Pathogen Identification, Antagonistic Microbe Screening, and Biocontrol Strategies for Aconitum carmichaelii Root Rot
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Grouping
2.2. Analysis of Soil Physicochemical Properties
2.3. Soil Genomic DNA Extraction and PCR Amplification
2.4. Isolation and Identification of Root Rot Pathogens from A. carmichaelii
2.5. Pathogenicity Assay of Putative Pathogens
2.6. Isolation and Functional Characterization of Rhizosphere Beneficial Microbes
2.6.1. Microbial Isolation and Preservation
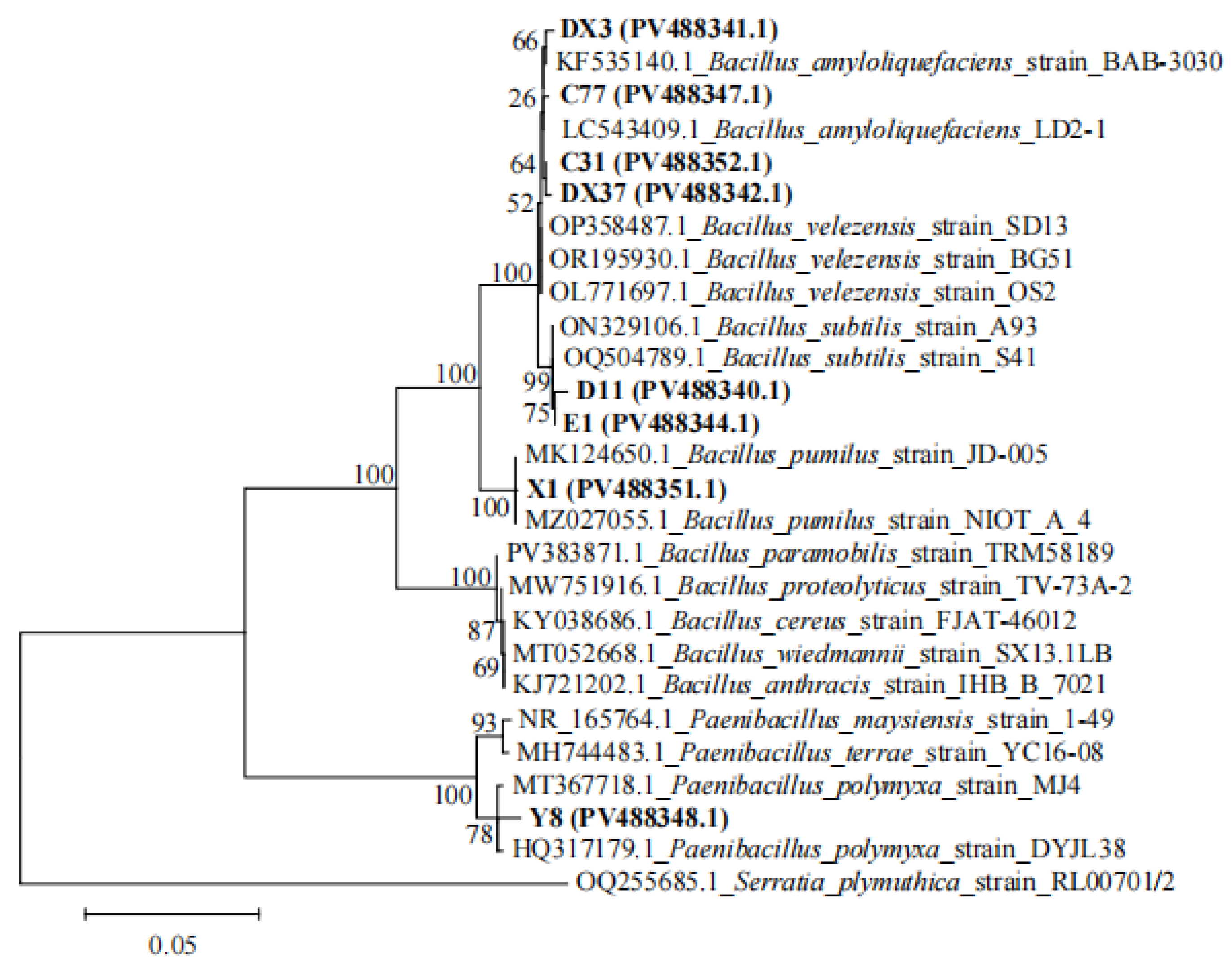
2.6.2. Molecular Identification
2.6.3. PGP Trait Screening
2.6.4. Antagonistic Activity Assay
2.7. Pot Experiment for Elite Strain Validation
2.7.1. Experimental Setup
2.7.2. PGP Effect Assessment
2.7.3. Biocontrol Efficacy Evaluation
2.7.4. Disease Assessment and Physiological Analysis
2.8. Statistical and Bioinformatics Analyses
3. Results
3.1. Rhizosphere Soil Properties of Healthy vs. Diseased A. carmichaelii
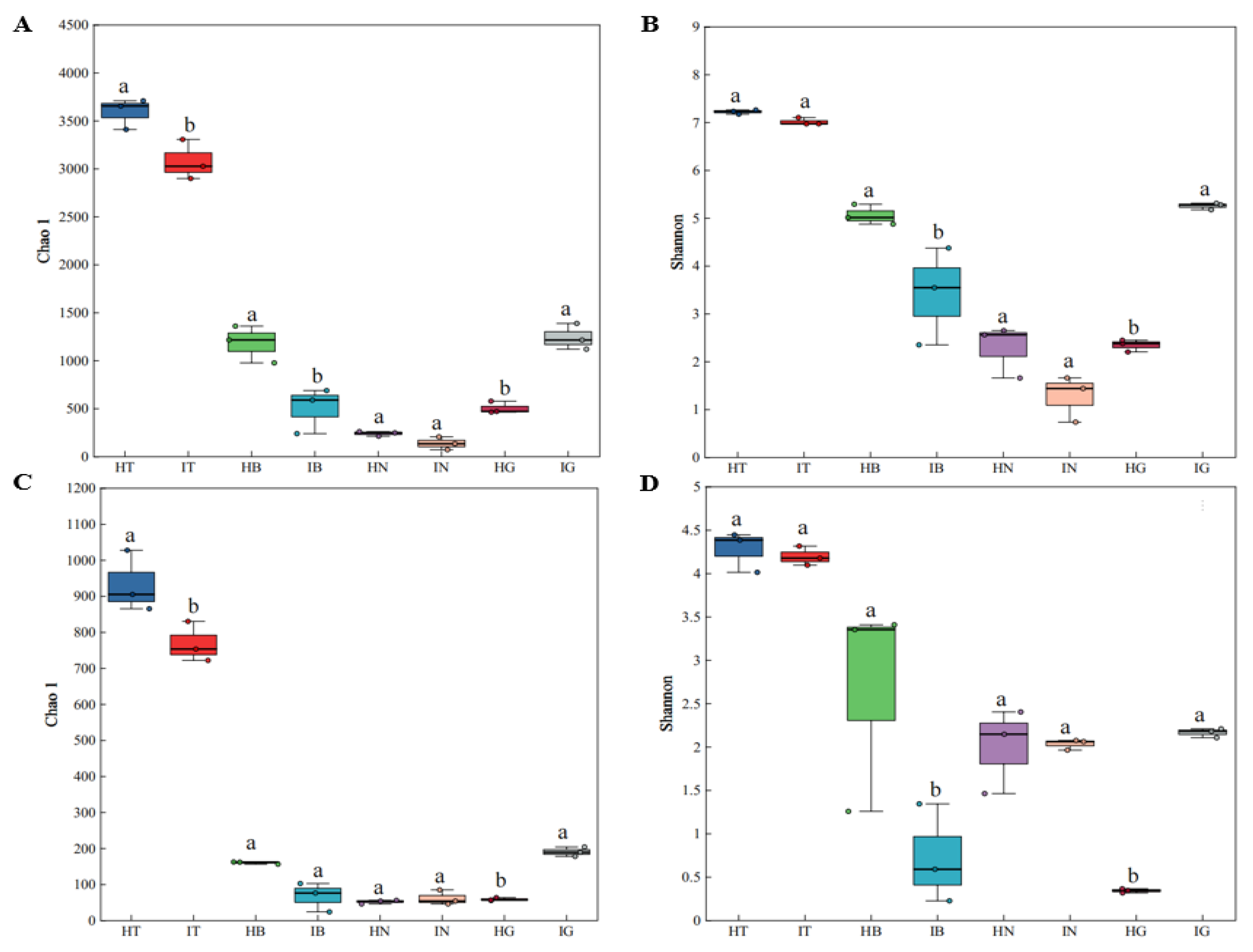
3.2. Microbial Diversity and Community Structure
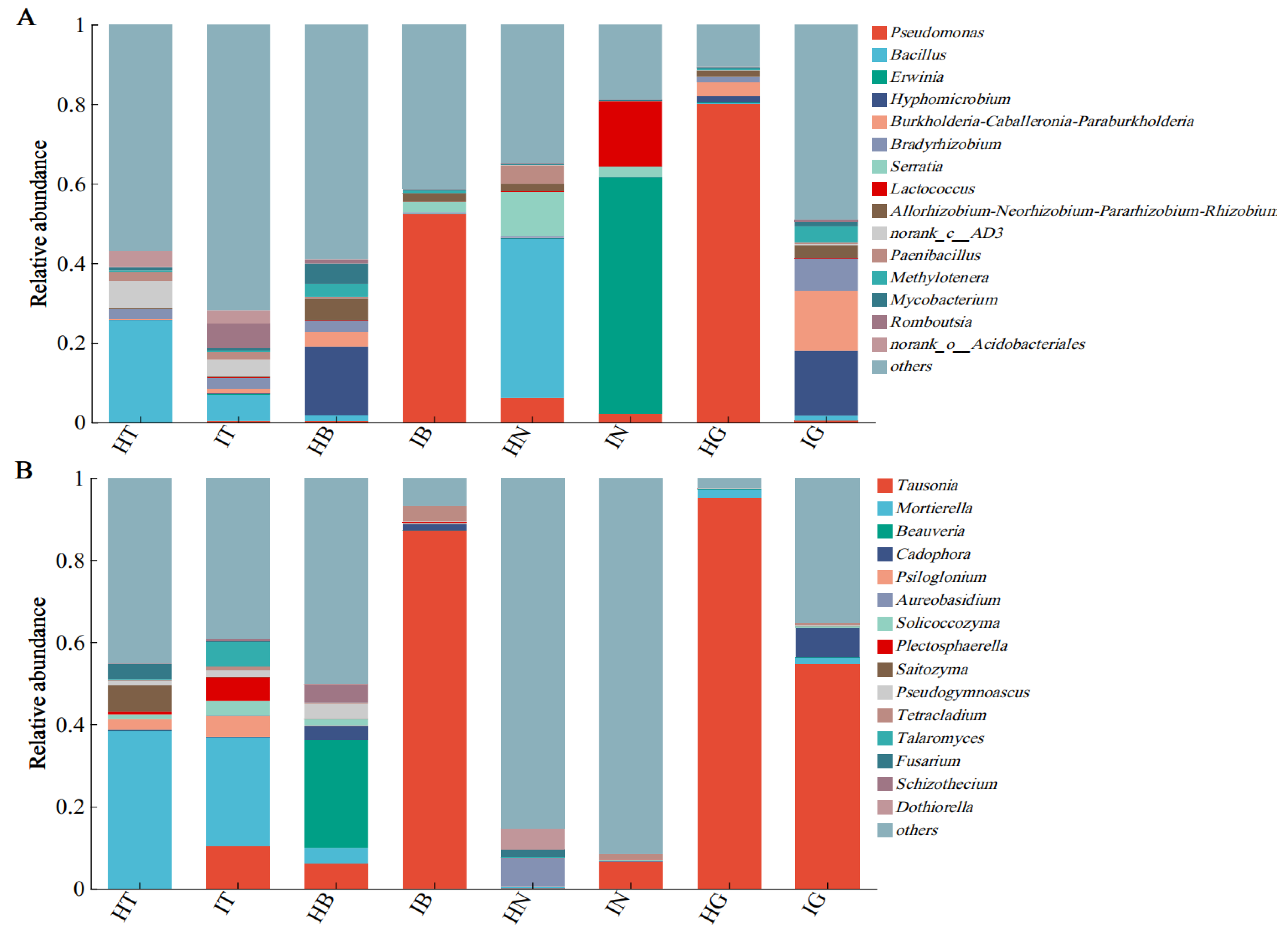
3.3. Microbial Community Composition
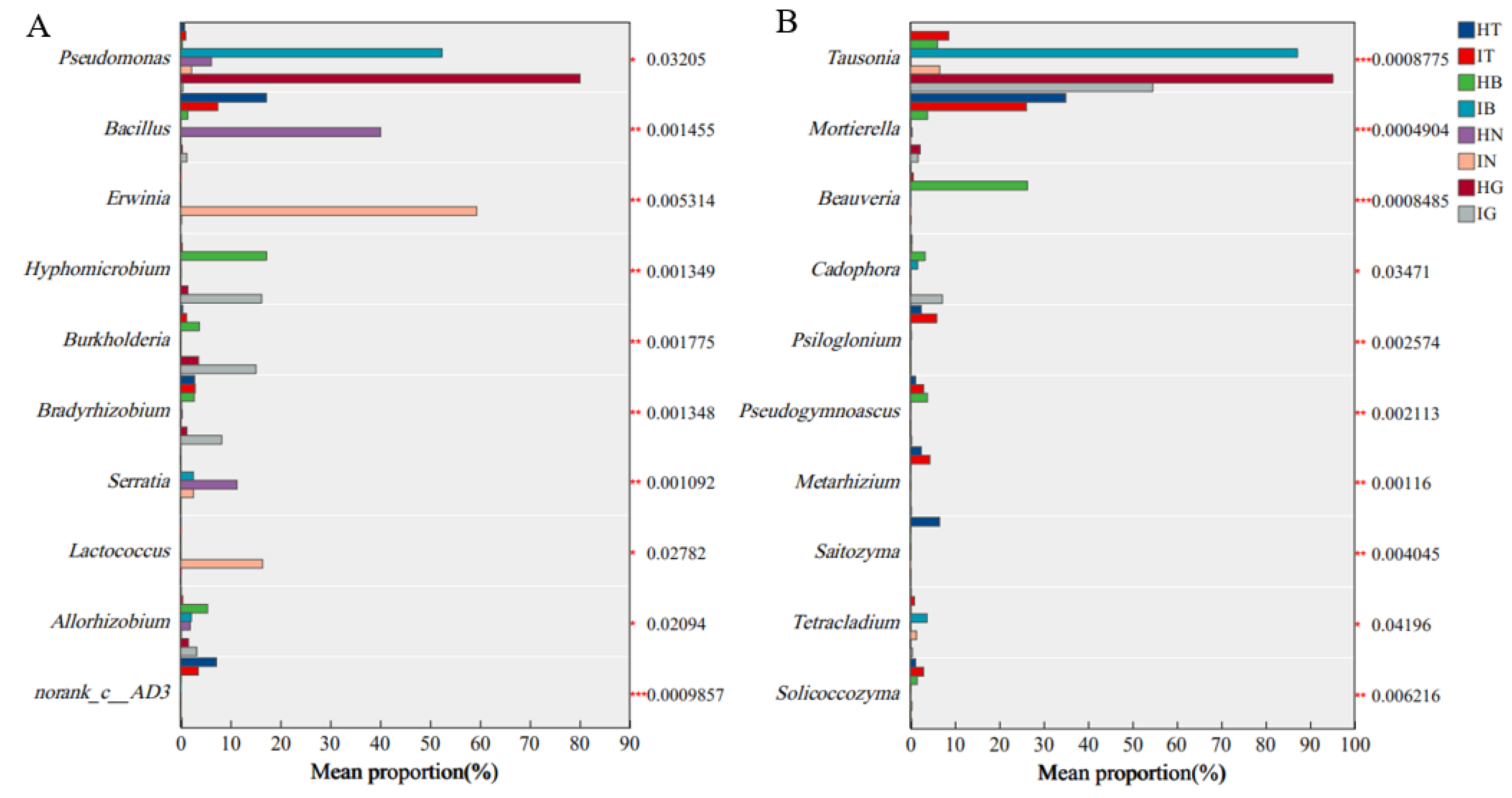
3.4. Differential Abundance Analysis
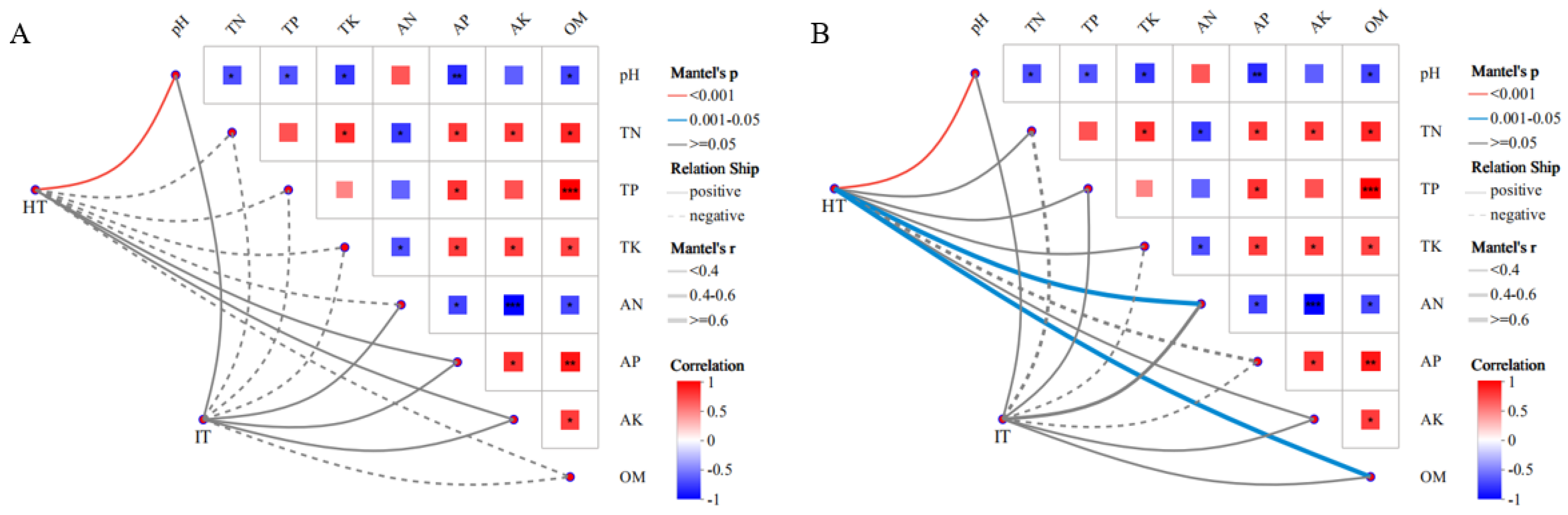
3.5. Correlations Between Soil Physicochemical Properties and A. carmichaelii Root Rot
3.6. Pathogenicity Assay and Identification of Potential Pathogens
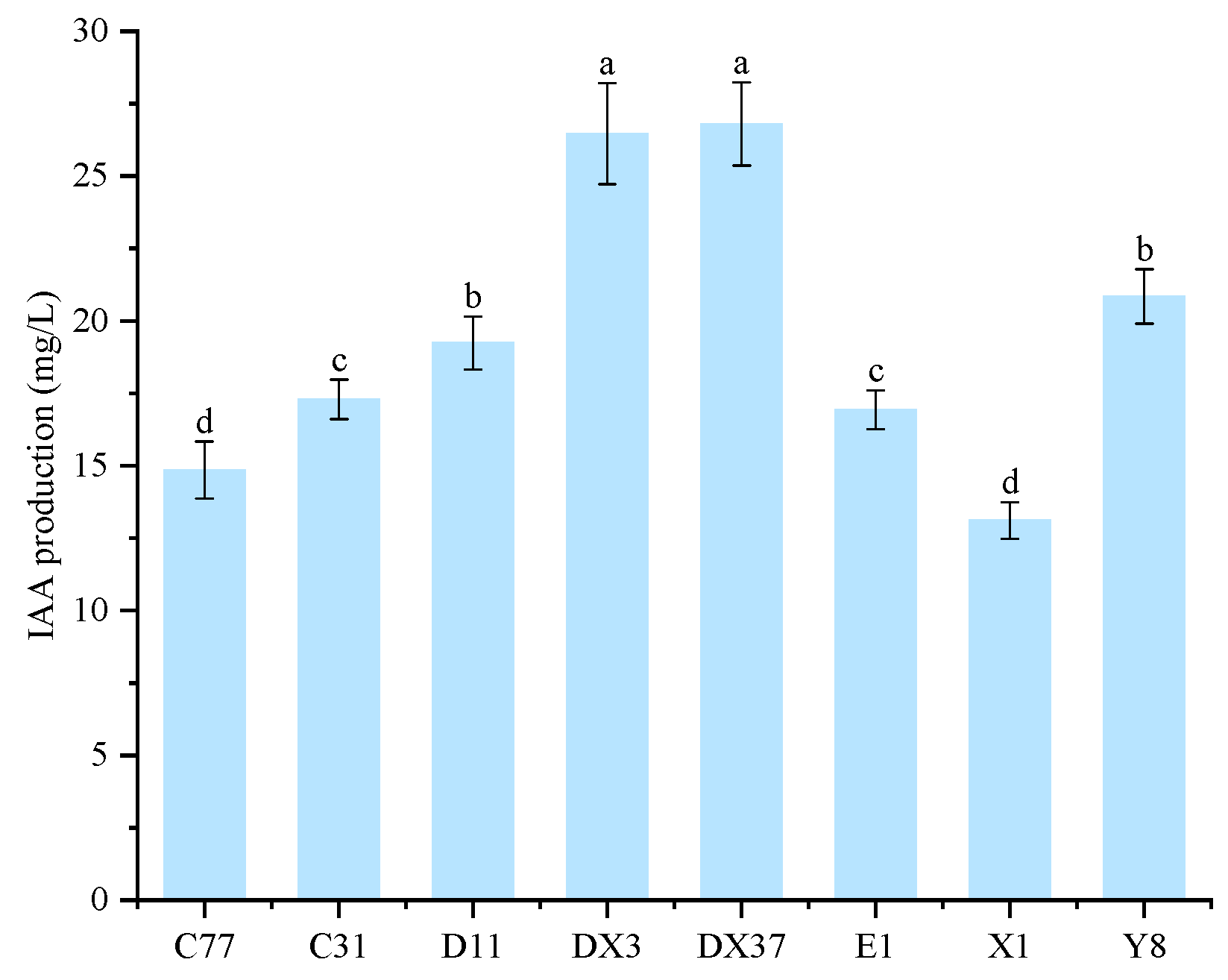
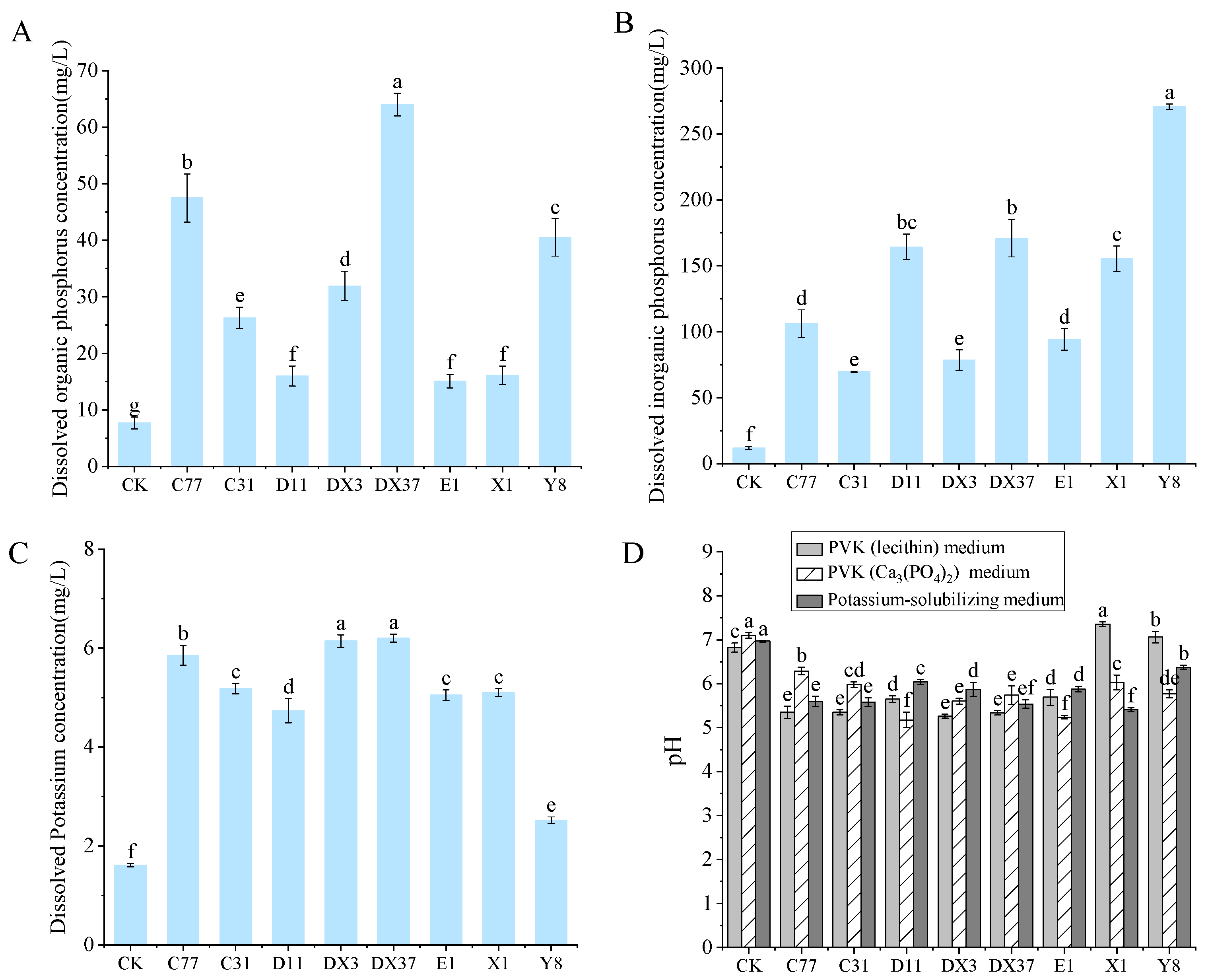
3.7. Plant Growth-Promoting Effects of Rhizosphere Beneficial Microorganisms
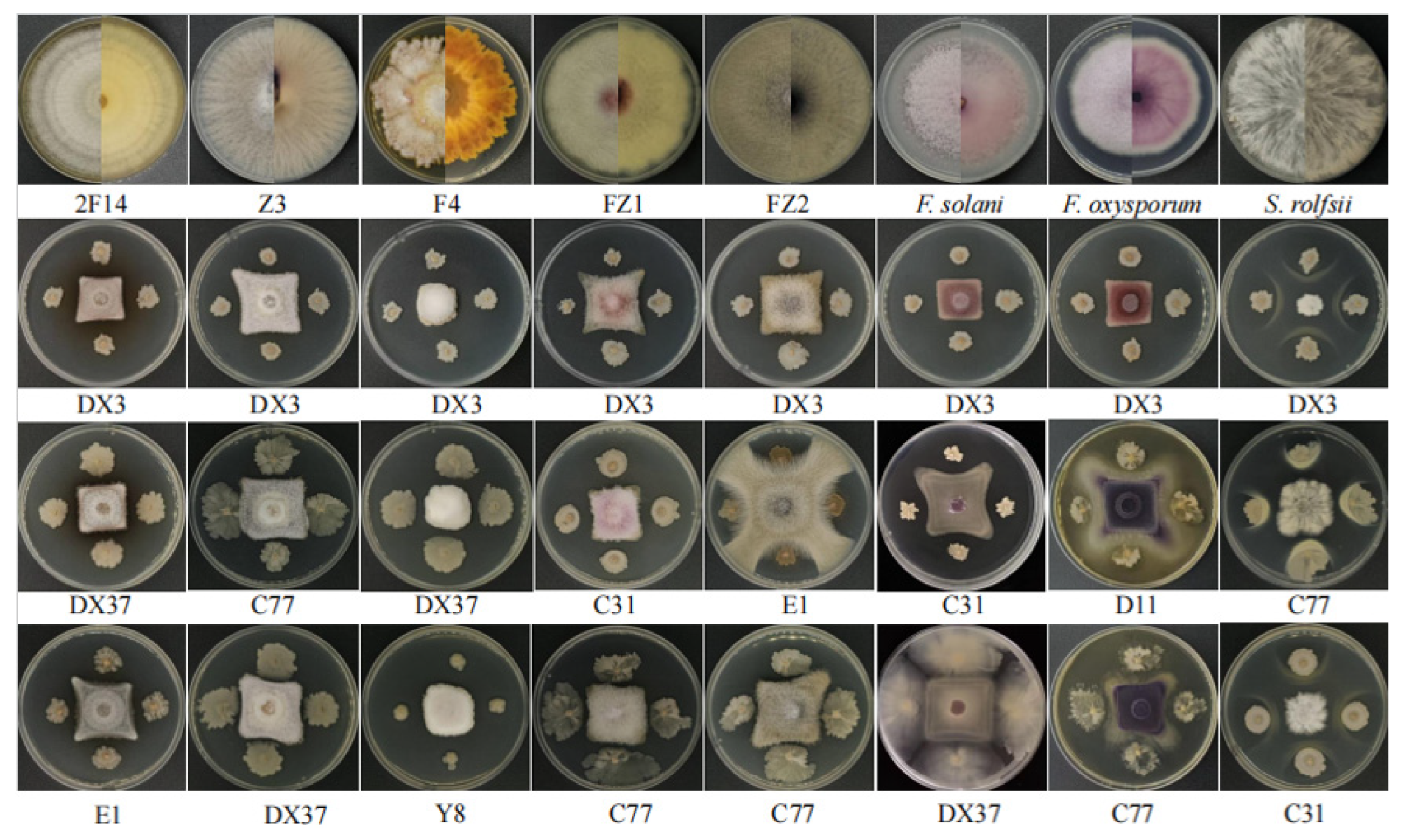
3.8. Antagonistic Activity of Rhizosphere Beneficial Microorganisms Against Pathogens
3.9. Quantification of Growth-Promoting Effects by Rhizosphere Beneficial Microorganisms
3.10. Biocontrol Efficacy of Strain DX3 Against A. carmichaelii Root Rot
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, L.L.; Zhou, Q.M.; Peng, C.; Meng, C.W.; Wang, X.Y.; Xiong, L. Discrimination of the geographical origin of the lateral roots of Aconitum carmichaelii using the fingerprint, multicomponent quantification, and chemometric methods. Molecules 2019, 24, 4124. [Google Scholar] [CrossRef]
- Xia, F.; Wang, L.N.; Chen, J.Y.; Fu, M.; Wang, G.D.; Yan, Y.P.; Cui, L.J. Variations of microbial community in Aconitum carmichaeli Debx. rhizosphere soilin a short-term continuous cropping system. J. Microbiol. 2021, 59, 481–490. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, X.; Chen, B.; Li, M.Z.; Zhang, G.Y.; Cui, L.J. Characteristics of isolates of Pseudomonas aeruginosa and Serratia marcescens associated with post-harvest Fuzi (Aconitum carmichaelii) rot and their novel loop-mediated isothermal amplification detection methods. Front. Microbiol. 2021, 12, 705329. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, P.D.; Liu, Z.Y.; Zhang, J.L.; Zhang, G.Y.; Cui, L.J. Strip intercropping with local crops increased Aconitum carmichaeli yield and soil quality. Front. Plant Sci. 2023, 14, 1147671. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Mei, Y.; Xu, S.Q.; Sun, M.Y.; Zhang, W.T.; Zhou, F.; Li, J.Y.; Wang, J.H. Research progress on continuous cropping obstacles of medicinal plants. Guangdong Agric. Sci. 2021, 48, 162–173. [Google Scholar]
- Zeeshan Ul Haq, M.; Yu, J.; Yao, G.L.; Yang, H.G.; Amina, L.H.; Tahir, H.; Cui, H.G.; Liu, Y.; Wu, Y.G. A systematic review on the continuous cropping obstacles and control strategies in medicinal plants. Int. J. Mol. Sci. 2023, 24, 12470. [Google Scholar] [CrossRef]
- Li, C.W.; Chen, G.Z.; Zhang, J.L.; Zhu, P.; Bai, X.F.; Hou, Y.P.; Zhang, X.X. The comprehensive changes in soil properties are continuous cropping obstacles associated with American ginseng (Panax quinquefolius) cultivation. Sci. Rep. 2021, 11, 5068. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Lin, W.X. Continuous cropping obstacle and allelopathic autotoxicity of medicinal plants. Chin. J. Eco-Agric. 2009, 17, 189–196. [Google Scholar] [CrossRef]
- Li, Y.L.; Guo, Q.; Wei, X.M.; Xue, Q.H.; Lai, H.X. Biocontrol effects of Penicillium griseofulvum against monkshood (Aconitum carmichaelii Debx.) root diseases caused by Sclerotium rolfsiii and Fusarium spp. J. Appl. Microbiol. 2019, 127, 1532–1545. [Google Scholar]
- Li, Y.L.; He, F.; Guo, Q.; Feng, Z.Y.; Zhang, M.; Ji, C.L.; Xue, Q.H.; Lai, H.X. Compositional and functional comparison on the rhizosphere microbial community between healthy and Sclerotium rolfsii-infected monkshood (Aconitum carmichaelii) revealed the biocontrol potential of healthy monkshood rhizosphere microorganisms. Biol. Control 2022, 165, 104790. [Google Scholar] [CrossRef]
- Martinko, K.; Ivanković, S.; Lazarević, B.; Đermić, E.; Đermić, D. Control of early blight fungus (Alternaria alternata) in tomato by boric and phenylboronic acid. Antibiotics 2022, 11, 320. [Google Scholar] [CrossRef]
- Pietri, J.C.A.; Brookes, P.C. Substrate inputs and pH as factors controlling microbial biomass, activity and community structure in an arable soil. Soil Biol. Biochem. 2009, 41, 1396–1405. [Google Scholar] [CrossRef]
- Bengtson, P.; Sterngren, A.E.; Rousk, J. Archaeal abundance across a pH gradient in an arable soil and its relationship to bacterial and fungal growth rates. Appl. Environ. Microbiol. 2012, 78, 5906–5911. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Achtenhagen, J.; Miltner, A.; Eckhardt, K.U.; Leinweber, P.; Emmerling, C.; Thiele, B.S. Microbial contribution to SOM quantity and quality in density fractions of temperate arable soils. Soil Biol. Biochem. 2015, 81, 311–322. [Google Scholar] [CrossRef]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef]
- Zou, L.; Wang, Q.; Wu, R.X.; Zhang, Y.P.; Wu, Q.S.; Li, M.Y.; Ye, K.H.; Dai, W.; Huang, J. Biocontrol and plant growth promotion potential of endophytic Bacillus subtilis JY-7-2L on Aconitum carmichaelii Debx. Front. Microbiol. 2023, 13, 1059549. [Google Scholar] [CrossRef]
- Wang, H.S.; Zhu, Y.X.; Lu, B.; He, W.J.; Lin, J.; Yang, Y.X.; Zhang, S.L.; Luo, B.; Zhang, X.; Fang, Q.M.; et al. First report of root rot caused by Ilyonectria robusta in the medicinal herb Aconitum carmichaelii in China. Plant Dis. 2023, 107, 3312. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, D.Y.; Wen, H.; Wang, Q.H.; Peng, C.; Gao, J.H. Soil fungal biodiversity and pathogen identification of rotten disease in Aconitum carmichaelii (Fuzi) roots. PLoS ONE 2018, 13, e0205891. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Zhao, D.; Yang, Y.W.; Zhao, T.C.; Guan, W.; Wang, T.L. First report of soft rot of Aconitum carmichaelii caused by Pectobacterium brasiliense in China. Plant Dis. 2022, 106, 1516. [Google Scholar] [CrossRef]
- Li, J.G.; Ren, G.D.; Jia, Z.J.; Dong, Y.H. Composition and activity of rhizosphere microbial communities associated with healthy and diseased greenhouse tomatoes. Plant Soil 2014, 380, 337–347. [Google Scholar] [CrossRef]
- Schlatter, D.C.; Hansen, J.; Carlson, B.; Leslie, I.N.; Huggins, D.R.; Paulitz, T.C. Are microbial communities indicators of soil health in a dryland wheat cropping system? Appl. Soil Ecol. 2022, 170, 104302. [Google Scholar] [CrossRef]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Han, S.Y.; Chen, J.X.; Zhao, Y.J.; Cai, H.S.; Guo, C.H. Bacillus subtilis HSY21 can reduce soybean root rot and inhibit the expression of genes related to the pathogenicity of Fusarium oxysporum. Pestic. Biochem. Physiol. 2021, 178, 104916. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D.W. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Fact. 2020, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Wang, W.H.; Zhang, S.K.; Chen, J.H.; Wu, J.S. Soil pH and potassium drive root rot in Torreya grandis via direct modulation and microbial taxa-mediated pathways. Ind. Crops Prod. 2025, 228, 120940. [Google Scholar] [CrossRef]
- Pu, T.T.; Liu, J.; Dong, J.J.; Qian, J.; Zhou, Z.Y.; Xia, C.L.; Wei, G.F.; Duan, B.Z. Microbial community diversity and function analysis of Aconitum carmichaelii Debeaux in rhizosphere soil of farmlands in Southwest China. Front. Microbiol. 2022, 13, 1055638. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Zhao, D.F.; Ma, W.J.; Guo, Y.D.; Wang, A.J.; Wang, Q.L.; Lee, D.J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef]
- Zhang, L.P.; Xu, M.; Yang, J.X.; Yang, J.Y.; Wang, J.; Xu, J.Q.; Kang, Y.B. Isolation of endophytic fungi from tobacco plants in Sanmenxia andscreening and identification of functional strains. Chin. J. Pestic. Sci. 2025, 27, 513–524. [Google Scholar]
- Wang, B.; Cui, W.Y.; He, P.J.; Wu, Y.X.; He, P.F.; Wang, Z.Q.; Li, X.Y.; He, P.B.; He, Y.Q. The inhibitory effect of crude protein from Bacillus amyloliquefaciens ASR-12 against various plant pathogens. J. Anhui Agric. Univ. 2019, 46, 865–869. [Google Scholar]
- Jia, Y.; Sun, R.K.; Wu, Y.X.; Mao, Z.C.; Sun, H.X.; He, Y.Q. Inhibition effect of Bacillus subtilis XF-1 extract on 11 fungal pathogens. J. Anhui Agric. Univ. 2011, 38, 753–756. [Google Scholar]
- Widawati, S. Isolation of Indole Acetic Acid (IAA) producing Bacillus siamensis from peat and optimization of the culture conditions for maximum IAA production. IOP Conf. Ser. Earth Environ. Sci. 2020, 572, 012025. [Google Scholar]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. The screening of potassium-and phosphate-solubilizing actinobacteria and the assessment of their ability to promote wheat growth parameters. Microorganisms 2021, 9, 470. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Wei, Y.H.; Chen, J.Q.; Dong, Y.H.; Hou, L.Y.; Jiao, R.Z. Screening, identification and growth-promotion products of multifunctional bacteria in a Chinese fir plantation. Forests 2021, 12, 120. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Guo, X.; Lei, Z.H.; Wang, Y.Y.; Yang, Z.Y.; Niu, J.P.; Liang, J.P. Screening of high-efficiency nitrogen-fixing bacteria from the traditional Chinese medicine plant Astragalus mongolicus and its effect on plant growth promotion and bacterial communities in the rhizosphere. BMC Microbiol. 2023, 23, 292. [Google Scholar] [CrossRef]
- Wang, J.J.; Bao, F.; Wei, H.X.; Zhang, Y. Screening of cellulose-degrading bacteria and optimization of cellulase production from Bacillus cereus A49 through response surface methodology. Sci. Rep. 2024, 14, 7755. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Liu, Z.; Han, F.; Chen, S.G.; Zhou, W.Z. Integrated application of phosphorus-accumulating bacteria and phosphorus-solubilizing bacteria to achieve sustainable phosphorus management in saline soils. Sci. Total Environ. 2023, 885, 163971. [Google Scholar]
- Sarikhani, M.R.; Oustan, S.; Ebrahimi, M.; Aliasgharzad, N. Isolation and identification of potassium-releasing bacteria in soil and assessment of their ability to release potassium for plants. Eur. J. Soil Sci. 2018, 69, 1078–1086. [Google Scholar] [CrossRef]
- Li, W.X.; Sun, L.L.; Wu, H.T.; Gu, W.J.; Lu, Y.S.; Liu, C.; Zhang, J.X.; Li, W.L.; Zhou, C.M.; Geng, H.Y.; et al. Bacillus velezensis YXDHD1-7 prevents early blight disease by promoting growth and enhancing defense enzyme activities in tomato plants. Microorganisms 2024, 12, 921. [Google Scholar] [CrossRef]
- Gao, S.; Ouyang, C.; Wang, S.; Xu, Y.; Tang, L.; Chen, F. Effects of salt stress on growth, antioxidant enzyme and phenylalanine ammonia-lyase activities in Jatropha curcas L. seedlings. Plant Soil Environ. 2008, 54, 374–381. [Google Scholar] [CrossRef]
- Chen, L.J.; Hu, Y.W.; Huang, L.; Chen, L.; Duan, X.L.; Wang, G.Z.; Ou, H. Comparative transcriptome revealed the molecular responses of Aconitum carmichaelii Debx. to downy mildew at different stages of disease development. BMC Plant Biol. 2024, 24, 332. [Google Scholar] [CrossRef]
- Myo, E.M.; Liu, B.H.; Ma, J.J.; Shi, L.M.; Jiang, M.G.; Zhang, K.C.; Ge, B.B. Evaluation of Bacillus velezensis NKG-2 for bio-control activities against fungal diseases and potential plant growth promotion. Biol. Control 2019, 134, 23–31. [Google Scholar] [CrossRef]
- Khan, M.; Salman, M.; Jan, S.A.; Shinwari, Z.K. Biological control of fungal phytopathogens: A comprehensive review based on Bacillus species. MOJ Biol. Med. 2021, 6, 90–92. [Google Scholar] [CrossRef]
- Gan, K.F.; Chen, M.; Liang, T.T.; Li, X.M.; Li, G.; Liu, D.Q. Biocontrol effects and underlying mechanism of Bacillus subtilis Pn1 on Panax notoginseng root rot caused by Fusarium solani. Ind. Crops Prod. 2025, 229, 120963. [Google Scholar]
- Kang, T.; Zhang, M.M.; Xia, M.Y.; Chen, K.; Zhai, Y.F.; Yan, B.B.; Wang, Y.P.; Wu, H.M. Rhizosphere regulation: Development and blueprint for soil-borne disease suppression in strawberry. Ann. Appl. Biol. 2025, 186, 27–37. [Google Scholar]
- Cardinale, B.J.; Wright, J.P.; Cadotte, M.W.; Weis, J.J. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl. Acad. Sci. USA 2007, 104, 18123–18128. [Google Scholar] [CrossRef]
- Armitage, D.W. Linking the development and functioning of a carnivorous pitcher plant’s microbial digestive community. ISME J. 2017, 11, 2439–2451. [Google Scholar] [CrossRef]
- Gong, B.; He, Y.; Luo, Z.B.; Peng, H.W.; Cai, H.Q.; Zhu, Y.N.; Bin, J.; Ding, M.J. Response of rhizosphere soil physicochemical properties and microbial community structure to continuous cultivation of tobacco. Ann. Microbiol. 2024, 74, 4. [Google Scholar] [CrossRef]
- Jia, C.B.; An, Y.R.; Du, Z.Y.; Gao, H.H.; Su, J.Y.; Xu, C.Y. Differences in soil microbial communities between healthy and diseased Lycium barbarum cv. Ningqi-5 plants with root rot. Microorganisms 2023, 11, 694. [Google Scholar] [CrossRef]
- Deng, Y.; Kong, W.Y.; Zhang, X.M.; Zhu, Y.; Xie, T.; Chen, M.; Zhu, L.; Sun, J.Z.; Zhang, Z.H.; Chen, C.Y.; et al. Rhizosphere microbial community enrichment processes in healthy and diseased plants: Implications of soil properties on biomarkers. Front. Microbiol. 2024, 15, 1333076. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Wróbel, B.; Górska, E.B. Taxonomy, ecology, and cellulolytic properties of the genus Bacillus and related genera. Agriculture 2023, 13, 1979. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Redmile-Gordon, M.; Zhang, J.B.; Zhang, C.Z.; Ning, Q.; Li, W. Mortierella elongata’s roles in organic agriculture and crop growth promotion in a mineral soil. Land Degrad. Dev. 2018, 29, 1642–1651. [Google Scholar] [CrossRef]
- Toscano-Verduzco, F.A.; Cedeño-Valdivia, P.A.; Chan-Cupul, W.; Hernández-Ortega, H.A.; Ruiz-Sánchez, E.; Galindo-Velasco, E.; Cruz-Crespo, E. Phosphates solubilization, indol-3-acetic acid and siderophores production by Beauveria brongniartii and its effect on growth and fruit quality of Capsicum chinense. J. Hortic. Sci. Biotechnol. 2020, 95, 235–246. [Google Scholar] [CrossRef]
- Mishra, D.S.; Kumar, A.; Prajapati, C.R.; Singh, A.K.; Sharma, S.D. Identification of compatible bacterial and fungal isolate and their effectiveness against plant disease. J. Environ. Biol. 2013, 34, 183. [Google Scholar]
- Lv, B.Y.; Yang, X.; Xue, H.L.; Nan, M.N.; Zhang, Y.; Liu, Z.G.; Bi, Y.; Shang, S.Q. Isolation of main pathogens causing postharvest disease in fresh Codonopsis pilosula during different storage stages and ozone control against disease and mycotoxin accumulation. J. Fungi 2023, 9, 146. [Google Scholar] [CrossRef]
- Qi, H.X.; Duan, X.M.; Xu, W.H.; Zhou, Y.T.; Ma, H.X.; Ma, W.L.; Ma, G.H. First report disease of Clonostachys rosea causing root rot on Astragalus membranaceus in China. Plant Dis. 2022, 106, 1752. [Google Scholar] [CrossRef]
- Ma, H.X.; Duan, X.M.; Xu, W.H.; Ma, G.H.; Ma, W.L.; Qi, H.X. Root rot of Angelica sinensis caused by Clonostachys rosea and Fusarium acuminatum in China. Plant Dis. 2022, 106, 2264. [Google Scholar] [CrossRef]
- Lee, S.A.; Kang, M.J.; Kim, T.D.; Park, E.J. First report of Clonostachys rosea causing root rot of Gastrodia elata in Korea. Plant Dis. 2020, 104, 3069. [Google Scholar] [CrossRef]
- Elnahal, A.S.M.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- de Almeida Leite, R.; Martins da Costa, E.; Cabral Michel, D.; do Amaral Leite, A.; de Oliveira-Longatti, S.M.; de Lima, W.; Konstantinidis, K.T.; de Souza Moreira, F.M. Genomic insights into organic acid production and plant growth promotion by different species of phosphate-solubilizing bacteria. World J. Microbiol. Biotechnol. 2024, 40, 311. [Google Scholar] [CrossRef]
- Delvasto, P.; Ballester, A.; Muñoz, J.A.; González, F.; Blázquez, M.L.; Igual, J.M.; Valverde, A.; García-Balboa, C. Mobilization of phosphorus from iron ore by the bacterium Burkholderia caribensis FeGL03. Miner. Eng. 2009, 22, 1–9. [Google Scholar] [CrossRef]
- Stajković-Srbinović, O.; Delić, D.; Nerandžić, B.; Andjelović, S.; Sikirić, B.; Kuzmanović, D.; Rasulić, N. Alfalfa yield and nutrient uptake as influenced by co-inoculation with rhizobium and rhizobacteria. Rom. Biotechnol. Lett. 2017, 22, 12834–12841. [Google Scholar]
- Yost, C.K.; Del Bel, K.L.; Quandt, J.; Hynes, M.F. Rhizobium leguminosarum methyl-accepting chemotaxis protein genes are down-regulated in the pea nodule. Arch. Microbiol. 2004, 182, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Yuan, Z.X.; Shi, Y.Q.; Cai, F.H.; Zhao, J.R.; Wang, J.Z.; Wang, Y.P. Bacillus amyloliquefaciens HG01 induces resistance in loquats against anthracnose rot caused by Colletotrichum acutatum. Postharvest Biol. Technol. 2020, 160, 111034. [Google Scholar] [CrossRef]
- Pei, D.L.; Zhang, Q.C.; Zhu, X.Q.; Zhang, L. Biological control of Verticillium wilt and growth promotion in tomato by rhizospheric soil-derived Bacillus amyloliquefaciens Oj-2.16. Pathogens 2023, 12, 37. [Google Scholar] [CrossRef]
- Pal, G.; Saxena, S.; Kumar, K.; Verma, A.; Kumar, D.; Shukla, P.; Pandey, A.; Verma, S.K. Seed endophytic bacterium Bacillus velezensis and its lipopeptides acts as elicitors of defense responses against Fusarium verticillioides in maize seedlings. Plant Soil 2023, 492, 109–124. [Google Scholar] [CrossRef]


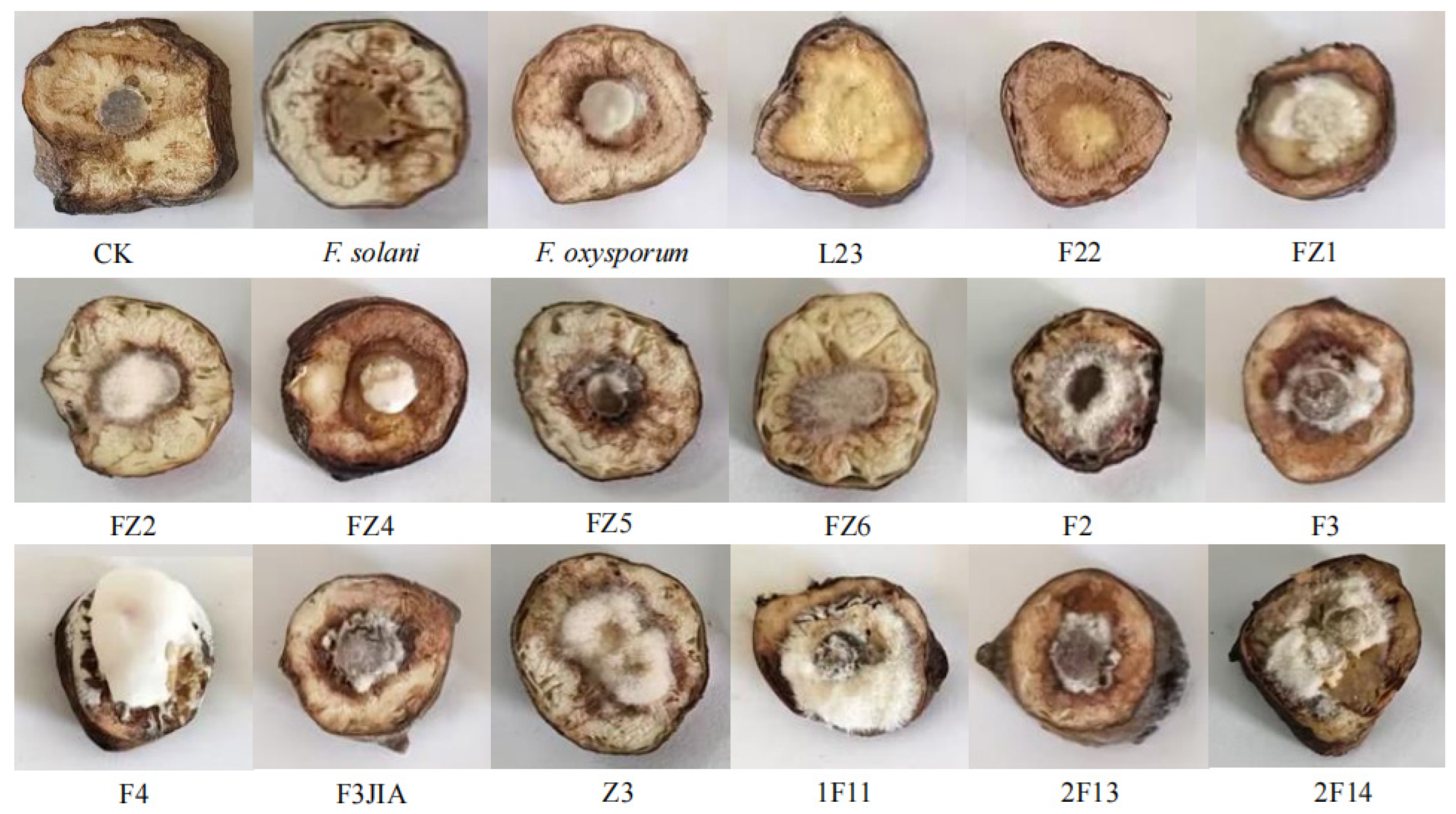
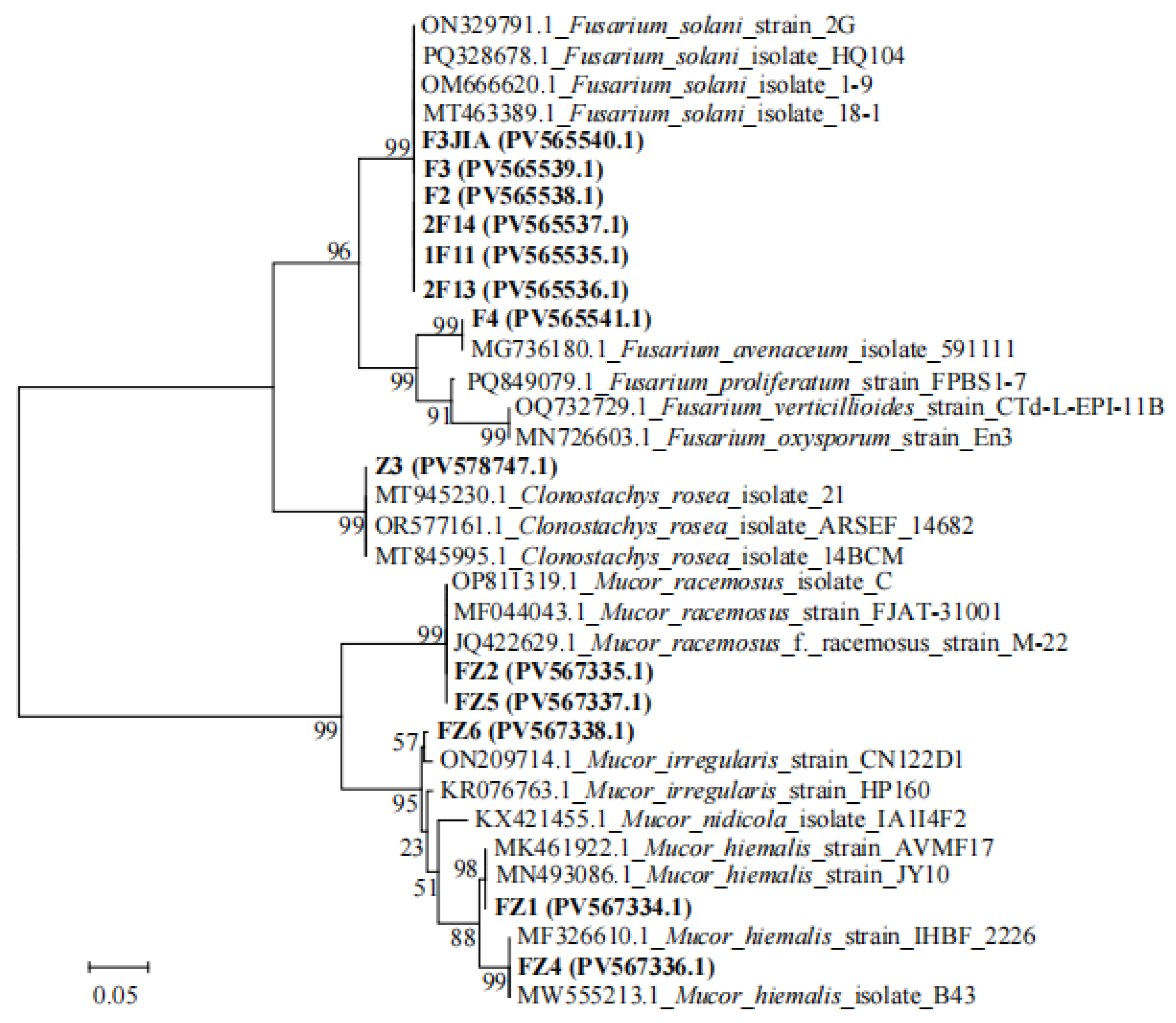
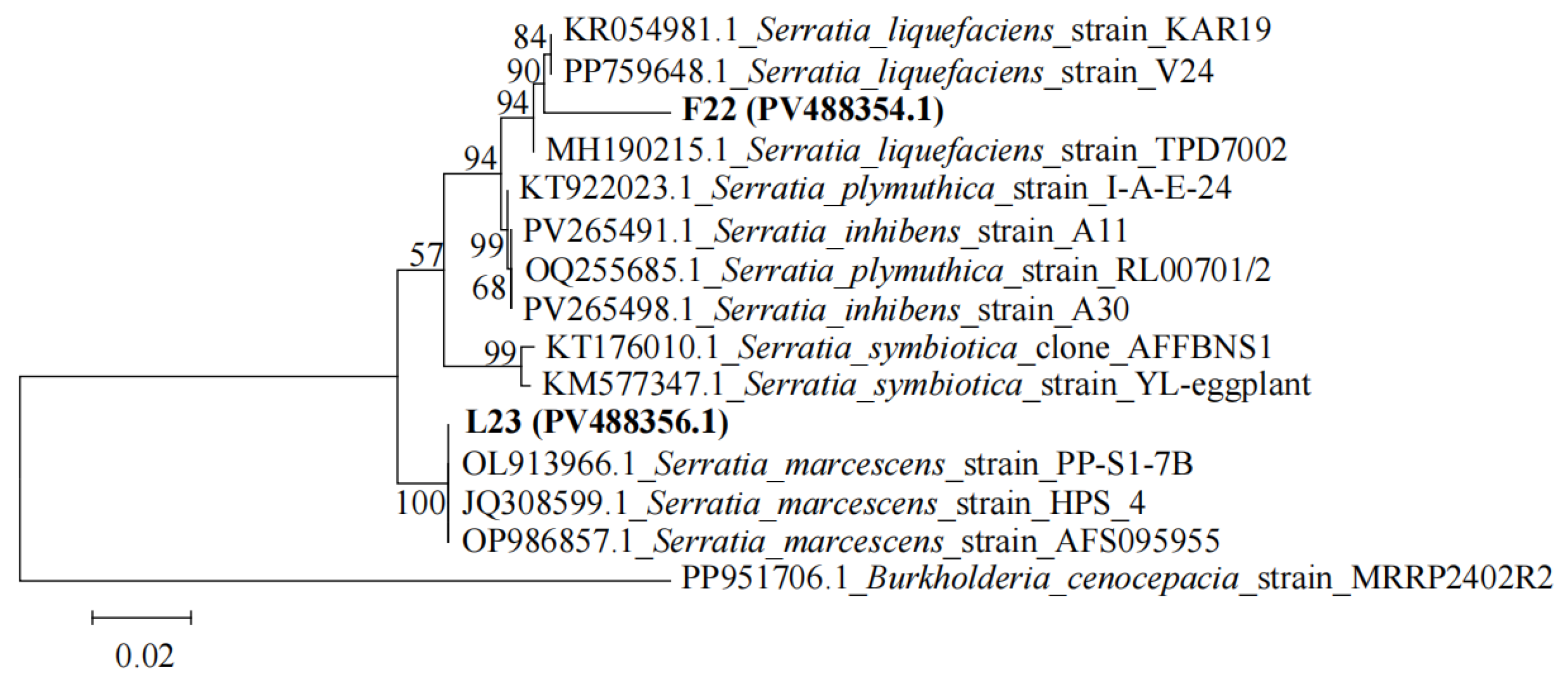
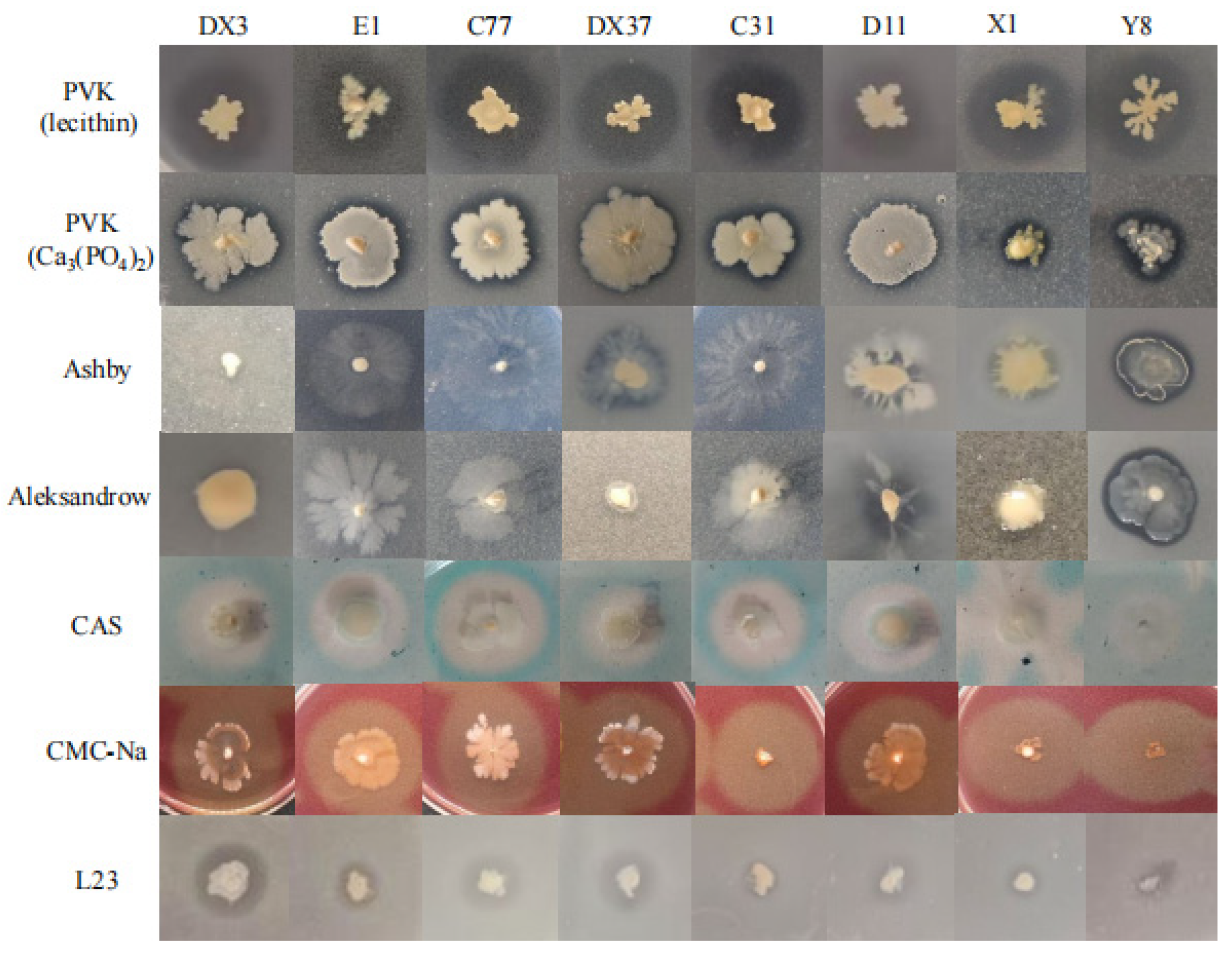


| Treatment | pH | TN (g/Kg) | TP (g/Kg) | TK (g/Kg) | AN (mg/Kg) | AP (mg/Kg) | AK (mg/Kg) | OM (g/Kg) |
|---|---|---|---|---|---|---|---|---|
| HT | 6.66 ± 0.06 a | 4.68 ± 0.04 b | 2.05 ± 0.15 b | 1.96 ± 0.06 b | 549.20 ± 5.40 a | 4.48 ± 0.31 b | 395.20 ± 20.33 b | 99.20 ± 3.56 b |
| IT | 5.52 ± 0.19 b | 5.03 ± 0.07 a | 2.36 ± 0.13 a | 2.24 ± 0.05 a | 505.60 ± 3.20 b | 7.44 ± 0.29 a | 1611.00 ± 61.89 a | 118.40 ± 3.85 a |
| Pathogen Inhibition Rate (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Strain | 2F14 | FZ1 | Z3 | F4 | FZ2 | Fusarium solani | Fusarium oxysporum | Sclerotium rolfsii |
| C77 | 67.27 ± 1.50 cd | 66.25 ± 2.43 a | 61.58 ± 1.00 b | 64.31 ± 1.89 bc | 63.27 ± 2.12 ab | 56.58 ± 1.19 cd | 59.91 ± 1.04 b | 66.50 ± 2.19 c |
| C31 | 71.17 ± 1.55 ab | 67.54 ± 1.97 a | 61.91 ± 1.20 ab | 61.46 ± 3.33 cd | 60.67 ± 2.19 bc | 58.02 ± 0.80 c | 62.78 ± 2.50 b | 72.33 ± 1.66 b |
| D11 | 61.81 ± 0.70 f | 51.77 ± 1.75 b | 53.96 ± 0.75 c | 52.92 ± 2.86 e | 55.77 ± 1.86 d | 55.08 ± 1.02 cd | 43.07 ± 3.00 e | / |
| DX3 | 72.80 ± 1.28 a | 68.23 ± 0.66 a | 65.10 ± 3.98 a | 70.64 ± 0.50 a | 65.79 ± 1.33 a | 72.75 ± 1.26 a | 67.82 ± 2.48 a | 83.09 ± 2.91 a |
| DX37 | 69.28 ± 1.60 bc | 65.66 ± 1.86 a | 62.10 ± 0.69 ab | 67.41 ± 2.63 ab | 65.41 ± 0.72 a | 65.11 ± 0.64 b | 60.30 ± 1.99 b | 50.44 ± 1.86 d |
| E1 | 65.17 ± 1.80 de | 55.29 ± 1.46 b | 56.41 ± 1.52 c | 59.95 ± 1.36 cd | 57.24 ± 2.73 cd | 52.93 ± 2.69 d | 48.15 ± 1.05 d | 52.76 ± 0.95 d |
| Y8 | 63.69 ± 2.27 ef | 54.17 ± 2.92 b | 54.02 ± 0.57 c | 58.71 ± 3.56 d | 46.22 ± 3.29 e | 54.03 ± 5.81 cd | 55.68 ± 2.97 c | / |
| Treatment | Plant Height (cm) | Stem Diameter (cm) | Leaf Number (count) | Chlorophyll Content (SPAD) | Fresh Shoot Weight (g) | Fresh Tuber Weight (g) |
|---|---|---|---|---|---|---|
| CK | 54.69 ± 3.69 b | 2.80 ± 0.24 d | 11.67 ± 1.53 d | 33.33 ± 1.17 b | 14.66 ± 1.96 c | 15.08 ± 4.02 c |
| DX3 | 69.31 ± 4.05 a | 3.82 ± 0.29 bc | 16.33 ± 1.53 a | 53.29 ± 4.62 a | 28.58 ± 4.55 a | 37.01 ± 9.60 a |
| C77 | 67.67 ± 6.60 a | 3.64 ± 0.33 bc | 14.00 ± 1.00 abcd | 49.91 ± 4.90 a | 20.34 ± 2.86 b | 26.43 ± 3.45 b |
| E1 | 63.35 ± 1.60 a | 3.52 ± 0.13 c | 13.33 ± 0.58 cd | 45.01 ± 4.13 a | 20.77 ± 2.83 b | 26.65 ± 2.14 b |
| C31 | 68.61 ± 5.02 a | 4.08 ± 0.34 b | 15.00 ± 1.00 abc | 48.51 ± 5.89 a | 21.24 ± 2.94 b | 26.33 ± 3.40 b |
| X1 | 67.20 ± 3.50 a | 3.38 ± 0.18 c | 13.67 ± 0.58 bcd | 50.18 ± 1.53 a | 20.59 ± 3.49 b | 23.75 ± 4.87 b |
| DX37 | 66.58 ± 4.12 a | 3.63 ± 0.31 bc | 14.33 ± 1.15 abc | 51.59 ± 8.96 a | 23.78 ± 2.49 ab | 26.33 ± 2.99 b |
| D11 | 66.37 ± 5.86 a | 4.73 ± 0.38 a | 16.00 ± 2.65 ab | 53.24 ± 4.05 a | 20.75 ± 4.63 b | 26.15 ± 5.74 b |
| Y8 | 70.40 ± 1.53 a | 3.85 ± 0.18 bc | 14.33 ± 0.58 abc | 50.66 ± 1.16 a | 21.03 ± 1.55 b | 21.04 ± 1.84 bc |
| Treatment | pH | AN (mg/Kg) | AP (mg/Kg) | AK (mg/Kg) | OM (g/Kg) |
|---|---|---|---|---|---|
| CK | 7.36 ± 0.04 a | 108.22 ± 5.36 c | 16.61 ± 1.40 b | 221.69 ± 5.78 f | 35.17 ± 0.57 c |
| DX3 | 7.19 ± 0.03 ef | 125.97 ± 2.13 ab | 20.82 ± 1.66 a | 335.74 ± 14.38 a | 39.16 ± 1.64 ab |
| C77 | 7.23 ± 0.02 de | 120.00 ± 1.58 b | 22.73 ± 1.23 a | 309.66 ± 17.52 bc | 40.13 ± 1.55 a |
| E1 | 7.29 ± 0.02 bc | 124.77 ± 3.78 ab | 23.13 ± 1.60 a | 330.77 ± 12.73 ab | 36.47 ± 1.49 bc |
| C31 | 7.25 ± 0.03 cd | 122.51 ± 4.72 ab | 18.07 ± 0.62 b | 259.51 ± 9.46 e | 37.87 ± 2.65 abc |
| X1 | 7.14 ± 0.03 f | 129.26 ± 2.66 a | 22.74 ± 0.86 a | 350.12 ± 14.91 a | 40.26 ± 1.12 a |
| DX37 | 7.24 ± 0.04 de | 110.81 ± 3.60 c | 23.01 ± 1.29 a | 293.98 ± 9.55 cd | 38.18 ± 0.95 ab |
| D11 | 7.26 ± 0.03 cd | 113.45 ± 2.89 c | 17.72 ± 1.32 b | 284.34 ± 14.07 d | 39.04 ± 1.63 ab |
| Y8 | 7.34 ± 0.04 ab | 129.17 ± 5.17 a | 17.17 ± 0.73 b | 245.05 ± 10.18 e | 38.68 ± 1.25 ab |
| Treatment | Disease Severity Index (DSI) (%) | Treatment | Disease Severity Index (DSI) (%) | Biocontrol Efficacy (BE) (%) |
|---|---|---|---|---|
| 2F14 | 53.78 ± 2.04 b | DX3+2F14 | 19.78 ± 3.01 a | 63.32 ± 4.27 ab |
| FZ1 | 46.22 ± 3.08 c | DX3+FZ1 | 20.00 ± 4.00 a | 56.60 ± 8.86 b |
| L23 | 60.44 ± 2.78 a | DX3+L23 | 21.33 ± 3.53 a | 64.63 ± 6.19 ab |
| 2F14+FZ1 | 40.00 ± 1.33 d | DX3+2F14+FZ1 | 18.22 ± 2.04 a | 54.48 ± 4.32 b |
| 2F14+L23 | 46.67 ± 4.81 c | DX3+2F14+L23 | 18.44 ± 2.52 a | 60.41 ± 4.21 ab |
| FZ1+L23 | 59.55 ± 2.04 a | DX3+FZ1+L23 | 18.45 ± 3.67 a | 69.10 ± 5.39 a |
| 2F14+FZ1+L23 | 39.55 ± 2.04 d | DX3+2F14+FZ1+L23 | 18.67 ± 3.53 a | 52.84 ± 8.26 b |
| Treatment | PPO (U/g) | POD (U/g) | PAL (U/g) |
|---|---|---|---|
| 2F14 | 208.87 ± 29.00 ef | 1672.70 ± 132.02 c | 35.04 ± 5.53 de |
| FZ1 | 259.52 ± 27.94 de | 1701.38 ± 45.44 c | 42.67 ± 4.76 d |
| L23 | 187.27 ± 28.16 f | 1580.15 ± 97.37 c | 27.50 ± 6.03 e |
| 2F14+FZ1 | 285.62 ± 30.79 d | 1545.22 ± 59.32 c | 34.76 ± 5.16 de |
| 2F14+L23 | 295.46 ± 30.39 d | 1707.44 ± 49.53 c | 32.53 ± 5.28 de |
| FZ1+L23 | 218.81 ± 40.50 ef | 1522.61 ± 132.43 c | 26.98 ± 4.60 e |
| 2F14+FZ1+L23 | 221.36 ± 18.41 ef | 1666.23 ± 134.14 c | 43.423.40 d |
| DX3+2F14 | 539.76 ± 37.45 b | 2160.38 ± 66.68 ab | 85.02 ± 10.01 b |
| DX3+FZ1 | 593.65 ± 27.79 a | 2243.80 ± 52.92 a | 71.10 ± 4.66 c |
| DX3+L23 | 524.77 ± 31.23 b | 2148.51 ± 89.83 ab | 97.60 ± 5.72 a |
| DX3+2F14+FZ1 | 555.40 ± 41.65 ab | 2018.11 ± 88.50 b | 76.33 ± 7.32 bc |
| DX3+2F14+L23 | 507.31 ± 11.66 bc | 2042.32 ± 153.50 ab | 76.92 ± 7.03 bc |
| DX3+FZ1+L23 | 458.57 ± 30.89 c | 2182.10 ± 127.96 ab | 97.86 ± 6.44 a |
| DX3+2F14+FZ1+L23 | 467.26 ± 22.76 c | 2187.23 ± 204.51 ab | 72.17 ± 7.84 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, X.; He, Y.; Su, Y.; Mo, H.; Li, W.; Li, W.; Zi, S.; Liu, L.; Di, Y. Pathogen Identification, Antagonistic Microbe Screening, and Biocontrol Strategies for Aconitum carmichaelii Root Rot. Microorganisms 2025, 13, 2202. https://doi.org/10.3390/microorganisms13092202
Dai X, He Y, Su Y, Mo H, Li W, Li W, Zi S, Liu L, Di Y. Pathogen Identification, Antagonistic Microbe Screening, and Biocontrol Strategies for Aconitum carmichaelii Root Rot. Microorganisms. 2025; 13(9):2202. https://doi.org/10.3390/microorganisms13092202
Chicago/Turabian StyleDai, Xingxun, Yuqin He, Yu Su, Huishu Mo, Weichun Li, Wanting Li, Shuhui Zi, Lufeng Liu, and Yining Di. 2025. "Pathogen Identification, Antagonistic Microbe Screening, and Biocontrol Strategies for Aconitum carmichaelii Root Rot" Microorganisms 13, no. 9: 2202. https://doi.org/10.3390/microorganisms13092202
APA StyleDai, X., He, Y., Su, Y., Mo, H., Li, W., Li, W., Zi, S., Liu, L., & Di, Y. (2025). Pathogen Identification, Antagonistic Microbe Screening, and Biocontrol Strategies for Aconitum carmichaelii Root Rot. Microorganisms, 13(9), 2202. https://doi.org/10.3390/microorganisms13092202






