Phytochemicals from Euclea natalensis Modulate Th17 Differentiation, HIV Latency, and Comorbid Pathways: A Systems Pharmacology and Thermodynamic Profiling Approach
Abstract
1. Introduction
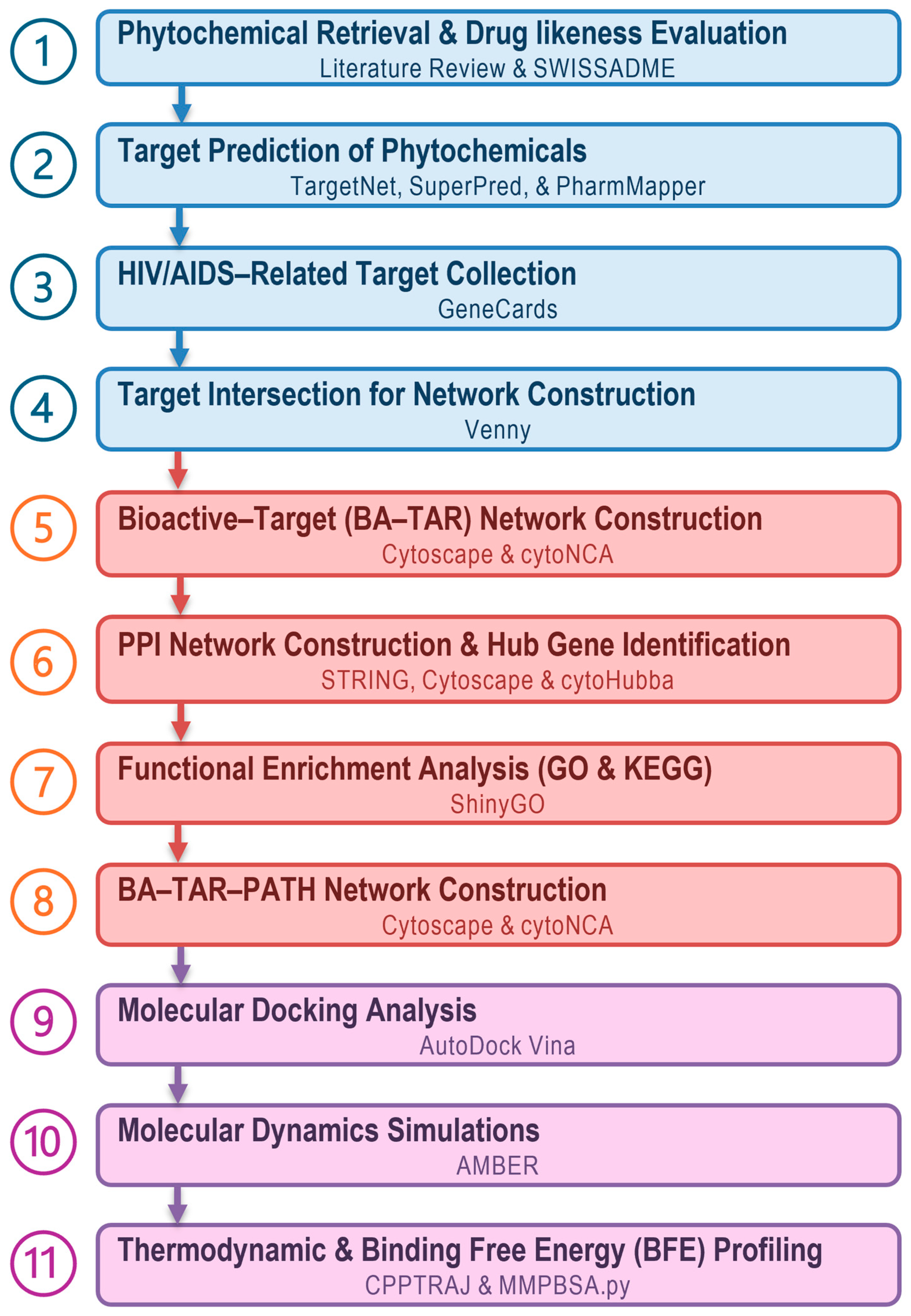
2. Materials and Methods
2.1. Collection of Bioactive Phytochemicals from Euclea natalensis
2.2. In Silico ADME Phytochemical Profiling
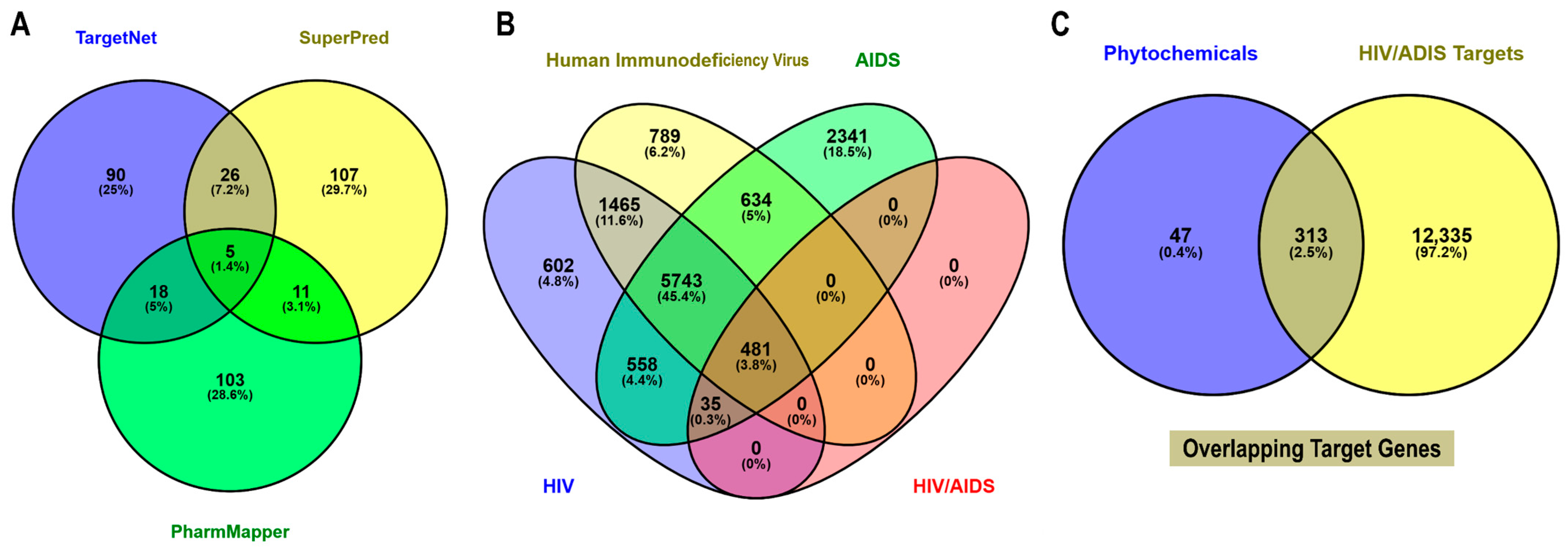
2.3. Prediction of Phytochemical Associated Human Targets
2.4. Collection of HIV/AIDS-Associated Protein Targets
2.5. Identification of HIV/AIDS-Related Host Targets
2.6. Bioactive–Target (BA-TAR) Network Construction
2.7. PPI Network Construction and Hub Gene Identification
2.8. GO and KEGG Pathway Enrichment Analysis
2.9. BAR-TAR-PATH Network Construction
2.10. Molecular Docking Calculations
2.11. MD Simulations
2.12. BFE Computations
3. Results
3.1. In Silico ADME Evaluation of Phytochemical Ligands
3.2. Identification of Potential Targets of Euclea natalensis Phytochemicals in HIV Pathogenesis
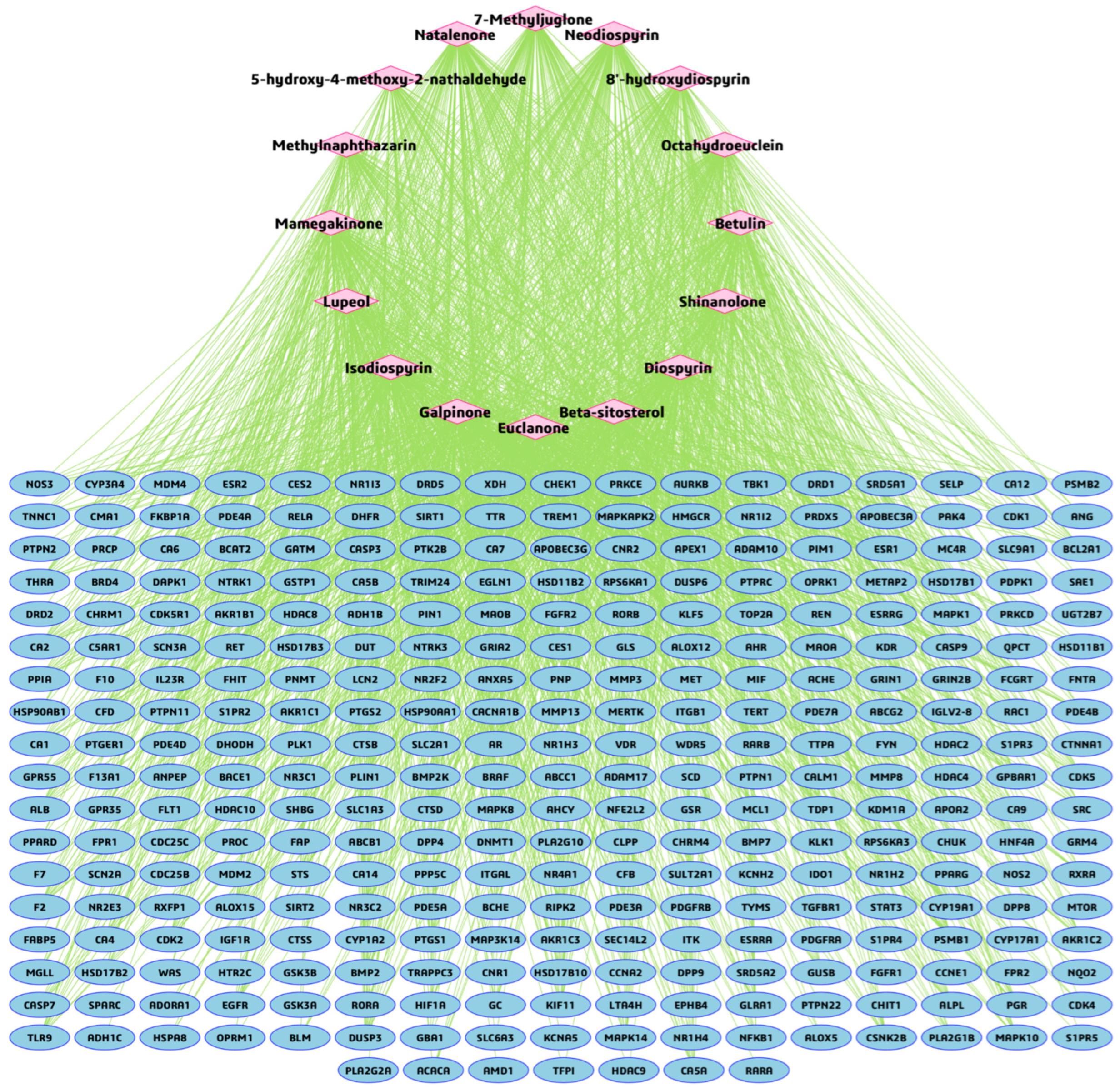
3.3. BA-TAR Network Construction and Phytochemical Topological Analysis
3.4. PPI Network and Hub Gene Analysis
3.5. GO and KEGG Pathway Enrichment Analyses of Euclea natalensis-HIV/AIDS Hub Targets
3.5.1. GO BP
3.5.2. GO CC
3.5.3. GO MF
3.5.4. KEGG Pathway Enrichment Analysis
3.6. BA-TAR-PATH Network Construction
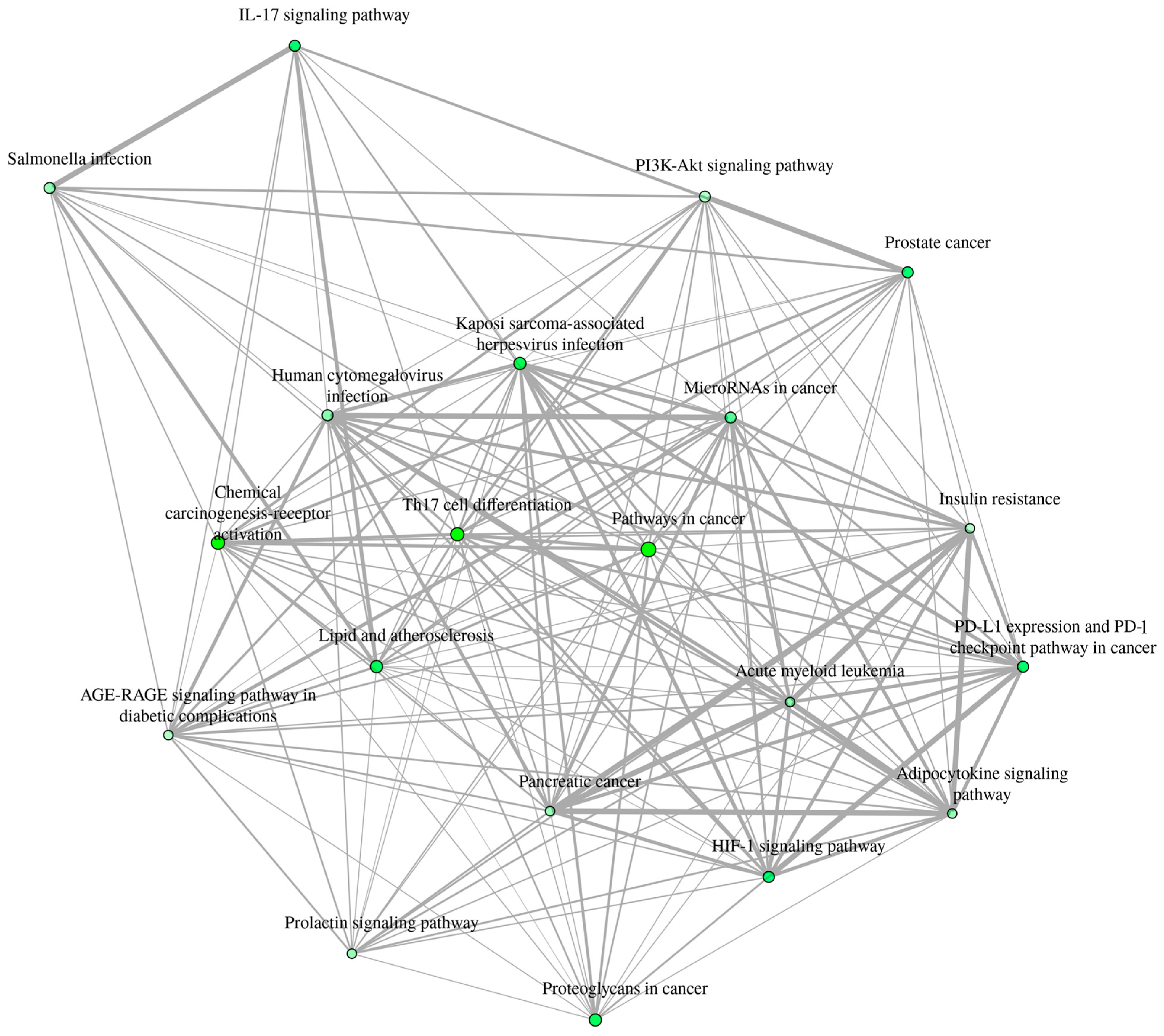
3.7. Inter-Pathway KEGG Network Connectivity
3.8. Molecular Docking Analysis
3.8.1. Binding Affinity Calculations
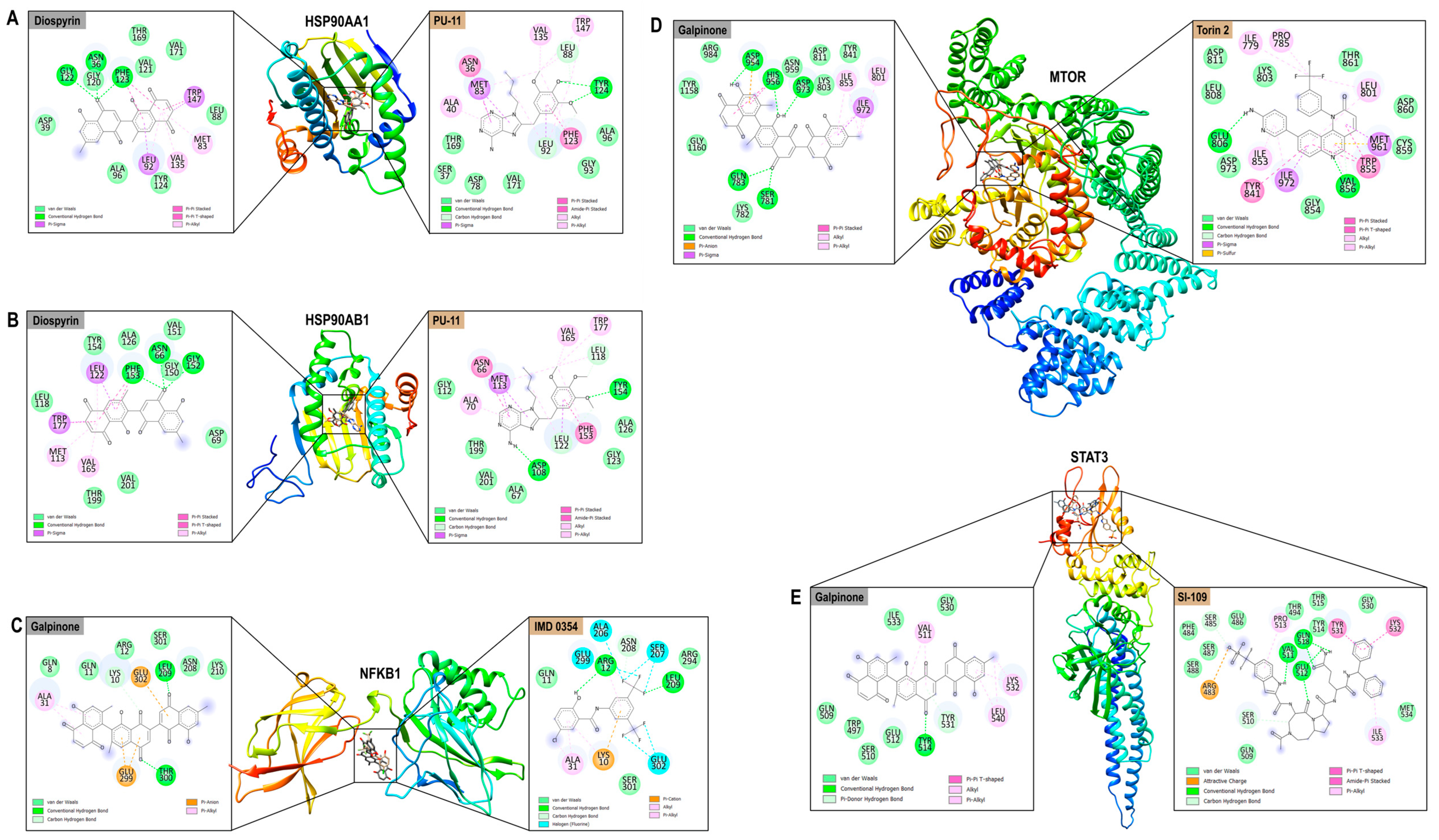
3.8.2. Protein–Ligand Interaction Analysis
3.9. Thermodynamic Analyses of Top-Ranking Complexes
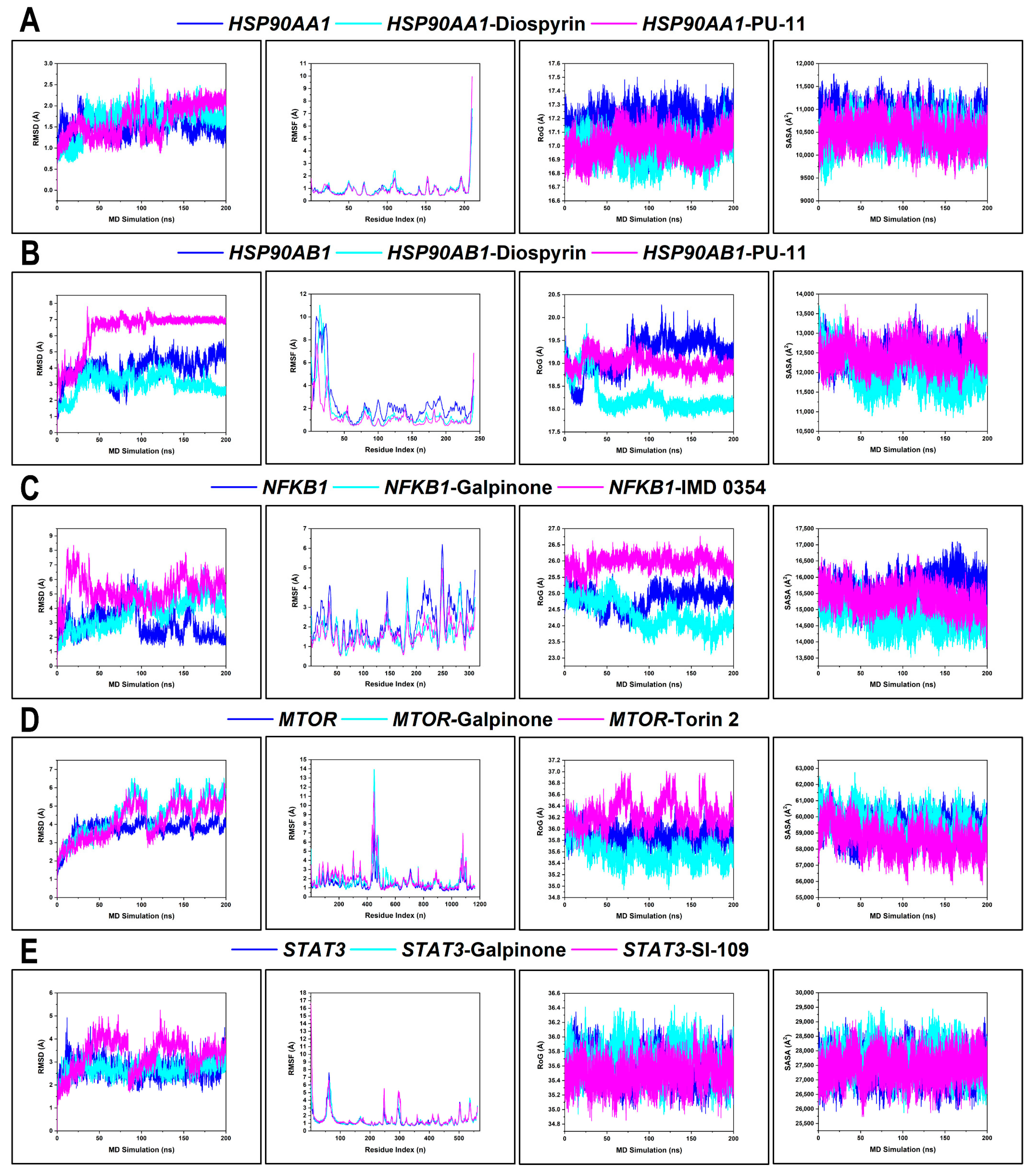
3.9.1. Conformational Profiling of Hub Targets
3.9.2. BFE Profiling
4. Discussion
Limitation of Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADME | Absorption, distribution, metabolism, and excretion |
| AIDS | Acquired immunodeficiency syndrome |
| ART | Antiretroviral therapy |
| BA-TAR | Bioactive-target |
| BA-TAR-PATH | Bioactive-target-pathway |
| BFE | Binding free energy |
| BP | Biological process |
| CC | Cellular component |
| GO | Gene ontology |
| GALT | Gut-associated lymphoid tissue |
| HIF-1 | Hypoxia-inducible factor 1 |
| HIV | Human immunodeficiency virus |
| KEGG | Kyoto encyclopedia of genes and genomes |
| IL | Interleukin |
| MCC | Maximal clique centrality |
| MD | Molecular dynamics |
| MM/GBSA | Molecular mechanics/generalized Born surface area |
| MF | Molecular function |
| PPI | Protein–protein interaction |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death ligand 1 |
| RMSD | Root mean square deviation |
| RMSF | Root mean square fluctuation |
| RoG | Radius of gyration |
| SASA | Solvent accessible surface area |
| Th17 | T helper 17 (CD4+ T-cell subtype) |
References
- Chen, Y.; Li, A.D.; Yang, Y.; Lu, J.; Xu, Y.; Ji, X.; Wu, L.; Han, L.; Zhu, B.; Xu, M. Global, Regional and National Burden of HIV/AIDS among Individuals Aged 15–79 from 1990 to 2021. AIDS Res. Ther. 2025, 22, 51. [Google Scholar] [CrossRef]
- Pasternak, A.O.; Berkhout, B. HIV Persistence: Silence or Resistance? Curr. Opin. Virol. 2023, 59, 101301. [Google Scholar] [CrossRef]
- Vaillant, A.A.J.; Naik, R. HIV-1–Associated Opportunistic Infections; StatPearls: Petersburg, FA, USA, 2023.
- Chawla, A.; Wang, C.; Patton, C.; Murray, M.; Punekar, Y.; de Ruiter, A.; Steinhart, C. A Review of Long-Term Toxicity of Antiretroviral Treatment Regimens and Implications for an Aging Population. Infect. Dis. Ther. 2018, 7, 183–195. [Google Scholar] [CrossRef]
- Mandal, A.; Biswas, D.; Hazra, B. Natural Products from Plants with Prospective Anti-HIV Activity and Relevant Mechanisms of Action. Stud. Nat. Prod. Chem. 2020, 66, 225–271. [Google Scholar]
- Wang, J.; Guo, N.; Hou, W. Antiviral Drug Carriers for Human Immunodeficiency Virus. Nano Trends 2023, 4, 100027. [Google Scholar] [CrossRef]
- Pellaers, E.; Denis, A.; Debyser, Z. New Latency-Promoting Agents for a Block-and-Lock Functional Cure Strategy. Curr. Opin. HIV AIDS 2024, 19, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Maina, E.K.; Adan, A.A.; Mureithi, H.; Muriuki, J.; Lwembe, R.M. A Review of Current Strategies Towards the Elimination of Latent HIV-1 and Subsequent HIV-1 Cure. Curr. HIV Res. 2020, 19, 14–26. [Google Scholar] [CrossRef]
- Sadowski, I.; Hashemi, F.B. Strategies to Eradicate HIV from Infected Patients: Elimination of Latent Provirus Reservoirs. Cell. Mol. Life Sci. 2019, 76, 3583–3600. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.R.; Mota, T.M.; Jones, R.B. Immunological Approaches to HIV Cure. Semin. Immunol. 2021, 51, 101412. [Google Scholar] [CrossRef] [PubMed]
- Maroyi, A. Review of Ethnomedicinal Uses, Phytochemistry and Pharmacological Properties of E. natalensis A.DC. Molecules 2017, 22, 2128. [Google Scholar] [CrossRef]
- Taye, A.D.; Bizuneh, G.K.; Kasahun, A.E. Ethnobotanical Uses, Phytochemistry and Biological Activity of the Genus Euclea: A Review. Front. Pharmacol. 2023, 14, 1170145. [Google Scholar] [CrossRef]
- Lall, N.; Weiganand, O.; Hussein, A.A.; Meyer, J.J.M. Antifungal Activity of Naphthoquinones and Triterpenes Isolated from the Root Bark of E. natalensis. S. Afr. J. Bot. 2006, 72, 579–583. [Google Scholar] [CrossRef]
- van der Kooy, F.; Meyer, J.J.M.; Lall, N. Antimycobacterial Activity and Possible Mode of Action of Newly Isolated Neodiospyrin and Other Naphthoquinones from E. natalensis. S. Afr. J. Bot. 2006, 72, 349–352. [Google Scholar] [CrossRef]
- Weigenand, O.; Hussein, A.A.; Lall, N.; Meyer, J.J.M. Antibacterial Activity of Naphthoquinones and Triterpenoids from E. natalensis Root Bark. J. Nat. Prod. 2004, 67, 1936–1938. [Google Scholar] [CrossRef]
- Tshikalange, T.E.; Lall, N.; Meyer, J.J.M.; Mahapatra, A. In Vitro HIV-1 Reverse Transcriptase Inhibitory Activity of Naphthoquinones and Derivatives from E. natalensis. S. Afr. J. Bot. 2007, 73, 339. [Google Scholar] [CrossRef]
- Bati, K.; Baeti, P.B.; Gaobotse, G.; Kwape, T.E. Leaf Extracts of E. natalensis A.D.C Ameliorate Biochemical Abnormalities in High-Fat-Low Streptozotocin-Induced Diabetic Rats through Modulation of the AMPK-GLUT4 Pathway. Egypt. J. Basic Appl. Sci. 2024, 11, 232–252. [Google Scholar] [CrossRef]
- Lall, N.; Kumar, V.; Meyer, D.; Gasa, N.; Hamilton, C.; Matsabisa, M.; Oosthuizen, C. In Vitro and In Vivo Antimycobacterial, Hepatoprotective and Immunomodulatory Activity of E. natalensis and Its Mode of Action. J. Ethnopharmacol. 2016, 194, 740–748. [Google Scholar] [CrossRef]
- Jain, N.K.; Chandrasekaran, B.; Khazaleh, N.; Jain, H.K.; Lal, M.; Joshi, G.; Jha, V. Computational Network Pharmacology, Molecular Docking, and Molecular Dynamics to Decipher Natural Compounds of Alchornea laxiflora for Liver Cancer Chemotherapy. Pharmaceuticals 2025, 18, 508. [Google Scholar] [CrossRef]
- Gois Leandro, C.; Senesi, P.; Singh Grewal, A.; Yan, Z.; Wei, C. Integrated Bioinformatics and in Silico Approaches Reveal the Biological Targets and Molecular Mechanisms of 1,25-Dihydroxyvitamin D against COVID-19 and Diabetes Mellitus. Front. Nutr. 2019, 9, 1060095. [Google Scholar]
- Akoonjee, A.; Lukman, H.Y.; Ajao, A.A.; Uthman, T.O.; Sabiu, S. A Network Pharmacology- and Molecular Dynamics Simulation-Based Bioprospection of Khaya grandifoliola C. DC. for diabetes care. J. Biomol. Struct. Dyn. 2024, 1–20. [Google Scholar] [CrossRef]
- Shao, C.; Wang, H.; Sang, F.; Xu, L. Study on the Mechanism of Improving HIV/AIDS Immune Function with Jian Aikang Concentrated Pill Based on Network Pharmacology Combined with Experimental Validation. Drug Des. Dev. Ther. 2022, 16, 2731–2753. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound Databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Oduro-Kwateng, E.; Soliman, M.E. DON/DRP-104 as Potent Serine Protease Inhibitors Implicated in SARS-CoV-2 Infection: Comparative Binding Modes with Human TMPRSS2 and Novel Therapeutic Approach. J. Cell. Biochem. 2024, 125, e30528. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Yao, Z.J.; Dong, J.; Che, Y.J.; Zhu, M.F.; Wen, M.; Wang, N.N.; Wang, S.; Lu, A.P.; Cao, D.S. TargetNet: A Web Service for Predicting Potential Drug–Target Interaction Profiling via Multi-Target SAR Models. J. Comput. Aided Mol. Des. 2016, 30, 413–424. [Google Scholar] [CrossRef]
- Gallo, K.; Goede, A.; Preissner, R.; Gohlke, B.O. SuperPred 3.0: Drug Classification and Target Prediction—A Machine Learning Approach. Nucleic Acids Res. 2022, 50, W726–W731. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 Update: A Web Server for Potential Drug Target Identification with a Comprehensive Target Pharmacophore Database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef] [PubMed]
- Breuza, L.; Poux, S.; Estreicher, A.; Famiglietti, M.L.; Magrane, M.; Tognolli, M.; Bridge, A.; Baratin, D.; Redaschi, N.; Xenarios, I.; et al. The UniProtKB Guide to the Human Proteome. Database 2016, 2016, bav120. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, M.; Wang, J.; Pan, Y.; Wu, F.X. CytoNCA: A Cytoscape Plugin for Centrality Analysis and Evaluation of Protein Interaction Networks. BioSystems 2015, 127, 67–72. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. CytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Johnston, R.C.; Yao, K.; Kaplan, Z.; Chelliah, M.; Leswing, K.; Seekins, S.; Watts, S.; Calkins, D.; Chief Elk, J.; Jerome, S.V.; et al. Epik: PKa and Protonation State Prediction through Machine Learning. J. Chem. Theory Comput. 2023, 19, 2380–2388. [Google Scholar] [CrossRef]
- Oduro-Kwateng, E.; Ali, M.; Kehinde, I.O.; Zhang, Z.; Soliman, M.E.S. De Novo Rational Design of Peptide-Based Protein–Protein Inhibitors (Pep-PPIs) Approach by Mapping the Interaction Motifs of the PP Interface and Physicochemical Filtration: A Case on P25-Cdk5-Mediated Neurodegenerative Diseases. J. Cell. Biochem. 2024, 125, e30633. [Google Scholar] [CrossRef]
- Oduro-Kwateng, E.; Kehinde, I.O.; Ali, M.; Kasumbwe, K.; Mzozoyana, V.; Parinandi, N.L.; Soliman, M.E.S. Computational Analysis of Plasmodium Falciparum DNA Damage Inducible Protein 1 (PfDdi1): Insights into Binding of Artemisinin and Its Derivatives and Implications for Antimalarial Drug Design. Cell Biochem. Biophys. 2025, 83, 3277–3297. [Google Scholar] [CrossRef]
- Salmon-Ferrer, R.; Goetz, A.W.; Poole, D.; Le Grand, S.; Walker, R.C. Routine Microsecond Molecular Dynamics Simulations with AMBER—Part II: Particle Mesh Ewald. J. Chem. Theory Comput. 2013, 9, 3878–3888. [Google Scholar] [CrossRef]
- Mark, P.; Nilsson, L. Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E., III. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Sun, H.; Wang, J.; Wang, Z.; Liu, H.; Zhang, J.Z.H.; Hou, T. End-Point Binding Free Energy Calculation with MM/PBSA and MM/GBSA: Strategies and Applications in Drug Design. Chem. Rev. 2019, 119, 9478–9508. [Google Scholar] [CrossRef]
- Miller, B.R.; McGee, T.D.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.Py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.; Rosen, N.; Twik, M.; BarShir, R.; Stein, T.I.; Dahary, D.; Fishilevich, S.; Lancet, D. The GeneCards Suite. In Practical Guide to Life Science Databases; Springer Nature: Singapore, 2022. [Google Scholar]
- Mu, W.; Patankar, V.; Kitchen, S.; Zhen, A. Examining Chronic Inflammation, Immune Metabolism, and T Cell Dysfunction in HIV Infection. Viruses 2024, 16, 219. [Google Scholar] [CrossRef]
- Moezpoor, M.R.; Stevenson, M. Help or Hinder: Protein Host Factors That Impact HIV-1 Replication. Viruses 2024, 16, 1281. [Google Scholar] [CrossRef]
- Hunt, P.W. Th17, Gut, and HIV: Therapeutic Implications. Curr. Opin. HIV AIDS 2010, 5, 189–193. [Google Scholar] [CrossRef]
- Khader, S.A.; Gaffen, S.L.; Kolls, J.K. Th17 Cells at the Crossroads of Innate and Adaptive Immunity against Infectious Diseases at the Mucosa. Mucosal Immunol. 2009, 2, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Renault, C.; Veyrenche, N.; Mennechet, F.; Bedin, A.S.; Routy, J.P.; Van de Perre, P.; Reynes, J.; Tuaillon, E. Th17 CD4+ T-Cell as a Preferential Target for HIV Reservoirs. Front. Immunol. 2022, 13, 822576. [Google Scholar] [CrossRef]
- Elhed, A.; Unutmaz, D. Th17 Cells and HIV Infection. Curr. Opin. HIV AIDS 2010, 5, 146–150. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Steward-Tharp, S.M.; Laurence, A.; Watford, W.T.; Wei, L.; Adamson, A.S.; Fan, S. Signal Transduction and Th17 Cell Differentiation. Microbes Infect. 2009, 11, 599–611. [Google Scholar] [CrossRef]
- Waickman, A.T.; Powell, J.D. MTOR, Metabolism, and the Regulation of T-Cell Differentiation and Function. Immunol. Rev. 2012, 249, 43–58. [Google Scholar] [CrossRef]
- Dang, E.V.; Barbi, J.; Yang, H.Y.; Jinasena, D.; Yu, H.; Zheng, Y.; Bordman, Z.; Fu, J.; Kim, Y.; Yen, H.R.; et al. Control of TH17/Treg Balance by Hypoxia-Inducible Factor 1. Cell 2011, 146, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Daniels, M.A.; Teixeiro, E. The NF-ΚB Signaling Network in the Life of T Cells. Front. Immunol. 2025, 16, 1559494. [Google Scholar] [CrossRef]
- Haase, M.; Fitze, G. HSP90AB1: Helping the Good and the Bad. Gene 2016, 575, 171–186. [Google Scholar] [CrossRef]
- Tukaj, S.; Zillikens, D.; Kasperkiewicz, M. Inhibitory Effects of Heat Shock Protein 90 Blockade on Proinflammatory Human Th1 and Th17 Cell Subpopulations. J. Inflamm. 2014, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, H.H.; Elfaki, E. The Immunomodulatory Role of Withania somnifera (L.) Dunal in Inflammatory Diseases. Front. Pharmacol. 2023, 14, 1084757. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, K.D.; Venkatesha, S.H. The Anti-Inflammatory and Immunomodulatory Activities of Natural Products to Control Autoimmune Inflammation. Int. J. Mol. Sci. 2023, 24, 95. [Google Scholar] [CrossRef]
- Butterfield, T.R.; Landay, A.L.; Anzinger, J.J. Dysfunctional Immunometabolism in HIV Infection: Contributing Factors and Implications for Age-Related Comorbid Diseases. Curr. HIV/AIDS Rep. 2020, 17, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Lisco, A.; Vanpouille, C.; Margolis, L. Coinfecting Viruses as Determinants of HIV Disease. Curr. HIV/AIDS Rep. 2009, 6, 5–12. [Google Scholar] [CrossRef]
- Anampa, J.; Barta, S.K.; Haigentz, M.; Sparano, J.A. Human Immunodeficiency Virus (HIV) Infection and Cancer. In Abeloff’s Clinical Oncology; IntechOpen: London, UK, 2019. [Google Scholar]
- Lin, X.; Song, B.; Cao, L.; Zhang, L.; Liu, S.; Wang, X.; Chen, X.; Li, S. PD-1 Suppression Enhances HIV Reactivation and T-Cell Immunity via MAPK/NF-ΚB Signaling. Eur. J. Med. Res. 2025, 30, 237. [Google Scholar] [CrossRef]
- Wang, F.; Cui, Y.; Shen, X.; Wang, S.; Yang, G.B. IL-17A and IL-17F Repair HIV-1 Gp140 Damaged Caco-2 Cell Barriers by Upregulating Tight Junction Genes. Microbes Infect. 2019, 21, 393–400. [Google Scholar] [CrossRef]
- Noorsaeed, S.; AlBurtamani, N.; Rokan, A.; Fassati, A. Heat Shock Protein 90 Is a Chaperone Regulator of HIV-1 Latency. PLoS Pathog. 2025, 21, e1012524. [Google Scholar] [CrossRef]
- Mbonye, U.; Karn, J. The Cell Biology of HIV-1 Latency and Rebound. Retrovirology 2024, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, Q.; Tan, L.; Xu, Y.; Xie, X.; Zhao, Y. The Regulatory Effects of MTOR Complexes in the Differentiation and Function of CD4+ T Cell Subsets. J. Immunol. Res. 2020, 2020, 3406032. [Google Scholar] [CrossRef]
- Aiken, C.; Chen, C.H. Betulinic Acid Derivatives as HIV-1 Antivirals. Trends Mol. Med. 2005, 11, 31–36. [Google Scholar] [CrossRef]
- Waki, K.; Durell, S.R.; Soheilian, F.; Nagashima, K.; Butler, S.L.; Freed, E.O. Structural and Functional Insights into the HIV-1 Maturation Inhibitor Binding Pocket. PLoS Pathog. 2012, 8, e1002997. [Google Scholar] [CrossRef]
- Saisin, S.; Panthong, K.; Hongthong, S.; Kuhakarn, C.; Thanasansurapong, S.; Chairoungdua, A.; Suksen, K.; Akkarawongsapat, R.; Napaswad, C.; Prabpai, S.; et al. Pyranonaphthoquinones and Naphthoquinones from the Stem Bark of Ventilago Harmandiana and Their Anti-HIV-1 Activity. J. Nat. Prod. 2023, 86, 498–507. [Google Scholar] [CrossRef]
- Tu, N.Q.; Richetta, C.; Putzu, F.; Delelis, O.; Ahmed, K.; Masand, V.H.; Schobert, R.; Tramontano, E.; Corona, A.; Biersack, B. Identification of HIV-1 Reverse Transcriptase-Associated Ribonuclease H Inhibitors Based on 2-Hydroxy-1,4-Naphthoquinone Mannich Bases. Molecules 2025, 30, 495. [Google Scholar] [CrossRef]
- Crosby, I.T.; Bourke, D.G.; Jones, E.D.; De Bruyn, P.J.; Rhodes, D.; Vandegraaff, N.; Cox, S.; Coates, J.A.V.; Robertson, A.D. Antiviral Agents 2. Synthesis of Trimeric Naphthoquinone Analogues of Conocurvone and Their Antiviral Evaluation against HIV. Bioorg. Med. Chem. 2010, 18, 6442–6450. [Google Scholar] [CrossRef]
- Sandur, S.K.; Ichikawa, H.; Sethi, G.; Kwang, S.A.; Aggarwal, B.B. Plumbagin (5-Hydroxy-2-Methyl-1,4-Naphthoquinone) Suppresses NF-ΚB Activation and NF-ΚB-Regulated Gene Products through Modulation of P65 and IκBα Kinase Activation, Leading to Potentiation of Apoptosis Induced by Cytokine and Chemotherapeutic Agents. J. Biol. Chem. 2006, 281, 17023–17033. [Google Scholar] [CrossRef]
- Andújar, I.; Recio, M.C.; Bacelli, T.; Giner, R.M.; Ríos, J.L. Shikonin Reduces Oedema Induced by Phorbol Ester by Interfering with IκBα Degradation Thus Inhibiting Translocation of NF-ΚB to the Nucleus. Br. J. Pharmacol. 2010, 160, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dai, X.; Wang, E. Plumbagin Inhibits Cell Proliferation and Promotes Apoptosis in Multiple Myeloma Cells through Inhibition of the PI3K/Akt-MTOR Pathway. Oncol. Lett. 2016, 12, 3614–3618. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, B.B.; Jamal, M.S.; Fischer, J.W.; Mustafa, A.; Verma, A.K. Plumbagin, a Plant Derived Natural Agent Inhibits the Growth of Pancreatic Cancer Cells in in Vitro and in Vivo via Targeting EGFR, Stat3 and NF-ΚB Signaling Pathways. Int. J. Cancer 2012, 131, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Kitson, R.R.A.; Kitsonová, D.; Siegel, D.; Ross, D.; Moody, C.J. Geldanamycin, a Naturally Occurring Inhibitor of Hsp90 and a Lead Compound for Medicinal Chemistry. J. Med. Chem. 2024, 67, 17946–17963. [Google Scholar] [CrossRef] [PubMed]
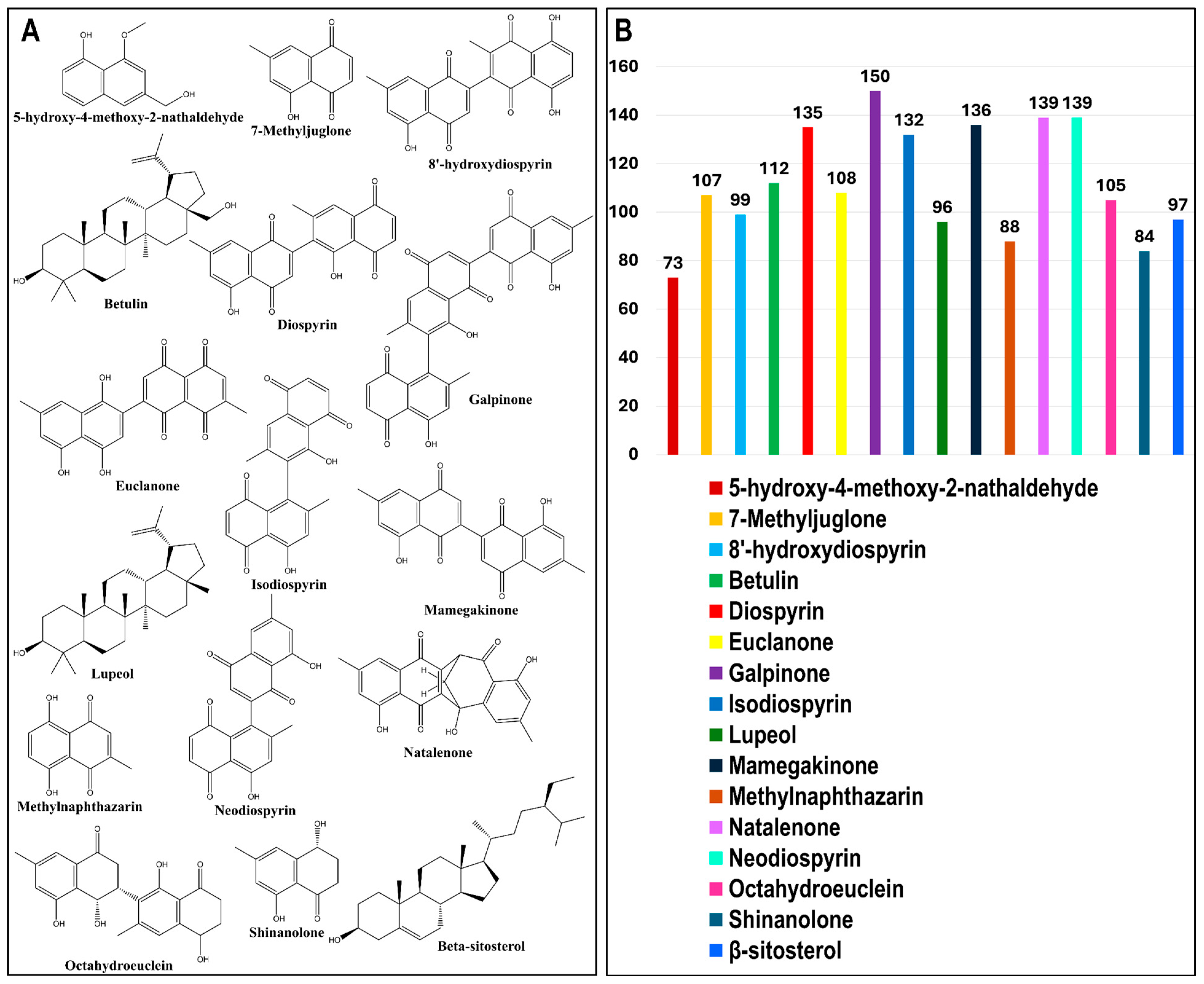
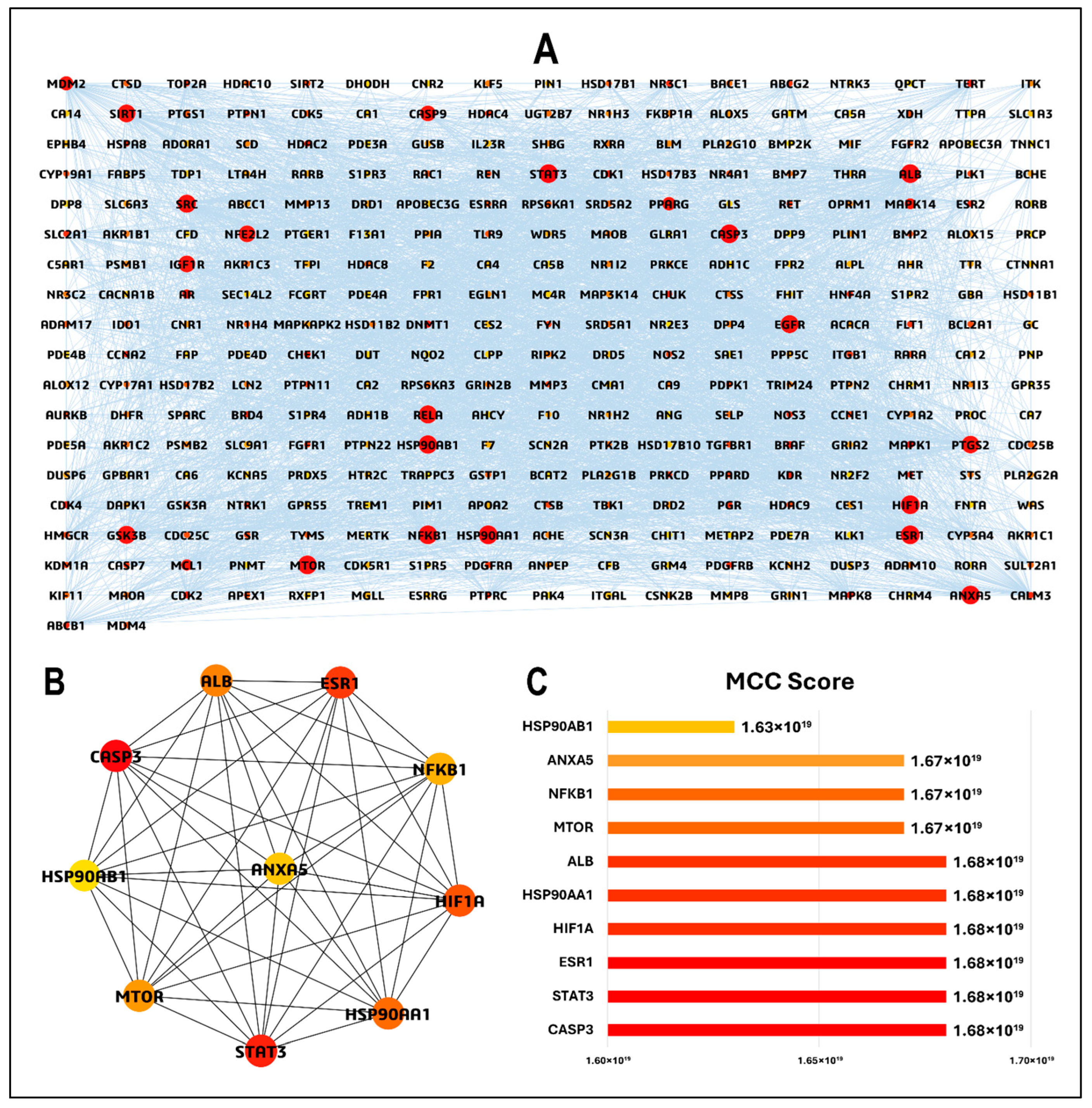
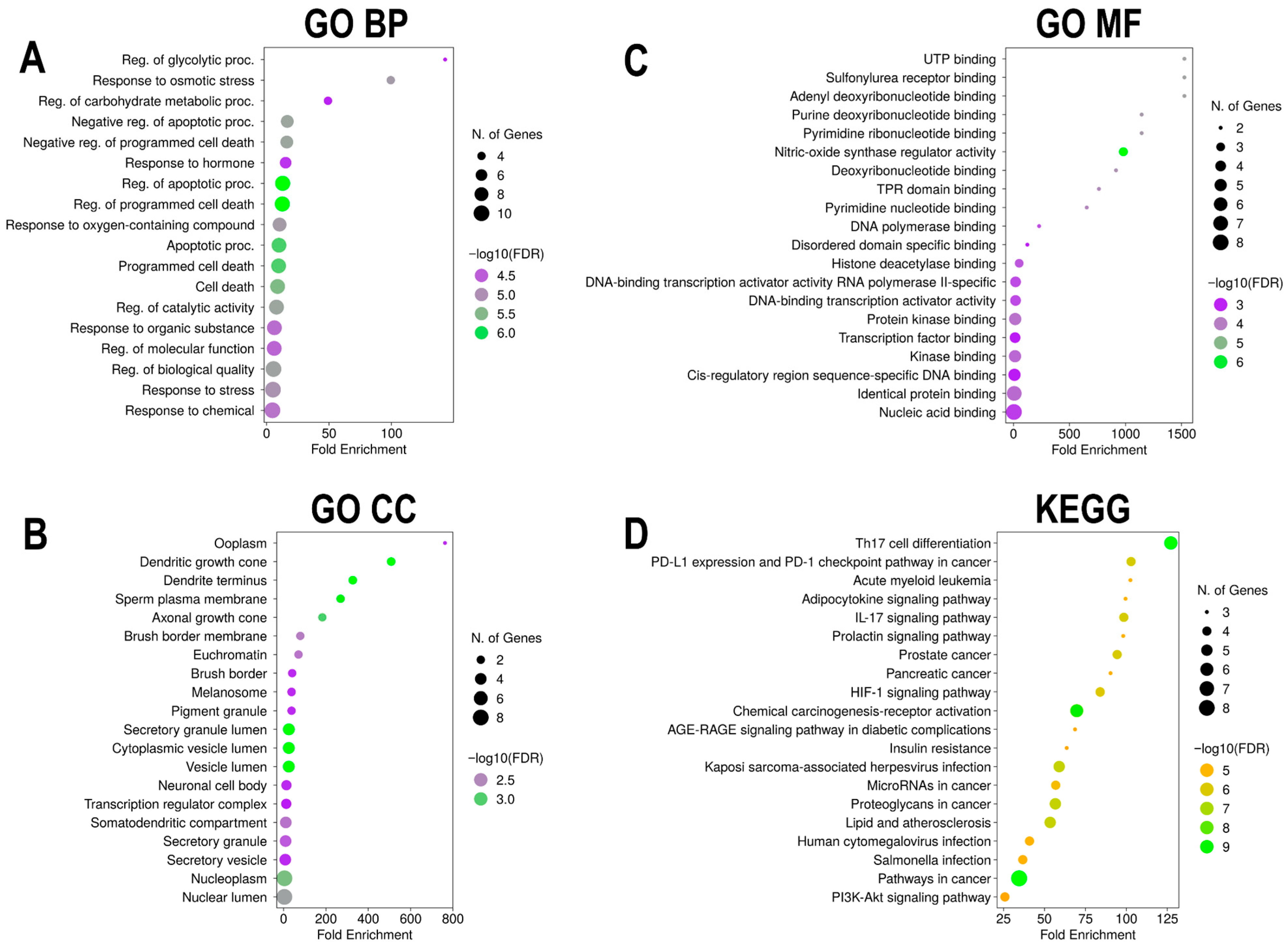
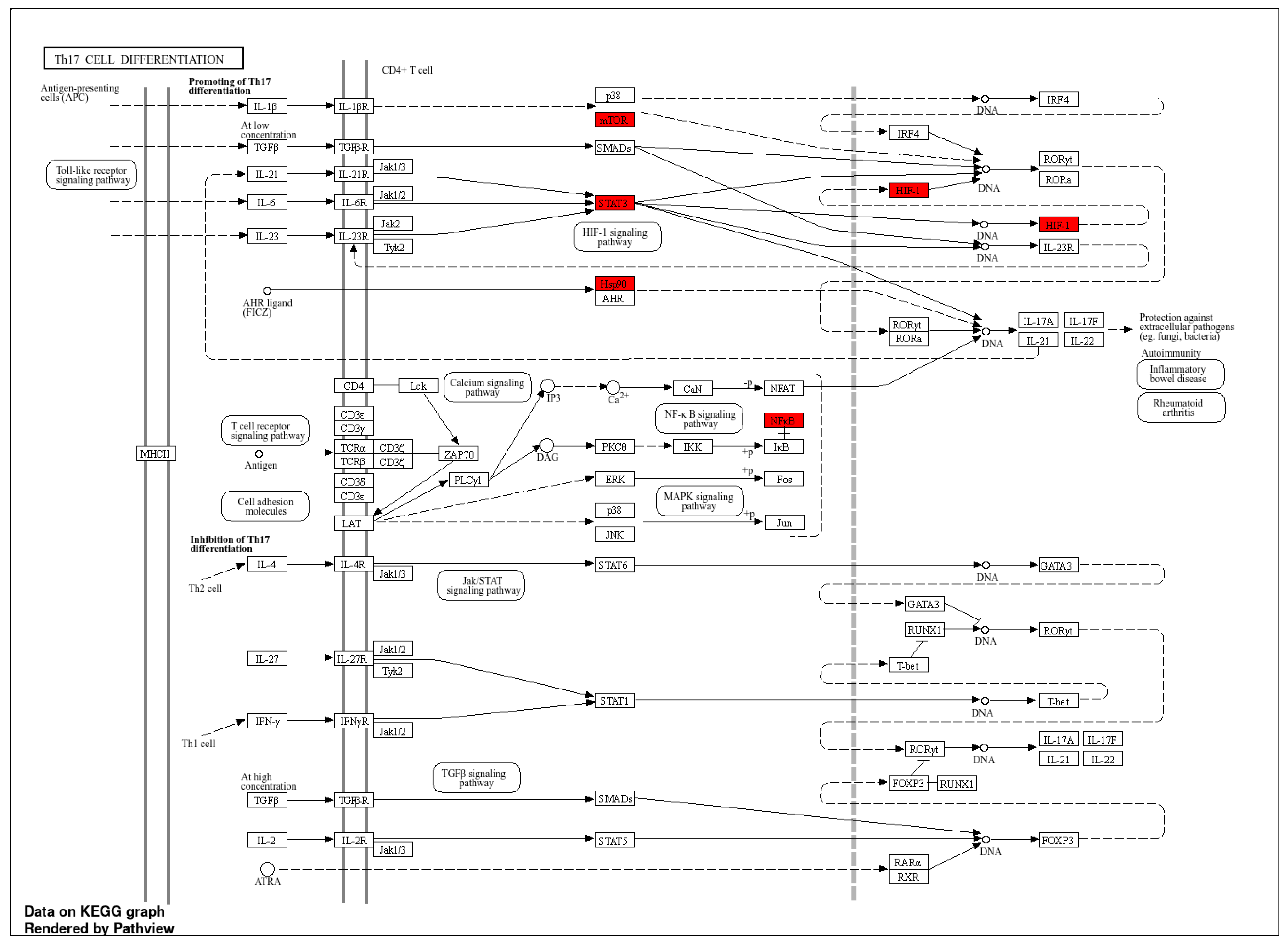
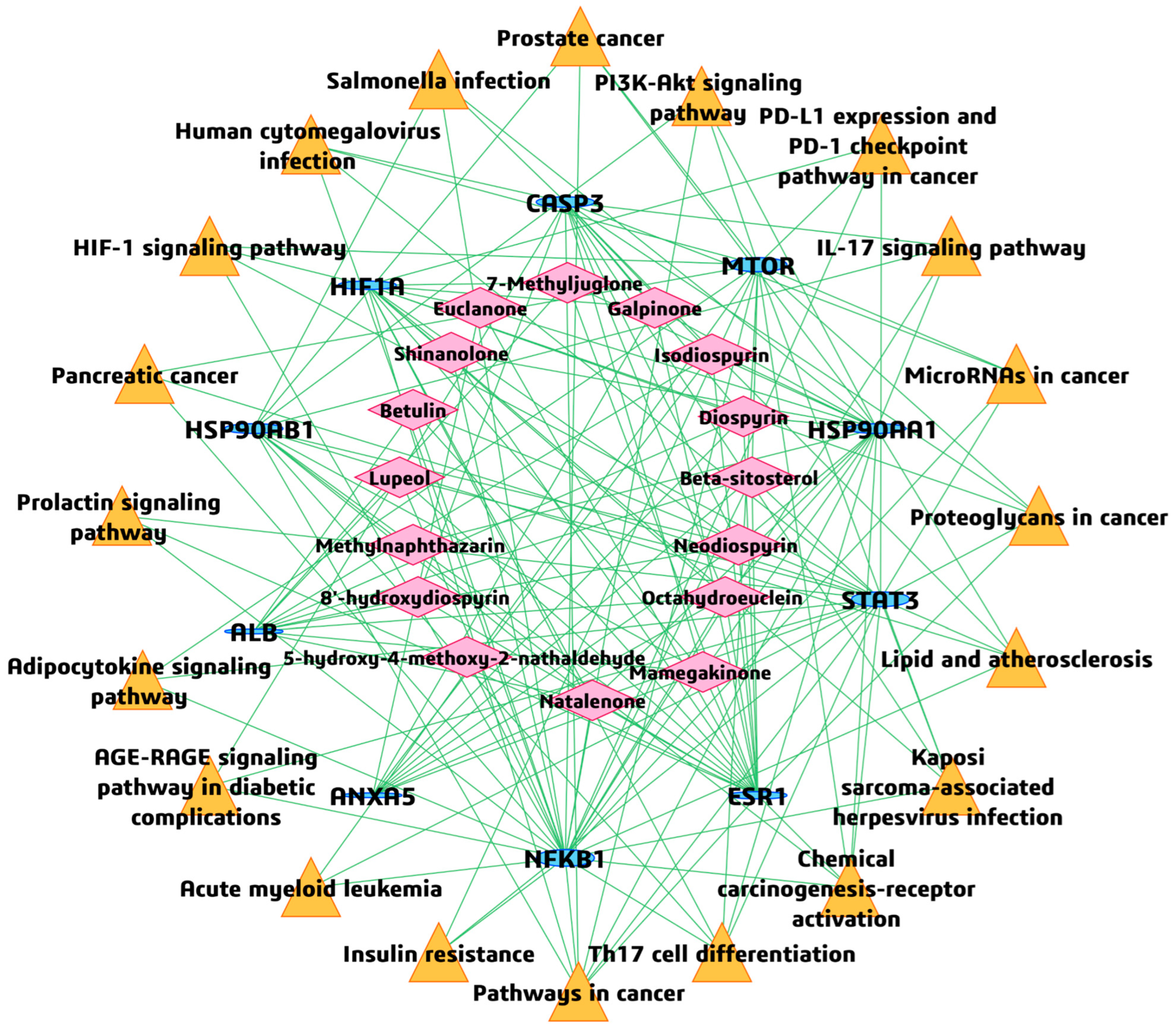
| Phytochemical | Canonical SMILES | PubChem CID | MF | BS | RO5 |
|---|---|---|---|---|---|
| 5-hydroxy-4-methoxy-2-nathaldehyde | COC1=CC(CO)=CC2=C1C(O)=CC=C2 | — | C12H12O3 | 0.55 | 0 |
| 7-Methyljuglone | CC1=CC2=C(C(=O)C=CC2=O)C(=C1)O | 26905 | C11H8O3 | 0.55 | 0 |
| 8′-hydroxydiospyrin | CC1=CC2=C(C(O)=C1)C(=O)C=C(C2=O)C1=C(C)C(=O)C2=C(C(O)=CC=C2O)C1=O | — | C22H14O7 | 0.55 | 0 |
| Betulin | CC(=C)[C@@H]1CC[C@]2([C@H]1[C@H]3CC[C@@H]4[C@]5(CC[C@@H](C([C@@H]5CC[C@]4([C@@]3(CC2)C)C)(C)C)O)C)CO | 72326 | C30H50O2 | 0.55 | 1 |
| Diospyrin | CC1=CC2=C(C(=C1)O)C(=O)C=C(C2=O)C3=C(C4=C(C=C3C)C(=O)C=CC4=O)O | 308140 | C22H14O6 | 0.55 | 0 |
| Euclanone | CC1=CC2=C(C(=CC(=C2C(=C1)O)O)C3=CC(=O)C4=C(C3=O)C(=O)C(=CC4=O)C)O | 633717 | C22H14O7 | 0.55 | 0 |
| Galpinone | CC1=CC2=C(C(=C1)O)C(=O)C(=CC2=O)C3=CC(=O)C4=C(C3=O)C(=C(C(=C4)C)C5=C6C(=O)C=CC(=O)C6=C(C=C5C)O)O | 635975 | C33H20O9 | 0.55 | 1 |
| Isodiospyrin | CC1=CC2=C(C(=O)C=CC2=O)C(=C1C3=C4C(=O)C=CC(=O)C4=C(C=C3C)O)O | 99298 | C22H14O6 | 0.55 | 0 |
| Lupeol | CC(=C)[C@@H]1CC[C@]2([C@H]1[C@H]3CC[C@@H]4[C@]5(CC[C@@H](C([C@@H]5CC[C@]4([C@@]3(CC2)C)C)(C)C)O)C)C | 259846 | C30H50O | 0.55 | 1 |
| Mamegakinone | CC1=CC2=C(C(=C1)O)C(=O)C(=CC2=O)C3=CC(=O)C4=C(C3=O)C(=CC(=C4)C)O | 167673 | C22H14O6 | 0.55 | 0 |
| Methylnaphthazarin | CC1=CC(=O)C2=C(C=CC(=C2C1=O)O)O | 271296 | C11H8O4 | 0.55 | 0 |
| Natalenone | [H]C1([H])C2C3=C(C(=O)C4=C(O)C=C(C)C=C4C3=O)C1(O)C1=C(C(O)=CC(C)=C1)C2=O | — | C22H16O6 | 0.55 | 0 |
| Neodiospyrin | CC1=CC2=C(C(=C1)O)C(=O)C(=CC2=O)C3=C4C(=O)C=CC(=O)C4=C(C=C3C)O | 16072922 | C22H14O6 | 0.55 | 0 |
| Octahydroeuclein | CC1=CC2=C([C@H]([C@H](CC2=O)C3=C(C4=C(C=C3C)C(CCC4=O)O)O)O)C(=C1)O | 5273355 | C22H22O6 | 0.55 | 0 |
| Shinanolone | CC1=CC2=C(C(=O)CC[C@H]2O)C(=C1)O | 5273357 | C11H12O3 | 0.55 | 0 |
| β-sitosterol | CC[C@H](CC[C@@H](C)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CC=C4[C@@]3(CC[C@@H](C4)O)C)C)C(C)C | 222284 | C29H50O | 0.55 | 1 |
| Phytochemical | DC | BC | CC | |
|---|---|---|---|---|
| 1 | Galpinone | 150 | 12,264.043 | 0.490 |
| 2 | Neodiospyrin | 139 | 7917.464 | 0.475 |
| 3 | Natalenone | 139 | 17,033.264 | 0.475 |
| 4 | Mamegakinone | 136 | 8877.140 | 0.471 |
| 5 | Diospyrin | 135 | 7291.925 | 0.469 |
| 6 | Isodiospyrin | 132 | 6207.231 | 0.465 |
| 7 | Betulin | 112 | 16,674.690 | 0.440 |
| 8 | Euclanone | 108 | 7403.342 | 0.436 |
| 9 | 7-Methyljuglone | 107 | 4261.511 | 0.434 |
| 10 | Octahydroeuclein | 105 | 7981.093 | 0.432 |
| 11 | 8’-hydroxydiospyrin | 99 | 6062.130 | 0.425 |
| 12 | β-sitosterol | 97 | 10,330.302 | 0.423 |
| 13 | Lupeol | 96 | 9744.878 | 0.422 |
| 14 | Methylnaphthazarin | 88 | 5105.912 | 0.414 |
| 15 | Shinanolone | 84 | 3258.515 | 0.409 |
| 16 | 5-hydroxy-4-methoxy-2-nathaldehyde | 73 | 8500.559 | 0.399 |
| Ligand | Docking Score (kcal/mol) | |||||||
|---|---|---|---|---|---|---|---|---|
| NFκβ1 | STAT3 | ESR1 | HSP90AA1 | MTOR | CASP3 | HIF1A | HSP90AB1 | |
| Natalenone | −7.4 | −6.8 | −5.7 | −10.8 | −8.0 | −8.5 | −8.8 | −10.5 |
| Mamegakinone | −7.2 | −7.4 | −6.0 | −9.0 | −9.5 | −8.7 | −8.7 | −8.8 |
| Octahydroeuclein | −6.6 | −6.8 | −5.9 | −9.8 | −8.7 | −7.6 | −7.9 | −9.5 |
| Neodiospyrin | −7.1 | −7.3 | −7.3 | −10.8 | −8.6 | −8.5 | −8.6 | −10.4 |
| β-sitosterol | −6.1 | −5.9 | −6.7 | −9.7 | −8.1 | −7.5 | −8.3 | −9.5 |
| Diospyrin | −7.6 | −6.8 | −5.4 | −11.6 | −9.4 | −8.3 | −9.1 | −11.3 |
| Isodiospyrin | −7.4 | −6.9 | −9.5 | −10.5 | −8.5 | −8.0 | −8.4 | −9.9 |
| Galpinone | −7.7 | −8.3 | −5.9 | −11.3 | −10.3 | −9.2 | −10.3 | −10.8 |
| Co-crystallized | - | SI-109 | Estradiol | PU-11 | Torin 2 | MSI | - | PU-11 |
| −9.8 | −10.6 | −8.1 | −11.6 | −8.5 | −8.0 | |||
| Reference | IMD 0354 | - | - | - | - | - | 2-ME2 | - |
| −6.5 | −7.7 | |||||||
| Protein–Ligand System | Energy Components (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|
| ∆EvdW | ∆Eelec | ∆Ggas | ∆GGB | ∆GSA | ∆Gsolv | ∆Gbind | |
| HSP90AA1–Diospyrin | −42.38 ±0.10 | −10.48 ±0.28 | −52.85 ±0.27 | 28.12 ±0.21 | −5.03 ±0.01 | 23.09 ±0.21 | −29.76 ±0.11 |
| HSP90AA1–PU-11 | −46.52 ±0.09 | −20.06 ±0.11 | −66.59 ±0.13 | 33.58 ±0.09 | −5.90 ±0.01 | 27.67 ±0.08 | −38.91 ±0.09 |
| HSP90AB1–Diospyrin | −44.52 ±0.07 | −10.66 ±0.25 | −55.17 ±0.25 | 29.89 ±0.20 | −5.37 ±0.01 | 24.52 ±0.20 | −30.66 ±0.09 |
| HSP90AB1–PU-11 | −45.99 ±0.09 | −25.18 ±0.16 | −71.17 ±0.17 | 36.63 ±0.12 | −5.92 ±0.01 | 30.71 ±0.11 | −40.46 ±0.10 |
| NFκβ1–Galpinone | −47.89 ±0.14 | −30.53 ±0.31 | −78.41 ±0.39 | 56.97 ±0.32 | −5.41 ±0.01 | 51.56 ±0.31 | −26.85 ±0.13 |
| NFκβ1–IMD 0354 | −22.16 ±0.11 | −42.15 ±0.34 | −64.31 ±0.34 | 50.62 ±0.28 | −3.67 ±0.01 | 46.95 ±0.27 | −17.36 ±0.11 |
| MTOR–Galpinone | −44.77 ±0.13 | −24.96 ±0.20 | −69.73 ±0.24 | 45.39 ±0.20 | −4.54 ±0.01 | 40.85 ±0.21 | −28.88 ±0.14 |
| MTOR–Torin 2 | −47.36 ±0.11 | −34.48 ±0.18 | −81.84 ±0.21 | 45.52 ±0.16 | −4.58 ±0.01 | 40.94 ±0.17 | −40.90 ±0.11 |
| STAT3–Galpinone | −32.43 ±0.15 | −10.08 ±0.13 | −42.51 ±0.20 | 24.63 ±0.12 | −4.03 ±0.02 | 20.60 ±0.11 | −21.91 ±0.13 |
| STAT3–SI-109 | −46.35 ±0.18 | −306.50 ±1.2 | −352.85 ±1.2 | 289.84 ±0.99 | −7.00 ±0.01 | 282.84 ±0.99 | −70.01 ±0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oduro-Kwateng, E.; Abo-Dya, N.E.; Soliman, M.E.; Mkhwanazi, N.P. Phytochemicals from Euclea natalensis Modulate Th17 Differentiation, HIV Latency, and Comorbid Pathways: A Systems Pharmacology and Thermodynamic Profiling Approach. Microorganisms 2025, 13, 2150. https://doi.org/10.3390/microorganisms13092150
Oduro-Kwateng E, Abo-Dya NE, Soliman ME, Mkhwanazi NP. Phytochemicals from Euclea natalensis Modulate Th17 Differentiation, HIV Latency, and Comorbid Pathways: A Systems Pharmacology and Thermodynamic Profiling Approach. Microorganisms. 2025; 13(9):2150. https://doi.org/10.3390/microorganisms13092150
Chicago/Turabian StyleOduro-Kwateng, Ernest, Nader E. Abo-Dya, Mahmoud E. Soliman, and Nompumelelo P. Mkhwanazi. 2025. "Phytochemicals from Euclea natalensis Modulate Th17 Differentiation, HIV Latency, and Comorbid Pathways: A Systems Pharmacology and Thermodynamic Profiling Approach" Microorganisms 13, no. 9: 2150. https://doi.org/10.3390/microorganisms13092150
APA StyleOduro-Kwateng, E., Abo-Dya, N. E., Soliman, M. E., & Mkhwanazi, N. P. (2025). Phytochemicals from Euclea natalensis Modulate Th17 Differentiation, HIV Latency, and Comorbid Pathways: A Systems Pharmacology and Thermodynamic Profiling Approach. Microorganisms, 13(9), 2150. https://doi.org/10.3390/microorganisms13092150








