The Reorganization of Rice Rhizosphere Microbial Communities Driven by Nitrogen Utilization Efficiency and the Regulatory Mechanism of Soil Nitrogen Cycling
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material and Design
2.2. Determination of the Agronomic Use Efficiency of Nitrogen Fertilizer
2.3. Measurement of Soil Physicochemical Properties
2.4. DNA Extraction and Sequencing of Rice Rhizosphere Soil Microorganisms
2.5. Statistical Analysis
3. Results
3.1. Determination of the Agronomic Use Efficiency of Nitrogen Fertilizer in Rice
3.2. Changes in Soil Physicochemical Properties
3.3. Changes in the α-Diversity of Rice Rhizosphere Soil Microbes
3.3.1. High-Throughput Sequencing Data Analysis and Microbial Community Composition
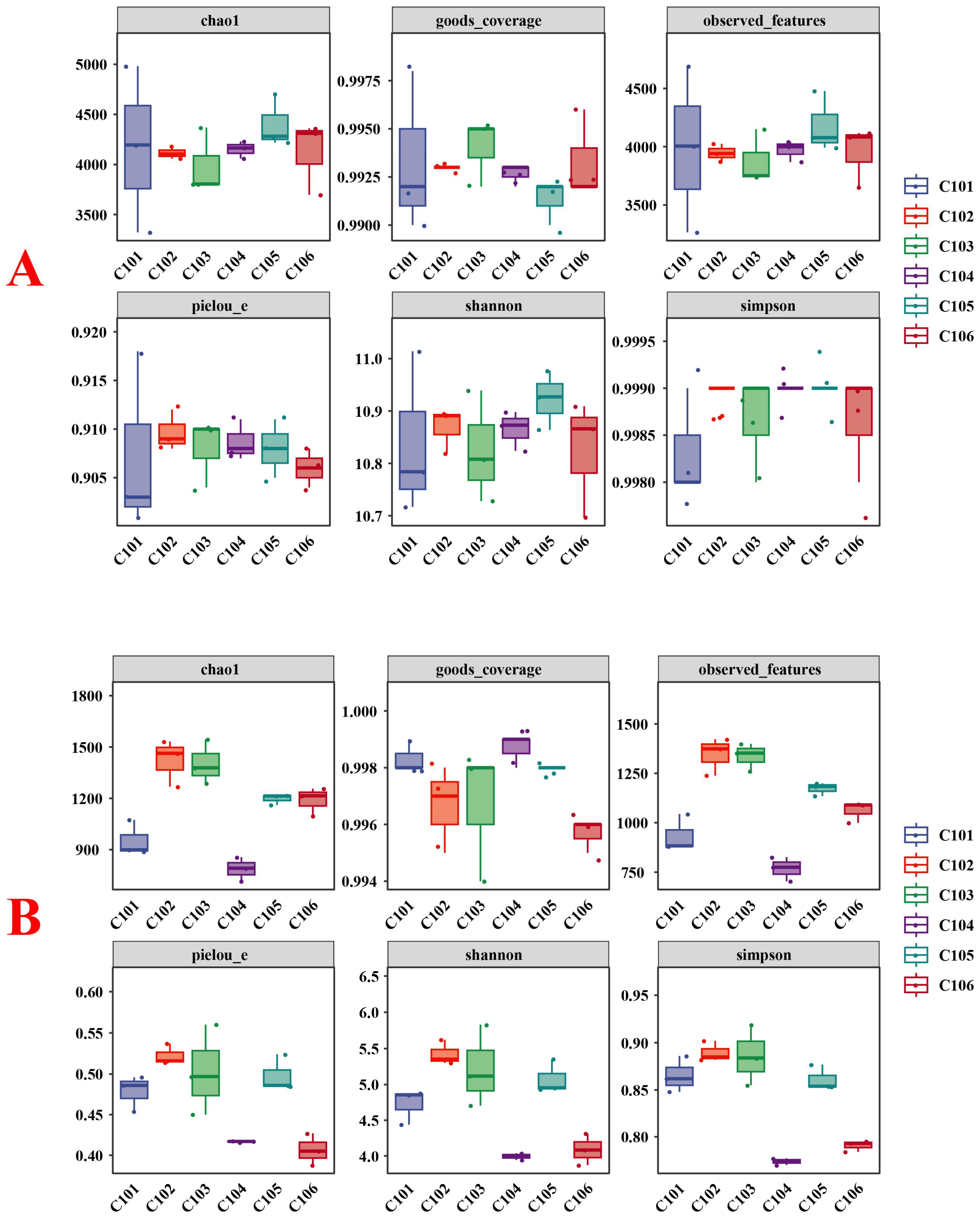
3.3.2. Changes in the α-Diversity of Soil Microbes in the Rice Rhizosphere
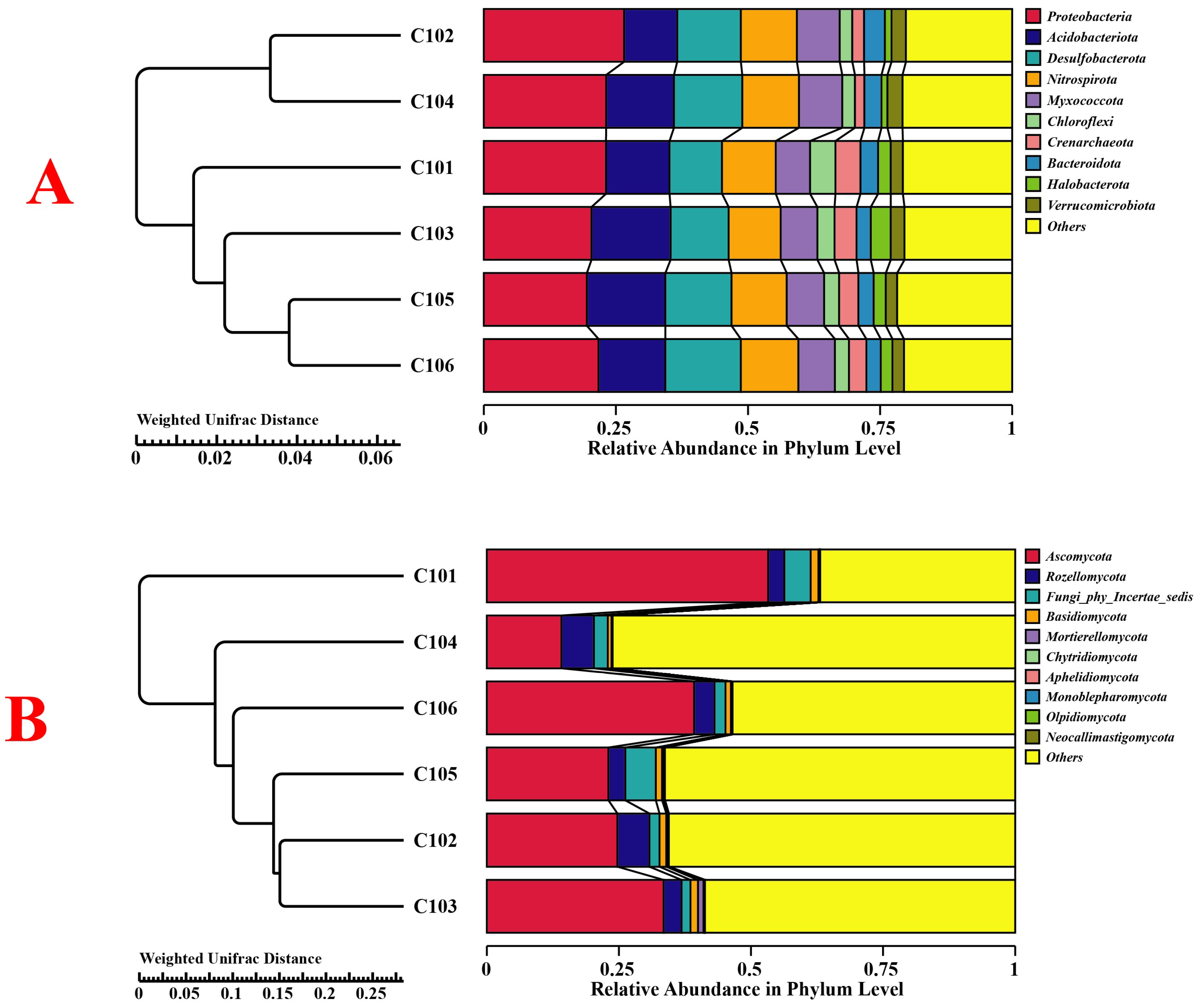
3.3.3. Composition and Abundance of Microbes in the Rice Rhizosphere
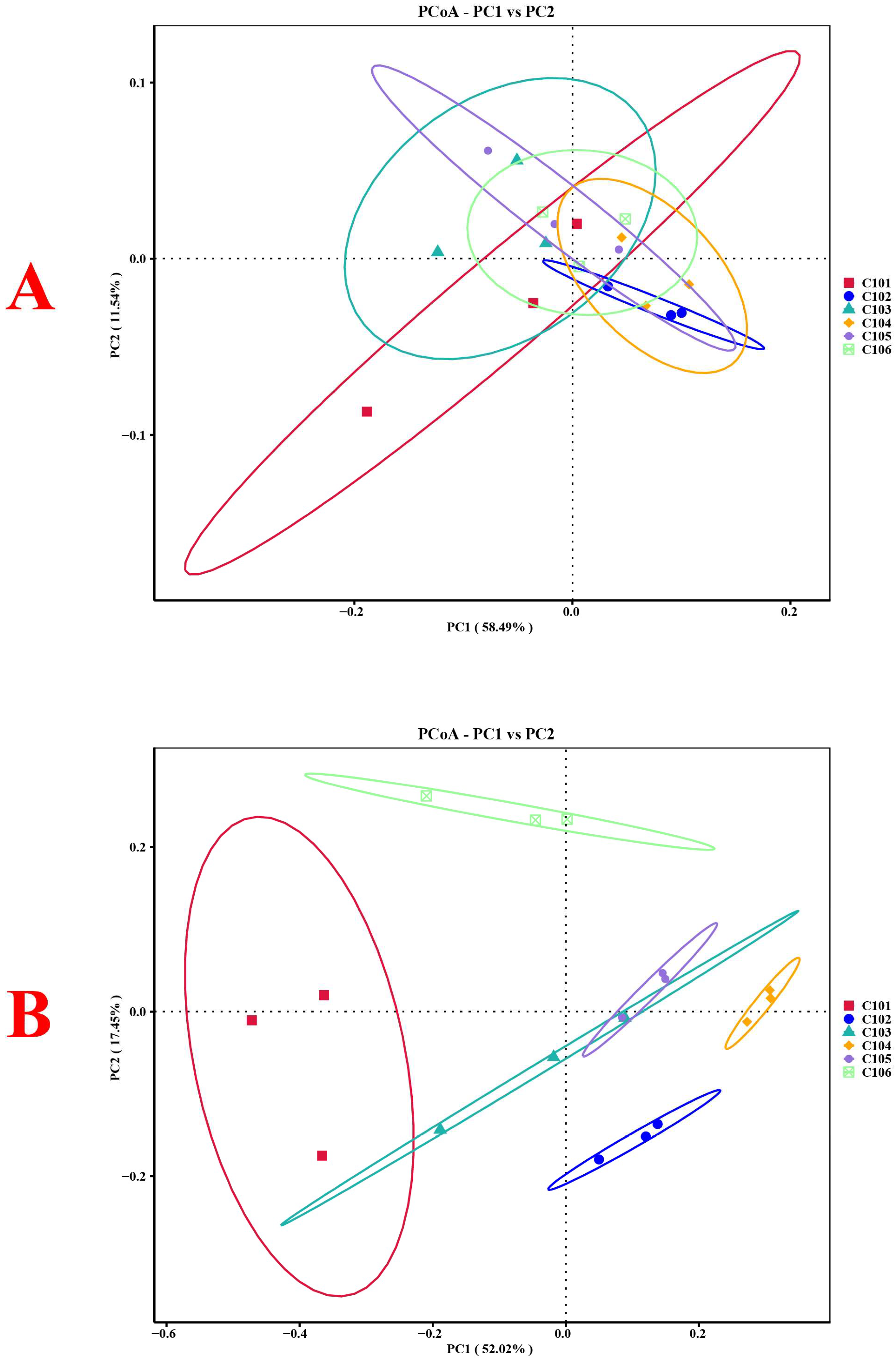
3.3.4. Structural Changes in the Microbial Communities in Rice Rhizosphere Soil
3.3.5. Functional Changes in Rice Rhizosphere Soil Microbes
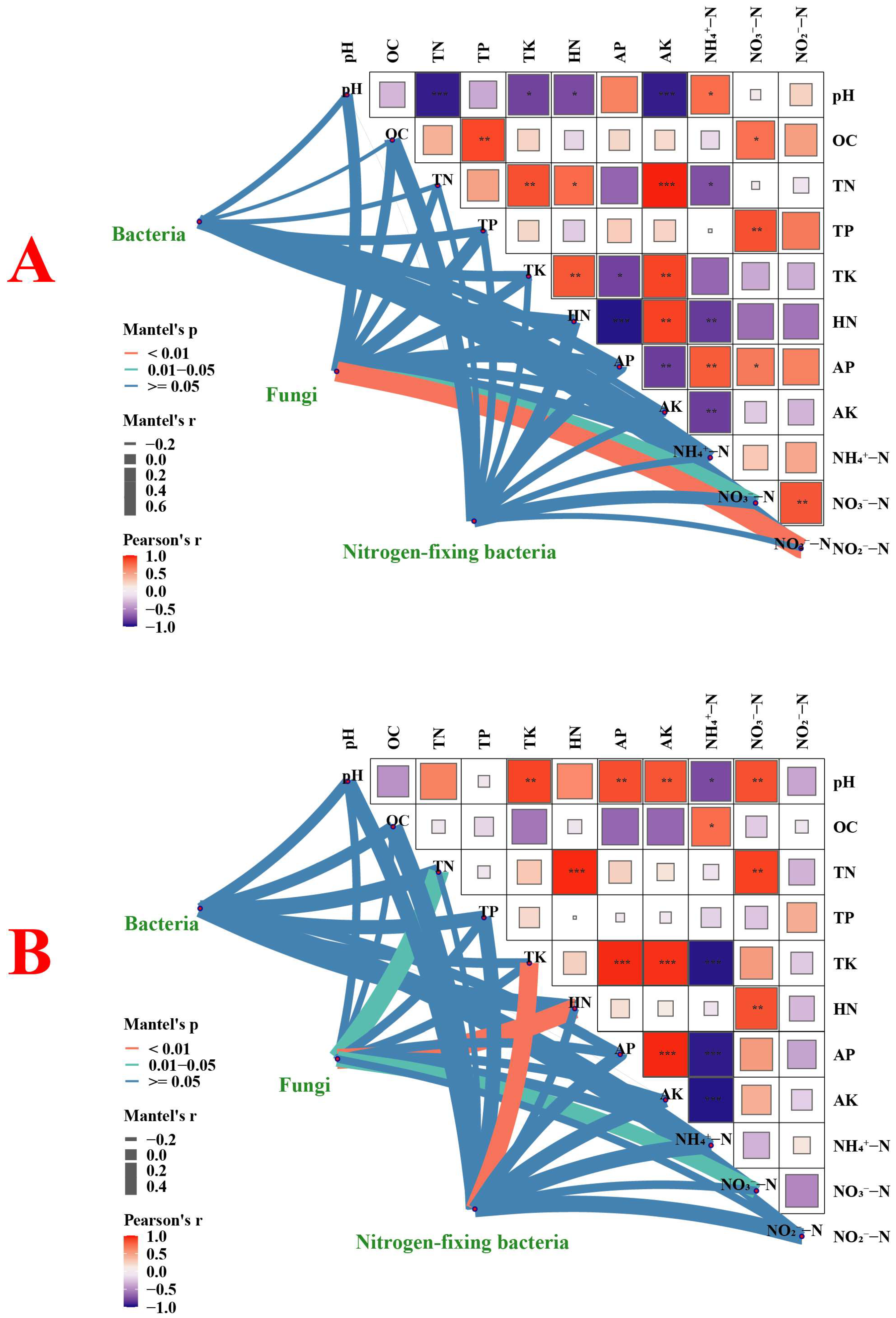
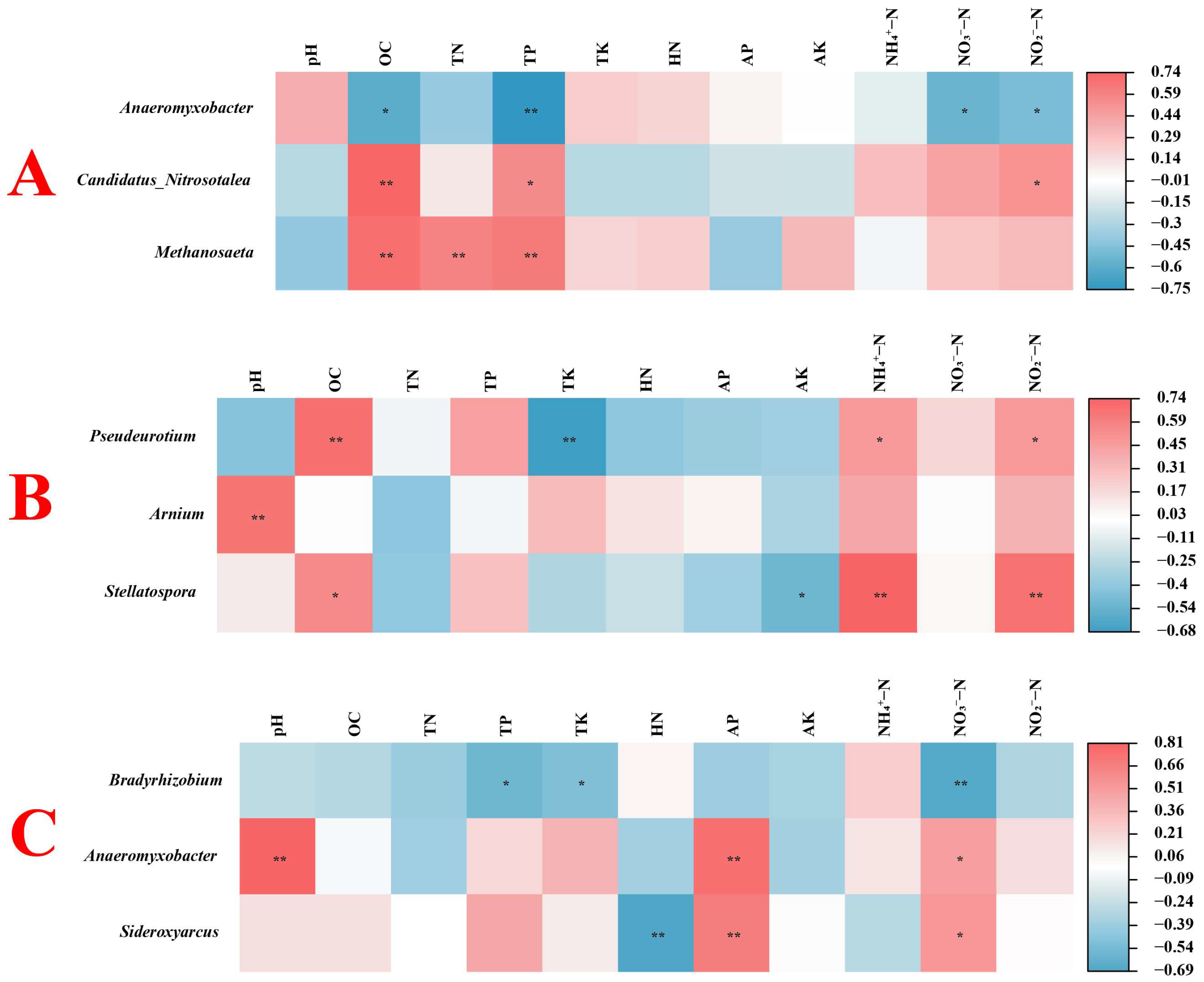
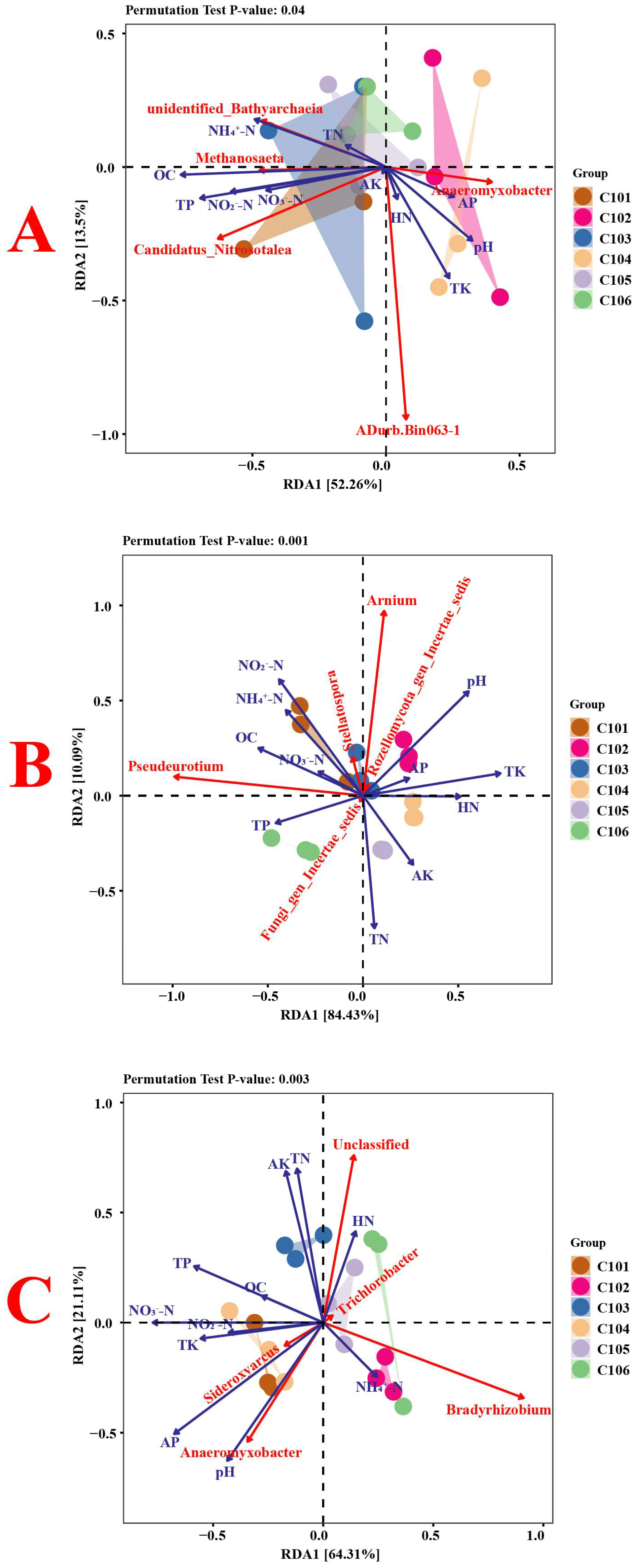
3.3.6. Correlation Analysis of Environmental Factors and Microbial Communities in Rice Rhizosphere Soil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otie, V.; Ping, A.; John, N.M.; Eneji, A.E. Interactive effects of plant growth regulators and nitrogen on corn growth and nitrogen use efficiency. J. Plant Nutr. 2016, 39, 1597–1609. [Google Scholar] [CrossRef]
- Guiné, R.P.F. The Challenges and Strategies of Food Security under Global Change. Foods 2024, 13, 2083. [Google Scholar] [CrossRef]
- Carciochi, W.D.; Sadras, V.O.; Pagani, A.; Ciampitti, I.A. Co-limitation and stoichiometry capture the interacting effects of nitrogen and sulfur on maize yield and nutrient use efficiency. Eur. J. Agron. 2020, 113, 125973. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.-L.; Li, Q.; Zeng, X.-P.; Liu, Y.; Li, Y.-R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef] [PubMed]
- Houlton, B.Z.; Almaraz, M.; Aneja, V.; Austin, A.T.; Bai, E.; Cassman, K.G.; Compton, J.E.; Davidson, E.A.; Erisman, J.W.; Galloway, J.N.; et al. A World of Cobenefits: Solving the Global Nitrogen Challenge. Earth’s Future 2019, 7, 865–872. [Google Scholar] [CrossRef]
- Kandel, S.L.; Herschberger, N.; Kim, S.H.; Doty, S.L. Diazotrophic Endophytes of Poplar and Willow for Growth Promotion of Rice Plants in Nitrogen-Limited Conditions. Crop Sci. 2015, 55, 1765–1772. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef]
- Ahmad, S.; Nadeem, M.Y.; Gao, S.; Li, Q.; Ding, Y.; Liu, Z.; Jiang, Y.; Li, W.; Li, G. Subsurface placement of controlled-release blended fertilizers mitigates ammonia volatilization by promoting nitrogen transformation in rice fields. Agric. Ecosyst. Environ. 2025, 386, 109624. [Google Scholar] [CrossRef]
- Weitzman, J.N.; Brooks, J.R.; Compton, J.E.; Faulkner, B.R.; Mayer, P.M.; Peachey, R.E.; Rugh, W.D.; Coulombe, R.A.; Hatteberg, B.; Hutchins, S.R. Deep soil nitrogen storage slows nitrate leaching through the vadose zone. Agric. Ecosyst. Environ. 2022, 332, 107949. [Google Scholar] [CrossRef]
- Pereira, V.V.; Morales, M.M.; Pereira, D.H.; de Rezende, F.A.; de Souza Magalhães, C.A.; de Lima, L.B.; Marimon-Junior, B.H.; Petter, F.A. Activated Biochar-Based Organomineral Fertilizer Delays Nitrogen Release and Reduces N2O Emission. Sustainability 2022, 14, 12388. [Google Scholar] [CrossRef]
- Woodley, A.L.; Drury, C.F.; Yang, X.Y.; Phillips, L.A.; Reynolds, D.W.; Calder, W.; Oloya, T.O. Ammonia volatilization; nitrous oxide emissions, and corn yields as influenced by nitrogen placement and enhanced efficiency fertilizers. Soil Sci. Soc. Am. J. 2020, 84, 1327–1341. [Google Scholar] [CrossRef]
- Galloway, J.N.; Cowling, E.B. Reactive Nitrogen and The World: 200 Years of Change. Ambio 2002, 31, 64–71. [Google Scholar] [CrossRef]
- Daba, N.A.; Huang, J.; Alam, M.A.; Li, J.; Shen, Z.; Tadesse, K.A.; Gilbert, N.; Han, T.; Kebede, E.; Legesse, T.G.; et al. Green manure substitution reduces carbon and nitrogen footprints and improves net ecosystem economic benefits in double rice systems. J. Clean. Prod. 2025, 521, 146266. [Google Scholar] [CrossRef]
- Sagehashi, Y.; Ikegaya, T.; Fujino, K. Integration of genetic engineering into conventional rice breeding programs for the next generation. Euphytica 2022, 218, 145. [Google Scholar] [CrossRef]
- Mahboob, W.; Yang, G.; Irfan, M. Crop nitrogen (N) utilization mechanism and strategies to improve N use efficiency. Acta Physiol. Plant. 2023, 45, 52. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Amelung, W.; Cao, Z.; Fiedler, S.; Frenzel, P.; Jahn, R.; Kalbitz, K.; Kölbl, A.; Schloter, M. Biogeochemistry of paddy soils. Geoderma 2010, 157, 1–14. [Google Scholar] [CrossRef]
- Wang, B.; Guo, X.; Qi, X.; Feng, F.; Zhu, X.; Hu, Y.; Li, J.; Zhao, Q.; Sun, H. OsSPL14 is involved in nitrogen-deficiency-induced root elongation in rice. Environ. Exp. Bot. 2022, 198, 104852. [Google Scholar] [CrossRef]
- Hou, L.; Chen, D.; Pan, X.; Jiang, S.; Liu, J.; Li, Q.; Liu, Y.; Tong, Y.; Zhu, L.; Hu, J.; et al. 9311 allele of OsNAR2.2 enhances nitrate transport to improve rice yield and nitrogen use efficiency. Plant Biotechnol. J. 2025, 23, 2501–2511. [Google Scholar] [CrossRef]
- Xiong, Q.; Hu, J.; Wei, H.; Zhang, H.; Zhu, J. Relationship between Plant Roots, Rhizosphere Microorganisms, and Nitrogen and Its Special Focus on Rice. Agriculture 2021, 11, 234. [Google Scholar] [CrossRef]
- Sun, C.; Wang, R.; Tang, G.; Cai, S.; Shi, H.; Liu, F.; Xie, H.; Zhu, J.; Xiong, Q. Integrated 16S and metabolomics revealed the mechanism of drought resistance and nitrogen uptake in rice at the heading stage under different nitrogen levels. Front. Plant Sci. 2023, 14, 1120584. [Google Scholar] [CrossRef]
- Farooq, M.S.; Uzair, M.; Maqbool, Z.; Fiaz, S.; Yousuf, M.; Yang, S.H.; Khan, M.R. Improving Nitrogen Use Efficiency in Aerobic Rice Based on Insights Into the Ecophysiology of Archaeal and Bacterial Ammonia Oxidizers. Front. Plant Sci. 2022, 13, 913204. [Google Scholar] [CrossRef]
- Chen, M.; Feng, S.; Lv, H.; Wang, Z.; Zeng, Y.; Shao, C.; Lin, W.; Zhang, Z. OsCIPK2 mediated rice root microorganisms and metabolites to improve plant nitrogen uptake. BMC Plant Biol. 2024, 24, 285. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, J.; Zhu, C.; Cao, W.; Luo, Y.; Zhang, M.; Zhang, S.; Jia, Z.; Yu, R.; Zhao, J.; et al. Elevated Atmospheric CO2 and Nitrogen Fertilization Affect the Abundance and Community Structure of Rice Root-Associated Nitrogen-Fixing Bacteria. Front. Microbiol. 2021, 12, 628108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.-X.; Zhang, N.; Hu, B.; Jin, T.; Xu, H.; Qin, Y.; Yan, P.; Zhang, X.; Guo, X.; et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 2019, 37, 676–684. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Leyser, O. A plant’s diet, surviving in a variable nutrient environment. Science 2020, 368, eaba0196. [Google Scholar] [CrossRef] [PubMed]
- Gaspareto, R.N.; Jalal, A.; Ito, W.C.N.; Oliveira, C.E.D.S.; Garcia, C.M.D.P.; Boleta, E.H.M.; Rosa, P.A.L.; Galindo, F.S.; Buzetti, S.; Ghaley, B.B.; et al. Inoculation with Plant Growth-Promoting Bacteria and Nitrogen Doses Improves Wheat Productivity and Nitrogen Use Efficiency. Microorganisms 2023, 11, 1046. [Google Scholar] [CrossRef]
- Liu, M.; Liu, T.; Zhang, Z.; Yao, J.; Xiao, X.; An, H.; Wei, P.; Luo, X.; Qin, S. Endophytes Enhance Rice Inorganic Nitrogen Use Efficiency and Mitigate Nitrogen Loss Via Dissimilatory Nitrate Reduction To Ammonium in Paddy Soils. Rice 2025, 18, 66. [Google Scholar] [CrossRef]
- Li, Z.; Henawy, A.R.; Halema, A.A.; Fan, Q.; Duanmu, D.; Huang, R. A Wild Rice Rhizobacterium Burkholderia cepacia BRDJ Enhances Nitrogen Use Efficiency in Rice. Int. J. Mol. Sci. 2022, 23, 10769. [Google Scholar] [CrossRef]
- Bashir, S.S.; Siddiqi, T.O.; Kumar, D.; Ahmad, A. Physio-biochemical, agronomical, and gene expression analysis reveals different responsive approach to low nitrogen in contrasting rice cultivars for nitrogen use efficiency. Mol. Biol. Rep. 2023, 50, 1575–1593. [Google Scholar] [CrossRef]
- Li, C.; Wen, H.; Wu, Y.; Li, Y.; Feng, X.; Li, X.; Xu, S.; Jiang, D.; Zhang, B.; Li, M.; et al. OsRF2b interacting with OsbZIP61 modulates nitrogen use efficiency and grain yield via heterodimers in rice. Plant Biotechnol. J. 2025, 23, 3300–3312. [Google Scholar] [CrossRef]
- Nazish, T.; Arshad, M.; Jan, S.U.; Javaid, A.; Khan, M.H.; Naeem, M.A.; Baber, M.; Ali, M. Transporters and transcription factors gene families involved in improving nitrogen use efficiency (NUE) and assimilation in rice (Oryza sativa L.). Transgenic Res. 2022, 31, 23–42. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhu, H.; Shi, A.; Wang, X. Optimizing Nitrogen Fertilizer Management Enhances Rice Yield, Dry Matter, and Nitrogen Use Efficiency. Agronomy 2024, 14, 919. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Duan, C.; Wang, X.; Zhang, X.; Ju, W.; Chen, H.; Yue, S.; Wang, Y.; Li, S.; et al. Ecoenzymatic stoichiometry reveals microbial phosphorus limitation decreases the nitrogen cycling potential of soils in semi-arid agricultural ecosystems. Soil Tillage Res. 2020, 197, 104463. [Google Scholar] [CrossRef]
- Li, B.; Ge, T.; Xiao, H.; Zhu, Z.; Li, Y.; Shibistova, O.; Liu, S.; Wu, J.; Inubushi, K.; Guggenberger, G. Phosphorus content as a function of soil aggregate size and paddy cultivation in highly weathered soils. Environ. Sci. Pollut. Res. 2016, 23, 7494–7503. [Google Scholar] [CrossRef]
- Zavyalova, N.E.; Vasbieva, M.T.; Shishkov, D.G.; Ivanova, O.V. Content of Potassium Forms in the Profile of Soddy-Podzolic Soil of the Cis-Ural Region. Eurasian Soil Sci. 2023, 56, 1083–1091. [Google Scholar] [CrossRef]
- Roberts, T.L.; Ross, W.J.; Norman, R.J.; Slaton, N.A.; Wilson, C.E., Jr. Predicting Nitrogen Fertilizer Needs for Rice in Arkansas Using Alkaline Hydrolyzable-Nitrogen. Soil Sci. Soc. Am. J. 2011, 75, 1161–1171. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Xing, J.; Brookes, P.C.; Xu, J. Soil available phosphorus content drives the spatial distribution of archaeal communities along elevation in acidic terrace paddy soils. Sci. Total Environ. 2019, 658, 723–731. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, S.; Fei, C.; Ding, X. Impacts of straw returning and N application on NH4+-N loss, microbially reducible Fe(III) and bacterial community composition in saline-alkaline paddy soils. Appl. Soil Ecol. 2021, 168, 104115. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, Y.; Chen, X.; Peng, L.; Zou, L.; Liu, B.; Li, Q. Mitigating the effects of polyethylene microplastics on Pisum sativum L. quality by applying microplastics-degrading bacteria: A Field study. Environ. Res. 2024, 263, 120201. [Google Scholar] [CrossRef]
- Kuang, X.; Su, H.; Li, W.; Lin, L.; Lin, W.; Luo, L. Effects of microbial community structure and its co-occurrence on the dynamic changes of physicochemical properties and free amino acids in the Cantonese soy sauce fermentation process. Food Res. Int. 2022, 156, 111347. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Chen, X.; Su, H.; Xing, A.; Chen, G.; Zhu, J.; Zhu, B.; Fang, J. Phosphorus addition decreases soil fungal richness and alters fungal guilds in two tropical forests. Soil Biol. Biochem. 2022, 175, 108836. [Google Scholar] [CrossRef]
- Li, Y.; Lei, S.; Cheng, Z.; Jin, L.; Zhang, T.; Liang, L.-M.; Cheng, L.; Zhang, Q.; Xu, X.; Lan, C.; et al. Microbiota and functional analyses of nitrogen-fixing bacteria in root-knot nematode parasitism of plants. Microbiome 2023, 11, 48. [Google Scholar] [CrossRef]
- Luo, J.; Liao, G.; Banerjee, S.; Gu, S.; Liang, J.; Guo, X.; Zhao, H.; Liang, Y.; Li, T. Long-term organic fertilization promotes the resilience of soil multifunctionality driven by bacterial communities. Soil Biol. Biochem. 2023, 177, 108922. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ai, J.; Chen, L.; Li, Y.; Tang, X.; Li, J. Nitrogen metabolism pathways and functional microorganisms in typical karst wetlands. Environ. Sci. Pollut. Res. 2024, 31, 22494–22506. [Google Scholar] [CrossRef]
- Shi, X.; Guo, P.; Shao, X.; Chen, Y.; Liu, C.; Yu, H.; Zou, H.; Zhou, Y. Nitrogen Supply Shapes the Nitrogen Cycling Microbiome in Sorghum Intercropped with Peanut. J. Plant Growth Regul. 2025. Correction in J. Plant Growth Regul. 2025, https://doi.org/10.1007/s00344-025-11746-z. [Google Scholar] [CrossRef]
- Shimoia, E.P.; Da-Silva, C.J.; Posso, D.A.; da Silva Martins, T.; Agualongo, D.A.P.; de Oliveira, A.C.B.; Amarante, L.D. Co-inoculation of Seeds with Bradyrhizobium, Azospirillum, and Rhizophagus Improves Nitrogen Assimilation and Growth in Soybean Plants Subjected to Waterlogging. Russ. J. Plant Physiol. 2023, 70, 146. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, X.; Li, B.; Sun, F.; Zhao, X.; Wang, Y.; Lu, Z.; Zhang, D.; Fang, J. Fungal communities are more sensitive to nitrogen fertilization than bacteria in different spatial structures of silage maize under short-term nitrogen fertilization. Appl. Soil Ecol. 2022, 170, 104275. [Google Scholar] [CrossRef]
- Li, J.; Nie, M.; Pendall, E. Soil physico-chemical properties are more important than microbial diversity and enzyme activity in controlling carbon and nitrogen stocks near Sydney, Australia. Geoderma 2020, 366, 114201. [Google Scholar] [CrossRef]
- Onley, J.R.; Ahsan, S.; Sanford, R.A.; Löffler, F.E. Denitrification by Anaeromyxobacter dehalogenans, a Common Soil Bacterium Lacking the Nitrite Reductase Genes nirS and nirK. Appl. Environ. Microbiol. 2018, 84, e01985-17. [Google Scholar] [CrossRef]
- Qiao, Y.; Huang, Q.; Guo, H.; Qi, M.; Zhang, H.; Xu, Q.; Shen, Q.; Ling, N. Nutrient status changes bacterial interactions in a synthetic community. Appl. Environ. Microbiol. 2024, 90, e0156623. [Google Scholar] [CrossRef]
- Scherer, H.W.; Feils, E.; Beuters, P.J.P. Soil, Environment, Ammonium fixation and release by clay minerals as influenced by potassium. Plant Soil Environ. 2014, 60, 325–331. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, Y.; Chen, X.; Sha, A.; Xiong, Z.; Luo, Y.; Peng, L.; Zou, L.; Zhao, C.; Li, Q. The Diversity and Community Composition of Three Plants’ Rhizosphere Fungi in Kaolin Mining Areas. J. Fungi 2024, 10, 306. [Google Scholar] [CrossRef]
- Xiong, Z.; Wu, P.; Xiang, P.; Chen, X.; Peng, L.; Zou, L.; Xu, J.; Li, Q. Application of Acinetobacter radioresistens to promote the growth of Cucumis sativus L. contaminated with polystyrene microplastics. J. Hazard. Mater. 2025, 488, 137388. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Gao, X.; Wang, J. The Intratumor Microbiota Signatures Associate With Subtype, Tumor Stage, and Survival Status of Esophageal Carcinoma. Front. Oncol. 2021, 11, 754788. [Google Scholar] [CrossRef]
| pH | OC (g/kg) | TN (g/kg) | TP (g/kg) | TK (g/kg) | HN (mg/kg) | AP (mg/kg) | AK (mg/kg) | NH4+-N (mg/kg) | NO3−-N (mg/kg) | NO2−-N (mg/kg) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C101 | 6.07 ± 0.05 a | 31.43 ± 0.84 a | 1.93 ± 0.02 e | 0.81 ± 0.03 a | 13.78 ± 0.3 c | 233.49 ± 1.91 d | 1.54 ± 0.04 b | 85.68 ± 1.46 e | 6.87 ± 0.54 a | 4.90 ± 0.23 a | 0.25 ± 0.02 a |
| C102 | 6.07 ± 0.03 a | 29.40 ± 0.64 b | 1.82 ± 0.03 f | 0.63 ± 0.02 c | 14.09 ± 0.2 c | 262.93 ± 2.34 b | 1.29 ± 0.07 cd | 96.28 ± 1.97 d | 6.42 ± 0.40 ab | 1.78 ± 0.11 f | 0.20 ± 0.01 b |
| C103 | 5.89 ± 0.04 c | 31.17 ± 0.73 a | 3.05 ± 0.04 a | 0.79 ± 0.03 ab | 14.79 ± 0.3 b | 282.53 ± 5.90 a | 1.18 ± 0.05 e | 142.14 ± 1.02 a | 5.60 ± 0.40 c | 3.20 ± 0.17 d | 0.22 ± 0.02 b |
| C104 | 6.06 ± 0.03 a | 29.51 ± 0.79 b | 2.82 ± 0.03 c | 0.76 ± 0.03 b | 15.31 ± 0.3 a | 250.20 ± 4.08 c | 1.64 ± 0.04 a | 125.20 ± 1.98 b | 4.38 ± 0.14 d | 3.55 ± 0.15 c | 0.19 ± 0.02 b |
| C105 | 5.98 ± 0.02 b | 30.31 ± 0.34 ab | 2.95 ± 0.03 b | 0.75 ± 0.02 b | 13.87 ± 0.3 c | 265.37 ± 4.52 b | 1.36 ± 0.04 c | 113.83 ± 2.99 c | 5.60 ± 0.35 c | 3.98 ± 0.13 b | 0.19 ± 0.01 b |
| C106 | 5.76 ± 0.03 d | 30.34 ± 0.54 ab | 2.71 ± 0.03 d | 0.76 ± 0.03 ab | 13.17 ± 0.3 d | 240.44 ± 6.23 d | 1.24 ± 0.03 de | 110.64 ± 1.63 c | 5.79 ± 0.18 bc | 2.45 ± 0.07 e | 0.20 ± 0.01 b |
| Normal Fertilization Yield (kg) | Nitrogen-Free Treatment Yield (kg) | NUE (kg/kg) | |
|---|---|---|---|
| C101 | 732.10 ± 18.30 | 412.08 ± 14.33 | 26.67 ± 1.72 a |
| C102 | 696.23 ± 16.61 | 374.62 ± 7.64 | 26.80 ± 0.99 a |
| C103 | 720.35 ± 7.15 | 403.19 ± 5.25 | 26.43 ± 0.50 a |
| C104 | 615.90 ± 6.34 | 475.26 ± 10.61 | 11.72 ± 1.39 c |
| C105 | 543.20 ± 20.55 | 364.15 ± 5.58 | 14.92 ± 1.25 b |
| C106 | 645.75 ± 23.81 | 507.96 ± 19.46 | 11.48 ± 2.37 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Z.; Li, Q.; Fu, R.; Wang, J.; Lu, D.; Chen, C. The Reorganization of Rice Rhizosphere Microbial Communities Driven by Nitrogen Utilization Efficiency and the Regulatory Mechanism of Soil Nitrogen Cycling. Microorganisms 2025, 13, 2215. https://doi.org/10.3390/microorganisms13092215
Xiong Z, Li Q, Fu R, Wang J, Lu D, Chen C. The Reorganization of Rice Rhizosphere Microbial Communities Driven by Nitrogen Utilization Efficiency and the Regulatory Mechanism of Soil Nitrogen Cycling. Microorganisms. 2025; 13(9):2215. https://doi.org/10.3390/microorganisms13092215
Chicago/Turabian StyleXiong, Zhuang, Qiang Li, Rongtao Fu, Jian Wang, Daihua Lu, and Cheng Chen. 2025. "The Reorganization of Rice Rhizosphere Microbial Communities Driven by Nitrogen Utilization Efficiency and the Regulatory Mechanism of Soil Nitrogen Cycling" Microorganisms 13, no. 9: 2215. https://doi.org/10.3390/microorganisms13092215
APA StyleXiong, Z., Li, Q., Fu, R., Wang, J., Lu, D., & Chen, C. (2025). The Reorganization of Rice Rhizosphere Microbial Communities Driven by Nitrogen Utilization Efficiency and the Regulatory Mechanism of Soil Nitrogen Cycling. Microorganisms, 13(9), 2215. https://doi.org/10.3390/microorganisms13092215







