Emerging Roles of the Gut Microbiome in Musculoskeletal Injury and Repair
Abstract
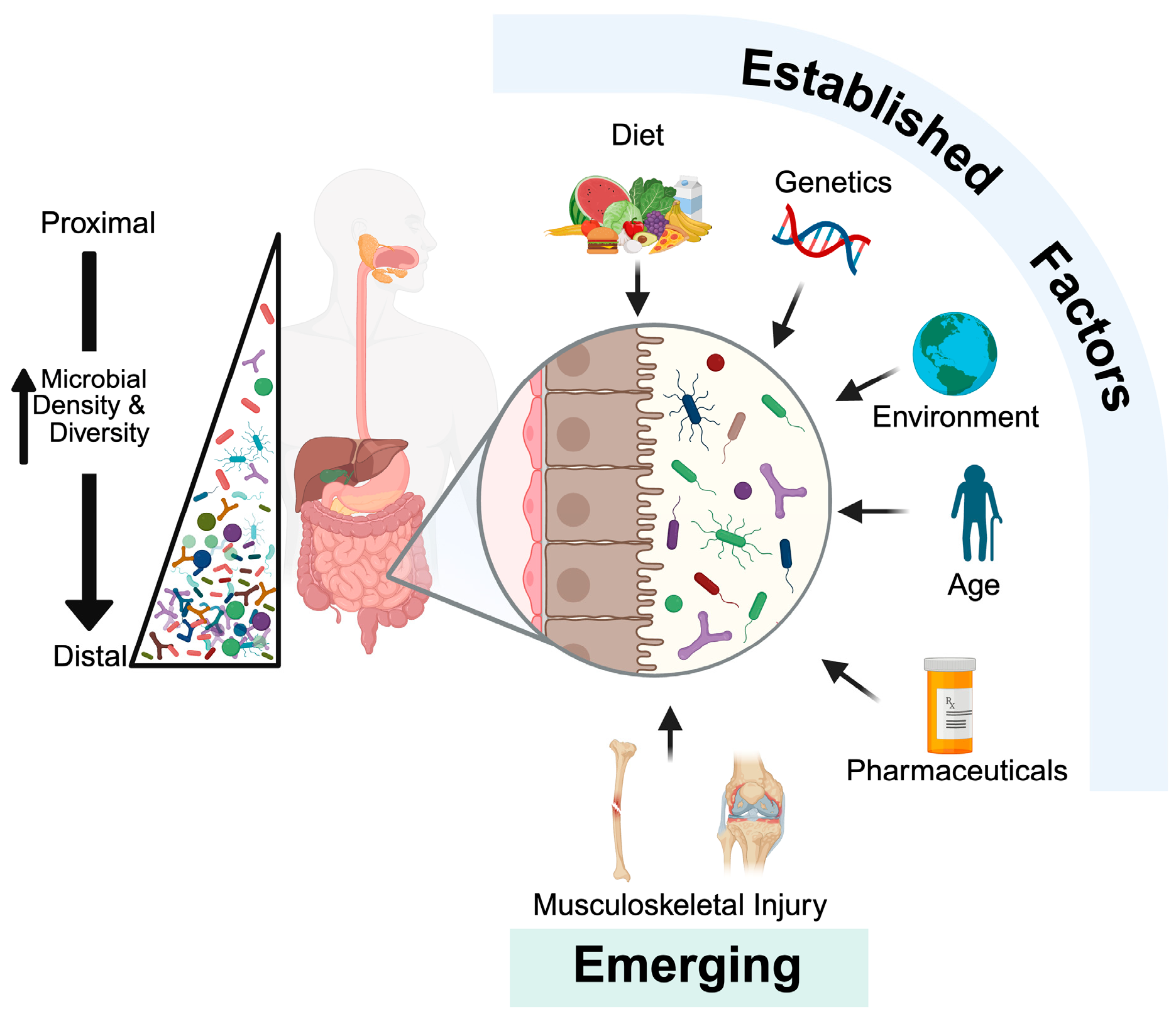
1. Introduction
2. Brief Overview of the Gut Microbiome
3. Impact of Musculoskeletal Injuries on Intestinal Function
4. Traumatic Musculoskeletal Injuries and Gut Microbiota Composition
4.1. Bone Fracture
4.2. Post-Traumatic Osteoarthritis
4.3. Tendon and Ligament Injury
5. Targeting the Gut Microbiota After Musculoskeletal Injury
5.1. Probiotics
5.2. Prebiotics
5.3. Fecal Microbiota Transplants (FMT)
| Injury Model | Subjects | Intervention | Key Results | Ref. |
|---|---|---|---|---|
| Fracture | ||||
| Closed unilateral femur fracture | C57BL/6J Male (10 weeks old) | Bifidobacterium adolescentis (ATCC 15703); 1 × 108 CFU 5 days per week oral gavage two weeks prior to fracture until sacrifice | ↑ Cartilaginous callus remodeling ↓ Gut permeability ↓ Inflammation | [34] |
| Open osteotomy femur fracture | C57BL/6 Female (8 weeks old) | Akkermansia muciniphila (ATCC BAA-835); 8 × 108 CFU twice per week oral gavage after fracture | ↑ Callus bone ↑ Biomechanical properties ↓ Gut permeability ↓ Inflammation | [72] |
| Open osteotomy femur fracture | C57BL/6 Female (8 weeks old) | Lactobacillus gasseri (ATCC 33323); 8 × 108 CFU twice per week oral gavage after fracture | ↑ Callus bone ↑ Biomechanical properties | [72] |
| Closed unilateral femur fracture | C57BL/6JN Female (18 months old) | Bifidobacterium longum (ATCC 15707); 1 × 108–1 × 109 CFU daily oral gavage two weeks prior to fracture until sacrifice | ↑ Callus bone ↑ Biomechanical properties ↓ Gut permeability ↓ Inflammation | [27] |
| Open osteotomy Femur Fracture | C57BL/6 Male (11 weeks old) | VSL#3 probiotic blend; 1 × 109 5 days per week for 5 weeks prior to fracture or for 4 weeks after fracture | Pre-treatment: ↑ callus bone and mechanical properties. Post-treatment: ↑ callus bone and mechanical properties. | [71] |
| Post-traumatic Osteoarthritis (PTOA) | ||||
| DMM | C57BL/6J (19 weeks old) | Prebiotic-supplemented high-fat diet containing 10% w/w Beneo-Orafti Orafti P95 Oligofructose | ↓ Cartilage damage | [84] |
| ACLT | Sprague-Dawley Rats | Diets containing 20% plant polysaccharides | ↓ Cartilage damage ↓ Systemic inflammation ↓ Pain | [85] |
| DMM | C57BL/6 Male (9 weeks old) | Prebiotic-supplemented high-fat diet containing 10% w/w Beneo-Orafti Orafti P95 Oligofructose | ↓ OARSI scores ↓ Osteophyte size ↓ Gut permeability | [36] |
| PMM | C57BL/6J Female (11 weeks old) | Lactobacillus acidophilus (ATCC 4356); 3 × 109 CFU twice per week oral gavage after PMM | ↓ OARSI scores ↓ Pain | [77] |
| ACLT | Sprague-Dawley Rats Male (8 weeks old) | Lactobacillus plantarum GKD7 (5 × 1010 CFU/kg bw) daily oral gavage after ACLT | ↓ OARSI scores ↑ Weight bearing ↓ Joint inflammation | [79] |
| ACLT | Male Wistar Rats (8 weeks old) | Streptococcus thermophilus TCI633 (5 × 109, 5 × 1010, or 5 × 1011 CFU/kg/day) daily oral gavage after ACLT | ↓ OARSI scores ↓ Knee swelling ↓ Mechanical allodynia ↓ Cartilage apoptosis | [80] |
| ACLT | Sprague-Dawley Rats (8 weeks old) | Clostridium butyricum GKB7; daily oral gavage after ACLT | ↓ OARSI scores ↑ Weight bearing ↓ Joint inflammation | [78] |
| DMM | C57BL/6 Male (8-weeks-old) | Lacticaseibacillus paracasei 8700:2 (DSM13434), Lactiplantibacillus plantarum HEAL9 (DSM15312), Lactiplantibacillus plantarum HEAL19 (DSM12313) in drinking water (108 CFU/mL) after DMM | ↓ OARSI score at medial femoral condyle ↑ Trabecular bone in femoral epiphysis | [88] |
| ACLT | C57BL mice (6–8 weeks-old) | Streptococcus thermophilus CICC 6222/ATCC 19258 or Lactobacillus pentosus CICC 24202 or combined; 2 × 1010 CFU/kg daily oral gavage starting two weeks prior to ACLT | ↓ OARSI scores ↓ Joint inflammation | [76] |
5.4. Central Mechanisms of Microbiota-Targeted Interventions
6. Future Directions
6.1. Defining the Optimal Microbiota Composition for Musculoskeletal Repair
6.2. Artificial Intelligence and Predictive Modeling of Healing Outcomes Using Microbiome Data
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACLT | Anterior cruciate ligament transection |
| AI | Artificial intelligence |
| DMM | Destabilization of the medial meniscus |
| FMT | Fecal microbiota transplant |
| LPS | Lipopolysaccharide |
| ML | Machine Learning |
| MLI | Meniscal/ligamentous injury |
| OA | Osteoarthritis |
| OARSI | Osteoarthritis Research Society International |
| PAMP | Pathogen-associated molecular patterns |
| PMM | Partial Meniscectomy Model |
| PRP | Platelet-Rich Plasma |
| PTOA | Post-traumatic osteoarthritis |
| SFB | Segmented Filamentous Bacteria |
| TLR4 | Toll-like receptor 4 |
References
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, D.L.; Lucas, J.W.; Clarke, T.C. Summary Health Statistics for U.S. Adults: National Health Interview Survey, 2012; Vital and Health Statistics, Series 10, Number 260; National Center for Health Statistics: Hyattsville, MD, USA, 2014.
- Briggs, A.M.; Cross, M.J.; Hoy, D.G.; Sanchez-Riera, L.; Blyth, F.M.; Woolf, A.D.; March, L. Musculoskeletal Health Conditions Represent a Global Threat to Healthy Aging: A Report for the 2015 World Health Organization World Report on Ageing and Health. Gerontologist 2016, 56 (Suppl. 2), S243–S255. [Google Scholar] [CrossRef]
- MacKay, C.; Canizares, M.; Davis, A.M.; Badley, E.M. Health care utilization for musculoskeletal disorders. Arthritis Care Res. 2010, 62, 161–169. [Google Scholar] [CrossRef]
- Nawar, E.W.; Niska, R.W.; Xu, J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv. Data 2007, 386, 1–32. [Google Scholar]
- Anderson, T.J.; Althausen, P.L. The Role of Dedicated Musculoskeletal Urgent Care Centers in Reducing Cost and Improving Access to Orthopaedic Care. J. Orthop. Trauma 2016, 30 (Suppl. 5), S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Nadeem-Tariq, A.; Kazemeini, S.; Michelberger, M.; Fang, C.; Maitra, S.; Nelson, K. The Role of Gut Microbiota in Orthopedic Surgery: A Systematic Review. Microorganisms 2025, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Hu, Y.; Guo, Y.; Liu, D. Modulation of bone remodeling by the gut microbiota: A new therapy for osteoporosis. Bone Res. 2023, 11, 31. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Bo, L.; Zhou, E.; Chen, Y.; Naranmandakh, S.; Xie, W.; Ru, Q.; Chen, L.; Zhu, Z.; et al. Progress of linking gut microbiota and musculoskeletal health: Casualty, mechanisms, and translational values. Gut Microbes 2023, 15, 2263207. [Google Scholar] [CrossRef]
- Li, R.; Boer, C.G.; Oei, L.; Medina-Gomez, C. The Gut Microbiome: A New Frontier in Musculoskeletal Research. Curr. Osteoporos. Rep. 2021, 19, 347–357. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Huang, X.; Wu, Y.; Shan, D. Microbiome’s role in musculoskeletal health through the gut-bone axis insights. Gut Microbes 2024, 16, 2410478. [Google Scholar] [CrossRef]
- Moore, A.M.; Mathias, M.; Valeur, J. Contextualising the microbiota-gut-brain axis in history and culture. Microb. Ecol. Health Dis. 2019, 30 (Suppl. 1), 1546267. [Google Scholar] [CrossRef]
- Liu, X. Microbiome. Yale J. Biol. Med. 2016, 89, 275–276. [Google Scholar]
- Gevers, D.; Knight, R.; Petrosino, J.F.; Huang, K.; McGuire, A.L.; Birren, B.W.; Nelson, K.E.; White, O.; Methe, B.A.; Huttenhower, C. The Human Microbiome Project: A community resource for the healthy human microbiome. PLoS Biol. 2012, 10, e1001377. [Google Scholar] [CrossRef] [PubMed]
- Ames, N.J.; Ranucci, A.; Moriyama, B.; Wallen, G.R. The Human Microbiome and Understanding the 16S rRNA Gene in Translational Nursing Science. Nurs. Res. 2017, 66, 184–197. [Google Scholar] [CrossRef]
- Yi, P.; Li, L. The germfree murine animal: An important animal model for research on the relationship between gut microbiota and the host. Vet. Microbiol. 2012, 157, 1–7. [Google Scholar] [CrossRef]
- Karimi, M.; Shirsalimi, N.; Hashempour, Z.; Salehi Omran, H.; Sedighi, E.; Beigi, F.; Mortezazadeh, M. Safety and efficacy of fecal microbiota transplantation (FMT) as a modern adjuvant therapy in various diseases and disorders: A comprehensive literature review. Front. Immunol. 2024, 15, 1439176. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Schoultz, I.; Claesson, M.J.; Dominguez-Bello, M.G.; Fak Hallenius, F.; Konturek, P.; Korpela, K.; Laursen, M.F.; Penders, J.; Roager, H.; Vatanen, T.; et al. Gut microbiota development across the lifespan: Disease links and health-promoting interventions. J. Intern. Med. 2025, 297, 560–583. [Google Scholar] [CrossRef]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.M.; Kornblith, L.Z.; Christie, S.A.; Conroy, A.S.; Nelson, M.F.; Campion, E.M.; Callcut, R.A.; Calfee, C.S.; Lamere, B.J.; Fadrosh, D.W.; et al. Characterizing the gut microbiome in trauma: Significant changes in microbial diversity occur early after severe injury. Trauma. Surg. Acute Care Open 2017, 2, e000108. [Google Scholar] [CrossRef]
- Roberts, J.L.; Chiedo, B.; Drissi, H. Systemic inflammatory and gut microbiota responses to fracture in young and middle-aged mice. Geroscience 2023, 45, 3115–3129. [Google Scholar] [CrossRef]
- Roberts, J.L.; Golloshi, M.; Harding, D.B.; Conduah, M.; Liu, G.; Drissi, H. Bifidobacterium longum supplementation improves age-related delays in fracture repair. Aging Cell 2023, 22, e13786. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E. Synergy of bone fractures, soft tissue disruption, and hemorrhagic shock in the genesis of postinjury immunochaos: The pathway to multiple organ failure. Crit. Care Med. 1998, 26, 1305–1306. [Google Scholar] [CrossRef]
- Guo, W.; Ding, J.; Huang, Q.; Jerrells, T.; Deitch, E.A. Alterations in intestinal bacterial flora modulate the systemic cytokine response to hemorrhagic shock. Am. J. Physiol. 1995, 269 Pt 1, G827–G832. [Google Scholar]
- Levy, R.M.; Prince, J.M.; Yang, R.; Mollen, K.P.; Liao, H.; Watson, G.A.; Fink, M.P.; Vodovotz, Y.; Billiar, T.R. Systemic inflammation and remote organ damage following bilateral femur fracture requires Toll-like receptor 4. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R970–R976. [Google Scholar] [CrossRef]
- Mollen, K.P.; Levy, R.M.; Prince, J.M.; Hoffman, R.A.; Scott, M.J.; Kaczorowski, D.J.; Vallabhaneni, R.; Vodovotz, Y.; Billiar, T.R. Systemic inflammation and end organ damage following trauma involves functional TLR4 signaling in both bone marrow-derived cells and parenchymal cells. J. Leukoc. Biol. 2008, 83, 80–88. [Google Scholar] [CrossRef]
- Napolitano, L.M.; Koruda, M.J.; Meyer, A.A.; Baker, C.C. The impact of femur fracture with associated soft tissue injury on immune function and intestinal permeability. Shock 1996, 5, 202–207. [Google Scholar] [CrossRef]
- Buzdon, M.M.; Napolitano, L.M.; Shi, H.J.; Ceresoli, D.M.; Rauniya, R.; Bass, B.L. Femur fracture induces site-specific changes in T-cell immunity. J. Surg. Res. 1999, 82, 201–208. [Google Scholar] [CrossRef]
- Roberts, J.L.; Liu, G.; Darby, T.M.; Fernandes, L.M.; Diaz-Hernandez, M.E.; Jones, R.M.; Drissi, H. Bifidobacterium adolescentis supplementation attenuates fracture-induced systemic sequelae. Biomed. Pharmacother. 2020, 132, 110831. [Google Scholar] [CrossRef]
- Dar, H.Y.; Perrien, D.S.; Pal, S.; Stoica, A.; Uppuganti, S.; Nyman, J.S.; Jones, R.M.; Weitzmann, M.N.; Pacifici, R. Callus gammadelta T cells and microbe-induced intestinal Th17 cells improve fracture healing in mice. J. Clin. Investig. 2023, 133, e166577. [Google Scholar] [CrossRef]
- Mi, Y.; Yi, N.; Xu, X.; Zeng, F.; Li, N.; Tan, X.; Gong, Z.; Yan, K.; Kuang, G.; Lu, M. Prebiotics alleviate cartilage degradation and inflammation in post-traumatic osteoarthritic mice by modulating the gut barrier and fecal metabolomics. Food Funct. 2023, 14, 4065–4077. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Langille, M.G.; Meehan, C.J.; Koenig, J.E.; Dhanani, A.S.; Rose, R.A.; Howlett, S.E.; Beiko, R.G. Microbial shifts in the aging mouse gut. Microbiome 2014, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, S.; Fite, A.; Macfarlane, G.T.; McMurdo, M.E. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 2004, 70, 3575–3581. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimaraes, V.; Sokol, H.; Dore, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Zwielehner, J.; Liszt, K.; Handschur, M.; Lassl, C.; Lapin, A.; Haslberger, A.G. Combined PCR-DGGE fingerprinting and quantitative-PCR indicates shifts in fecal population sizes and diversity of Bacteroides, bifidobacteria and Clostridium cluster IV in institutionalized elderly. Exp. Gerontol. 2009, 44, 440–446. [Google Scholar] [CrossRef]
- de Sousa Valente, J. The Pharmacology of Pain Associated with the Monoiodoacetate Model of Osteoarthritis. Front. Pharmacol. 2019, 10, 974. [Google Scholar] [CrossRef]
- Dieleman, J.L.; Baral, R.; Birger, M.; Bui, A.L.; Bulchis, A.; Chapin, A.; Hamavid, H.; Horst, C.; Johnson, E.K.; Joseph, J.; et al. US Spending on Personal Health Care and Public Health, 1996–2013. JAMA 2016, 316, 2627–2646. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborators. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Johnston, R.C.; Saltzman, C.L.; Marsh, J.L.; Buckwalter, J.A. Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma. 2006, 20, 739–744. [Google Scholar] [CrossRef]
- Narez, G.E.; Fischenich, K.M.; Donahue, T.L.H. Experimental animal models of post-traumatic osteoarthritis of the knee. Orthop Rev. 2020, 12, 8448. [Google Scholar] [CrossRef]
- Villani, D.A.; Ishii, T.; Hendesi, H.; Landgrave, S.H.; Gill, A.; Brenner, L.; Favazzo, L.; Gill, S.R.; Zuscik, M. Murine Post Traumatic Osteoarthritis Does Not Strongly Impact Gut Microbiome Community Structure, But Significantly Alters Thefunctional Output of Resident Taxa. Osteoarthr. Cartil. 2023, 31, S215–S216. [Google Scholar] [CrossRef]
- Guan, Z.; Jin, X.; Guan, Z.; Liu, S.; Tao, K.; Luo, L. The gut microbiota metabolite capsiate regulate SLC2A1 expression by targeting HIF-1alpha to inhibit knee osteoarthritis-induced ferroptosis. Aging Cell 2023, 22, e13807. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.E.; Murugesh, D.K.; Sebastian, A.; Hum, N.R.; McCloy, S.A.; Kuhn, E.A.; Christiansen, B.A.; Loots, G.G. Antibiotic Treatment Prior to Injury Improves Post-Traumatic Osteoarthritis Outcomes in Mice. Int. J. Mol. Sci. 2020, 21, 6424. [Google Scholar] [CrossRef]
- Ulici, V.; Kelley, K.L.; Azcarate-Peril, M.A.; Cleveland, R.J.; Sartor, R.B.; Schwartz, T.A.; Loeser, R.F. Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthr. Cartil. 2018, 26, 1098–1109. [Google Scholar] [CrossRef]
- Prinz, E.; Schlupp, L.; Dyson, G.; Barrett, M.; Szymczak, A.; Velasco, C.; Izda, V.; Dunn, C.M.; Jeffries, M.A. OA susceptibility in mice is partially mediated by the gut microbiome, is transferrable via microbiome transplantation and is associated with immunophenotype changes. Ann. Rheum. Dis. 2024, 83, 382–393. [Google Scholar] [CrossRef]
- Hahn, A.K.; Wallace, C.W.; Welhaven, H.D.; Brooks, E.; McAlpine, M.; Christiansen, B.A.; Walk, S.T.; June, R.K. The microbiome mediates epiphyseal bone loss and metabolomic changes after acute joint trauma in mice. Osteoarthr. Cartil. 2021, 29, 882–893. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, J.; Li, B.; Zeng, B.; Chou, C.H.; Zheng, X.; Xie, J.; Li, H.; Hao, Y.; Chen, G.; et al. Faecal microbiota transplantation from metabolically compromised human donors accelerates osteoarthritis in mice. Ann. Rheum. Dis. 2020, 79, 646–656. [Google Scholar] [CrossRef]
- Schlupp, L.; Prinz, E.; Dyson, G.; Barrett, M.; Izda, V.; Dunn, C.M.; Jeffries, M.A. Sex-Linked Discrepancies in C57BL6/J Mouse Osteoarthritis are Associated with the Gut Microbiome and are Transferrable by Microbiome Transplantation. Arthritis Rheumatol. 2024, 76, 231–237. [Google Scholar] [CrossRef]
- Wu, F.; Nerlich, M.; Docheva, D. Tendon injuries: Basic science and new repair proposals. EFORT Open Rev. 2017, 2, 332–342. [Google Scholar] [CrossRef]
- Leong, N.L.; Kator, J.L.; Clemens, T.L.; James, A.; Enamoto-Iwamoto, M.; Jiang, J. Tendon and Ligament Healing and Current Approaches to Tendon and Ligament Regeneration. J. Orthop. Res. 2020, 38, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Dietrich-Zagonel, F.; Hammerman, M.; Eliasson, P.; Aspenberg, P. Response to mechanical loading in rat Achilles tendon healing is influenced by the microbiome. PLoS ONE 2020, 15, e0229908. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.J. Musculoskeletal Microbiology: The Microbiome in Orthopaedic Biomechanics. Curr. Opin. Biomed. Eng. 2021, 19, 100290. [Google Scholar] [CrossRef] [PubMed]
- Schepull, T.; Kvist, J.; Norrman, H.; Trinks, M.; Berlin, G.; Aspenberg, P. Autologous platelets have no effect on the healing of human achilles tendon ruptures: A randomized single-blind study. Am. J. Sports Med. 2011, 39, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, F.; Hammerman, M.; Blomgran, P.; Tatting, L.; Bampi, V.F.; Silva, J.B.; Aspenberg, P. Effect of platelet-rich plasma on rat Achilles tendon healing is related to microbiota. Acta Orthop. 2017, 88, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Dietrich-Zagonel, F.; Hammerman, M.; Tatting, L.; Dietrich, F.; Kozak Ljunggren, M.; Blomgran, P.; Eliasson, P.; Aspenberg, P. Stimulation of Tendon Healing with Delayed Dexamethasone Treatment Is Modified by the Microbiome. Am. J. Sports Med. 2018, 46, 3281–3287. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Gahche, J.J.; Herrick, K.A.; Davis, C.D.; Potischman, N.; Vargas, A.J. Nonfood Prebiotic, Probiotic, and Synbiotic Use Has Increased in US Adults and Children From 1999 to 2018. Gastroenterology 2021, 161, 476–486.e3. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674, Erratum in Front. Microbiol. 2024, 15, 1378225. [Google Scholar] [CrossRef]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, S.; Wang, Y.; Yu, D.; Hua, L.; Guo, C.; Wang, D.; Lei, M. Oral administration of Lactobacillus casei Shirota improves recovery of hand functions after distal radius fracture among elder patients: A placebo-controlled, double-blind, and randomized trial. J. Orthop. Surg. Res. 2019, 14, 257. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Hua, L.M.; Wang, D.W. The effect of probiotic treatment on elderly patients with distal radius fracture: A prospective double-blind, placebo-controlled randomised clinical trial. Benef. Microbes 2016, 7, 631–637. [Google Scholar] [CrossRef]
- Wang, Y.; Agenor, A.; Clement, A.; Hopfgartner, A.; Whyne, C.; Nam, D. Probiotics: Can it modulate fracture healing? PLoS ONE 2023, 18, e0290738. [Google Scholar] [CrossRef]
- Liu, J.H.; Yue, T.; Luo, Z.W.; Cao, J.; Yan, Z.Q.; Jin, L.; Wan, T.F.; Shuai, C.J.; Wang, Z.G.; Zhou, Y.; et al. Akkermansia muciniphila promotes type H vessel formation and bone fracture healing by reducing gut permeability and inflammation. Dis. Model. Mech. 2020, 13, dmm043620. [Google Scholar] [CrossRef]
- Rahman, S.O.; Bariguian, F.; Mobasheri, A. The Potential Role of Probiotics in the Management of Osteoarthritis Pain: Current Status and Future Prospects. Curr. Rheumatol. Rep. 2023, 25, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Guo, C.; Wang, D.; Zhang, C.; Hua, L. The effect of probiotic Lactobacillus casei Shirota on knee osteoarthritis: A randomised double-blind, placebo-controlled clinical trial. Benef. Microbes 2017, 8, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Dolatkhah, N.; Jafari, A.; Eslamian, F.; Toopchizadeh, V.; Saleh, P.; Hashemian, M. Saccharomyces boulardii improves clinical and paraclinical indices in overweight/obese knee osteoarthritis patients: A randomized triple-blind placebo-controlled trial. Eur. J. Nutr. 2024, 63, 2291–2305. [Google Scholar] [CrossRef] [PubMed]
- Amin, U.; Jiang, R.; Raza, S.M.; Fan, M.; Liang, L.; Feng, N.; Li, X.; Yang, Y.; Guo, F. Gut-joint axis: Oral Probiotic ameliorates Osteoarthritis. J. Tradit. Complement. Med. 2024, 14, 26–39. [Google Scholar] [CrossRef]
- O-Sullivan, I.; Anbazhagan, A.N.; Singh, G.; Ma, K.; Green, S.J.; Singhal, M.; Wang, J.; Kumar, A.; Dudeja, P.K.; Unterman, T.G.; et al. Lactobacillus acidophilus Mitigates Osteoarthritis-Associated Pain, Cartilage Disintegration and Gut Microbiota Dysbiosis in an Experimental Murine OA Model. Biomedicines 2022, 10, 1298. [Google Scholar] [CrossRef]
- Chang, S.L.; Lin, Y.Y.; Liu, S.C.; Tsai, Y.S.; Lin, S.W.; Chen, Y.L.; Chen, C.C.; Ko, C.Y.; Chen, H.T.; Chen, W.C.; et al. Oral Administration of Clostridium butyricum GKB7 Ameliorates Signs of Osteoarthritis in Rats. Cells 2022, 11, 2169. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Chang, S.L.; Liu, S.C.; Achudhan, D.; Tsai, Y.S.; Lin, S.W.; Chen, Y.L.; Chen, C.C.; Chang, J.W.; Fong, Y.C.; et al. Therapeutic Effects of Live Lactobacillus plantarum GKD7 in a Rat Model of Knee Osteoarthritis. Nutrients 2022, 14, 3170. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Chen, N.F.; Yang, S.N.; Jean, Y.H.; Kuo, H.M.; Chen, P.C.; Feng, C.W.; Liu, Y.W.; Lai, Y.C.; Wen, Z.H. Effects of Streptococcus thermophilus on anterior cruciate ligament transection-induced early osteoarthritis in rats. Exp. Ther. Med. 2021, 21, 222. [Google Scholar] [CrossRef]
- Quagliani, D.; Felt-Gunderson, P. Closing America’s Fiber Intake Gap: Communication Strategies from a Food and Fiber Summit. Am. J. Lifestyle Med. 2017, 11, 80–85. [Google Scholar] [CrossRef]
- Whisner, C.M.; Castillo, L.F. Prebiotics, Bone and Mineral Metabolism. Calcif. Tissue Int. 2018, 102, 443–479. [Google Scholar] [CrossRef]
- Fortuna, R.; Wang, W.; Mayengbam, S.; Tuplin, E.W.N.; Sampsell, K.; Sharkey, K.A.; Hart, D.A.; Reimer, R.A. Effect of prebiotic fiber on physical function and gut microbiota in adults, mostly women, with knee osteoarthritis and obesity: A randomized controlled trial. Eur. J. Nutr. 2024, 63, 2149–2161. [Google Scholar] [CrossRef]
- Schott, E.M.; Farnsworth, C.W.; Grier, A.; Lillis, J.A.; Soniwala, S.; Dadourian, G.H.; Bell, R.D.; Doolittle, M.L.; Villani, D.A.; Awad, H.; et al. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight 2018, 3, e95997. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Meng, H.; Wang, Y.; Sheng, P.; Dong, Y.; Yang, J.; Chen, B.; Wang, X. Dietary fiber may benefit chondrocyte activity maintenance. Front. Cell Infect. Microbiol. 2024, 14, 1401963. [Google Scholar] [CrossRef] [PubMed]
- Biazzo, M.; Deidda, G. Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. J. Clin. Med. 2022, 11, 4119. [Google Scholar] [CrossRef]
- Hamamah, S.; Gheorghita, R.; Lobiuc, A.; Sirbu, I.O.; Covasa, M. Fecal microbiota transplantation in non-communicable diseases: Recent advances and protocols. Front. Med. 2022, 9, 1060581. [Google Scholar] [CrossRef]
- Sophocleous, A.; Azfer, A.; Huesa, C.; Stylianou, E.; Ralston, S.H. Probiotics Inhibit Cartilage Damage and Progression of Osteoarthritis in Mice. Calcif. Tissue Int. 2023, 112, 66–73. [Google Scholar] [CrossRef]
- Wolfarth, B.; Speed, C.; Raymuev, K.; Vanden Bossche, L.; Migliore, A. Managing pain and inflammation associated with musculoskeletal disease: Time for a change? Curr. Med. Res. Opin. 2022, 38, 1695–1701. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xie, G.; Zhao, A.; Zhao, L.; Yao, C.; Chiu, N.H.; Zhou, Z.; Bao, Y.; Jia, W.; Nicholson, J.K.; et al. The footprints of gut microbial-mammalian co-metabolism. J. Proteome Res. 2011, 10, 5512–5522. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef] [PubMed]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar]
- Roberts, J.L.; Drissi, H. Advances and Promises of Nutritional Influences on Natural Bone Repair. J. Orthop. Res. 2020, 38, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Nakamura, M.; Miclau, T.; Marcucio, R. Effects of Aging on Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 601–608. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, B.R.; Lee, S.Y.; Beom, J.; Choi, J.H.; Lim, J.Y. Influence of comorbidities on functional outcomes in patients with surgically treated fragility hip fractures: A retrospective cohort study. BMC Geriatr. 2021, 21, 283. [Google Scholar] [CrossRef]
- Hernandez, R.K.; Do, T.P.; Critchlow, C.W.; Dent, R.E.; Jick, S.S. Patient-related risk factors for fracture-healing complications in the United Kingdom General Practice Research Database. Acta Orthop. 2012, 83, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Barnes, M.R.; Sugito, H.R.; Sin, J.M.; Henderson, E.R.; Levy, J.J. Applications of artificial intelligence in orthopaedic surgery. Front. Med. Technol. 2022, 4, 995526. [Google Scholar] [CrossRef]
- Hernandez Medina, R.; Kutuzova, S.; Nielsen, K.N.; Johansen, J.; Hansen, L.H.; Nielsen, M.; Rasmussen, S. Machine learning and deep learning applications in microbiome research. ISME Commun. 2022, 2, 98. [Google Scholar] [CrossRef]
- Dean, M.C.; Oeding, J.F.; Diniz, P.; Seil, R.; Samuelsson, K.; Group, E.A.I.W. Leveraging digital twins for improved orthopaedic evaluation and treatment. J. Exp. Orthop. 2024, 11, e70084. [Google Scholar] [CrossRef]
- Sizemore, N.; Oliphant, K.; Zheng, R.; Martin, C.R.; Claud, E.C.; Chattopadhyay, I. A digital twin of the infant microbiome to predict neurodevelopmental deficits. Sci. Adv. 2024, 10, eadj0400. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef] [PubMed]
- Kok, C.R.; Rose, D.; Hutkins, R. Predicting Personalized Responses to Dietary Fiber Interventions: Opportunities for Modulation of the Gut Microbiome to Improve Health. Annu. Rev. Food Sci. Technol. 2023, 14, 157–182. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, J.L.; Park, C.C. Emerging Roles of the Gut Microbiome in Musculoskeletal Injury and Repair. Microorganisms 2025, 13, 2193. https://doi.org/10.3390/microorganisms13092193
Roberts JL, Park CC. Emerging Roles of the Gut Microbiome in Musculoskeletal Injury and Repair. Microorganisms. 2025; 13(9):2193. https://doi.org/10.3390/microorganisms13092193
Chicago/Turabian StyleRoberts, Joseph L., and Connor C. Park. 2025. "Emerging Roles of the Gut Microbiome in Musculoskeletal Injury and Repair" Microorganisms 13, no. 9: 2193. https://doi.org/10.3390/microorganisms13092193
APA StyleRoberts, J. L., & Park, C. C. (2025). Emerging Roles of the Gut Microbiome in Musculoskeletal Injury and Repair. Microorganisms, 13(9), 2193. https://doi.org/10.3390/microorganisms13092193






