, B.; Baliyarsingh, B. Microbial Remediation of Persistent Agro-Chemicals by Soil Bacteria: An Overview. In Microbial Biotechnology: Volume 2. Application in Food and Pharmacology; Patra, J.K., Das, G., Shin, H.-S., Eds.; Springer: Singapore, 2018; pp. 275–301. ISBN 978-981-10-7140-9. [Google Scholar]
- Qattan, S.Y.A. Harnessing Bacterial Consortia for Effective Bioremediation: Targeted Removal of Heavy Metals, Hydrocarbons, and Persistent Pollutants. Environ. Sci. Eur. 2025, 37, 85. [Google Scholar] [CrossRef]
- Odukkathil, G.; Vasudevan, N. Toxicity and Bioremediation of Pesticides in Agricultural Soil. Rev. Environ. Sci. Biotechnol. 2013, 12, 421–444. [Google Scholar] [CrossRef]
- Refaey, M.; Abdel-Azeem, A.M.; Abo Nahas, H.H.; Abdel-Azeem, M.A.; El-Saharty, A.A. Role of Fungi in Bioremediation of Soil Contaminated with Heavy Metals. In Industrially Important Fungi for Sustainable Development: Volume 1: Biodiversity and Ecological Perspectives; Abdel-Azeem, A.M., Yadav, A.N., Yadav, N., Usmani, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 509–540. ISBN 978-3-030-67561-5. [Google Scholar]
- Akpasi, S.O.; Anekwe, I.M.S.; Tetteh, E.K.; Amune, U.O.; Shoyiga, H.O.; Mahlangu, T.P.; Kiambi, S.L. Mycoremediation as a Potentially Promising Technology: Current Status and Prospects—A Review. Appl. Sci. 2023, 13, 4978. [Google Scholar] [CrossRef]
- Khatoon, Z.; Orozco-Mosqueda, M.d.C.; Santoyo, G. Microbial Contributions to Heavy Metal Phytoremediation in Agricultural Soils: A Review. Microorganisms 2024, 12, 1945. [Google Scholar] [CrossRef]
- Verma, S.; Kuila, A. Bioremediation of Heavy Metals by Microbial Process. Environ. Technol. Innov. 2019, 14, 100369. [Google Scholar] [CrossRef]
- Karigar, C.S.; Rao, S.S. Role of Microbial Enzymes in the Bioremediation of Pollutants: A Review. Enzym. Res. 2011, 2011, 805187. [Google Scholar] [CrossRef]
- Tyagi, M.; da Fonseca, M.M.R.; de Carvalho, C.C.C.R. Bioaugmentation and Biostimulation Strategies to Improve the Effectiveness of Bioremediation Processes. Biodegradation 2011, 22, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.; Tawari-Fufeyin, P.; Okoro, S. (PDF) Bioremediation, Biostimulation and Bioaugmention: A Review. Int. J. Environ. Bioremediation Biodegrad. 2015, 3, 28–39. [Google Scholar] [CrossRef]
- Yaman, C. Performance and Kinetics of Bioaugmentation, Biostimulation, and Natural Attenuation Processes for Bioremediation of Crude Oil-Contaminated Soils. Processes 2020, 8, 883. [Google Scholar] [CrossRef]
- Ape, F.; Manini, E.; Quero, G.M.; Luna, G.M.; Sarà, G.; Vecchio, P.; Brignoli, P.; Ansferri, S.; Mirto, S. Biostimulation of in Situ Microbial Degradation Processes in Organically-Enriched Sediments Mitigates the Impact of Aquaculture. Chemosphere 2019, 226, 715–725. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef]
- Raffa, C.M.; Chiampo, F. Bioremediation of Agricultural Soils Polluted with Pesticides: A Review. Bioengineering 2021, 8, 92. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Sofy, M.R.; Almoneafy, A.A.; Abdelhamid, M.T.; Basit, A.; Sofy, A.R.; Lone, R.; Abou-El-Enain, M.M. Role of Microorganisms in Managing Soil Fertility and Plant Nutrition in Sustainable Agriculture. In Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management; Mohamed, H.I., El-Beltagi, H.E.-D.S., Abd-Elsalam, K.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 93–114. ISBN 978-3-030-66587-6. [Google Scholar]
- Tejada, M.; Hernandez, M.T.; Garcia, C. Soil Restoration Using Composted Plant Residues: Effects on Soil Properties. Soil Tillage Res. 2009, 102, 109–117. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen Fixing Azotobacter Species as Potential Soil Biological Enhancers for Crop Nutrition and Yield Stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef] [PubMed]
- Kaschuk, G.; Hungria, M. Diversity and Importance of Diazotrophic Bacteria to Agricultural Sustainability in the Tropics. In Diversity and Benefits of Microorganisms from the Tropics; de Azevedo, J.L., Quecine, M.C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 269–292. ISBN 978-3-319-55804-2. [Google Scholar]
- Ibrahim, M.; Iqbal, M.; Tang, Y.-T.; Khan, S.; Guan, D.-X.; Li, G. Phosphorus Mobilization in Plant–Soil Environments and Inspired Strategies for Managing Phosphorus: A Review. Agronomy 2022, 12, 2539. [Google Scholar] [CrossRef]
- Deubel, A.; Merbach, W. Influence of Microorganisms on Phosphorus Bioavailability in Soils. In Microorganisms in Soils: Roles in Genesis and Functions; Varma, A., Buscot, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 177–191. ISBN 978-3-540-26609-9. [Google Scholar]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef]
- Hart, M.M.; Forsythe, J.A. Using Arbuscular Mycorrhizal Fungi to Improve the Nutrient Quality of Crops; Nutritional Benefits in Addition to Phosphorus. Sci. Hortic. 2012, 148, 206–214. [Google Scholar] [CrossRef]
- Bhantana, P.; Rana, M.S.; Sun, X.; Moussa, M.G.; Saleem, M.H.; Syaifudin, M.; Shah, A.; Poudel, A.; Pun, A.B.; Bhat, M.A.; et al. Arbuscular Mycorrhizal Fungi and Its Major Role in Plant Growth, Zinc Nutrition, Phosphorous Regulation and Phytoremediation. Symbiosis 2021, 84, 19–37. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of Arbuscular Mycorrhizal Fungi, Phosphate–Solubilizing Bacteria, and Silicon to P Uptake by Plant. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.d.C.; Santoyo, G.; Glick, B.R. Recent Advances in the Bacterial Phytohormone Modulation of Plant Growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef]
- Amara, U.; Khalid, R.; Hayat, R. Soil Bacteria and Phytohormones for Sustainable Crop Production. In Bacterial Metabolites in Sustainable Agroecosystem; Maheshwari, D.K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 87–103. ISBN 978-3-319-24654-3. [Google Scholar]
- Njaramanana, N.M.R.; Rahetlah, V.B.; Trap, J.; Autfray, P. Field Arbuscular Mycorrhizal Inoculation Increased Plant Performance without Phosphorus Fertilizer Supply of Four Promoted Upland Rice Varieties in Madagascar. Exp. Agric. 2022, 58, e57. [Google Scholar] [CrossRef]
- Rog, I.; van der Heijden, M.G.A.; Bender, F.; Boussageon, R.; Lambach, A.; Schlaeppi, K.; Bodenhausen, N.; Lutz, S. Mycorrhizal Inoculation Success Depends on Soil Health and Crop Productivity. FEMS Microbiol. Lett. 2025, 372, fnaf031. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.M.; Jones, D.L.; Chadwick, D.R.; Qi, X.; Cotta, S.R.; Araújo, V.L.V.P.; Matteoli, F.P.; Lacerda-Júnior, G.V.; Pereira, A.P.A.; Fernandes-Júnior, P.I.; et al. Can Arbuscular Mycorrhizal Fungi and Rhizobacteria Facilitate 33P Uptake in Maize Plants under Water Stress? Microbiol. Res. 2023, 271, 127350. [Google Scholar] [CrossRef]
- Semenov, V.M.; Tulina, A.S.; Semenova, N.A.; Ivannikova, L.A. Humification and Nonhumification Pathways of the Organic Matter Stabilization in Soil: A Review. Eurasian Soil Sci. 2013, 46, 355–368. [Google Scholar] [CrossRef]
- Tang, J.; Mo, Y.; Zhang, J.; Zhang, R. Influence of Biological Aggregating Agents Associated with Microbial Population on Soil Aggregate Stability. Appl. Soil Ecol. 2011, 47, 153–159. [Google Scholar] [CrossRef]
- Jiang, Z.; Shi, M.; Shi, L. Degradation of Organic Contaminants and Steel Corrosion by the Dissimilatory Metal-Reducing Microorganisms Shewanella and Geobacter Spp. Int. Biodeterior. Biodegrad. 2020, 147, 104842. [Google Scholar] [CrossRef]
- Khanpour-Alikelayeh, E.; Partovinia, A. Synergistic and Antagonistic Effects of Microbial Co-Culture on Bioremediation of Polluted Environments. In Microbial Rejuvenation of Polluted Environment: Volume 2; Panpatte, D.G., Jhala, Y.K., Eds.; Springer: Singapore, 2021; pp. 229–265. ISBN 978-981-15-7455-9. [Google Scholar]
- Olivera, N.L.; Nievas, M.L.; Lozada, M.; del Prado, G.; Dionisi, H.M.; Siñeriz, F. Isolation and Characterization of Biosurfactant-Producing Alcanivorax Strains: Hydrocarbon Accession Strategies and Alkane Hydroxylase Gene Analysis. Res. Microbiol. 2009, 160, 19–26. [Google Scholar] [CrossRef]
- Medić, A.B.; Karadžić, I.M. Pseudomonas in Environmental Bioremediation of Hydrocarbons and Phenolic Compounds-Key Catabolic Degradation Enzymes and New Analytical Platforms for Comprehensive Investigation. World J. Microbiol. Biotechnol. 2022, 38, 165. [Google Scholar] [CrossRef] [PubMed]
- Rakić, I.Z.; Đurović, A.D.; Kevrešan, Ž.S.; Kovač, R.M.; Kravić, S.Ž.; Panić, S.N.; Svirčev, Z.B.; Stojanović, Z.S. Exploring Biosorption and Bioaccumulation Capacities of Cyanobacteria Nostoc and Anabaena for Remediation of Heavy Metals in Wastewater. Int. J. Environ. Sci. Technol. 2025, 22, 12905–12922. [Google Scholar] [CrossRef]
- Rana, S.; Handa, S.; Aggarwal, Y.; Puri, S.; Chatterjee, M. Role of Candida in the Bioremediation of Pollutants: A Review. Lett. Appl. Microbiol. 2023, 76, ovad103. [Google Scholar] [CrossRef]
- Sannino, F.; Nuzzo, A.; Ventorino, V.; Pepe, O.; Piccolo, A. Effective Degradation of Organic Pollutants in Aqueous Media by Microbial Strains Isolated from Soil of a Contaminated Industrial Site. Chem. Biol. Technol. Agric. 2016, 3, 2. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, H. Microbial Consortia Are Needed to Degrade Soil Pollutants. Microorganisms 2022, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Agarwal, S.; Agarwal Malik, R.; Kumar, G.; Pal, D.B.; Mandal, M.; Sarkar, A.; Bantun, F.; Haque, S.; Singh, P.; et al. Recent Advances in Microbial Engineering Approaches for Wastewater Treatment: A Review. Bioengineered 2023, 14, 2184518. [Google Scholar] [CrossRef]
- Altomare, C.; Tringovska, I. Beneficial Soil Microorganisms, an Ecological Alternative for Soil Fertility Management. In Genetics, Biofuels and Local Farming Systems; Lichtfouse, E., Ed.; Sustainable Agriculture Reviews; Springer Netherlands: Dordrecht, The Netherlands, 2011; Volume 7, pp. 161–214. ISBN 978-94-007-1520-2. [Google Scholar]
- Chaudhary, P.; Xu, M.; Ahamad, L.; Chaudhary, A.; Kumar, G.; Adeleke, B.S.; Verma, K.K.; Hu, D.-M.; Širić, I.; Kumar, P.; et al. Application of Synthetic Consortia for Improvement of Soil Fertility, Pollution Remediation, and Agricultural Productivity: A Review. Agronomy 2023, 13, 643. [Google Scholar] [CrossRef]
- Wang, G.; Ren, Y.; Bai, X.; Su, Y.; Han, J. Contributions of Beneficial Microorganisms in Soil Remediation and Quality Improvement of Medicinal Plants. Plants 2022, 11, 3200. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Singh, S.; Chaudhary, A.; Sharma, A.; Kumar, G. Overview of Biofertilizers in Crop Production and Stress Management for Sustainable Agriculture. Front. Plant Sci. 2022, 13, 930340. [Google Scholar] [CrossRef] [PubMed]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for Beneficial Microorganisms Inocula Used as Biofertilizers. Sci. World J. 2012, 2012, 491206. [Google Scholar] [CrossRef] [PubMed]
- Sumbul, A.; Ansari, R.A.; Rizvi, R.; Mahmood, I. Azotobacter: A Potential Bio-Fertilizer for Soil and Plant Health Management. Saudi J. Biol. Sci. 2020, 27, 3634–3640. [Google Scholar] [CrossRef]
- Nosheen, S.; Ajmal, I.; Song, Y. Microbes as Biofertilizers, a Potential Approach for Sustainable Crop Production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]
- Zaman, W.; Ayaz, A.; Puppe, D. Biogeochemical Cycles in Plant–Soil Systems: Significance for Agriculture, Interconnections, and Anthropogenic Disruptions. Biology 2025, 14, 433. [Google Scholar] [CrossRef]
- Misu, I.J.; Kayess, M.O.; Siddiqui, M.N.; Gupta, D.R.; Islam, M.N.; Islam, T. Microbiome Engineering for Sustainable Rice Production: Strategies for Biofertilization, Stress Tolerance, and Climate Resilience. Microorganisms 2025, 13, 233. [Google Scholar] [CrossRef]
- Ibáñez, A.; Garrido-Chamorro, S.; Vasco-Cárdenas, M.F.; Barreiro, C. From Lab to Field: Biofertilizers in the 21st Century. Horticulturae 2023, 9, 1306. [Google Scholar] [CrossRef]
- Giri, S.; Shitut, S.; Kost, C. Harnessing Ecological and Evolutionary Principles to Guide the Design of Microbial Production Consortia. Curr. Opin. Biotechnol. 2020, 62, 228–238. [Google Scholar] [CrossRef]
- Ghosh, S.; Chowdhury, R.; Bhattacharya, P. Mixed Consortia in Bioprocesses: Role of Microbial Interactions. Appl. Microbiol. Biotechnol. 2016, 100, 4283–4295. [Google Scholar] [CrossRef] [PubMed]
- Jehani, M.D.; Singh, S.; Kumar, D.; Kumar, G. Azospirillum—A Free-Living Nitrogen-Fixing Bacterium. In Rhizobiome; Parray, J.A., Shameem, N., Egamberdieva, D., Sayyed, R.Z., Eds.; Microbiome Research in Plants and Soil; Academic Press: Cambridge, MA, USA, 2023; pp. 285–308. ISBN 978-0-443-16030-1. [Google Scholar]
- Wani, S.A.; Chand, S.; Wani, M.A.; Ramzan, M.; Hakeem, K.R. Azotobacter Chroococcum–A Potential Biofertilizer in Agriculture: An Overview. In Soil Science: Agricultural and Environmental Prospectives; Hakeem, K.R., Akhtar, J., Sabir, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 333–348. ISBN 978-3-319-34451-5. [Google Scholar]
- Abd-Alla, M.H.; Al-Amri, S.M.; El-Enany, A.-W.E. Enhancing Rhizobium–Legume Symbiosis and Reducing Nitrogen Fertilizer Use Are Potential Options for Mitigating Climate Change. Agriculture 2023, 13, 2092. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Olaniyan, F.T.; Alori, E.T.; Adekiya, A.O.; Ayorinde, B.B.; Daramola, F.Y.; Osemwegie, O.O.; Babalola, O.O. The Use of Soil Microbial Potassium Solubilizers in Potassium Nutrient Availability in Soil and Its Dynamics. Ann. Microbiol. 2022, 72, 45. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Soleymani, A. The Roles of Plant-Growth-Promoting Rhizobacteria (PGPR)-Based Biostimulants for Agricultural Production Systems. Plants 2024, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Negi, R.; Yadav, N.; Yadav, A.N. Microbial Biofertilizers: A Paradigm Shift towards Agricultural Sustainability. Biologia 2025, 80, 389–414. [Google Scholar] [CrossRef]
- Zeng, W.; Xiang, D.; Li, X.; Gao, Q.; Chen, Y.; Wang, K.; Qian, Y.; Wang, L.; Li, J.; Mi, Q.; et al. Effects of Combined Inoculation of Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizosphere Bacteria on Seedling Growth and Rhizosphere Microecology. Front. Microbiol. 2025, 15, 1475485. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, M.V.; Poudyal, R.S.; Tiwari, I.; Voloshin, R.A.; Zharmukhamedov, S.K.; Nam, H.G.; Zayadan, B.K.; Bruce, B.D.; Hou, H.J.M.; Allakhverdiev, S.I. Biofuel Production: Challenges and Opportunities. Int. J. Hydrog. Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Wen, F.; Nair, N.U.; Zhao, H. Protein Engineering in Designing Tailored Enzymes and Microorganisms for Biofuels Production. Curr. Opin. Biotechnol. 2009, 20, 412–419. [Google Scholar] [CrossRef]
- Elshahed, M.S. Microbiological Aspects of Biofuel Production: Current Status and Future Directions. J. Adv. Res. 2010, 1, 103–111. [Google Scholar] [CrossRef]
- Alfenore, S.; Molina-Jouve, C. Current Status and Future Prospects of Conversion of Lignocellulosic Resources to Biofuels Using Yeasts and Bacteria. Process Biochem. 2016, 51, 1747–1756. [Google Scholar] [CrossRef]
- Ni, Y.; Sun, Z. Recent Progress on Industrial Fermentative Production of Acetone–Butanol–Ethanol by Clostridium Acetobutylicum in China. Appl. Microbiol. Biotechnol. 2009, 83, 415–423. [Google Scholar] [CrossRef]
- Vamsi Krishna, K.; Bharathi, N.; George Shiju, S.; Alagesan Paari, K.; Malaviya, A. An Updated Review on Advancement in Fermentative Production Strategies for Biobutanol Using Clostridium Spp. Environ. Sci. Pollut. Res. 2022, 29, 47988–48019. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Yadav, A.N.; Khannam, K.S.; Saxena, A.K.; Suman, A. Potassium-Solubilizing Microbes: Diversity, Distribution, and Role in Plant Growth Promotion. In Microorganisms for Green Revolution: Volume 1: Microbes for Sustainable Crop Production; Panpatte, D.G., Jhala, Y.K., Vyas, R.V., Shelat, H.N., Eds.; Springer: Singapore, 2017; pp. 125–149. ISBN 978-981-10-6241-4. [Google Scholar]
- Khan, S.; Siddique, R.; Sajjad, W.; Nabi, G.; Hayat, K.M.; Duan, P.; Yao, L. Biodiesel Production From Algae to Overcome the Energy Crisis. HAYATI J. Biosci. 2017, 24, 163–167. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Yasin, N.H.M.; Derek, C.J.C.; Lim, J.K. Microalgae as a Sustainable Energy Source for Biodiesel Production: A Review. Renew. Sustain. Energy Rev. 2011, 15, 584–593. [Google Scholar] [CrossRef]
- Al-Hammadi, M.; Güngörmüşler, M. New Insights into Chlorella Vulgaris Applications. Biotechnol. Bioeng. 2024, 121, 1486–1502. [Google Scholar] [CrossRef] [PubMed]
- Mutungwazi, A.; Ijoma, G.N.; Matambo, T.S. The Significance of Microbial Community Functions and Symbiosis in Enhancing Methane Production during Anaerobic Digestion: A Review. Symbiosis 2021, 83, 1–24. [Google Scholar] [CrossRef]
- Lin, L. Bottom-up Synthetic Ecology Study of Microbial Consortia to Enhance Lignocellulose Bioconversion. Biotechnol. Biofuels Bioprod. 2022, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Rudra, S.; Khatun, I.; Sinha, N.; Sen, M.; Ghosh, D. Concise Review on Lignocellulolytic Microbial Consortia for Lignocellulosic Waste Biomass Utilization: A Way Forward? Microbiology 2023, 92, 301–317. [Google Scholar] [CrossRef]
- Vinzelj, J.; Joshi, A.; Insam, H.; Podmirseg, S.M. Employing Anaerobic Fungi in Biogas Production: Challenges & Opportunities. Bioresour. Technol. 2020, 300, 122687. [Google Scholar] [CrossRef]
- Merlin Christy, P.; Gopinath, L.R.; Divya, D. A Review on Anaerobic Decomposition and Enhancement of Biogas Production through Enzymes and Microorganisms. Renew. Sustain. Energy Rev. 2014, 34, 167–173. [Google Scholar] [CrossRef]
- Nzila, A. Mini Review: Update on Bioaugmentation in Anaerobic Processes for Biogas Production. Anaerobe 2017, 46, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Das, S.R.; Basak, N. Molecular Biohydrogen Production by Dark and Photo Fermentation from Wastes Containing Starch: Recent Advancement and Future Perspective. Bioprocess Biosyst. Eng. 2021, 44, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-H.; Chang, Y.-T.; Chang, Y.-J. Roles of Microorganisms Other than Clostridium and Enterobacter in Anaerobic Fermentative Biohydrogen Production Systems—A Review. Bioresour. Technol. 2011, 102, 8437–8444. [Google Scholar] [CrossRef] [PubMed]
- Torres de Souza, I.; Moreira, F.S.; de Souza Ferreira, J.; Cardoso, V.L.; Batista, F.R.X. Technological Advances in Hydrogen Production by Enterobacter Bacteria upon Substrate, Luminosity and Anaerobic Conditions. Int. J. Hydrog. Energy 2019, 44, 16190–16198. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Mohd Soom, M.A. Trends in Bio-Hydrogen Generation—A Review. Environ. Sci. 2006, 3, 255–271. [Google Scholar] [CrossRef]
- Lau, M.W.; Gunawan, C.; Balan, V.; Dale, B.E. Comparing the Fermentation Performance of Escherichia Coli KO11, Saccharomyces Cerevisiae 424A(LNH-ST) and Zymomonas Mobilis AX101 for Cellulosic Ethanol Production. Biotechnol. Biofuels 2010, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Dianursanti; Santoso, A. Increasing Lipid Accumulation of Chlorella vulgaris Using Spirulina platensis in Flat Plate Reactor for Synthesizing Biodiesel. Energy Procedia 2015, 65, 58–66. [Google Scholar] [CrossRef]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of Temperature and Nitrogen Concentration on the Growth and Lipid Content of Nannochloropsis oculata and Chlorella vulgaris for Biodiesel Production. Chem. Eng. Process. Process Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Senila, L.; Kovacs, E.; Roman, C. Chemical Characterization, Lipid Profile, and Volatile Compounds in Chlorella Sp. and Spirulina Platensis: A Promising Feedstock for Various Applications. Molecules 2025, 30, 1499. [Google Scholar] [CrossRef] [PubMed]
- Zagrodnik, R.; Łaniecki, M. Hydrogen Production from Starch by Co-Culture of Clostridium acetobutylicum and Rhodobacter sphaeroides in One Step Hybrid Dark- and Photofermentation in Repeated Fed-Batch Reactor. Bioresour. Technol. 2017, 224, 298–306. [Google Scholar] [CrossRef]
- Torzillo, G.; Scoma, A.; Faraloni, C.; Giannelli, L. Advances in the Biotechnology of Hydrogen Production with the Microalga Chlamydomonas Reinhardtii. Crit. Rev. Biotechnol. 2015, 35, 485–496. [Google Scholar] [CrossRef]
- McKinlay, J.B.; Harwood, C.S. Photobiological Production of Hydrogen Gas as a Biofuel. Curr. Opin. Biotechnol. 2010, 21, 244–251. [Google Scholar] [CrossRef]
- Jourdain, L.; Gu, W. Designing Synthetic Microbial Communities for Enhanced Anaerobic Waste Treatment. Appl. Environ. Microbiol. 2025, 91, e00404-25. [Google Scholar] [CrossRef]
- Wang, S.; Xu, C.; Song, L.; Zhang, J. Anaerobic Digestion of Food Waste and Its Microbial Consortia: A Historical Review and Future Perspectives. Int. J. Environ. Res. Public Health 2022, 19, 9519. [Google Scholar] [CrossRef]
- Clomburg, J.M.; Gonzalez, R. Biofuel Production in Escherichia Coli: The Role of Metabolic Engineering and Synthetic Biology. Appl. Microbiol. Biotechnol. 2010, 86, 419–434. [Google Scholar] [CrossRef]
- Pabbathi, N.P.P.; Velidandi, A.; Pogula, S.; Gandam, P.K.; Baadhe, R.R. Advancements in Metabolic Engineering: Enhancing Biofuel Production Through Escherichia Coli and Saccharomyces Cerevisiae Models. Processes 2025, 13, 2115. [Google Scholar] [CrossRef]
- Intasit, R.; Kim, B.S. Sustainable Biodiesel Production from Agricultural Lignocellulosic Waste via Oleaginous Microbial Processes. BMC Biotechnol. 2025, 25, 84. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Ali, M.M.; Osman, A.I.; Elgarahy, A.M.; Samer, M.; Xu, Y.; Liu, Z. A Critical Review on Conversion Technology for Liquid Biofuel Production from Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2025, 217, 115726. [Google Scholar] [CrossRef]
- Antoszewski, M.; Mierek-Adamska, A.; Dąbrowska, G.B. The Importance of Microorganisms for Sustainable Agriculture—A Review. Metabolites 2022, 12, 1100. [Google Scholar] [CrossRef]
- Du, J.; Shao, Z.; Zhao, H. Engineering Microbial Factories for Synthesis of Value-Added Products. J. Ind. Microbiol. Biotechnol. 2011, 38, 873–890. [Google Scholar] [CrossRef] [PubMed]
- Möhle, S.; Zirbes, M.; Rodrigo, E.; Gieshoff, T.; Wiebe, A.; Waldvogel, S.R. Modern Electrochemical Aspects for the Synthesis of Value-Added Organic Products. Angew. Chem. Int. Ed. 2018, 57, 6018–6041. [Google Scholar] [CrossRef]
- Vishnu, D.; Dhandapani, B.; Mahadevan, S. Recent Advances in Organic Acid Production from Microbial Sources by Utilizing Agricultural By-Products as Substrates for Industrial Applications. In Bioprocess Engineering for Bioremediation: Valorization and Management Techniques; Jerold, M., Arockiasamy, S., Sivasubramanian, V., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 67–87. ISBN 978-3-030-57911-1. [Google Scholar]
- Di Lorenzo, R.D.; Serra, I.; Porro, D.; Branduardi, P. State of the Art on the Microbial Production of Industrially Relevant Organic Acids. Catalysts 2022, 12, 234. [Google Scholar] [CrossRef]
- Fatih Demirbas, M. Biorefineries for Biofuel Upgrading: A Critical Review. Appl. Energy 2009, 86, S151–S161. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.U.; Park, J.H.; Park, J.M.; Kim, T.Y. Metabolic Engineering of Microorganisms: General Strategies and Drug Production. Drug Discov. Today 2009, 14, 78–88. [Google Scholar] [CrossRef]
- Krivoruchko, A.; Nielsen, J. Production of Natural Products through Metabolic Engineering of Saccharomyces cerevisiae. Curr. Opin. Biotechnol. 2015, 35, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Cao, Y.; Zou, H.; Xian, M. Metabolic Engineering of Escherichia Coli for Biotechnological Production of High-Value Organic Acids and Alcohols. Appl. Microbiol. Biotechnol. 2011, 89, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Yafetto, L.; Odamtten, G.T.; Wiafe-Kwagyan, M. Valorization of Agro-Industrial Wastes into Animal Feed through Microbial Fermentation: A Review of the Global and Ghanaian Case. Heliyon 2023, 9, e14814. [Google Scholar] [CrossRef]
- Mahmoud, Y.A.-G.; El-Naggar, M.E.; Abdel-Megeed, A.; El-Newehy, M. Recent Advancements in Microbial Polysaccharides: Synthesis and Applications. Polymers 2021, 13, 4136. [Google Scholar] [CrossRef]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current Status of Biotechnological Production and Applications of Microbial Exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Coban, H.B. Organic Acids as Antimicrobial Food Agents: Applications and Microbial Productions. Bioprocess Biosyst. Eng. 2020, 43, 569–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Song, Y.; An, Y.; Lu, Y.; Zhong, G. Soil Microorganisms: Their Role in Enhancing Crop Nutrition and Health. Diversity 2024, 16, 734. [Google Scholar] [CrossRef]
- Singh nee’ Nigam, P. Production of Organic Acids from Agro-Industrial Residues. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Singh nee’ Nigam, P., Pandey, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 37–60. ISBN 978-1-4020-9942-7. [Google Scholar]
- Schneider, J.; Niermann, K.; Wendisch, V.F. Production of the Amino Acids L-Glutamate, l-Lysine, l-Ornithine and l-Arginine from Arabinose by Recombinant Corynebacterium glutamicum. J. Biotechnol. 2011, 154, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Takeno, S. Amino Acid Production by Corynebacterium Glutamicum. In Corynebacterium glutamicum: Biology and Biotechnology; Yukawa, H., Inui, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 107–147. ISBN 978-3-642-29857-8. [Google Scholar]
- Razak, M.A.; Viswanath, B. Optimization of Fermentation Upstream Parameters and Immobilization of Corynebacterium Glutamicum MH 20-22 B Cells to Enhance the Production of l-Lysine. 3 Biotech 2015, 5, 531–540. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a Promising Alternative to Synthetic Pesticides: A Case for Microbial Pesticides, Phytopesticides, and Nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef]
- Seenivasagan, R.; Babalola, O.O. Utilization of Microbial Consortia as Biofertilizers and Biopesticides for the Production of Feasible Agricultural Product. Biology 2021, 10, 1111. [Google Scholar] [CrossRef]
- Maharana, C.; Padala, V.K.; Hubballi, A.B.; Nikhil Raj, M.; Paschapur, A.; Bhat, C.; Singh, A.K.; Subbanna, A.R.N.S. Secondary Metabolites of Microbials as Potential Pesticides. In Sustainable Management of Potato Pests and Diseases; Chakrabarti, S.K., Sharma, S., Shah, M.A., Eds.; Springer: Singapore, 2022; pp. 111–142. ISBN 978-981-16-7695-6. [Google Scholar]
- Chouhan, A.; Tiwari, A. Production of Polyhydroxyalkanoate (PHA) Biopolymer from Crop Residue Using Bacteria as an Alternative to Plastics: A Review. RSC Adv. 2025, 15, 11845–11862. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, V.; Singh, P.K.; Mahata, C.; Jeon, J.-M.; Kumar, G.; Yang, Y.-H.; Bhatia, S.K. A Review on Microbes Mediated Resource Recovery and Bioplastic (Polyhydroxyalkanoates) Production from Wastewater. Microb. Cell Factories 2024, 23, 187. [Google Scholar] [CrossRef] [PubMed]
- Akinsemolu, A.; Onyeaka, H.; Fagunwa, O.; Adenuga, A.H. Toward a Resilient Future: The Promise of Microbial Bioeconomy. Sustainability 2023, 15, 7251. [Google Scholar] [CrossRef]
- González-Rojo, S.; Paniagua-García, A.I.; Díez-Antolínez, R. Advances in Microbial Biotechnology for Sustainable Alternatives to Petroleum-Based Plastics: A Comprehensive Review of Polyhydroxyalkanoate Production. Microorganisms 2024, 12, 1668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lyu, S. Microbial Interactions in a Vitamin C Industrial Fermentation System: Novel Insights and Perspectives. Appl. Environ. Microbiol. 2022, 88, e01212-22. [Google Scholar] [CrossRef]
- Salman, A.; Dhanashree, B.; Kotian, H. Antimicrobial Effect of Common Bacterial Pigments on Clinically Significant Microorganisms. Scientifica 2025, 2025, 3951925. [Google Scholar] [CrossRef]
- Singh, T.; Pandey, V.K.; Dash, K.K.; Zanwar, S.; Singh, R. Natural Bio-Colorant and Pigments: Sources and Applications in Food Processing. J. Agric. Food Res. 2023, 12, 100628. [Google Scholar] [CrossRef]
- Elazzazy, A.M.; Baeshen, M.N.; Alasmi, K.M.; Alqurashi, S.I.; Desouky, S.E.; Khattab, S.M.R. Where Biology Meets Engineering: Scaling Up Microbial Nutraceuticals to Bridge Nutrition, Therapeutics, and Global Impact. Microorganisms 2025, 13, 566. [Google Scholar] [CrossRef]
- Knychala, M.M.; Boing, L.A.; Ienczak, J.L.; Trichez, D.; Stambuk, B.U. Precision Fermentation as an Alternative to Animal Protein, a Review. Fermentation 2024, 10, 315. [Google Scholar] [CrossRef]
- Yan, D.; Wang, C.; Zhou, J.; Liu, Y.; Yang, M.; Xing, J. Construction of Reductive Pathway in Saccharomyces cerevisiae for Effective Succinic Acid Fermentation at Low pH Value. Bioresour. Technol. 2014, 156, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zha, J.; Koffas, M.A. Microbial Production of Bioactive Chemicals for Human Health. Curr. Opin. Food Sci. 2020, 32, 9–16. [Google Scholar] [CrossRef]
- Bajić, B.; Vučurović, D.; Vasić, Đ.; Jevtić-Mučibabić, R.; Dodić, S. Biotechnological Production of Sustainable Microbial Proteins from Agro-Industrial Residues and By-Products. Foods 2023, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Nadar, C.G.; Fletcher, A.; Moreira, B.R.d.A.; Hine, D.; Yadav, S. Waste to Protein: A Systematic Review of a Century of Advancement in Microbial Fermentation of Agro-Industrial Byproducts. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13375. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.A.; Yaverino-Gutierrez, M.A.; Monteiro, M.C.; Souza, B.A.; Bachheti, R.K.; Chandel, A.K. Precision Fermentation in the Realm of Microbial Protein Production: State-of-the-Art and Future Insights. Food Res. Int. 2025, 200, 115527. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, G.; Wijffels, R.H.; Marzocchella, A.; Russo, M.E. Bioreactor and Bioprocess Design Issues in Enzymatic Hydrolysis of Lignocellulosic Biomass. Catalysts 2021, 11, 680. [Google Scholar] [CrossRef]
- Palladino, F.; Marcelino, P.R.F.; Schlogl, A.E.; José, Á.H.M.; Rodrigues, R.d.C.L.B.; Fabrino, D.L.; Santos, I.J.B.; Rosa, C.A. Bioreactors: Applications and Innovations for a Sustainable and Healthy Future—A Critical Review. Appl. Sci. 2024, 14, 9346. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Liyaskina, E.V.; Kurgaeva, I.V.; Liyaskina, A.U.; Yang, G.; Revin, V.V. Exopolysaccharides Producing Bacteria: A Review. Microorganisms 2023, 11, 1541. [Google Scholar] [CrossRef]
- Revin, V.V.; Liyaskina, E.V.; Parchaykina, M.V.; Kurgaeva, I.V.; Efremova, K.V.; Novokuptsev, N.V. Production of Bacterial Exopolysaccharides: Xanthan and Bacterial Cellulose. Int. J. Mol. Sci. 2023, 24, 14608. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Division of Behavioral and Social Sciences and Education; Board on Environmental Change and Society; Health and Medicine Division; Food and Nutrition Board; Division on Earth and Life Studies; Water Science and Technology Board; Board on Life Sciences; Board on Atmospheric Sciences and Climate; Board on Agriculture and Natural Resources. Science Breakthroughs to Advance Food and Agricultural Research by 2030; National Academies Press: Cambridge, MA, USA, 2019; ISBN 978-0-309-47395-8. [Google Scholar]
- Glockow, T.; Kaster, A.-K.; Rabe, K.S.; Niemeyer, C.M. Sustainable Agriculture: Leveraging Microorganisms for a Circular Economy. Appl. Microbiol. Biotechnol. 2024, 108, 452. [Google Scholar] [CrossRef]
- Buyel, J.F. Plant Molecular Farming—Integration and Exploitation of Side Streams to Achieve Sustainable Biomanufacturing. Front. Plant Sci. 2019, 9, 1893. [Google Scholar] [CrossRef]
- Clomburg, J.M.; Crumbley, A.M.; Gonzalez, R. Industrial Biomanufacturing: The Future of Chemical Production. Science 2017, 355, aag0804. [Google Scholar] [CrossRef]
- Ploll, U.; Arato, M.; Börner, J.; Hartmann, M. Sustainable Innovations: A Qualitative Study on Farmers’ Perceptions Driving the Diffusion of Beneficial Soil Microbes in Germany and the UK. Sustainability 2022, 14, 5749. [Google Scholar] [CrossRef]
- Galanakis, C.M. The Future of Food. Foods 2024, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.G.; Koontz, J.L.; Goddard, J.M. Hurdles to Commercial Translation of next Generation Active Food Packaging Technologies. Curr. Opin. Food Sci. 2017, 16, 40–48. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Ong, H.C.; Yong, A.M.H.; Fattori, V.; Mukherjee, K. Addressing the Safety of New Food Sources and Production Systems. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13341. [Google Scholar] [CrossRef]
- Shilomboleni, H.; De Plaen, R. Scaling up Research-for-Development Innovations in Food and Agricultural Systems. Dev. Pract. 2019, 29, 723–734. [Google Scholar] [CrossRef]
- Ververis, E.; Ackerl, R.; Azzollini, D.; Colombo, P.A.; de Sesmaisons, A.; Dumas, C.; Fernandez-Dumont, A.; Ferreira da Costa, L.; Germini, A.; Goumperis, T.; et al. Novel Foods in the European Union: Scientific Requirements and Challenges of the Risk Assessment Process by the European Food Safety Authority. Food Res. Int. 2020, 137, 109515. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Zhao, Z.; Fan, S. Research on Government Regulation, Agricultural Socialization Service and Green Treatment Behavior of Mushroom Residue by Mushroom Farmers—Based on Research Data from Gutian County, Fujian Province, China. Sustainability 2025, 17, 767. [Google Scholar] [CrossRef]
- Liu, Y.; Aimutis, W.R.; Drake, M. Dairy, Plant, and Novel Proteins: Scientific and Technological Aspects. Foods 2024, 13, 1010. [Google Scholar] [CrossRef]
- Fernández-López, L.; González-García, P.; Fernández-Ríos, A.; Aldaco, R.; Laso, J.; Martínez-Ibáñez, E.; Gutiérrez-Fernández, D.; Pérez-Martínez, M.M.; Marchisio, V.; Figueroa, M.; et al. Life Cycle Assessment of Single Cell Protein Production–A Review of Current Technologies and Emerging Challenges. Clean. Circ. Bioeconomy 2024, 8, 100079. [Google Scholar] [CrossRef]
- Zhuang, Z.; Wan, G.; Lu, X.; Xie, L.; Yu, T.; Tang, H. Metabolic Engineering for Single-Cell Protein Production from Renewable Feedstocks and Its Applications. Adv. Biotechnol. 2024, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- The Good Food Institute. The State of Global Policy: Alternative Proteins. Available online: https://gfi.org/resource/alternative-proteins-state-of-global-policy/ (accessed on 11 September 2025).
- Chapman, J.; Power, A.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.E.; Truong, V.K.; Cozzolino, D. Challenges and Opportunities of the Fourth Revolution: A Brief Insight into the Future of Food. Crit. Rev. Food Sci. Nutr. 2022, 62, 2845–2853. [Google Scholar] [CrossRef] [PubMed]
- Çakmakçı, S.; Polatoğlu, B.; Çakmakçı, R. Foods of the Future: Challenges, Opportunities, Trends, and Expectations. Foods 2024, 13, 2663. [Google Scholar] [CrossRef]
- Singh, B.K.; Trivedi, P. Microbiome and the Future for Food and Nutrient Security. Microb. Biotechnol. 2017, 10, 50–53. [Google Scholar] [CrossRef]
- Arshad, M.; Arshad, I.; Aslam, H.; Sukmawati, D.; Anvar, A.H.; Shakir, H.A.; Khan, M.; Franco, M.; Irfan, M. Microbial Bioproducts: Current Advances, Industrial Applications, and Future Perspectives. J. Umm Al Qura Univ. Appl. Sci. 2025, 11, 545–560. [Google Scholar] [CrossRef]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.A.; Mendes, M.A. Chlorella and Spirulina Microalgae as Sources of Functional Foods, Nutraceuticals, and Food Supplements; an Overview. MOJ Food Process Technol. 2018, 6, 45–58. [Google Scholar] [CrossRef]
- Barreto, J.V.d.O.; Casanova, L.M.; Junior, A.N.; Reis-Mansur, M.C.P.P.; Vermelho, A.B. Microbial Pigments: Major Groups and Industrial Applications. Microorganisms 2023, 11, 2920. [Google Scholar] [CrossRef]
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A.M. Carotenoids and Some Other Pigments from Fungi and Yeasts. Metabolites 2021, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Kaur, M.; Kalra, R.; Rene, E.R.; Goel, M. Application of Microbial Resources in Biorefineries: Current Trend and Future Prospects. Heliyon 2024, 10, e28615. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Chin, J. Survival and Therapeutic Potential of Probiotic Organisms with Reference to Lactobacillus Acidophilus and Bifidobacterium Spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef]
- Li, Y.P.; Ahmadi, F.; Kariman, K.; Lackner, M. Recent Advances and Challenges in Single Cell Protein (SCP) Technologies for Food and Feed Production. Npj Sci. Food 2024, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lin, H.; Peng, X.; Xu, C.; Sun, Z.; Jiang, K.; Huang, A.; Wu, X.; Tang, N.; Salvioli, A.; et al. Arbuscular Mycorrhizal Symbiosis Requires a Phosphate Transceptor in the Gigaspora Margarita Fungal Symbiont. Mol. Plant 2016, 9, 1583–1608. [Google Scholar] [CrossRef]
- Patakova, P.; Linhova, M.; Rychtera, M.; Paulova, L.; Melzoch, K. Novel and Neglected Issues of Acetone–Butanol–Ethanol (ABE) Fermentation by Clostridia: Clostridium Metabolic Diversity, Tools for Process Mapping and Continuous Fermentation Systems. Biotechnol. Adv. 2013, 31, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Raina, N.; Chuetor, S.; Elalami, D.; Tayibi, S.; Barakat, A. Biomass Valorization for Bioenergy Production: Current Techniques, Challenges, and Pathways to Solutions for Sustainable Bioeconomy. BioEnergy Res. 2024, 17, 1999–2028. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; da Fonseca, E.M. Biofertilization and Bioremediation—How Can Microbiological Technology Assist the Ecological Crisis in Developing Countries? Micro 2025, 5, 18. [Google Scholar] [CrossRef]
- Adegboye, M.F.; Ojuederie, O.B.; Talia, P.M.; Babalola, O.O. Bioprospecting of Microbial Strains for Biofuel Production: Metabolic Engineering, Applications, and Challenges. Biotechnol. Biofuels 2021, 14, 5. [Google Scholar] [CrossRef]
- Jagadevan, S.; Banerjee, A.; Banerjee, C.; Guria, C.; Tiwari, R.; Baweja, M.; Shukla, P. Recent Developments in Synthetic Biology and Metabolic Engineering in Microalgae towards Biofuel Production. Biotechnol. Biofuels 2018, 11, 185. [Google Scholar] [CrossRef]
- Astudillo, Á.; Rubilar, O.; Briceño, G.; Diez, M.C.; Schalchli, H. Advances in Agroindustrial Waste as a Substrate for Obtaining Eco-Friendly Microbial Products. Sustainability 2023, 15, 3467. [Google Scholar] [CrossRef]
- de Farias Silva, C.E.; Bertucco, A. Bioethanol from Microalgae and Cyanobacteria: A Review and Technological Outlook. Process Biochem. 2016, 51, 1833–1842. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Atiyeh, H.; Mariano, A.P.; Rakshit, S.K. Editorial: Innovative Bioconversion of Non-Food Substrates to Fuels. Front. Bioeng. Biotechnol. 2023, 11, 1163513. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, H.R. Biorefineries from the Perspective of Sustainability: Feedstocks, Products, and Processes. Renew. Sustain. Energy Rev. 2011, 15, 4042–4052. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Xiong, C.; Egidi, E.; Singh, B.K. Formulation Challenges Associated with Microbial Biofertilizers in Sustainable Agriculture and Paths Forward. J. Sustain. Agric. Environ. 2024, 3, e70006. [Google Scholar] [CrossRef]
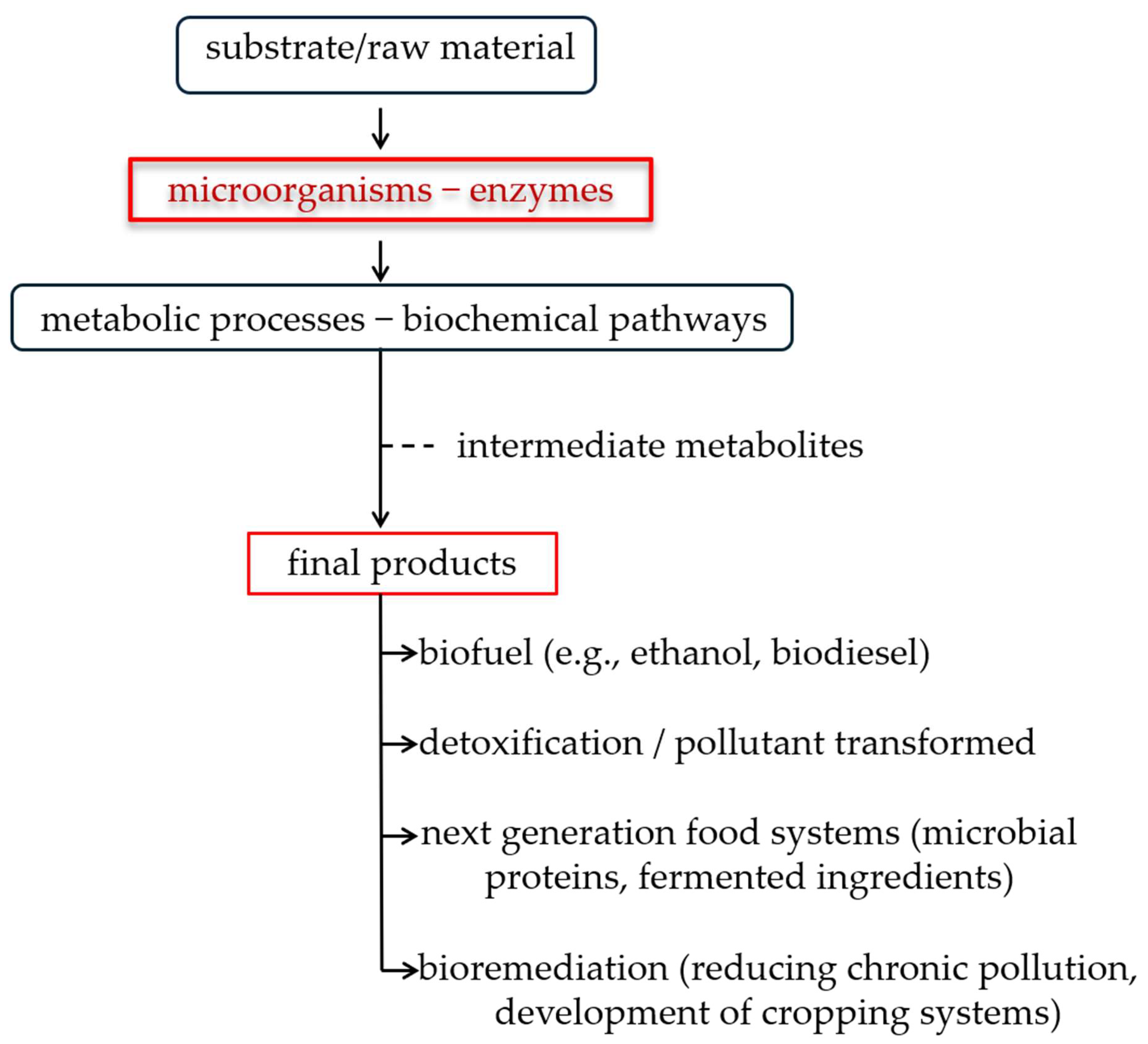





| Microorganism | Role in Bioremediation | Example | Key Takeaways | Refs. |
|---|---|---|---|---|
| hydrocarbon degrading bacteria | metabolize petroleum hydrocarbons and transform them into less toxic compounds | Pseudomonas Alcanivorax | efficiently degrades petroleum hydrocarbons, reduces environmental toxicity, contributes to soil regeneration | [50,52] |
| metal-reducing bacteria | reduce and immobilize heavy metals in contaminated soils and waters | Geobacter Shewanella | facilitate heavy metal detoxification | [53,54] |
| fungi and yeasts | degrade complex organic compounds and pollutants | Aspergillus Candida | improves soil and wastewater bioremediation | [55,56] |
| cyanobacteria | absorb and fix inorganic pollutants, contributing to wastewater treatment | Anabaena Nostoc | removes nutrients and pollutants from wastewater, contributes to nitrogen fixation | [57] |
| microbial consortia | synergistic action of multiple species for simultaneous degradation of mixed pollutants | mixed bacteria–fungi consortia in contaminated soils | pollutant degradation due to synergistic metabolic interactions among species | [58,59,60] |
| Microorganism | Role in Bioremediation | Example | Key Takeaways | Refs. |
|---|---|---|---|---|
| nitrogen-fixing bacteria | convert atmospheric nitrogen (N2) into ammonia, making it available to plants and improving soil fertility | Rhizobium Azotobacter Azospirillum | enhance soil nitrogen content, support plant growth, reduce the need for chemical fertilizers | [74,75,76] |
| phosphate-solubilizing bacteria | release insoluble phosphates in soil through organic acid production, enhancing plant phosphorus uptake | Pseudomonas Bacillus | improves phosphorus bioavailability, promotes better plant nutrition and increased crop yields | [77] |
| mycorrhizal fungi | form symbiotic associations with plant roots, improving water and nutrient uptake, especially phosphorus | Glomus Gigaspora | enhance plant nutrient, water uptake, improve plant growth and stress resilience | [44,74] |
| potassium-solubilizing microorganisms | mobilize potassium from insoluble minerals, increasing plant growth and stress tolerance | Bacillus mucilaginosus Aspergillus niger | increase potassium availability, improve plant development and tolerance to abiotic stress | [75,76,78] |
| plant growth-promoting rhizobacteria | produce phytohormones (auxins, gibberellins, cytokinins), suppress pathogens, and enhance root growth | Burkholderia Enterobacter | promote root development, enhance plant resistance to pathogens, stimulate plant growth | [79] |
| Biofuel | Microorganisms | Role | Examples | Key Takeaways | Refs. |
|---|---|---|---|---|---|
| bioethanol | bacteria and yeasts | fermentation of sugars to produce ethanol | Saccharomyces cerevisiae Zymomonas mobilis | efficiently converts sugars into ethanol, provides a scalable and renewable biofuel source | [102] |
| biodiesel | algae and cyanobacteria | accumulation of lipids that can be converted into biodiesel | Chlorella vulgaris Nannochloropsis Spirulina platensis | high lipid content, sustainable alternative to fossil diesel | [103,104,105] |
| biogas | methanogenic and anaerobic bacteria | anaerobic decomposition of organic matter to produce CH4 (methane) | Methanobacterium Methanosarcina anaerobic microbial consortia | converts organic waste into methane, supports waste-to-energy strategies | [101,102] |
| biohydrogen | bacteria and photosynthetic algae | photobiological or fermentative production of H2 (hydrogen) from organic substrates or light | Clostridium Rhodobacter sphaeroides Chlamydomonas reinhardtii | produces hydrogen sustainably, provides a clean energy alternative | [106,107,108] |
| biobutanol | acetobutylic bacteria | fermentation of carbohydrates to produce butanol | Clostridium acetobutylicum Clostridium beijerinckii | produces butanol efficiently, offers higher energy density than ethanol | [82,109] |
| microbial biomass (feedstock) | bacteria and yeasts | produced biomass can be further converted into solid or liquid fuels | Escherichia coli Saccharomyces cerevisiae | serves as a versatile feedstock for biofuels, enables multiple conversion pathways and added value | [106,110,111,112] |
| Product | Role/Application | Microorganisms | Key Takeaways | Refs. |
|---|---|---|---|---|
| biodegradable polymers | alternative to petroleum-based plastics, plastic waste reduction | Cupriavidus necator Halomonas | sustainable alternative to plastics, reduce environmental pollution, and support circular economy approaches | [139] |
| nutraceuticals and pharmacological compounds | production of resveratrol, naringenin, and curcuminoids, use of agro-industrial waste | Saccharomyces cerevisiae E. coli | high-value nutraceuticals from renewable substrates, provide scalable and sustainable production routes | [133,134] |
| vitamin precursors | intermediate for vitamin C production, scalable, efficient bioprocesses | Gluconobacter oxydans Ketogulonicigenium B. megaterium | enable cost-effective and scalable production of vitamin precursors, enhance industrial supply, reduce chemical synthesis dependency | [140] |
| waste-to-protein systems | conversion of agro-industrial residues into microbial protein for animal feed | mixed microbial consortia | convert agro-industrial waste into protein-rich biomass, support sustainable animal nutrition and waste valorization | [117,141,142,143] |
| fermentative biopolymers | production of exopolysaccharides (xanthan, pullulan, curdlan, bacterial cellulose) for food and industrial applications | Aspergillus Bacillus Xanthomonas Aureobasidium | provide functional biopolymers for food and industrial use, offer environmentally friendly alternatives to synthetic polymers | [136,137] |
| Application Area | Advantages | Disadvantages |
|---|---|---|
| bioremediation | degradation of toxic pollutants into less harmful compounds, eco-friendly alternative to chemical methods, cost-effective for large-scale contaminated sites | slow degradation rates for some pollutants, sensitivity to environmental conditions, incomplete mineralization can generate secondary products |
| biofertilization | improved nutrient availability (N, P, K), enhanced soil health and fertility, reduced dependence on chemical fertilizers, and promotion of plant growth | variable efficiency under field conditions, competition with native soil microbiota, limited shelf life of microbial inoculants |
| biofuel production | renewable energy source, potential use of agro-industrial waste as feedstock, reduction in greenhouse gas emissions | high production costs, difficulties in large-scale scalability, technical barriers in downstream processing |
| biochemical synthesis | production of biodegradable polymers, nutraceuticals, vitamins, and bioplastics, valorization of agricultural residues, contribution to circular economy | low yields for some target compounds, complex metabolic engineering required, regulatory and safety concerns for new bioproducts |
| next-generation food systems (precision fermentation, microbial protein) | sustainable protein alternatives, reduced land and water use, decoupling from traditional livestock, alignment with climate goals | consumer acceptance issues, high production costs, strict regulatory frameworks, scale-up and infrastructure limitations |
| Category | Commercialized Product | Pilot-Stage Product | Refs. |
|---|---|---|---|
| Biofuel | ethanol from fermentation | hydrogen from microbial fermentation (e.g., Clostridium spp.) | [171,172] |
| Microbial protein | microalgae proteins (e.g., Spirulina, Chlorella) | single-cell proteins from methanotrophic bacteria | [171,172,173] |
| Pigment | carotenoids (e.g., β-carotene, astaxanthin) from fungal and bacterial cultures | pigments from metal-tolerant bacterial cultures | [174,175] |
| Bioplastic | polyhydroxyalkanoates (PHAs) from bacterial cultures | PHAs from methanotrophic bacteria or organic waste | [136] |
| Biofertilizer | Azotobacter, Rhizobium, and Bacillus spp. for agriculture | microorganisms for bioremediation of soils contaminated with heavy metals or pesticides | [67,79,176] |
| Food ingredient | probiotic bacteria (Lactobacillus, Bifidobacterium) | microbial proteins from methanotrophic bacteria for alternative foods | [172,177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birgovan, A.L.; Lakatos, E.S.; Cioca, L.I.; Paul, N.L.; Vatca, S.D.; Kis, E.; Pacurariu, R.L. Harnessing Microbial Power for a Sustainable Future Food System. Microorganisms 2025, 13, 2217. https://doi.org/10.3390/microorganisms13092217
Birgovan AL, Lakatos ES, Cioca LI, Paul NL, Vatca SD, Kis E, Pacurariu RL. Harnessing Microbial Power for a Sustainable Future Food System. Microorganisms. 2025; 13(9):2217. https://doi.org/10.3390/microorganisms13092217
Chicago/Turabian StyleBirgovan (Rhazzali), Andreea Loredana, Elena Simina Lakatos, Lucian Ionel Cioca, Natalia Lorela Paul, Sorin Daniel Vatca, Erzsebeth Kis, and Roxana Lavinia Pacurariu. 2025. "Harnessing Microbial Power for a Sustainable Future Food System" Microorganisms 13, no. 9: 2217. https://doi.org/10.3390/microorganisms13092217
APA StyleBirgovan, A. L., Lakatos, E. S., Cioca, L. I., Paul, N. L., Vatca, S. D., Kis, E., & Pacurariu, R. L. (2025). Harnessing Microbial Power for a Sustainable Future Food System. Microorganisms, 13(9), 2217. https://doi.org/10.3390/microorganisms13092217











