The Ecological Trap: Biodegradable Mulch Film Residue Undermines Soil Fungal Network Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Experimental Design and Treatments
2.3. Sample Collection
2.4. Analysis of Film Degradation and Microplastic Concentration
2.5. Measurement of Soil Properties
2.6. DNA Extraction and Fungal ITS High-Throughput Sequencing
2.7. Bioinformatic and Statistical Analysis
2.8. Co-Occurrence Network Analysis
3. Results
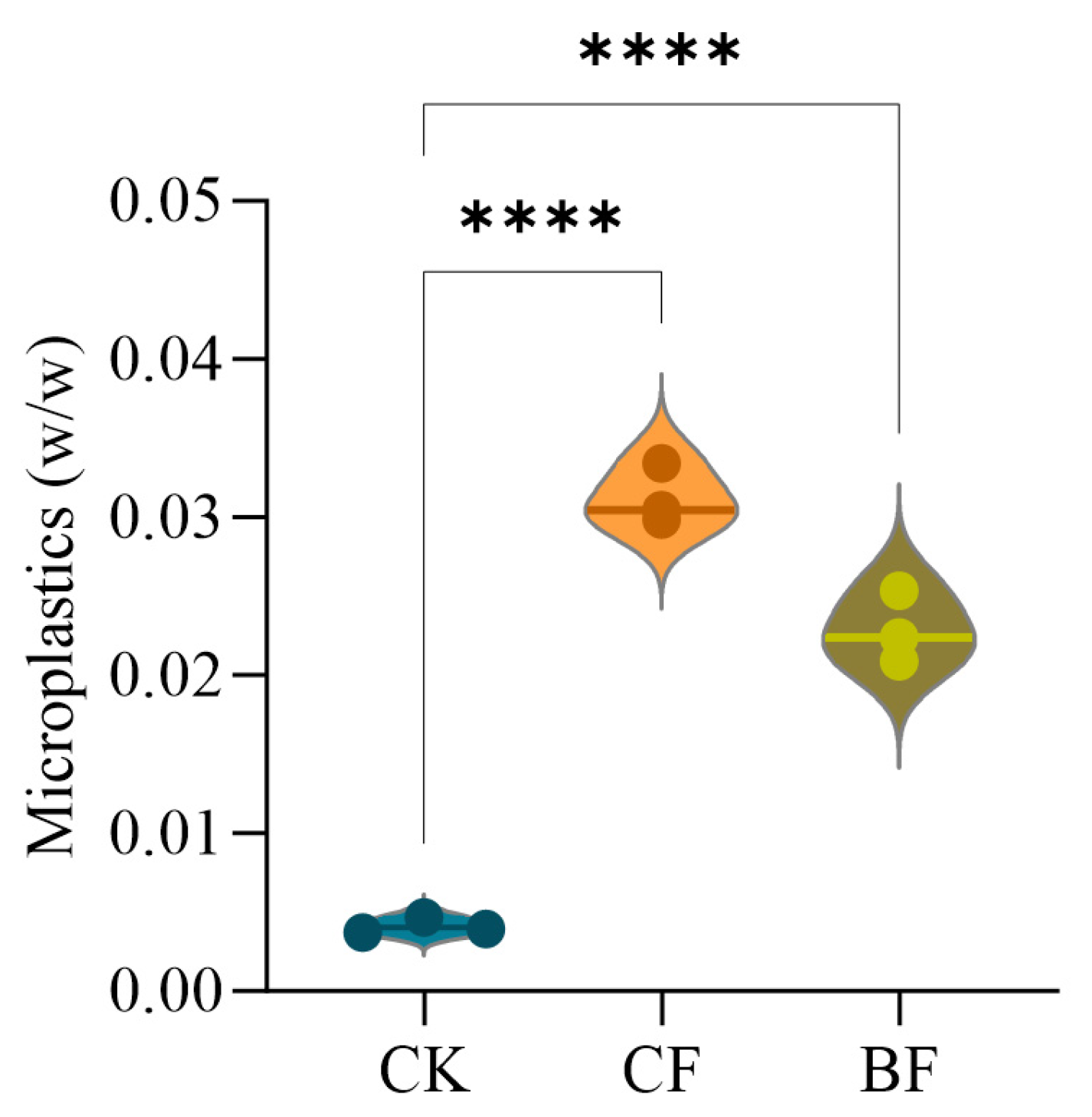
3.1. SEM Analysis of Film Change and Soil Microplastic Concentration
3.2. Differences in Soil Microenvironment Induced by Film Residues
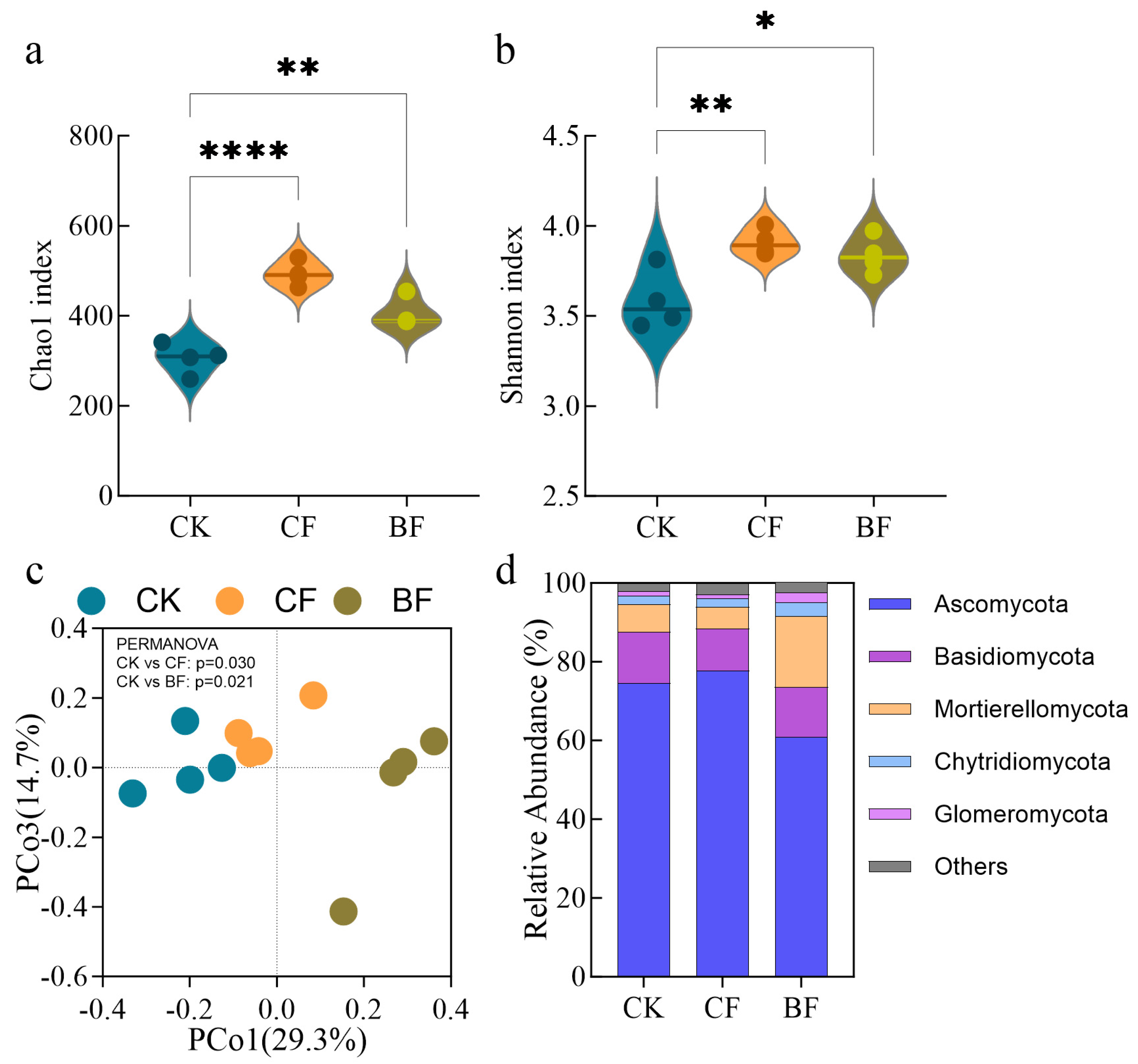
3.3. Response of Rhizosphere Fungal Community to Film Residues
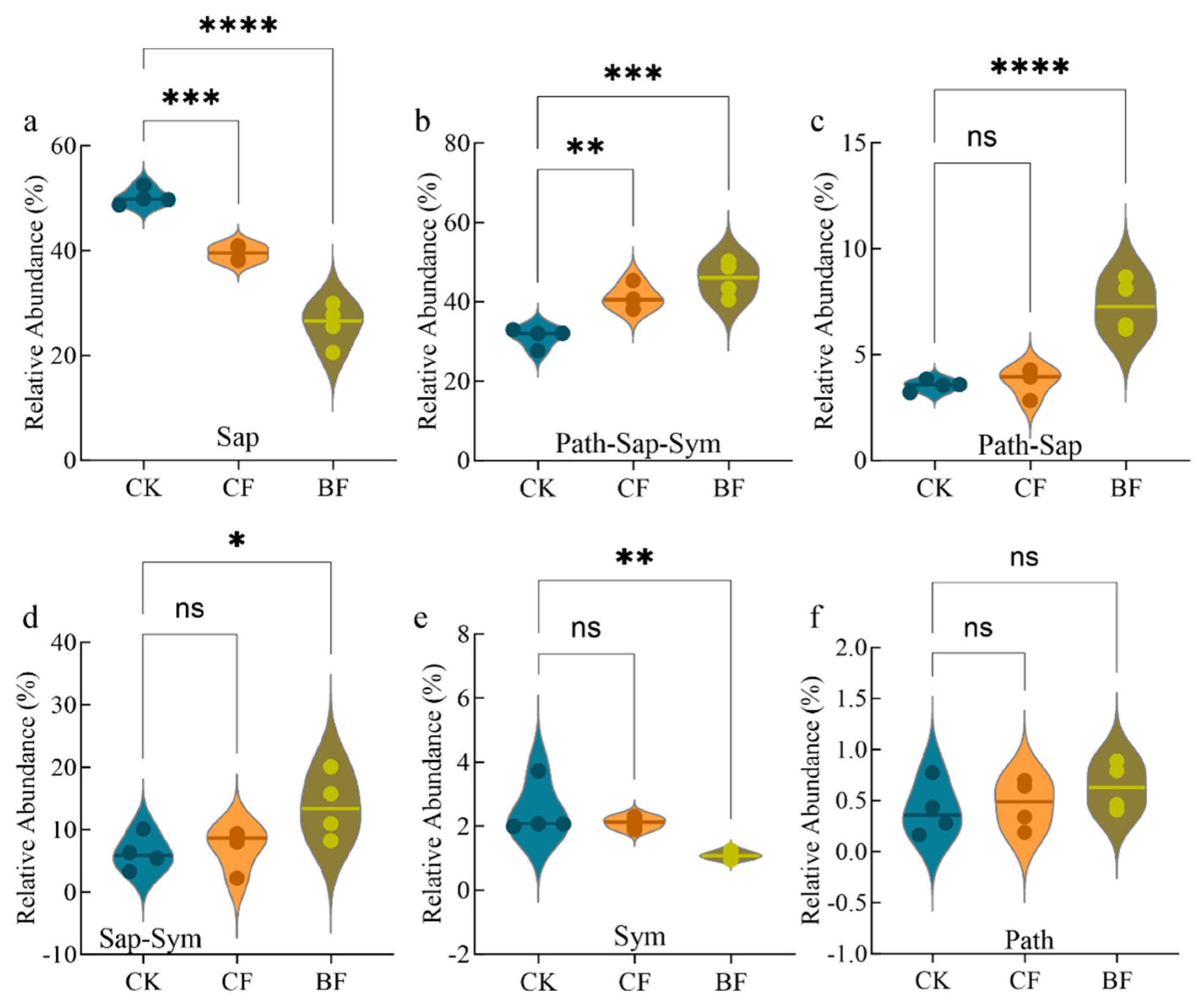
3.4. Differentiated Response of Fungal Functional Guilds in Maize Rhizosphere Soil
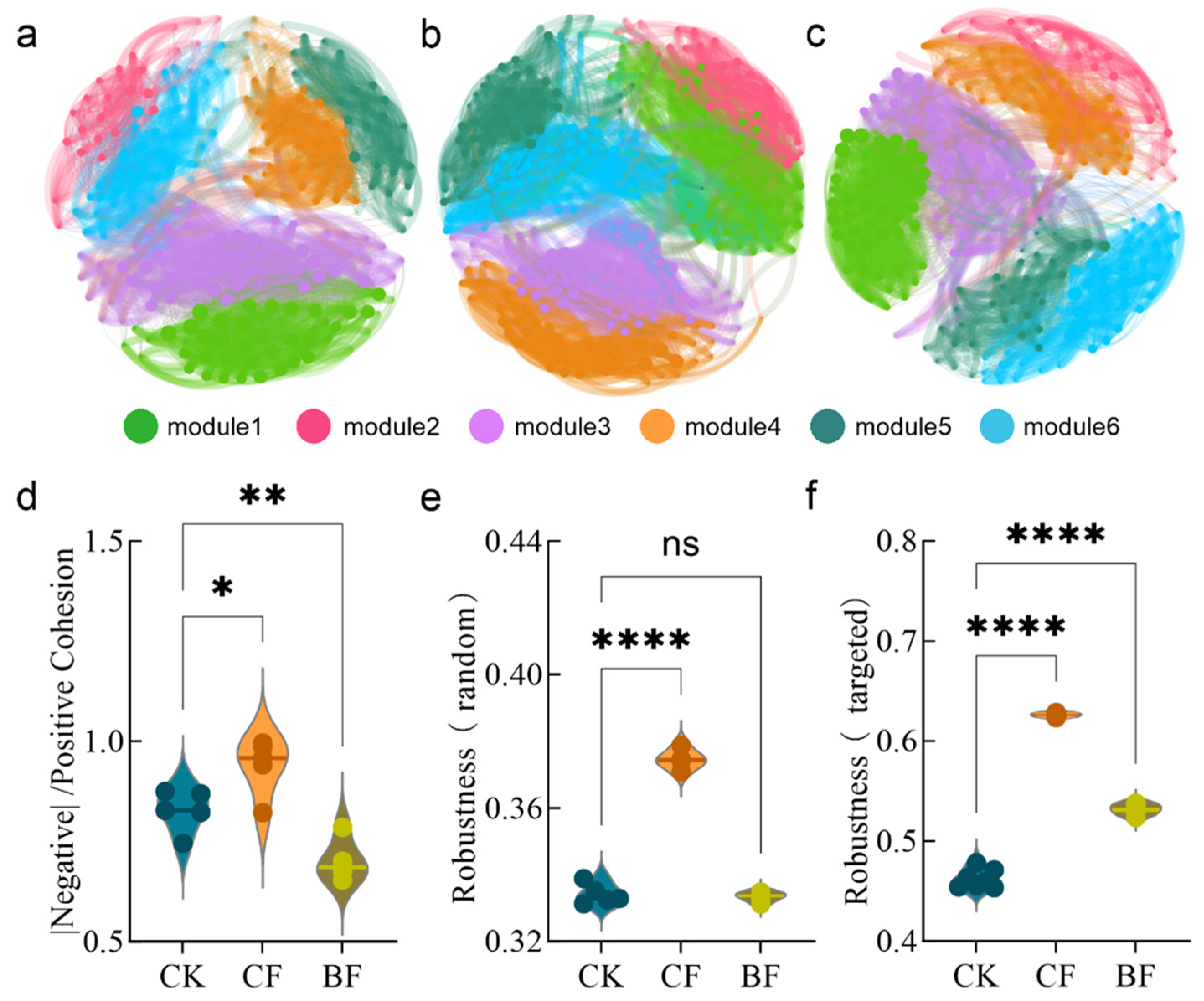
3.5. Ecological Reshaping of Fungal Co-Occurrence Networks by Different Film Residues
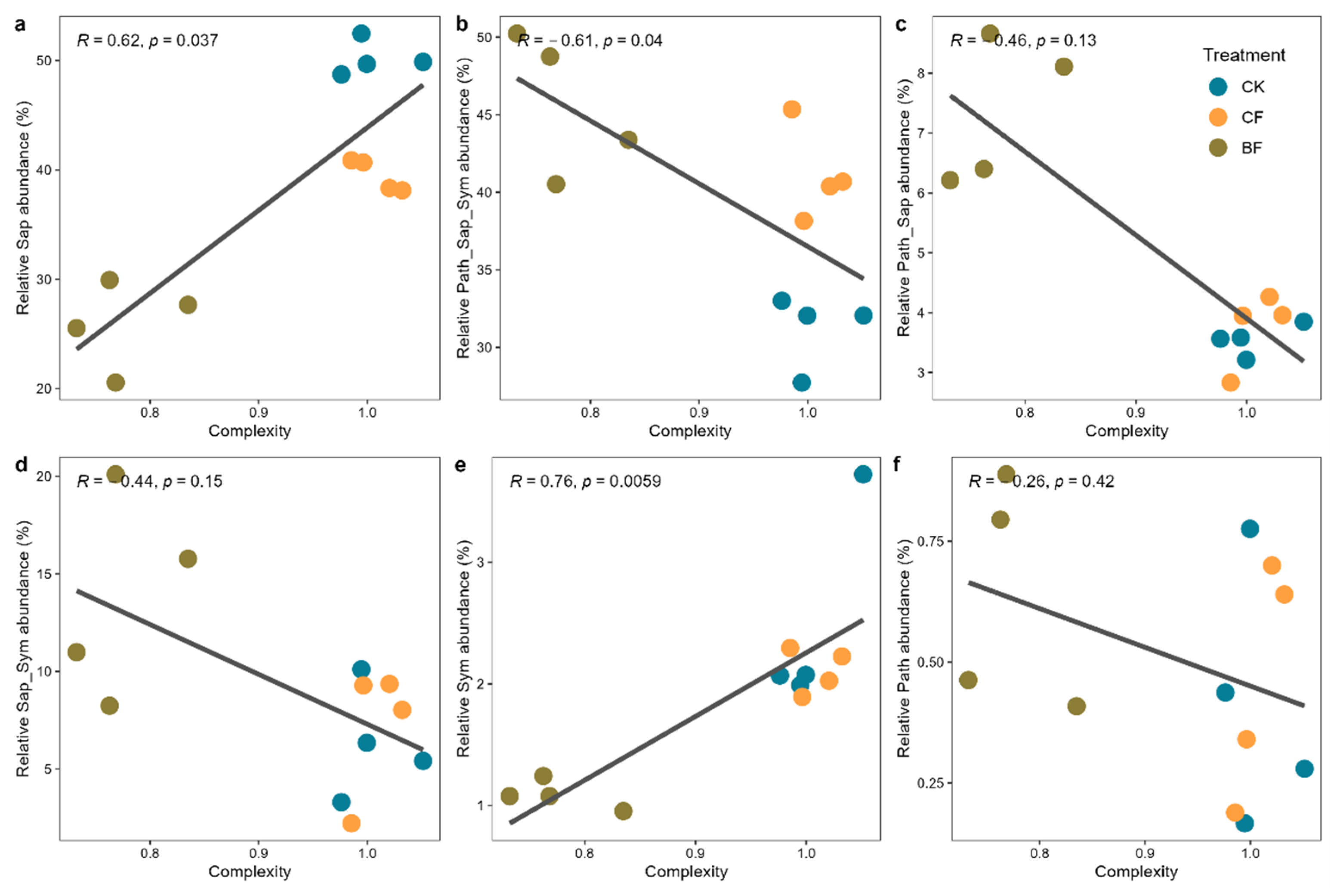
3.6. Correlation Analysis of Fungal Functional Guilds and Network Stability
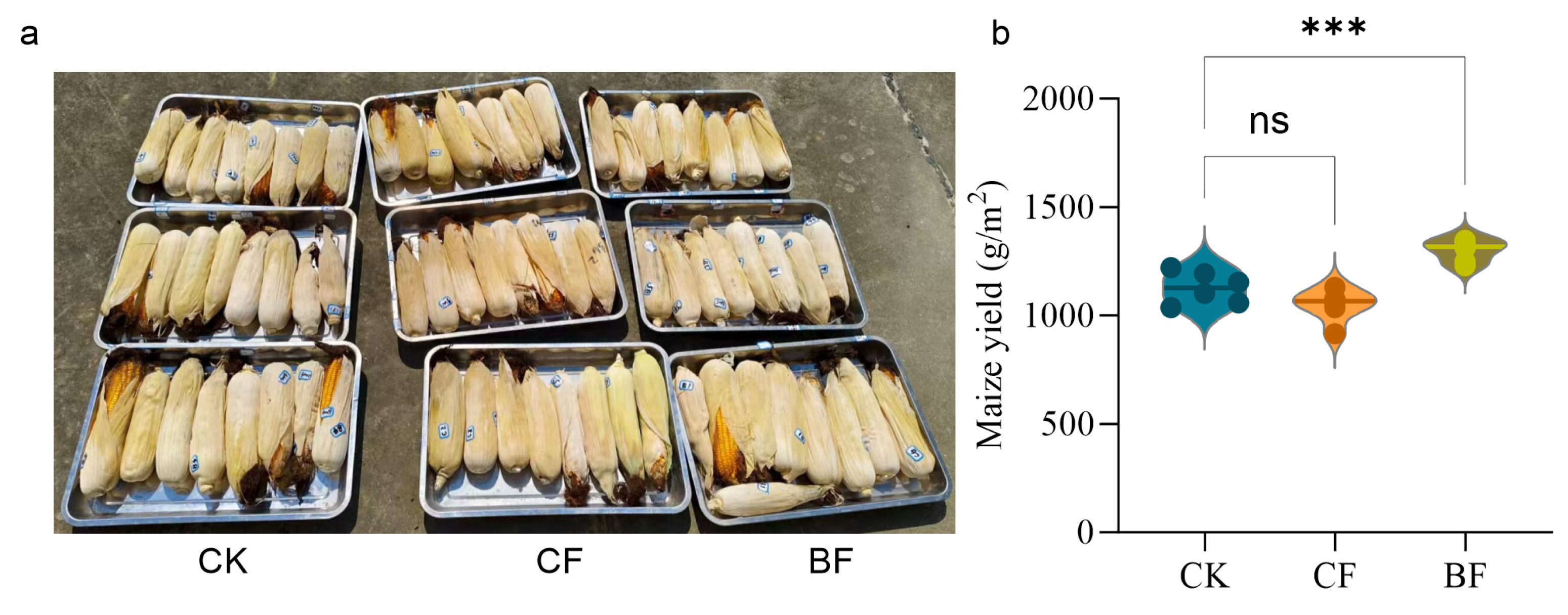
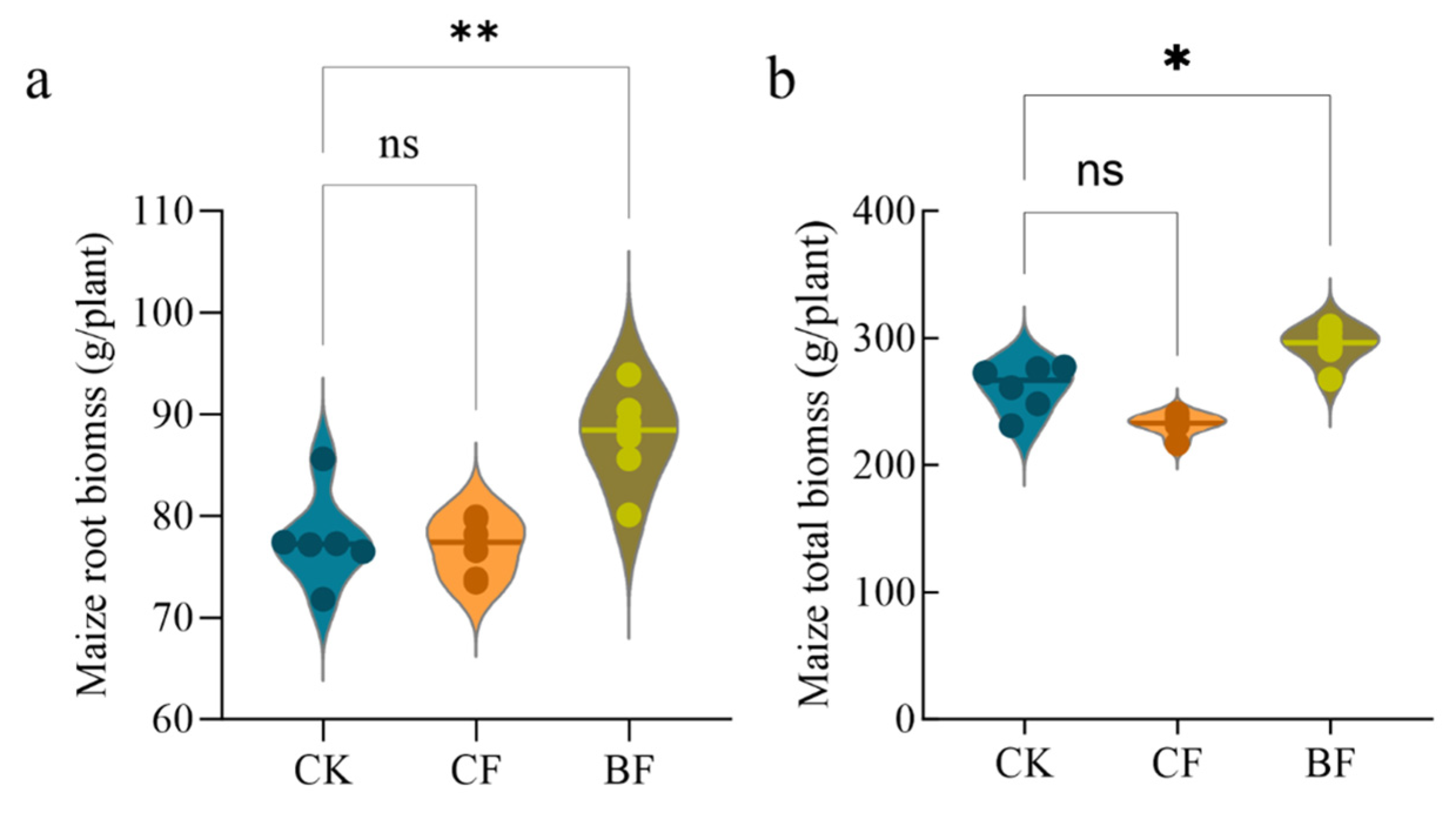
3.7. Differences in Plant Growth Induced by Film Residues
4. Discussion
4.1. Biodegradable Mulch as a Significant Source of Soil Microplastic Pollution
4.2. Impacts of Biodegradable Mulch on Soil Fungal Community Structure and Functional Guilds
4.3. Decreased Stability and Complexity of the Fungal Ecological Network
4.4. An Ecological Trap: The Contradiction Between Short-Term Yield Increase and Long-Term Soil Health Risks
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Huang, T.; Wu, Q.; Yuan, Y.; Zhang, X.; Sun, R.; Hao, R.; Yang, X.; Li, C.; Qin, X.; Song, F.; et al. Effects of plastic film mulching on yield, water use efficiency, and nitrogen use efficiency of different crops in China: A meta-analysis. Field Crops Res. 2024, 312, 109407. [Google Scholar] [CrossRef]
- Sun, D.; Li, H.; Wang, E.; He, W.; Hao, W.; Yan, C.; Li, Y.; Mei, X.; Zhang, Y.; Sun, Z.; et al. An overview of the use of plastic-film mulching in China to increase crop yield and water-use efficiency. Natl. Sci. Rev. 2020, 7, 1523–1526. [Google Scholar] [CrossRef]
- Batista, T.; Cansado, I.P.d.P.; Tita, B.; Ilhéu, A.; Metrogos, L.; Mourão, P.A.M.; Nabais, J.M.V.; Castanheiro, J.E.; Borges, C.; Matos, G. Dealing with Plastic Waste from Agriculture Activity. Agronomy 2022, 12, 134. [Google Scholar] [CrossRef]
- Fu, F.; Long, B.; Huang, Q.; Li, J.; Zhou, W.; Yang, C. Integrated effects of residual plastic films on soil-rhizosphere microbe-plant ecosystem. J. Hazard. Mater. 2023, 445, 130420. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Pan, X.; Zhang, D. The persistently breaking trade-offs of three-decade plastic film mulching: Microplastic pollution, soil degradation and reduced cotton yield. J. Hazard. Mater. 2022, 439, 129586. [Google Scholar] [CrossRef]
- Koskei, K.; Munyasya, A.N.; Wang, Y.-B.; Zhao, Z.-Y.; Zhou, R.; Indoshi, S.N.; Wang, W.; Cheruiyot, W.K.; Mburu, D.M.; Nyende, A.B.; et al. Effects of increased plastic film residues on soil properties and crop productivity in agro-ecosystem. J. Hazard. Mater. 2021, 414, 125521. [Google Scholar] [CrossRef]
- Somanathan, H.; Sathasivam, R.; Sivaram, S.; Mariappan Kumaresan, S.; Muthuraman, M.S.; Park, S.U. An update on polyethylene and biodegradable plastic mulch films and their impact on the environment. Chemosphere 2022, 307, 135839. [Google Scholar] [CrossRef]
- Fan, P.; Yu, H.; Xi, B.; Tan, W. A review on the occurrence and influence of biodegradable microplastics in soil ecosystems: Are biodegradable plastics substitute or threat? Environ. Int. 2022, 163, 107244. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bary, A.I.; Hayes, D.G.; Wadsworth, L.C.; Anunciado, M.B.; English, M.E.; Bandopadhyay, S.; Schaeffer, S.M.; DeBruyn, J.M.; Miles, C.A.; et al. In situ degradation of biodegradable plastic mulch films in compost and agricultural soils. Sci. Total Environ. 2020, 727, 138668. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Q.; Zhang, C.; Zhang, J.; Dong, Z.; Xu, Q. Aging simulation of thin-film plastics in different environments to examine the formation of microplastic. Water Res. 2021, 202, 117462. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Li, Y.; Gao, J.; Cao, R.; Shang, E.; Zhang, W. ROS-mediated photoaging pathways of nano- and micro-plastic particles under UV irradiation. Water Res. 2022, 216, 118320. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic Shape, Polymer Type, and Concentration Affect Soil Properties and Plant Biomass. Front. Plant Sci. 2021, 12, 616645. [Google Scholar] [CrossRef]
- Qi, Y.; Ossowicki, A.; Yang, X.; Huerta Lwanga, E.; Dini-Andreote, F.; Geissen, V.; Garbeva, P. Effects of plastic mulch film residues on wheat rhizosphere and soil properties. J. Hazard. Mater. 2020, 387, 121711. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lu, L.; Sun, Y.; Rillig, M.C.; Peng, Y.; Duan, Z.; Xiao, K.; Adyel, T.M.; Zhu, D.; Ding, J.; et al. A Double-Edged Sword of Biodegradable Microplastics on the Soil Microbial Carbon Pump. Glob. Change Biol. 2025, 31, e70313. [Google Scholar] [CrossRef]
- Zhou, A.; Ji, Q.; Kong, X.; Zhu, F.; Meng, H.; Li, S.; He, H. Response of soil property and microbial community to biodegradable microplastics, conventional microplastics and straw residue. Appl. Soil Ecol. 2024, 196, 105302. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, W.; Liu, J.; Wu, H. Discrepant responses of bacterial community and enzyme activities to conventional and biodegradable microplastics in paddy soil. Sci. Total Environ. 2024, 909, 168513. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, X.; Liu, Y.; Cui, W.; Sun, Y.; Zhang, S.; Wang, F. Effects of microplastics and carbon nanotubes on soil geochemical properties and bacterial communities. J. Hazard. Mater. 2022, 433, 128826. [Google Scholar] [CrossRef]
- Sun, Y.; Duan, C.; Cao, N.; Li, X.; Li, X.; Chen, Y.; Huang, Y.; Wang, J. Effects of microplastics on soil microbiome: The impacts of polymer type, shape, and concentration. Sci. Total Environ. 2022, 806, 150516. [Google Scholar] [CrossRef]
- Song, W.; Wu, H.; Xiang, Z.; Fan, Y.; Wang, S.; Guo, J. Effects of Plastic Mulch Residue on Soil Fungal Communities in Cotton. Agriculture 2024, 14, 1365. [Google Scholar] [CrossRef]
- Yang, J.; Song, K.; Tu, C.; Li, L.; Feng, Y.; Li, R.; Xu, H.; Luo, Y. Distribution and weathering characteristics of microplastics in paddy soils following long-term mulching: A field study in Southwest China. Sci. Total Environ. 2023, 858, 159774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Peng, W.; Duan, C.; Zhu, X.; Wu, H.; Zhang, X.; Fang, L. Microplastics pollution from different plastic mulching years accentuate soil microbial nutrient limitations. Gondwana Res. 2022, 108, 91–101. [Google Scholar] [CrossRef]
- Xing, J.; Wang, X.; Hu, C.; Wang, L.; Xu, Z.; He, X.; Wang, Z.; Zhao, P.; Liu, Q. Effects of residual mulching films with different mulching years on the diversity of soil microbial communities in typical regions. Heliyon 2022, 8, e12180. [Google Scholar] [CrossRef]
- Yi, M.; Zhou, S.; Zhang, L.; Ding, S. The effects of three different microplastics on enzyme activities and microbial communities in soil. Water Environ. Res. 2020, 93, 24–32. [Google Scholar] [CrossRef]
- Hou, J.; Xu, X.; Yu, H.; Xi, B.; Tan, W. Comparing the long-term responses of soil microbial structures and diversities to polyethylene microplastics in different aggregate fractions. Environ. Int. 2021, 149, 106398. [Google Scholar] [CrossRef]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, Z.; Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Effect of Polyvinyl Chloride Microplastics on Bacterial Community and Nutrient Status in Two Agricultural Soils. Bull. Environ. Contam. Toxicol. 2020, 107, 602–609. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Li, K.; Xu, L.; Bai, X.; Zhang, G.; Zhang, M.; Huang, Y. Differential fungal assemblages and functions between the plastisphere of biodegradable and conventional microplastics in farmland. Sci. Total Environ. 2024, 906, 167478. [Google Scholar] [CrossRef]
- Kumari, A.; Dash, M.; Singh, S.K.; Jagadesh, M.; Mathpal, B.; Mishra, P.K.; Pandey, S.K.; Verma, K.K. Soil microbes: A natural solution for mitigating the impact of climate change. Environ. Monit. Assess. 2023, 195, 1436. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Jin, T.; Zhang, H.; Peng, J.; Zuo, N.; Huang, Y.; Han, Y.; Tian, C.; Yang, Y.; Peng, K.; et al. Deciphering the diversity and functions of plastisphere bacterial communities in plastic-mulching croplands of subtropical China. J. Hazard. Mater. 2022, 422, 126865. [Google Scholar] [CrossRef]
- Shi, Z.; Xiong, L.; Liu, T.; Wu, W. Alteration of bacterial communities and co-occurrence networks as a legacy effect upon exposure to polyethylene residues under field environment. J. Hazard. Mater. 2022, 426, 128126. [Google Scholar] [CrossRef]
- Wu, C.; Ma, Y.; Wang, D.; Shan, Y.; Song, X.; Hu, H.; Ren, X.; Ma, X.; Cui, J.; Ma, Y. Integrated microbiology and metabolomics analysis reveal plastic mulch film residue affects soil microorganisms and their metabolic functions. J. Hazard. Mater. 2022, 423, 127258. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, B.; Yang, Y.; Yang, Y.; Jiang, G.; Tian, Y. Effects of biodegradable (PBAT/PLA) and conventional (LDPE) mulch film residues on bacterial communities and metabolic functions in different agricultural soils. J. Hazard. Mater. 2024, 472, 134425. [Google Scholar] [CrossRef]
- Meng, F.; Harkes, P.; Van Steenbrugge, J.J.M.; Geissen, V. Effects of microplastics on common bean rhizosphere bacterial communities. Appl. Soil Ecol. 2023, 181, 104649. [Google Scholar] [CrossRef]
- Liu, L.; Zou, G.; Zuo, Q.; Li, C.; Gu, J.; Kang, L.; Ma, M.; Liang, K.; Liu, D.; Du, L. Soil bacterial community and metabolism showed a more sensitive response to PBAT biodegradable mulch residues than that of LDPE mulch residues. J. Hazard. Mater. 2022, 438, 129507. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zheng, Y.; Sui, X.; Zhang, Z.; Wang, E.; Liu, Y.; Yu, T.; Yang, J.; Wu, Y. Comparative analysis of the effects of conventional and biodegradable plastic mulching films on soil-peanut ecology and soil pollution. Chemosphere 2023, 334, 139044. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sun, Q.; Wei, M.; Xie, M.; Shen, T.; Liu, D. Plastic Film Residue Reshaped Protist Communities and Induced Soil Nutrient Deficiency Under Field Conditions. Agronomy 2025, 15, 419. [Google Scholar] [CrossRef]
- Xie, M.; Wei, M.; Sun, Q.; Wang, G.; Shen, T.; He, X.; Liu, D. Comparative impacts of polyethylene and biodegradable film residues on soil microbial communities and rapeseed performance under field conditions. Front. Microbiol. 2025, 16, 1553807. [Google Scholar] [CrossRef]
- Li, S.; Ding, F.; Flury, M.; Wang, Z.; Xu, L.; Li, S.; Jones, D.L.; Wang, J. Macro- and microplastic accumulation in soil after 32 years of plastic film mulching. Environ. Pollut. 2022, 300, 118945. [Google Scholar] [CrossRef]
- Sun, Q.; Shen, T.; Wei, M.; Xie, M.; Wang, G.; Liu, D. Evaluating the Impact of Traditional and Biodegradable Mulch Film Residues on Heavy Metal Dynamics and Maize Productivity: Insights from Arbuscular Mycorrhizal Fungi Community Analysis. Agronomy 2025, 15, 780. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Kuai, J.; Ullah, S.; Fahad, S.; Zhou, G. Optimization of Nitrogen Rate and Planting Density for Improving Yield, Nitrogen Use Efficiency, and Lodging Resistance in Oilseed Rape. Front. Plant Sci. 2017, 8, 532. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.; Li, J.; Lu, J.; Ren, T.; Cong, R.; Fahad, S.; Li, X. Effects of fertilization on crop production and nutrient-supplying capacity under rice-oilseed rape rotation system. Sci. Rep. 2017, 7, 1270. [Google Scholar] [CrossRef] [PubMed]
- Kakade, A.; Zhang, Q.; Wu, T.; Yang, X.; Mi, J.; Jing, X.; Long, R. An integrated evaluation of potentially toxic elements and microplastics in the highland soils of the northeastern Qinghai-Tibetan Plateau. J. Hazard. Mater. 2025, 489, 137453. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Zeng, F.X.; Jiang, H. Determination of Total Nitrogen in Solid Samples by Two-Step Digestion–Ultraviolet Spectrophotometry Method. Commun. Soil Sci. Plant Anal. 2013, 44, 1080–1091. [Google Scholar] [CrossRef]
- Liu, X.; Wu, D.; Abid, A.A.; Liu, Y.; Zhou, J.; Zhang, Q. Determination of Paddy Soil Ammonia Nitrogen Using Rapid Detection Kit Coupled with Microplate Reader. Toxics 2022, 10, 725. [Google Scholar] [CrossRef]
- Liu, D.; Nishida, M.; Takahashi, T.; Asakawa, S. Transcription of mcrA Gene Decreases Upon Prolonged Non-flooding Period in a Methanogenic Archaeal Community of a Paddy-Upland Rotational Field Soil. Microb. Ecol. 2018, 75, 751–760. [Google Scholar] [CrossRef]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-Coverage ITS Primers for the DNA-Based Identification of Ascomycetes and Basidiomycetes in Environmental Samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Lv, J.; Wang, Z.; Peng, Y.; Shang, J.; Wang, X. Microplastic additions alter soil organic matter stability and bacterial community under varying temperature in two contrasting soils. Sci. Total Environ. 2022, 838, 156471. [Google Scholar] [CrossRef]
- Herren, C.M.; McMahon, K.D. Cohesion: A method for quantifying the connectivity of microbial communities. ISME J. 2017, 11, 2426–2438. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef] [PubMed]
- Mercier, A.; Gravouil, K.; Aucher, W.; Brosset-Vincent, S.; Kadri, L.; Colas, J.; Bouchon, D.; Ferreira, T. Fate of Eight Different Polymers under Uncontrolled Composting Conditions: Relationships Between Deterioration, Biofilm Formation, and the Material Surface Properties. Environ. Sci. Technol. 2017, 51, 1988–1997. [Google Scholar] [CrossRef]
- Beltrán-Sanahuja, A.; Benito-Kaesbach, A.; Sánchez-García, N.; Sanz-Lázaro, C. Degradation of conventional and biobased plastics in soil under contrasting environmental conditions. Sci. Total Environ. 2021, 787, 147678. [Google Scholar] [CrossRef]
- Qin, M.; Chen, C.; Song, B.; Shen, M.; Cao, W.; Yang, H.; Zeng, G.; Gong, J. A review of biodegradable plastics to biodegradable microplastics: Another ecological threat to soil environments? J. Clean. Prod. 2021, 312, 127816. [Google Scholar] [CrossRef]
- Liao, J.; Chen, Q. Biodegradable plastics in the air and soil environment: Low degradation rate and high microplastics formation. J. Hazard. Mater. 2021, 418, 126329. [Google Scholar] [CrossRef]
- Wu, X.; Lyu, X.; Li, Z.; Gao, B.; Zeng, X.; Wu, J.; Sun, Y. Transport of polystyrene nanoplastics in natural soils: Effect of soil properties, ionic strength and cation type. Sci. Total Environ. 2020, 707, 136065. [Google Scholar] [CrossRef]
- Li, K.; Jia, W.; Xu, L.; Zhang, M.; Huang, Y. The plastisphere of biodegradable and conventional microplastics from residues exhibit distinct microbial structure, network and function in plastic-mulching farmland. J. Hazard. Mater. 2023, 442, 130011. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Qi, Y.; Beriot, N.; Gort, G.; Huerta Lwanga, E.; Gooren, H.; Yang, X.; Geissen, V. Impact of plastic mulch film debris on soil physicochemical and hydrological properties. Environ. Pollut. 2020, 266, 115097. [Google Scholar] [CrossRef]
- Wei, X.-F.; Capezza, A.J.; Cui, Y.; Li, L.; Hakonen, A.; Liu, B.; Hedenqvist, M.S. Millions of microplastics released from a biodegradable polymer during biodegradation/enzymatic hydrolysis. Water Res. 2022, 211, 118068. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, J.; Wang, X.; Ding, C.; Wang, J.; Poretsky, R. Deciphering the Mechanisms Shaping the Plastisphere Microbiota in Soil. mSystems 2022, 7, e0035222. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, X.; Qiu, X.; Sun, T.; Cao, J.; Lv, M.; Sui, Z.; Wang, Z.; Jiao, S.; Xu, Y.; et al. Discrepant soil microbial community and C cycling function responses to conventional and biodegradable microplastics. J. Hazard. Mater. 2024, 470, 134176. [Google Scholar] [CrossRef]
- Sánchez, C. Fungal potential for the degradation of petroleum-based polymers: An overview of macro- and microplastics biodegradation. Biotechnol. Adv. 2020, 40, 107501. [Google Scholar] [CrossRef]
- Carmen, S. Microbial capability for the degradation of chemical additives present in petroleum-based plastic products: A review on current status and perspectives. J. Hazard. Mater. 2021, 402, 123534. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhang, Z. Occurrence, effects, and biodegradation of plastic additives in sludge anaerobic digestion: A review. Environ. Pollut. 2021, 287, 117568. [Google Scholar] [CrossRef]
- Peng, C.; Wang, J.; Liu, X.; Wang, L. Differences in the Plastispheres of Biodegradable and Non-biodegradable Plastics: A Mini Review. Front. Microbiol. 2022, 13, 849147. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Duan, C.; Cao, N.; Ding, C.; Huang, Y.; Wang, J. Biodegradable and conventional microplastics exhibit distinct microbiome, functionality, and metabolome changes in soil. J. Hazard. Mater. 2022, 424, 127282. [Google Scholar] [CrossRef]
- Machado, D.; Maistrenko, O.M.; Andrejev, S.; Kim, Y.; Bork, P.; Patil, K.R.; Patil, K.R. Polarization of microbial communities between competitive and cooperative metabolism. Nat. Ecol. Evol. 2021, 5, 195–203. [Google Scholar] [CrossRef]
- Thumarat, U.; Nakamura, R.; Kawabata, T.; Suzuki, H.; Kawai, F. Biochemical and genetic analysis of a cutinase-type polyesterase from a thermophilic Thermobifida alba AHK119. Appl. Microbiol. Biotechnol. 2012, 95, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Biundo, A.; Hromic, A.; Pavkov-Keller, T.; Gruber, K.; Quartinello, F.; Haernvall, K.; Perz, V.; Arrell, M.S.; Zinn, M.; Ribitsch, D.; et al. Characterization of a poly(butylene adipate-co-terephthalate)-hydrolyzing lipase from Pelosinus fermentans. Appl. Microbiol. Biotechnol. 2015, 100, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lu, Y.; Chen, F.; Li, X.; Xiao, D.; Wang, H. Molecular Ecological Network Complexity Drives Stand Resilience of Soil Bacteria to Mining Disturbances among Typical Damaged Ecosystems in China. Microorganisms 2020, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, X.; Wu, D.; Peng, L.; Fan, C.; Zhang, W.; Li, Q.; Ge, C. Addition of biodegradable microplastics alters the quantity and chemodiversity of dissolved organic matter in latosol. Sci. Total Environ. 2022, 816, 151960. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Walder, F.; Buchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Chen, X.; Meng, Z.; Xu, R.; Duoji, D.; Zhang, J.; He, J.; Wang, Z.; Chen, J.; et al. Soil microbial network complexity predicts ecosystem function along elevation gradients on the Tibetan Plateau. Soil Biol. Biochem. 2022, 172, 108766. [Google Scholar] [CrossRef]
- Wagg, C.; Hautier, Y.; Pellkofer, S.; Banerjee, S.; Schmid, B.; van der Heijden, M.G. Diversity and asynchrony in soil microbial communities stabilizes ecosystem functioning. eLife 2021, 10, e62813. [Google Scholar] [CrossRef]
- Cornell, C.R.; Zhang, Y.; Ning, D.; Xiao, N.; Wagle, P.; Xiao, X.; Zhou, J. Land use conversion increases network complexity and stability of soil microbial communities in a temperate grassland. ISME J. 2023, 17, 2210–2220. [Google Scholar] [CrossRef]

| Parameter | CK | CF | BF |
|---|---|---|---|
| pH | 6.31 ± 0.06 a | 6.14 ± 0.03 b | 5.79 ± 0.15 c |
| SWC (%) | 20% ± 0.02 a | 19% ± 0.02 a | 19% ± 0.04 a |
| NH4+-N (mg/kg) | 6.05 ± 1.27 ab | 4.53 ± 0.86 b | 6.77 ± 0.51 a |
| NO3−-N (mg/kg) | 12.86 ± 1.87 b | 11.01 ± 1.43 b | 14.27 ± 1.54 a |
| DON (g/kg) | 0.20 ± 0.03 a | 0.13 ± 0.05 b | 0.23 ± 0.02 a |
| DOC (g/kg) | 7.83 ± 0.93 a | 8.58 ± 0.93 a | 8.20 ± 2.2 a |
| TC (g/kg) | 45.89 ± 2.21 b | 47.08 ± 1.76 b | 51.36 ± 1.17 a |
| TN (g/kg) | 2.68 ± 0.4 a | 2.63 ± 0.23 a | 2.63 ± 0.2 a |
| Network Indexes | CK | CF | BF |
|---|---|---|---|
| Nodes | 236 | 324 | 254 |
| Edges | 2968 | 4960 | 2808 |
| Degree | 25.15 | 30.62 | 22.11 |
| Path length | 0.07 | 0.06 | 0.07 |
| Diameter | 0.15 | 0.12 | 0.14 |
| Density | 0.11 | 0.095 | 0.087 |
| Clustering coefficient | 0.29 | 0.25 | 0.22 |
| Modularity | 0.65 | 0.66 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, M.; Wang, Y.; Xie, F.; Sun, Q.; Shao, H.; Cheng, X.; Wang, X.; Tao, X.; He, X.; Yong, B.; et al. The Ecological Trap: Biodegradable Mulch Film Residue Undermines Soil Fungal Network Stability. Microorganisms 2025, 13, 2137. https://doi.org/10.3390/microorganisms13092137
Wei M, Wang Y, Xie F, Sun Q, Shao H, Cheng X, Wang X, Tao X, He X, Yong B, et al. The Ecological Trap: Biodegradable Mulch Film Residue Undermines Soil Fungal Network Stability. Microorganisms. 2025; 13(9):2137. https://doi.org/10.3390/microorganisms13092137
Chicago/Turabian StyleWei, Maolu, Yiping Wang, Feiyu Xie, Qian Sun, Huanhuan Shao, Xiaojie Cheng, Xiaoyan Wang, Xiang Tao, Xinyi He, Bin Yong, and et al. 2025. "The Ecological Trap: Biodegradable Mulch Film Residue Undermines Soil Fungal Network Stability" Microorganisms 13, no. 9: 2137. https://doi.org/10.3390/microorganisms13092137
APA StyleWei, M., Wang, Y., Xie, F., Sun, Q., Shao, H., Cheng, X., Wang, X., Tao, X., He, X., Yong, B., & Liu, D. (2025). The Ecological Trap: Biodegradable Mulch Film Residue Undermines Soil Fungal Network Stability. Microorganisms, 13(9), 2137. https://doi.org/10.3390/microorganisms13092137







