Abstract
White line disorders represent the most prevalent claw horn disruption lesion in dairy cattle. Recent studies have yielded new insights into the appropriate treatment modalities for these lesions. The aims of this study are to elucidate the pathogenesis of white line disorders and its associated claw lesions, such as toe tip necrosis, and to discuss practical treatment applications. In Western Europe, many herds are endemically infected with digital dermatitis. White line disorders in zone 3 and toe tip necrosis starting in zone 1—often beginning as axial white line lesions—frequently exhibit a suboptimal response to standard treatments, including corrective trimming, the application of a hoof block on the healthy claw and the administration of NSAIDs, due to secondary infections with Treponema spp. This study addresses the current perspectives on the aetiopathogenesis of white line disorders and the therapeutic challenges in promoting complete recovery and the correct use of antibiotics, along with preventive measures, such as good flooring. An important factor of its pathogenesis is a decrease in body condition around parturition, Correct diagnosis can be made via the use of regular locomotion scoring and good diagnostic tools, and thin soles by among others overtrimming should be prevented. Current therapeutic methods consist of the prompt application of a block and an NSAID and, in some circumstances, a parenteral injection with antibiotics when there is no good response to the applied therapies.
1. Introduction
Lameness in dairy cattle, alongside mastitis and decreased fertility, represents a significant herd health issue. Due to the severe pain and prolonged duration associated with most claw disorders, lameness adversely impacts animal welfare and leads to substantial economic losses. These economic impacts primarily stem from reduced milk production, premature culling, the loss of body weight before slaughter, and increased labour requirements [1,2,3,4,5,6]. Furthermore, the estimated prevalence of lameness is high, affecting approximately 30% of cows in the Netherlands, and managing this condition is time-consuming, with additional labour often yielding suboptimal results. Due to less flexibility at the moment of lying down and standing up in cubicles, claw lesions are also frequently associated with hock lesions and lesions in the carpal region [7]. A Danish investigation concluded that locomotor disorders are responsible for approximately 40% of all euthanised cows, making them the most frequent cause of on-farm euthanasia in dairy cows [8]. Recently, the benchmarking of claw health has been introduced, enabling the comparison of individual herd claw health with that of numerous other dairy farms exhibiting similar performance levels. This benchmarking may further support analyses of the improvement potential of farm herds, encourage collaboration between farmers and veterinarians to enhance animal welfare, and assist in minimising economic losses due to lameness [9]. Additionally, maintaining good claw health can contribute to increased job satisfaction among farmers.
The primary cause of lameness in dairy cattle originates predominantly from the hoof and surrounding tissues, and it can be of either infectious or non-infectious origin. Infectious claw disorders are typically associated with effects on the skin around the hoof and in the interdigital space. The most frequently observed infectious lesions include digital dermatitis (DD) and interdigital phlegmon (IP). Digital dermatitis is associated with infections by bacteria such as Treponema spp., while interdigital phlegmon is commonly linked to Fusobacterium necrophorum. Additionally, bacteria such as Dichelobacter nodosus, Porphyromonas levii, and Prevotella melaninogenica are frequently found in IP lesions [10,11]. The most frequently noted non-infectious claw disorders, also known as claw horn disruption lesions (CHDLs), include white line disorders in zone 3 (WLDs), sole ulcers (SUs), and toe tip necrosis in zone 1 (TTN) (zone classification according to van der Tol et al., 2002 [12]), which have prevalence rates of approximately 18%, 9%, and 2–3%, respectively, in The Netherlands [13]. Similar prevalence data have been estimated in other Western European countries, such as the United Kingdom and Switzerland [14,15], as well as in Canada (Alberta) [16]. The aetiopathogenesis of CHDLs is multifactorial and often related to sole horn thickness, sole horn moisture content [17], and management practices, including housing conditions, bedding, exercise patterns (e.g., sharp turning around corners), and grooved floors [14,16,17]. Other frequently mentioned risk factors for CHDLs are: the sinking of the pedal bone [18] around parturition; a decrease in claw horn cushion thickness as a consequence of a decrease in total body condition [19,20,21]; fatty liver-related diseases, such as mastitis and endometritis; nutrition (e.g., barley grain, protein, and fibre); housing and feeding management; calving; season; age; growth rate; genetics; conformation; and behaviour [22,23,24,25]. Nutritional deficiencies, particularly of trace elements and the vitamin biotin, have also been implicated in the development of CHDLs, as confirmed by longitudinal field studies [26,27]. These studies demonstrated that, among cattle with laminitis-related CHDLs, the survival rate was higher in cows supplemented daily with biotin (20 mg/kg).
About fifteen years ago, a study conducted in Liverpool identified an association between Treponema bacteria involved in DD and three “non-healing” claw horn lesions: TTN, “non-healing white line disorders” (NH-WLDs), and non-healing sole ulcers. Treponema bacteria were identified in these types of lesions in DD-infected herds; in regular infections, pyrogenic bacteria such as Arcanobaterium pyogenes (formerly called Trueperella pyogenes) are usually identified. These lesions are characterised by penetration through the horn and most probably subsequent infection of the corium by Treponema bacteria [28]. This association was later corroborated by studies on cattle with similar “non-healing” horn lesions in Austria [29] and Switzerland [30], as well as in various investigations involving goats with severe lameness issues in the UK and Germany [31,32].
The objective of this contribution is to discuss current perspectives on the aetiopathogenesis of and therapeutic approaches for DD-associated WLD abscesses in zone 3 and toe necrosis in zone 1 in dairy cattle, as presented in the ICAR Claw Health Atlas Appendix A (January 2020), including the correct use of antibiotics. Although, new insights into these aspects of sole ulcers, these were excluded from this paper.
2. Materials and Methods
Scope of the Papers Examined in This Review
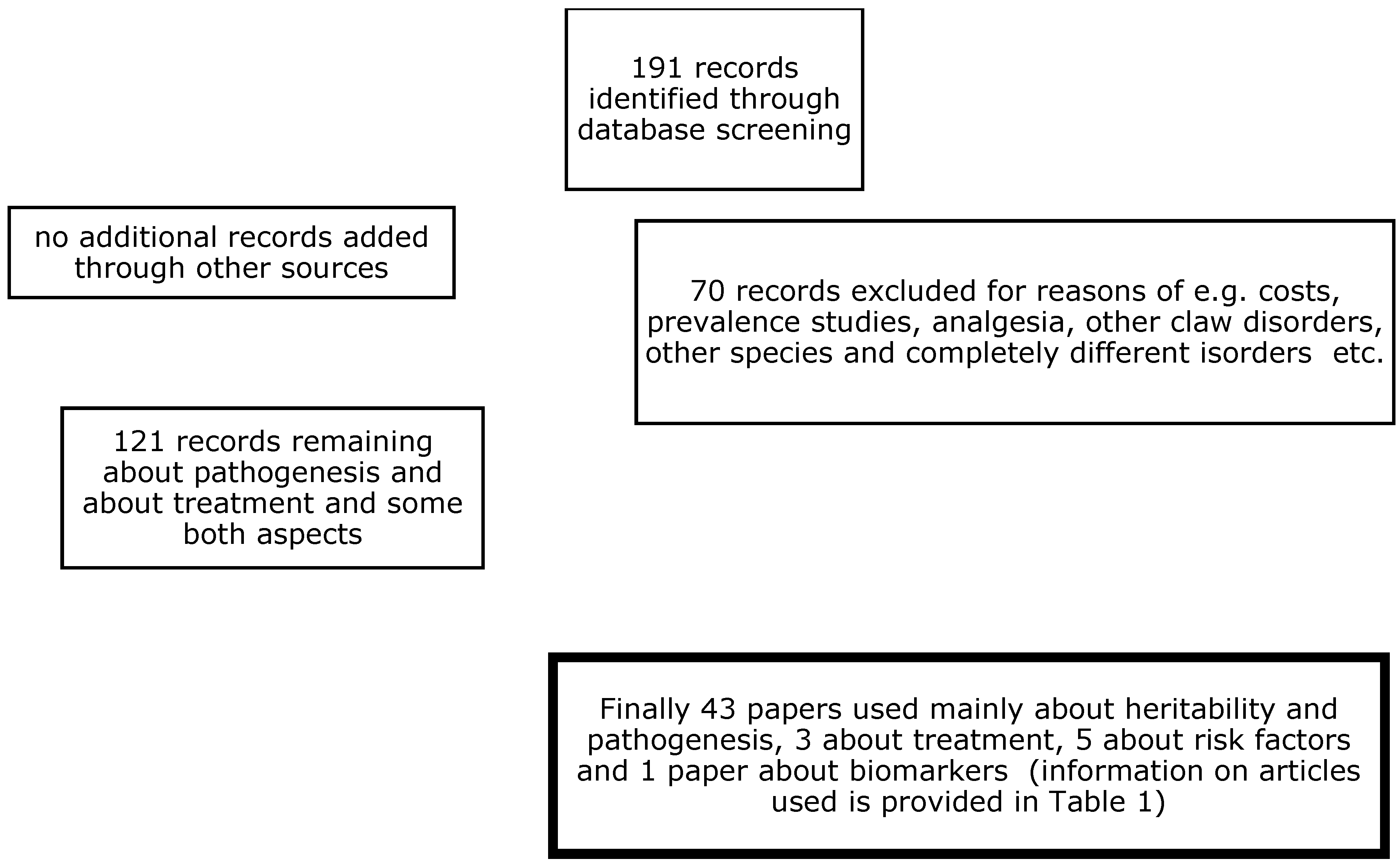
Articles from countries with modern dairy production systems were selected, where lameness was related to claw horn lesions and more specific to WLD in zone 3 and TTN in zone 1. A preliminary database search for scientific articles (described below) returned 43 papers related to the pathogenesis of these disorders and 6 papers related to their treatment, with nearly all being from Western countries. This comprehensive literature search was conducted in 2025 to identify relevant studies involving white line and toe necrosis lesions in dairy cows. The search terms included “white line”, “pathogenesis”, or “treatment”, and “dairy cows” in the title, in the abstract, and/or as a keyword. The listed search terms were entered into three databases:
Web of Science PubMed and ScienceDirect. To perform this systematic review in a reasonable period of time, the publication period was set to the last two decennia, that is, from 2000 to 2024. This period was chosen because new insights into the aetiopathogenesis of CHDLs have been obtained since that time, as well as new insights into the correct treatment of CHDLs, which has recently changed. Thus, the outcome parameters of interest in these publications were the aetiopathogenesis and treatment of WLD and TTN, which are frequently related to each other [33].
A total of 121 papers were retrieved from the electronic database search for pathogenesis-related studies, and the first selection of potentially interesting papers was made (see Scheme 1). Among these papers, 39 (33.9%) contained pathogenesis investigations, and, of these, the majority (16/39 = 41.0%) investigated heritability. The estimated h2 in almost all of the papers was between 0.06 and 0.15 for TTN and CHDLs, respectively [34]. The excluded papers contained prevalence studies, estimations of the costs of claw disorders (including white line disorders), or analyses of other bovine diseases or disorders. Table 1 presents the selected papers, along with the first author, year of publication, no. of cows included, study design, journal of publication, and the subject of the study. The main results of these studies are presented in Appendix A. Among them, 15 (38%) were case-control studies, and 15 (38%) were cross-sectional studies; regarding those remaining, 3 (8%) were observational cohort studies, 2 (5%) were prospective confirmation studies, 2 were prospective observational studies, 1 was a retrospective cohort study, and 1 was a retrospective confirmation study. Furthermore, 22 (56%) were published in the Journal of Dairy Science, 5 were published in the British Veterinary Journal, and 3 were published in the Canadian Veterinary Journal; regarding those remaining, 2 were published in Animals, 2 were published in Veterinary Record, 1 was published in Veterinary Pathology, 1 was published in the New Zealand Veterinary Journal, 1 was published in in Veterinary and Animal Science, 1 was published in Research and Veterinary Science, 1 was published in the Journal of Animal Breeding and Genetics, and 1 was published in Tropical Animal Health and Production.
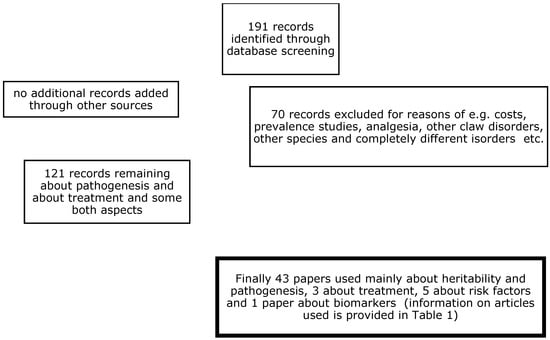
Scheme 1.
Flow diagram for the analyses systematically reviewed.

Table 1.
First author, year of publication, originating country, no. of cows used, study design, and journal of publication.
3. Pathogenesis
As stated above, the development of WLD in zone 3 and TTN in zone 1 as part of the CHDL complex is considered to be multifactorial. Both WLD and TTN are part of the bovine laminitis complex, and they have been found to originate from a combination of heritability (h2: 0.05–0.15) [34], nutrition [68], and housing management [69]. As previously mentioned, factors mainly related to nutrition are the sinking of the pedal bone [18] and the loss of condition around parturition [19,70], and good rumen function and good mineral supply are important [71,72]. Regarding housing, factors such as pasturing when possible, cubicles with a good size and bedding, the prevention of overcrowding, and the use of rubber flooring are important [70,73]. The most widely accepted explanation for the development of TTN in feedlot cattle is probably “abrasion theory”, which posits that it is caused by variations in the hardness and elasticity [23,74] and, consequently, increasing moisture content of the solar horn and the thinning of the soles, as well as indirectly by white line separation [17]. Excessive wear of the solar horn leads to separation along the apical portion of the white line, which likely allows for secondary bacterial infections. In areas endemic for DD in herds, infections with Treponema spp. may penetrate the corium and progress to P3 osteitis, P2 osteomyelitis, tendonitis, tenosynovitis, cellulitis, and, in some cases, septicaemia [28]. In the absence of DD, infections with pyrogenic bacteria may cause problems. According to Jelinsky et al., this theory explains the main risk factors for TTN [75]. Although Western Europe does not have feedlot cattle, the same problems are also seen in dairy herds endemically infected with DD with proven Treponema infections [28]; the prevalence of WLD has doubled compared to that 20 years ago (9% vs. 18%, respectively) [13,76], and the prevalence of TTN varies from 1 to 3% and even up to 10% [77]. To the best of our knowledge, comparable infections with Treponema spp. are not seen in herds without DD. In line with a Canadian study, our experience is that TTN starts with a disorder or separation of the axial white line (zone 1), allowing for secondary bacterial infection [48,78]. In dairy cattle, CHDLs are related to certain genetic factors, greater production and ration [34,71,72], and housing factors, such as grooved slats, sharp turns, and a high stocking density. Research has shown that CHDL rates are lower in cattle kept in free stalls [79] and on rubber-covered slatted floors than in those kept on uncovered slatted and concrete floors [80,81,82]. Furthermore, attention should be paid to overtrimming by unexperienced claw trimmers [83].
4. Treatment
After observing a lame cow, identifying which leg is the most probable cause of lameness, and determining that the cause originates from the claw, the cow is brought into the trimming chute, and, normally, one starts with the trimming of the affected foot. It is advised to start with the seven steps of prophylactic claw trimming, as developed about fifty years ago by Toussaint Raven [84]. After completing these steps, the horn shoe is checked for a pain response with a hoof tester to determine the location of the WLD lesion, thereby allowing for a conclusion to be drawn as to whether a white line disorder is the most probable cause of lameness [85]. Many people such as those from the Nottingham Research Group and the Faculty of Veterinary Medicine in Malaysia have found a positive effect with the use of a wooden block on the contralateral claw of the same leg (the inner claw of the hindleg in most cases) and the parenteral application of an NSAID [66,86]. So, the next step is correctly applying the block before the therapeutic trimming of the affected outer claw. The goal of the block is to reduce the weight on the diseased claw. This approach only covers cases where the infection is limited to the dermis and not those with deep digital sepsis. In the case of sepsis, the parenteral application of antibiotics should be considered to limit the consequences of the infection (e.g., to prevent the joints from being affected) [87].
4.1. White Line Disorder in Zone 3
After all these preparations, one has to make the correct diagnosis, therapeutically trim the affected claw, and treat the cow using the following steps:
- Examination and Diagnosis:Sometimes, the exact location of the lesion is not clear, and, in such cases, it is advisable to use a hoof tester to locate the specific area of pain and separation.The affected hoof should be carefully investigated to determine the extent of the damage and to ensure that there is no deeper infection. This allows for the differentiation between a white line fissure and a white line abscess.
- Trimming and Cleaning:The affected area of the hoof should be trimmed by removing any loose or damaged horn and exposing the affected area. Sometimes, part of the hoof wall has to be removed to allow the lesion to be relieved of pus and pressure from the corium, which also relieves the cow of pain. In the case of WLD type I (see Figure 1), this treatment on the affected hoof is usually sufficient. Besides the cleaning of the separated area to thoroughly remove any debris, dirt, or manure that might be trapped in the crack, no additional topical application of any therapeutic agent has been proven to be helpful. However, the parenteral application of an NSAID is helpful [66,83,86].
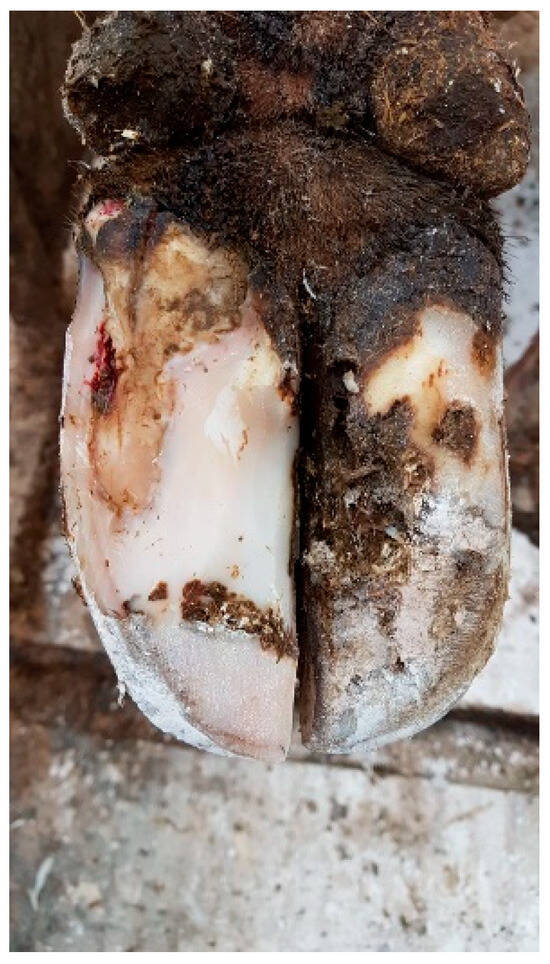 Figure 1. White line disorder grade 1, affecting a small area of the dermis, according to the ICAR Claw Health Atlas.Figure 1. White line disorder grade 1, affecting a small area of the dermis, according to the ICAR Claw Health Atlas.
Figure 1. White line disorder grade 1, affecting a small area of the dermis, according to the ICAR Claw Health Atlas.Figure 1. White line disorder grade 1, affecting a small area of the dermis, according to the ICAR Claw Health Atlas.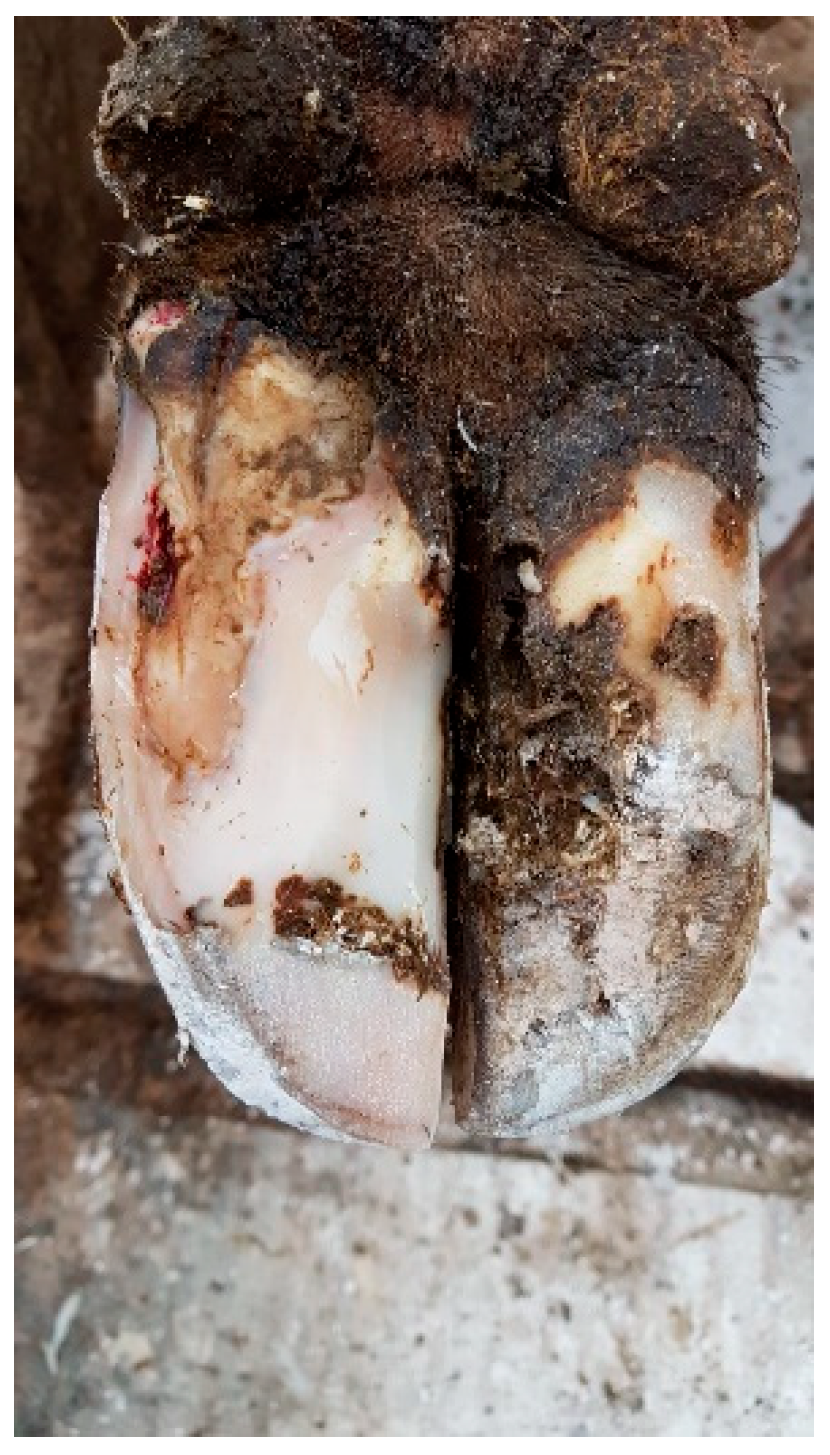
- Debridement:If the local infection is more extensive (“non-healing white line abscesses”, ICAR Claw Health Atlas), it may be necessary to remove more of the claw horn, and this often becomes complicated. Thus, if standard treatment does not improve or cure the lesion, then it is advisable to let this step be performed by an experienced foot trimmer or veterinarian with the support of anaesthesia.All necrotic (dead and contaminated) tissue and any foreign material that might be present should be removed. Again, this may involve careful removal of the hoof wall to ensure that all damaged tissue is removed. This surgical debridement should always be performed using local anaesthesia. This is a time-consuming process that is usually not carried out at the same time as regular claw trimming of the whole herd (see Figure 2A–D). For topical treatment, one can consider products based on copper and zinc chelate or salicylic acid and methyl salicylate; other disinfecting products might also be recommended.
 Figure 2. Presentations of cows with serious non-healing white line disorders at zone 3 before (A,C) and 3 months after (B,D) topical treatment and a parenteral injection with tilmycosin (10 mg/kg BW. SC). We also used in these cases hard plastic blocks beneath the inner claw, that hardly do not wear.Figure 2. Presentations of cows with serious non-healing white line disorders at zone 3 before (A,C) and 3 months after (B,D) topical treatment and a parenteral injection with tilmycosin (10 mg/kg BW. SC). We also used in these cases hard plastic blocks beneath the inner claw, that hardly do not wear.
Figure 2. Presentations of cows with serious non-healing white line disorders at zone 3 before (A,C) and 3 months after (B,D) topical treatment and a parenteral injection with tilmycosin (10 mg/kg BW. SC). We also used in these cases hard plastic blocks beneath the inner claw, that hardly do not wear.Figure 2. Presentations of cows with serious non-healing white line disorders at zone 3 before (A,C) and 3 months after (B,D) topical treatment and a parenteral injection with tilmycosin (10 mg/kg BW. SC). We also used in these cases hard plastic blocks beneath the inner claw, that hardly do not wear.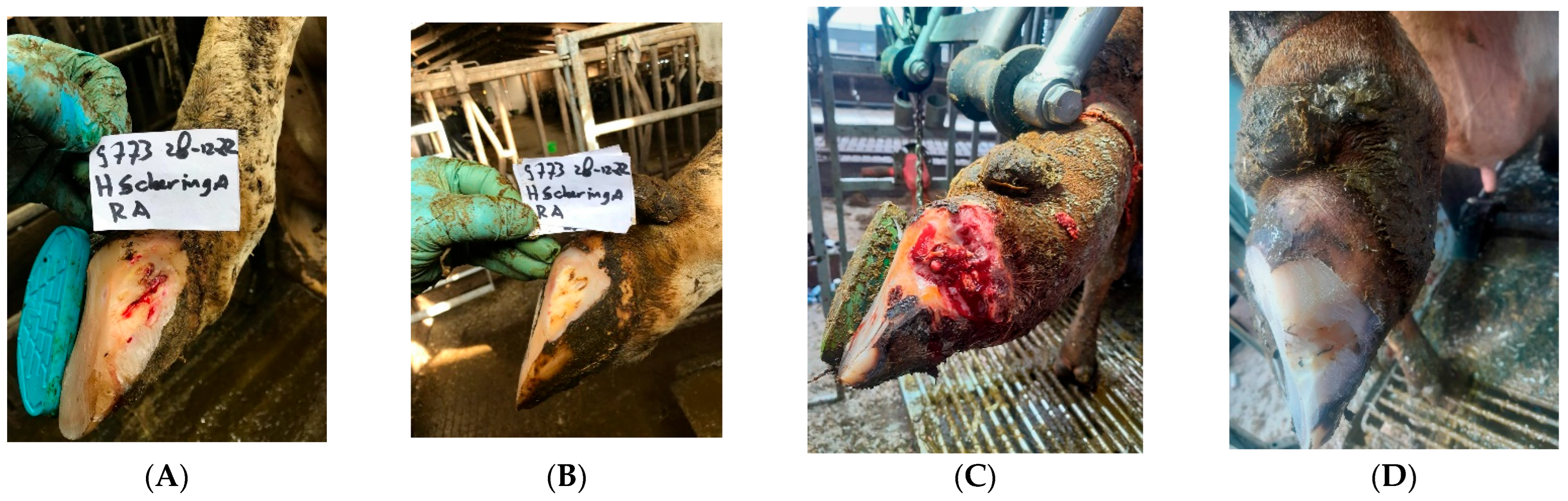
- NSAIDs and AntibioticsIf repeated remedial trimming with topical treatment does not result in clinical cure, a single parenteral antibiotic treatment (e.g., 10 mg/kg BW. SC of tilmycosin) can help to achieve complete clinical cure, and most cows will be suitable for further milk production [67].
4.2. Toe Tip Necrosis (See Figure 3)
- Examination and DiagnosisThe toe tip should be carefully inspected to determine the extent of the damage, and the depth of the infection should be determined with a probe. Samples should be sent to a laboratory for further investigation. It is not worthwhile to only send in a swab or horn sample for bacteriology, and Polymerase Chain Reaction (PCR) for Treponema is only performed in a small no. of laboratories in Europe. Infections may penetrate the corium and progress to P3 osteitis; P2 osteomyelitis; tendonitis; tenosynovitis; cellulitis (caused by E. coli or Arcanobaterium pyogenes); and, in some cases, septicaemia, which leads to an embolic event that culminates in death [31]. According to the sparse literature on the background of TTN, a low intake of both selenium and magnesium may play a role [68].
- Trimming and cleaningIn terms of TTN, different therapeutic options are now available, as solely claw trimming and the topical application of a product such as tetracycline powder or spray are usually not sufficient and also undesirable with the risk of developing antibiotic resistance. More effective options are as follows:
- Loco-regional/regional anaesthesia and the removal of the entire affected horn and necrotic bone tissue with a grinder, as proposed by Kofler [88];
- The removal of necrotic tissue using local anaesthesia and surgical intervention or amputation of the tip of the claw [89];
- Loco-regional/regional anaesthesia and the removal of the entire affected horn and necrotic bone tissue, in combination with topical application of a disinfectant non-antibiotic unguent and a single parenteral antibiotic treatment, e.g., tilmycosin [67].

Figure 3.
Serious toe tip necrosis.
Figure 3.
Serious toe tip necrosis.

All options should be combined with correct pain management such as a block under the claw (the inner claw in most cases) and the parenteral application of an NSAID. In a study performed in 2024, plastic foot blocks were used, which, unlike wooden blocks, do not wear unevenly.
5. Prevention
To prevent CHDLs and especially WLD lesions (both WLD in zone 3 and TTN in zone 1), it is necessary to perform regular locomotion scoring of the herd at two-week intervals in order to detect lameness and WLD lesions at an early stage [90], perhaps before the secondary infection of the corium by Treponema spp.; additionally, attention should be paid to proper preventive claw trimming, nutrition, and the environment. Proper claw trimming refers to the correct dorsal wall length; a sole horn thickness of at least 5–7 mm; the correct heel height, especially of the medial hind claw; and the proper removal of load from the claw affected by WLD separation or other CHDLs (step 4 of functional hoof trimming). Research from Sweden showed that, if CHDLs are the main cause of lameness, then strategic claw trimming (of lame cows, cows at the start of the dry standing period, and cows around 3 months in milk) is preferable to the trimming of all cows twice a year [91]. This advice is related to the greatest risk period (1–3 months pp.) of these disorders [92]. Good nutrition for the prevention of CHDLs is mainly based on good rumen function [71,72], which results in maximal endogenous biotin production, and a good supply of essential minerals such as zinc, copper, manganese, and selenium [68]. Good housing management for the prevention of CHDLs involves good cow comfort, which means the prevention of overcrowding, good adjustment of the cubicles, preferably the use of rubber-coated floors [73], and a clean and dry environment to prevent further contamination and to promote healing. Good cow comfort can be monitored using the cow standing index, as proposed by Cook et al. [93]. It is important to consult a bovine veterinarian to determine the appropriate course of treatment for white line disease in zone 3 in cattle. These practitioners can provide guidance and perform underfoot anaesthesia, and they may need to intervene if the condition is severe or if there are complications such as deep infections. This will hopefully prevent long-lasting lameness, improve cattle welfare and longevity, and enhance job satisfaction for dairy farmers and bovine practitioners.
6. Discussion
This review is limited to studies providing insights into the aetiopathogenesis and treatment of dairy cattle with white line lesions in zone 3 and toe tip necrosis conducted during the period of 2000–2025. This period was chosen because many studies conducted around the turn of the century examined the displacement of the pedal bone within the horn shoe, and those conducted more recently provided insights into the optimal treatment of claw horn lesions, which should contribute to the better welfare and longevity of dairy cows.
White line disorders in zone 3 are one of the most prevalent CHDLs in dairy cattle in Western Europe and the USA [14,70]. An investigation in the UK showed that increased parity, increased herd size, cows at pasture by day and housed at night, and solid grooved concrete floors in yards or alleys were the main risk factors for WLD in zone 3 [14], while a study from the USA focused on unbalanced weight bearing and metabolic, enzymatic, and hormonal changes [94]. Our experience is that WLD in zone 3 and TTN, which almost always starts as an axial WLD, are part of the bovine laminitis complex and a consequence of a combination of factors such as: the sinking of the pedal bone [18]; a decrease in body condition around the dry period [53,95]; poor horn quality; and sharp turns in walkways, which is more relevant for herds kept on grooved concrete and/or in permanent housing. In addition, sole thickness should be evaluated as a risk factor, as too-small thickness can be caused by excessive abrasion of the sole horn, overtrimming, and long walking distances from the pasture to the milking parlour. This is often observed in large herds with over 1000 cows, where long walking distances are common [83].
For welfare and recovery reasons, it is advisable to trim the lesion promptly and correctly and to apply an orthopaedic foot block under the healthy claw of the same leg. In many areas free from DD and Treponema spp. infections (e.g., in central and northern Europe), WLD and TTN lesions are frequently secondarily infected with pyrogenic bacteria, such as Arcanobaterium pyogenes. In areas with endemic DD-infected herds, investigations by both the Nottingham Lameness Expert Group and the University of Malaysia have shown that the presence of WLD in zone 3 is associated with a reduced likelihood of recovery, with cows that have been severely lame for a long time [96]. Regarding therapy, the current opinion is that an additional parenteral injection with an NSAID (e.g., an injection of Ketofen 10% solution at 3 mg/kg IM) results in even better recovery [86]. Herd veterinarians may choose another registered NSAID with a longer duration of action and possibly a more positive result, but this often also has consequences for the meat withdrawal period [97]. However, with the current legislation and considerations of animal welfare, farmers cannot cull such animals [98]. The positive effect of the combination of a block and an NSAID on lameness was also found in a recent review of the literature from Asia [66].
This is the best approach for the majority of CHDLs; however, it is not sufficient for the treatment of “non-healing” WLD in zone 3 and TTN, especially in areas endemic for infections with Treponema causing DD. TTN is almost always related to a secondary disorder and infection of the tip of the pedal bone [28]. Microbial investigations conducted at Liverpool University using PCR showed that, although the clinical and pathological presentations differ [78], Treponema bacteria are involved in both TTN and “non-healing” WLD [28]. TTN is, in our opinion, an osteitis of the pedal bone (P3), while “non-healing” WLD in zone 3 is a consequence of an infection of the pododerma of the hoof wall [78]. In both cases (non-healing white line lesions and TTN), the involvement of Arcanobaterium pyogenes and/or Treponema spp. in DD-endemic areas was proven [28], and tilmycosin treatment was found to have a positive effect. This research showed that a single treatment was always combined with surgical debridement of the infected area or resection of the infected tip of the pedal bone, of course, both under anaesthesia, and with an NSAID and a block under the contralateral claw. In cases of such complicated hoof lesions, this approach showed a very positive long-lasting effect. This treatment was also remarkable in cows affected by TTN or “non-healing” WLD in zone 3 for more than a year, who showed complete recovery. For farmers, the most important points were that the cows did not need attention after the removal of the foot block, and milk production was almost completely restored [67]. Tilmycosin, an antibiotic with a small molecular size, which is necessary for penetration into bone tissue, has been officially registered for use in cattle and claw disorders. Its therapeutic effect may be the consequence of a combination of antibacterial activity and an exaggerated immune response, such as that implicated in severe inflammatory reactions [99], which is responsible for the positive effect [67]. From a welfare perspective, it is irresponsible to send such lame cows to slaughter. The relative disadvantage of this approach is the additional use of antibiotics and the long withdrawal time for milk (35 days); therefore, it is advisable to use it restrictively. In the case of serious infection of the tip of the pedal bone, alternative treatments for TTN are, e.g., the removal of necrotic tissue with a grinder and partial amputation of the claw, both of which are performed under local anaesthesia with good pain management and with limited withdrawal times for milk and slaughter [88,89]. It is the responsibility of herd veterinarians to provide dairy farmers with the right information so that they can make a good decision together.
This study can be summarised as follows:
Prevention: The prevention of CHDLs starts with regular locomotion scoring at two-week intervals to detect WLD and TTN as early as possible [100,101], and, specifically, to detect WLD in zone 3 and TTN in cattle. Attention must be paid to proper preventive measures, including claw trimming; nutrition, for example, by employing the body condition score and achieving good ruminal function and a good mineral supply; and environmental housing management.
Claw Trimming: Research from Sweden indicates that, if CHDLs are the primary cause of lameness, strategic claw trimming—targeting lame cows, cows at the start of the dry standing period, and cows around three months in milk—is more effective than trimming all cows twice a year [91]. This strategy aligns with the greatest risk period for these disorders, which is between one and three months postpartum [92].
Nutrition: Good nutrition is crucial for preventing CHDLs, primarily by maintaining optimal rumen function. This promotes maximal endogenous biotin production; ensures an adequate supply of essential minerals, such as zinc, copper, manganese, and selenium [68,71,72]; and reduces the risk of sinking of the pedal bone within the hoof capsule, which subsequently leads to the development of CHDLs [18].
Environmental Management: Effective housing management to prevent CHDLs involves ensuring good cow comfort. This includes preventing overcrowding, properly adjusting cubicles, using rubber-coated floors when possible [73], and maintaining a clean and dry environment to prevent further contamination and promote healing. Cow comfort can be monitored using the cow standing index, as proposed by Cook et al. [93].
Veterinary Consultation: It is important to consult a bovine veterinarian for the appropriate treatment of complicated CHDLs. Veterinarians can provide guidance, perform underfoot anaesthesia, and intervene in cases of severe conditions or complications such as deep infections. This approach aims to prevent long-lasting lameness, improve cattle welfare and longevity, and enhance job satisfaction for dairy farmers and bovine practitioners.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Relevant studies related to aetiopathogenesis and treatment of claw-horn lesions.
In a study from Bristol University, it was proven that primary causal events associated with calving weaken the connective tissue of the hoof suspensory apparatus, leading to increased susceptibility to clinical lameness associated with sole ulcers and white line disease [18].
In a study from the Universities in South-East of North America (Universities of Tennessee, Florida, and Georgia), the moisture content and thickness of the sole horn was estimated in relation to lesions of the sole horn. They concluded that cows with thin-soled claws and higher risk of suffering from lesions had higher levels of moisture than cows with normal sole thickness from the same herd. The highest claw moisture levels were recorded in the rear feet [17].
In a study from the Norwegian School of Veterinary Science, the influence of different types of flooring was evaluated and both haemorrhages and fissures were more prevalent on slatted and solid concrete floors than in those housed on solid rubber [82].
In a study from Liverpool University (UK), the association between bovine digital dermatitis treponemes and three “non-healing” hoof horn lesions, “toe necrosis”, “non-healing white line disease” and “non-healing sole ulcer”, was confirmed [28].
In a paper of Bicalho and Oikonomou [102], it was stated that sole ulcers and white line disease are the most prevalent diseases associated with lameness and pain, representing over 65% of all lesions diagnosed in visually lame cows and causing the largest economic losses amongst several lameness-related diseases. Recent research results regarding the pathogenesis of CHDLs cast some light on a complex matter and highlighted the importance of prevention of the disease through improved management and housing. Preventing intra-claw trauma by improvements to housing systems to enhance cow comfort, as well as through management strategies that reduce total standing periods and increase resting time, is also more likely to yield immediate results.
In a study of the university of Halle (Germany), the relationships between bovine claw disorders, body condition traits, and test-day yields were evaluated. They concluded that increased bio-mechanical stress caused by different factors (weight, social rank, standing time) is a presumed effect that leads to an increased susceptibility for claw disorders. From a management perspective, under- and over-conditioning should be avoided. Milk production traits differed between cows with and without laminitis-related claw horn lesions. Fat percentage and fat-to-protein ratio in the first month before trimming were significantly decreased for cows with a positive laminitis status. Hence, this finding points to metabolic disorders being associated with a higher risk for hoof diseases. Estimates of heritability for laminitis, dermatitis digitalis, dermatitis interdigitalis, white line disease, and sole ulcer were 0.09, 0.14, 0.10, 0.11, and 0.06 when applying a threshold model with a probit link function [42].
In a study from Melbourne University, Australia, the effects of feeding a supplement to grazing dairy cows were investigated. The results showed that for the most prevalent lesions (white line disease, haemorrhage, and bruising), there was no effect of feeding system or amount of supplement on the presence of the moderate to severe forms in early lactation, but cows were more likely to have a particular lesion at the second assessment if it was present in early lactation [41].
An investigation from the University of Vienna confirmed the presence of Treponema DNA in 42 bovine “non-healing” and 25 DD lesions and 15 common sole ulcers and white line disorders. The type of lesions differed with the identified Treponema phylotypes. It is suggested that specifically T. medium may have an active role in the pathogenesis of the “non-healing” lesions [29].
In a paper from Iowa State University, Ames (USA), the best strategy to mitigate lameness pain in cattle was discussed. In their opinion, a multi-modal approach is advised including the following: (1) use of intravenous regional or ring block anaesthesia when needed for painful conditions, (2) careful and thorough corrective trimming without damage to adjacent healthy corium tissues, (3) use of an orthopaedic foot block to relieve weight bearing on injured claws, (4) avoidance of topical therapies that increase discomfort and prolong recovery, (5) administration of analgesics including local anaesthetics, NSAIDs, and sedative-analgesics, and finally (6) comfortable housing and thoughtful management of lame cows in the post-treatment period [83].
A study from University of Saskatchewan (Alberta, Canada) was performed to characterize the lesions of toe tip necrosis (TTN) and provide insights into the pathogenesis of the disease. Different forms of evidence were found that TTN starts as a defect at the white line at the apex of the claw and that the presence of this defect allows for the entry of bacteria into the corium that can then spread, by extension, proximally to P3. Bacteria within the claw may then serve as a nidus for the local or more distant spread of bacteria and the lesions of TTN [48].
In study from Nottingham, the treatments of CHDLs in dairy cows were evaluated and the outcome was that lameness cure is maximized with NSAID treatment in addition to the common practices of therapeutic trimming and elevation of the diseased claw using a block when cows are newly and predominantly mildly lame [86].
In another study from 2016 from Nottingham University, more about the pathogenesis of claw horn disruption lesions (CHDLs) was elucidated, whereby histology demonstrated that new bone development was osteoma, also termed “exostosis.” Age explained much of the variation in bone development. One of the conclusions was to stop irreparable anatomical damage within the foot, early identification of CHDLs, and effective treatment could be critical [53].
In a study from the University of Calgary the prevalence and risk factors were estimated in different housing systems. The odds of CHDLs were >2 times higher in cows housed in free stalls than those housed in deep-bedded packs and later lactation stages were estimated as a risk factor [103].
In a paper about pathogenesis and treatment of sole ulcers and white line disorders [94], the high prevalence of these type of lesions is emphasized and risk factors are among others the weakening of the suspensory apparatus of the third phalanx, increased metalloproteinase enzymes, peripartum hormonal activity, cow comfort, and horn overgrowth. They consider corrective trimming in combination with an orthopaedic foot block as correct therapy and proper healing takes at least 24–30 days. The routine use of bandages is controversial.
From the University of Malaysia, a paper evaluated among other things the prevalence and risk factors of claw lesions in dairy farms. White line disorders were found in 61.2% of the lame cows and claw lesions were associated with dirty legs, poor hygiene, and overgrown claw. To reduce claw disorders it was advised to improve management of cows with overgrown claws, injured hocks, routine claw trimming, and efficient stall design [55].
In a paper from the University of Vienna (Austria), the pathogenesis and treatment of toe lesions in cattle, including “non-healing lesions”, are discussed. Toe lesions in this paper include apical white line disorders, thin soles, toe ulcers, toe necrosis, and DD-associated toe ulcers/necrosis. For anatomic reasons these lesions are at high risk of rapidly developing into a bone infection. Treatment should be based on careful evaluation of the soles of all claws by examination of the coronet and by checking other vital parameters of the animal. Toe lesions can be expected to have a good prognosis if diagnosed at an early stage. Prevention should be based on regular monitoring for mild lameness and prompt treatment by trained hoof trimmers or personnel [88].
In another study from University of Saskatchewan (Alberta, Canada), the concentrations of eight minerals in cows with TTN were evaluated in 16 feedlots. Selenium was most correlated and the cases had significantly lower Mg concentrations in the horn tissue [104].
In a pilot study from 2020, DD and “non-healing” claw lesions were evaluated for the presence of several bacterial agents. The data from that pilot suggest that Porphyromonas endodontalis and Fusobacterium necrophorum should be considered as potential aetiological agents of “non-healing” claw lesions [59].
In a study from Teagasc, Cork (Ireland), cow- and herd-level prevalence of hoof lesions were estimated during both the grazing and the housing periods and among other things established the prevalence of lesions always associated with pain. They concluded among other things that that the non-infectious lesions white line separation, sole haemorrhages, and overgrown claws were the most prevalent lesions at both the cow and herd level. All lesion types had a similar prevalence between grazing and housing. Toe necrosis and digital dermatitis had the strongest correlation of all lesion types and identifying the main causes of lameness in a partly housed, pasture-based system helps provide a focus for treating and preventing these lesion types [61].
In a study from the University of Copenhagen (Denmark), two different trimming methods were used to evaluate the prevalence of CHDLs in dairy cows. They found a significantly lower prevalence of sole ulcer and white line lesions in cows trimmed with the White Line Atlas method compared to the Danish method. They stated that further studies using a randomised experimental design with control groups are required to establish a possible causal relation between the incidence of CHDLs and trimming method [62]
In a study from the University of Helsinki (Finland), attention was paid to the biochemical laboratory diagnostic possibilities in case of claw disorders and specifically serum amyloid A and interleukin seem to be of interest [60].
In a study from the University of Miyazaki (Japan), an analysis of the bacterial population in non-healing claw lesions was performed. Twelve cases of non-healing lesions were collected from five dairy farms. The predominant bacterial genera in the lesions differed among the lesions examined, suggesting that Treponema species present predominantly in DD were in that study not predominant in the claw-horn lesions and that the bacterial population in CHDLs may vary among individual cattle and/or farms [65].
In a recent paper from the Guelph University about lameness, prevalence risk factors, and treatment, among other things attention was paid to both extrinsic and intrinsic barriers to addressing lameness and injuries on dairy farms. It is the combination of advisors and the farmer him- or herself who are influencing and implementing on-farm decisions related to lameness prevention, treatment, and control [64].
A systematic review was performed on the treatment modalities for CHDLs and their effects on locomotion scores, gait properties, lesion progression, and nociceptive threshold in dairy cows. This paper is mainly an elaboration of the study of Thomas et al. [86] whereby the effect on the parameters mentioned is poorly understood and more empirical studies and evidence-based data are necessary [66].
In a Dutch study from Royal GD Animal Health, a multi-approach therapy was investigated in case of non-healing claw-disorders (NH-CD). Treatments included claw trimming, pain management, use of an orthopaedic foot block (hard plastics), topical application of an salicylic acid unguent, and a single parenteral antibiotic injection (tilmycosin 10 mg/kg B.W.). An evaluation was conducted 3 months after treatment using locomotion scoring (LS), a clinical observation of the lesion, and the lactation value (the lactation value is the net profit of the individual animal divided by the average net profit of the entire herd). The mean is 100, so >100 is related to better production (combination of kg milk, %fat, and protein) before and after treatment. The mean LS improved significantly from 4.0 (SD: 0.2) before treatment to 1.2 (SD: 0.4; p < 0.001). The clinical presentation showed that all of the cows were cured from horn shoe infection (NH-CD) [67].
References
- Bruijnis, M.R.N.; Hogeveen, H.; Stassen, E.N. Assessing economic consequences of foot disorders in dairy cattle using a dynamic stochastic simulation model. J. Dairy Sci. 2010, 93, 2419–2432. [Google Scholar] [CrossRef]
- Dolecheck, K.; Bewley, J. Animal board invited review: Dairy cow lameness expenditures, losses and total cost. Animal 2018, 12, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Robcis, R.; Ferchiou, A.; Berrada, M.; Ndiaye, Y.; Herman, N.; Lhermie, G.; Raboisson, D. Cost of lameness in dairy herds: An integrated bioeconomic modelling approach. J. Dairy Sci. 2023, 106, 2519–2534. [Google Scholar] [CrossRef] [PubMed]
- Bruijnis, M.R.N.; Beerda, B.; Hogeveen, H.; Stassen, E.N. Foot disorders in dairy cattle: Impact on cow and dairy farmer. Anim. Welf. 2012, 21, 33–40. [Google Scholar] [CrossRef]
- Shearer, J.K.; Stock, M.L.; Van Amstel, S.R.; Coetzee, J.F. Assessment and management of pain associated with lameness in cattle. Vet. Clin. N. Am. 2013, 29, 135–156. [Google Scholar] [CrossRef]
- Puerto, M.A.; Shepley, E.; Cue, R.I.; Warner, D.; Dubuc, J.; Vasseur, E. The hidden cost of disease: II. Impact of the first incidence of lameness on production and economic indicators of primiparous dairy cows. J. Dairy Sci. 2021, 104, 7944–7955. [Google Scholar] [CrossRef]
- Kester, E.; Holzhauer, M.; Frankena, K. A descriptive review of the prevalence and risk factors of hock lesions in dairy cows. Vet. J. 2014, 202, 222–228. [Google Scholar] [CrossRef]
- Thomsen, P.T.; Shearer, J.K.; Houe, H. Prevalence of lameness in dairy cows: A literature review. Vet. J. 2023, 295, 105975. [Google Scholar] [CrossRef]
- Kofler, J.; Suntinger, M.; Mayerhofer, M.; Linke, K.; Maurer, L.; Hund, A.; Fiedler, A.; Duda, J.; Egger-Danner, C. Benchmarking Based on Regularly Recorded Claw Health Data of Austrian Dairy Cattle for Implementation in the Cattle Data Network (RDV). Animals 2022, 12, 808. [Google Scholar] [CrossRef]
- Rasmussen, M.; Capion, N.; Klitgaard, K.; Rogdo, T.; Fjeldaas, T.; Boye, M.; Jensen, T.K. Bovine digital dermatitis: Possible pathogenic consortium consisting of Dichelobacter nodosus and multiple Treponema species. Vet. Micr. 2012, 160, 151–161. [Google Scholar] [CrossRef]
- Kontturi, M.; Junni, R.; Simojoki, H.; Malinen, E.; Seuna, E.; Klitgaard, K.; Kujala-Wirth, M.; Soveri, T.; Pelkonen, S. Bacterial species associated with interdigital phlegmon outbreaks in Finnish dairy herds. BMC. Vet. Res. 2019, 15, 44. [Google Scholar] [CrossRef]
- van der Tol, P.P.J.; Metz, J.H.M.; Noordhuizen-Stassen, E.N.; Back, W.; Braam, C.R.; Weijs, W.A. The pressure distribution under the bovine claw during square standing on a flat surface. J. Dairy Sci. 2002, 85, 1476–1481. [Google Scholar] [CrossRef]
- De Roos, S.; Holzhauer, M. Claw Health Recording in The Netherlands; EuroTier: Hannover, Germany, 2016. [Google Scholar]
- Barker, Z.E.; Amory, J.R.; Wright, J.L.; Mason, S.A.; Blowey, R.W.; Green, L.E. Risk factors for increased rates of sole ulcers, white line disease, and digital dermatitis in dairy cattle from twenty-seven farms in England and Wales. J. Dairy Sci. 2009, 92, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Fürmann, A.; Syring, C.; Becker, J.; Sarbach, A.; Weber, J.; Welham Ruiters, M.; Steiner, A. Prevalence of Painful Lesions of the Digits and Risk Factors Associated with Digital Dermatitis, Ulcers and White Line Disease on Swiss Cattle Farms. Animals 2024, 14, 153. [Google Scholar] [CrossRef]
- Jelinski, M.; Marti, S.; Janzen, E.; Schwartzkopf-Genswein, K. A longitudinal investigation of an outbreak of toe tip necrosis syndrome in Western Canadian feedlot cattle. Can. Vet. J. 2018, 59, 1202–1208. [Google Scholar] [PubMed] [PubMed Central]
- van Amstel, S.R.; Shearer, J.K.; Palin, F.L. Moisture Content, Thickness, and Lesions of Sole Horn Associated with Thin Soles in Dairy Cattle. J. Dairy Sci. 2004, 87, 757–763. [Google Scholar] [CrossRef]
- Tarlton, J.F.; Holah, D.E.; Evans, K.M.; Jones, S.; Pearson, G.R.; Webster, A.J. Biomechanical and histopathological changes in the support structures of bovine hooves around the time of first calving. Vet. J. 2002, 163, 196–204. [Google Scholar] [CrossRef]
- Machado, V.S.; Caixeta, L.S.; McArt, J.A.A.; Bicalho, R.C. The effect of claw horn disruption lesions and body condition score at dry-off on survivability, reproductive performance, and milk production in the subsequent lactation. J. Dairy Sci. 2010, 93, 4071–4078. [Google Scholar] [CrossRef] [PubMed]
- Foditsch, C.; Oikonomou, G.; Machado, V.S.; Bicalho, M.L.; Ganda, E.K. Lameness Prevalence and Risk Factors in Large Dairy Farms in Upstate New York. Model Development for the Prediction of Claw Horn Disruption Lesions. PLoS ONE 2016, 11, e0146718. [Google Scholar] [CrossRef]
- Griffiths, B.E.; Barden, M.; Anagnostopoulos, A.; Bedford, C.; Higgins, H.; Psifidi, A.; Banos, G.; Oikonomou, G. A prospective cohort study examining the association of claw anatomy and sole temperature with the development of claw horn disruption lesions in dairy cattle. J. Dairy Sci. 2024, 107, 2483–2498. [Google Scholar] [CrossRef]
- Greenough, P.R.; Bergsten, C.; Brizzi, A.; Mülling, C.K.W.; Nordlund, K. Bovine Laminitis and Lameness; W.B. Saunders: Philadelphia, PA, USA, 2007; ISBN 9780702027802. [Google Scholar]
- Vermunt, J.J.; Greenough, P.R. Lesions associated with subclinical laminitis of the claws of dairy calves in two management systems. Vet. J. 1995, 151, 391–399. [Google Scholar] [CrossRef]
- Bergsten, C. Laminitis and Sole Lesions in Dairy Cows; Pathogenesis, Risk Factors, and Precautions. Acta Vet. Scand. 2003, 44 (Suppl. S1), P58. [Google Scholar] [CrossRef]
- Ingvartsen, K.L. Feeding- and management-related diseases in the transition cow Physiological adaptations around calving and strategies to reduce feeding-related diseases. Anim. Feed Sci. Technol. 2006, 126, 175–213. [Google Scholar] [CrossRef]
- Hedges, V.; Blowey, R.W.; Packington, A.J.; O’Callaghan, C.J.; Green, L.E. A longitudinal field trial of the effect of biotin supplementation on lameness in dairy cows. J. Dairy Sci. 2001, 84, 1969–1975. [Google Scholar] [CrossRef] [PubMed]
- Pötzsch, C.J.; Collis (née Hedges), V.J.; Blowey, R.W.; Packington, A.J.; Green, L.E. The Impact of Parity and Duration of Biotin Supplementation on White Line Disease Lameness in Dairy Cattle. J. Dairy Sci. 2003, 86, 2577–2582. [Google Scholar] [CrossRef]
- Evans, N.J.; Blowey, R.W.; Timofte, D.; Isherwood, D.R.; Brown, J.M.; Murray, R.; Paton, R.J.; Carter, S.D. Association between bovine digital dermatitis treponemes and a range of ‘non-healing’ bovine hoof disorders. Vet. Rec. 2011, 168, 214. [Google Scholar] [CrossRef] [PubMed]
- Sykora, S.; Kofler, J.; Golonegger-reichert, J.; Dietrich, J.; Auersperg, G.; Brandt, S. Treponema DNA in bovine “non-healing” versus common sole ulcers and white line disease. Vet. J. 2015, 205, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Alsaaod, M.; Weber, J.; Jensen, T.; Brandt, S.; Gurtner, C.; Devaux, D.; Studer, E.; Steiner, A. “Non-healing” claw horn lesions in dairy cows: Clinical, histopathological and molecular biological characterization of four cases. Front in Vet. Sci. 2022, 9, 1041215. [Google Scholar] [CrossRef]
- Groenevelt, M.; Anzuino, K.; Smith, S.; Lee, M.R.; Grogono-Thomas, R. A case report of lameness in two dairy goat herds; a suspected combination of nutritional factors concurrent with treponeme infection. BMC Res. Notes 2015, 8, 791. [Google Scholar] [CrossRef]
- Tegtmeyer, P.C.; Staton, G.J.; Evans, N.J.; Rohde, J.; Punsmann, T.M.; Ganter, M. First cases of contagious ovine digital dermatitis in Germany. Acta Vet. Scand. 2020, 62, 46. [Google Scholar] [CrossRef]
- Holzhauer, M.; Hardenberg, C.; Bartels, C.J.M. Herd and cow-level prevalence of sole ulcers in The Netherlands and associated-risk factors. Prev. Vet. Med. 2008, 85, 125–135. [Google Scholar] [CrossRef]
- Heringstad, B.; Egger-Danner, C.; Charfeddine, N.; Pryce, J.E.; Stock, K.F.; Kofler, J.; Sogstad, A.M.; Holzhauer, M.; Fiedler, A.; Müller, K.; et al. Invited review: Genetics and claw health: Opportunities to enhance claw health by genetic selection. J. Dairy Sci. 2018, 101, 4801–4821. [Google Scholar] [CrossRef]
- van der Waaij, E.H.; Holzhauer, M.; Ellen, E.; Kamphuis, C.; de Jong, G. Genetic parameters for claw disorders in Dutch dairy cattle and correlations with conformation traits. J. Dairy Sci. 2005, 88, 3672–3678. [Google Scholar] [CrossRef]
- Chesterton, R.N.; Lawrence, K.E.; Laven, R.A. A descriptive analysis of the foot lesions identified during veterinary treatment for lameness on dairy farms in north Taranaki. N. Z. Vet. J. 2008, 56, 130–138. [Google Scholar] [CrossRef]
- Kujala, M.; Dohoo, I.R.; Soveri, T. White-line disease and haemorrhages in hooves of Finnish dairy cattle. Prev. Vet. Med. 2010, 94, 18–27. [Google Scholar] [CrossRef] [PubMed]
- van der Linde, C.; de Jong, G.; Koenen, E.P.; Eding, H. Claw health index for Dutch dairy cattle based on claw trimming and conformation data. J. Dairy Sci. 2010, 93, 4883–4891. [Google Scholar] [CrossRef]
- Al-Qudah, K.M.; Ismail, Z.B. The relationship between serum biotin and oxidant/antioxidant activities in bovine lameness. Res. Vet. Sci. 2012, 92, 138–141. [Google Scholar] [CrossRef]
- Mason, W.A.; Laven, L.J.; Laven, R.A. An outbreak of toe ulcers, sole ulcers and white line disease in a group of dairy heifers immediately after calving. N. Z. Vet. J. 2012, 60, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Coombe, J.E.; Pyman, M.F.; Mansell, P.D.; Auldist, M.J.; Anderson, G.A.; Wales, W.J.; Malmo, J.; Conley, M.J.; Fisher, A.D. The effects on claw health of supplement feeding grazing dairy cows on feed pads. Vet. J. 2013, 198, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Schöpke, K.; Weidling, S.; Pijl, R.; Swalve, H.H. Relationships between bovine hoof disorders, body condition traits, and test-day yields. J. Dairy Sci. 2013, 96, 679–689. [Google Scholar] [CrossRef]
- Oberbauer, A.M.; Berry, S.L.; Belanger, J.M.; McGoldrick, R.M.; Pinos-Rodriquez, J.M.; Famula, T.R. Determining the heritable component of dairy cattle foot lesions. J. Dairy Sci. 2013, 96, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Ødegård, C.; Svendsen, M.; Heringstad, B. Genetic analyses of claw health in Norwegian Red cows. J. Dairy Sci. 2013, 96, 7274–7283. [Google Scholar] [CrossRef] [PubMed]
- Häggman, J.; Juga, J.; Sillanpää, M.J.; Thompson, R. Genetic parameters for claw health and feet and leg conformation traits in Finnish Ayrshire cows. J. Anim. Breed. Genet. 2013, 130, 89–97. [Google Scholar] [CrossRef]
- Chapinal, N.; Koeck, A.; Sewalem, A.; Kelton, D.F.; Mason, S.; Cramer, G.; Miglior, F. Genetic parameters for hoof lesions and their relationship with feet and leg traits in Canadian Holstein cows. J. Dairy Sci. 2013, 96, 2596–2604. [Google Scholar] [CrossRef]
- Oikonomou, G.; Banos, G.; Machado, V.; Caixeta, L.; Bicalho, R.C. Short communication: Genetic characterization of digital cushion thickness. J. Dairy Sci. 2014, 97, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Gyan, L.A.; Paetsch, C.D.; Jelinski, M.D.; Allen, A.L. The lesions of toe tip necrosis in southern Alberta feedlot cattle provide insight into the pathogenesis of the disease. Can. Vet. J. 2015, 56, 1134–1139. [Google Scholar] [PubMed]
- Pérez-Cabal, M.A.; Charfeddine, N. Models for genetic evaluations of claw health traits in Spanish dairy cattle. J. Dairy Sci. 2015, 98, 8186–8194. [Google Scholar] [CrossRef]
- van der Spek, D.; van Arendonk, J.A.; Bovenhuis, H. Genome-wide association study for claw disorders and trimming status in dairy cattle. J. Dairy Sci. 2015, 98, 1286–1295. [Google Scholar] [CrossRef]
- van der Spek, D.; van Arendonk, J.A.; Bovenhuis, H. Genetic relationships between claw health traits of dairy cows in different parities, lactation stages, and herds with different claw disorder frequencies. J. Dairy Sci. 2015, 98, 6564–6571. [Google Scholar] [CrossRef]
- Burgstaller, J.; Raith, J.; Kuchling, S.; Mandl, V.; Hund, A.; Kofler, J. Claw health and prevalence of lameness in cows from compost bedded and cubicle freestall dairy barns in Austria. Vet. J. 2016, 216, 81–86. [Google Scholar] [CrossRef]
- Newsome, R.; Green, M.J.; Bell, N.J.; Chagunda, M.G.G.; Mason, C.S.; Rutland, C.S.; Sturrock, C.J.; Whay, H.R.; Huxley, J.N. Linking bone development on the caudal aspect of the distal phalanx with lameness during life. J. Dairy Sci. 2016, 99, 4512–4525. [Google Scholar] [CrossRef]
- Malchiodi, F.; Koeck, A.; Mason, S.; Christen, A.M.; Kelton, D.F.; Schenkel, F.S.; Miglior, F. Genetic parameters for hoof health traits estimated with linear and threshold models using alternative cohorts. J. Dairy Sci. 2017, 100, 2828–2836. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.B.; Ramanoon, S.Z.; Mansor, R.; Syed-Hussain, S.S.; Shaik Mossadeq, W.M. Prevalence of lameness, claw lesions, and associated risk factors in dairy farms in Selangor, Malaysia. Trop. Anim. Health Prod. 2017, 49, 1741–1748. [Google Scholar] [CrossRef]
- Ring, S.C.; Twomey, A.J.; Byrne, N.; Kelleher, M.M.; Pabiou, T.; Doherty, M.L.; Berry, D.P. Genetic selection for hoof health traits and cow mobility scores can accelerate the rate of genetic gain in producer-scored lameness in dairy cows. J. Dairy Sci. 2018, 101, 10034–10047. [Google Scholar] [CrossRef]
- Croué, I.; Michenet, A.; Leclerc, H.; Ducrocq, V. Genomic analysis of claw lesions in Holstein cows: Opportunities for genomic selection, quantitative trait locus detection, and gene identification. J. Dairy Sci. 2019, 102, 6306–6318. [Google Scholar] [CrossRef]
- Somers, J.R.; Huxley, J.N.; Doherty, M.L.; O’Grady, L.E. Routine Herd Health Data as Cow-Based Risk Factors Associated with Lameness in Pasture-Based, Spring Calving Irish Dairy Cows. Animals 2019, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Staton, G.J.; Sullivan, L.E.; Blowey, R.W.; Carter, S.D.; Evans, N.J. Surveying bovine DD and non-healing bovine foot lesions for the presence of Fusobacterium necrophorum, Porphyromonas endodontalis and Treponema pallidum. Vet. Rec. 2020, 186, 450. [Google Scholar] [CrossRef] [PubMed]
- Pirkkalainen, H.; Talvio, I.; Kujala-Wirth, M.; Soveri, T.; Orro, T. Acute phase response of sole ulcer, white line disease and digital dermatitis in dairy cows. Vet. Anim. Sci. 2022, 17, 100253. [Google Scholar] [CrossRef]
- Browne, N.; Hudson, C.D.; Crossley, R.E.; Sugrue, K.; Huxley, J.N.; Conneely, M. Hoof lesions in partly housed pasture-based dairy cows. J. Dairy Sci. 2022, 105, 9038–9053. [Google Scholar] [CrossRef]
- Capion, N.; Cannings, E.S.; Krogh, M.A. Comparison of claw horn disruption lesions in four dairy herds using two different trimming techniques: A case study. Vet. J. 2022, 287, 105886. [Google Scholar] [CrossRef]
- Li, B.; Barden, M.; Kapsona, V.; Sánchez-Molano, E.; Anagnostopoulos, A.; Griffiths, B.E.; Bedford, C.; Dai, X.; Coffey, M.; Psifidi, A.; et al. Single-step genome-wide association analyses of claw horn lesions in Holstein cattle using linear and threshold models. Genet. Sel. Evol. 2023, 55, 16. [Google Scholar] [CrossRef]
- Roche, S.M.; Renaud, D.L.; Saraceni, J.; Kelton, D.F.; DeVries, T.J. Invited review: Prevalence, risk factors, treatment, and barriers to best practice adoption for lameness and injuries in dairy cattle-A narrative review. J. Dairy Sci. 2024, 107, 3347–3366. [Google Scholar] [CrossRef]
- Hori, K.; Taniguchi, T.; Elpita, T.; Khemgaew, R.; Sasaki, S.; Gotoh, Y.; Yasutomi, I.; Misawa, N. Comprehensive Analyses of the Bacterial Population in Non-Healing Claw Lesions of Dairy Cattle. Animals 2022, 12, 3584. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.B.; Ramanoon, S.Z.; Syed-Hussain, S.S.; Mansor, R.; Mossadeq, W.M.S.; Degu, N.Y. Treatment modalities for claw horn lesions and their effects on locomotion scores, gait properties, lesion progression, and nociceptive threshold in dairy cows: A systematic review. Res. Vet. Sci. 2024, 181, 105448. [Google Scholar] [CrossRef] [PubMed]
- Holzhauer, M.; Boersma, S.J.; Boon, D.; de Leeuw, H. An Evaluation of a Parenteral Antibiotic Treatment of Cattle with Non-Healing Claw Horn Lesions. Animals 2024, 14, 1396. [Google Scholar] [CrossRef]
- Tomlinson, D.J.; Műlling, C.H.; Fakler, T.M. Invited Review: Formation of Keratins in the Bovine Claw: Roles of Hormones, Minerals, and Vitamins in Functional Claw Integrity. J. Dairy Sci. 2004, 87, 797–809. [Google Scholar] [CrossRef]
- Paetsch, C.; Fenton, K.; Perrett, T.; Janzen, E.; Clark, T.; Shearer, J.; Jelinski, M. Prospective case-control study of toe tip necrosis syndrome (TTNS) in western Canadian feedlot cattle. Can. Vet. J. 2017, 58, 247–254. [Google Scholar] [PubMed]
- Jewell, M.T.; Cameron, M.; Spears, J.; McKenna, S.L.; Cockram, M.S.; Sanchez, J.; Keefe, G.P. Prevalence of lameness and associated risk factors on dairy farms in the Maritime Provinces of Canada. J. Dairy Sci. 2019, 102, 3392–3405. [Google Scholar] [CrossRef]
- Offer, J.E.; Leach, K.A.; Brocklehurst, S.; Logue, D.N. Effect of Forage Type on Claw Horn Lesion Development in Dairy Heifers. Vet. J. 2003, 165, 221–227. [Google Scholar] [CrossRef]
- Offer, J.E.; Logue, D.N.; Brockelhurst, S.; Mason, C. Effect of an incident of overfeeding of concentrate on claw horn lesion development in first lactation dairy heifers. In Proceedings of the 13th International Symposium and 5th Conference on Lameness in Ruminants, Maribor, Slovenia, 11–15 February 2004; pp. 175–178. [Google Scholar]
- Bergsten, C.; Telezhenko, E.; Ventorp, M. Influence of Soft or Hard Floors before and after First Calving on Dairy Heifer Locomotion, Claw and Leg Health. Animals 2015, 5, 662–686. [Google Scholar] [CrossRef]
- Hinterhofer, C.; Apprich, V.; Ferguson, J.C.; Stanek, C. Elastic properties of hoof horn on different positions in the bovine claw. Dtsch. Tierärztl Wschr. 2005, 112, 142–146. [Google Scholar] [PubMed]
- Jelinski, M.; Fenton, K.; Perrett, T.; Paetsch, C. Epidemiology of toe tip necrosis syndrome (TTNS) of North American feedlot cattle. Can. Vet. J. 2016, 57, 829–834. [Google Scholar] [PubMed]
- Holzhauer, M.; Bartels, C.J.; van den Borne, B.H.; van Schaik, G. Intra-class correlation attributable to claw trimmers scoring common hind-claw disorders in Dutch dairy herds. Prev. Vet. Med. 2006, 75, 47–55. [Google Scholar] [CrossRef]
- Holzhauer, M.; van Egmond, M.J. A new structural approach to improve cow hoof health on dairy farms. J. Dairy Res. 2021, 88, 388–395. [Google Scholar] [CrossRef]
- Holzhauer, M.; Vos, J.H. Claw health in Dutch dairy herds, an update of some recent disorders. Tijdschr. Diergeneeskd. 2009, 134, 200–205. [Google Scholar] [PubMed]
- Somers, J.G.C.J.; Frankena, K.; Noordhuizen-Stassen, E.N.; Metz, J.H.M. Prevalence of Claw Disorders in Dutch Dairy Cows Exposed to Several Floor Systems. J. Dairy Sci. 2003, 86, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Ouweltjes, W.; Holzhauer, M.; van der Tol, P.P.J.; van der Werf, J. Effects of two trimming methods of dairy cattle on concrete or rubber-covered slatted floors. J. Dairy Sci. 2009, 92, 960–971. [Google Scholar] [CrossRef]
- Vanegas, J.; Overton, M.; Lilly, E.; Berry, S.L.; Sischo, W.M. Effect of Rubber Flooring on Claw Health in Lactating Dairy Cows Housed in Free-Stall Barns. J. Dairy Sci. 2006, 89, 4251–4258. [Google Scholar] [CrossRef] [PubMed]
- Fjeldaas, T.; Sogstad, Å.M.; Østerås, O. Locomotion and claw disorders in Norwegian dairy cows housed in freestalls with slatted concrete, solid concrete, or solid rubber flooring in the alleys. J. Dairy Sci. 2010, 94, 1243–1255. [Google Scholar] [CrossRef]
- Shearer, J.K.; Plummer, P.J.; Schleining, J.A. Perspectives on the treatment of claw lesions in cattle. Vet. Med. 2015, 6, 273–292. [Google Scholar] [CrossRef]
- Toussaint-Raven, E. The principles of claw trimming. Vet. Clin. N. Am. Food Anim. Pract. 1985, 1, 93–107. [Google Scholar] [CrossRef]
- Cramer, G.; Solano, L.; Constable, P. Lameness Originating in the Hoof in Cattle. In Merck Veterinary Manual; Musculoskeletal System/Lameness in Cattle/lameness Originating in the Hoof in Cattle; Merck & Company, Incorporated: White Station, NJ, USA, 2025. [Google Scholar]
- Thomas, H.J.; Miguel-Pacheco, G.G.; Bollard, N.J.; Archer, S.C.; Bell, N.J.; Mason, C.; Maxwell, O.J.R.; Remnant, J.G.; Sleeman, P.; Whay, H.R.; et al. Evaluation of treatments for claw horn lesions in dairy cows in a randomized controlled trial. J. Dairy Sci. 2015, 98, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Laschinger, J.; Kofler, J. Chronic pain management in a dairy cow with deep digital sepsis. Vet. Rec. 2025, 13, e70011. [Google Scholar] [CrossRef]
- Kofler, J. Pathogenesis and Treatment of Toe Lesions in Cattle Including “Nonhealing” Toe Lesions. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 301–328. [Google Scholar] [CrossRef]
- Holzhauer, M. Surgical Intervention in Case of Apex Necrosis of Bovine Claw. J. Surg. Tech. Proced. 2021, 5, 1048. [Google Scholar]
- Alaweh, J.I.; Laven, R.A.; Stevenson, M.A. Interval between detection of lameness by locomotion scoring and treatment for lameness: A survival analysis. Vet. J. 2012, 193, 622–625. [Google Scholar] [CrossRef]
- Bergsten, C.; Åkerström, F.; Nyman, A. Prevention of claw disorders by strategic maintenance trimming in relation to calving time. In Proceedings of the 20th International Symposium and 12th International Conference on Lameness in Ruminants, Asakusa View Hotel, Tokio, Japan, 10–13 March 2019. [Google Scholar]
- Bergsten, C.; Frank, B. Sole haemorrhages in tied heifers in early gestation as an indicator of laminitis: Effects of diet and flooring. Acta Vet. Scand. 1996, 37, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.B.; Bennett, T.B.; Nordlund, K.V. Monitoring Indices of Cow Comfort in Free-Stall-Housed Dairy Herds. J. Dary Sci. 2005, 88, 3876–3885. [Google Scholar] [CrossRef]
- Shearer, J.K.; van Amstel, S.R. Pathogenesis and Treatment of Sole Ulcers and White Line Disease. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 283–300. [Google Scholar] [CrossRef]
- Sadiq, M.B.; Ramanoon, S.Z.; Shaik, M.W.M.; Mansor, R.; Syed-Hussain, S.S. Treatment protocols for claw horn lesions and their impact on lameness recovery, pain sensitivity, and lesion severity in moderately lame primiparous dairy cows. Front. Vet. Sci. 2022, 9, 1060520. [Google Scholar] [CrossRef]
- Miguel-Pacheco, G.G.; Thomas, H.J.; Huxley, J.N.; Newsome, R.F.; Kaler, J. Effect of claw horn lesion type and severity at the time of treatment on outcome of lameness in dairy cows. Vet. J. 2017, 225, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.W.; Davis, J.L.; Tell, L.A.; Webb, A.I.; Riviere, J.E. Extra label use of nonsteroidal anti-inflammatory drugs in cattle. JAVMA 2008, 232, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Browne, N.; Conneely, M.; Hudson, C. Use of Non-Steroidal Anti-Inflammatory Drugs and Attitudes to Pain in Pasture-Based Dairy Cows: A Comparative Study of Farmers and Veterinarians. Front. Vet. Sci. 2022, 9, 912564. [Google Scholar] [CrossRef]
- Buret, A.G. Immuno-modulation and anti-inflammatory benefits of antibiotics: The example of tilmicosin. Can. J. Vet. Res. 2010, 74, 1–10. [Google Scholar] [PubMed]
- ICAR 2022: ICAR Guidelines: Section 7-Functional Traits in Dairy Cattle. Available online: https://www.icar.org/Guidelines/07-Bovine-Functional-Traits.pdf (accessed on 1 May 2022).
- Eriksson, H.K.; Daros, R.R.; Von Keyserlingk, M.A.G.; Weary, D.M. Effects of case definition and assessment frequency on lameness incidence estimates. J. Dairy Sci. 2020, 103, 638–648. [Google Scholar] [CrossRef]
- Bicalho, R.C.; Oikonomou, G. Control and prevention of lameness associated with claw 470 lesions in dairy cows. Livest. Sci. 2013, 156, 96–105. [Google Scholar] [CrossRef]
- Solano, L.; Barkema, H.W.; Pajor, E.A.; Mason, S.; LeBlanc, S.J.; Zaffino Heyerhoff, J.C.; Nash, C.G.R.; Haley, D.B.; Vasseur, E.; Pellerin, D.; et al. Prevalence of lameness and associated risk factors in Canadian Holstein-Friesian cows housed in freestall barns. J. Dairy Sci. 2015, 98, 6978–6991. [Google Scholar] [CrossRef]
- Jelinski, M.; Waldner, C.; Penner, G. Case-control study of mineral concentrations of hoof horn tissue derived from feedlot cattle with toe necrosis. Can. Vet. J. 2018, 59, 254–260. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).