Bacillus amyloliquefaciens SC06 Ameliorated Intestinal Mucosal Injury by Regulated Intestinal Stem Cells Proliferation and Differentiation via Activating Wnt/β-Catenin Signal Pathway in Clostridium perfringens-Challenged Mouse
Abstract
1. Introduction
2. Materials and Methods
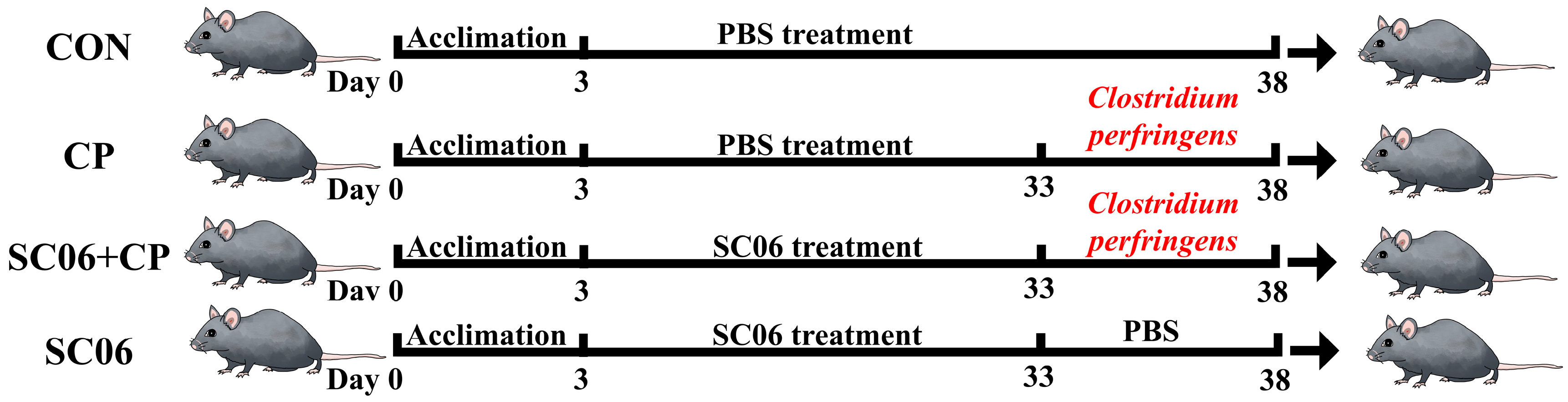
2.1. Animal and Probiotic Strain
2.2. Biochemical Assays
2.3. Hematoxylin and Eosin (H&E) Staining
2.4. Morphology of Jejunum Microvilli
2.5. Crypt Isolation and Intestinal Organoid Culture
2.6. Total RNA Extraction, Reverse Transcription, and Relative Quantitative Real-Time PCR
2.7. Immunofluorescence and Tunel Analysis
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
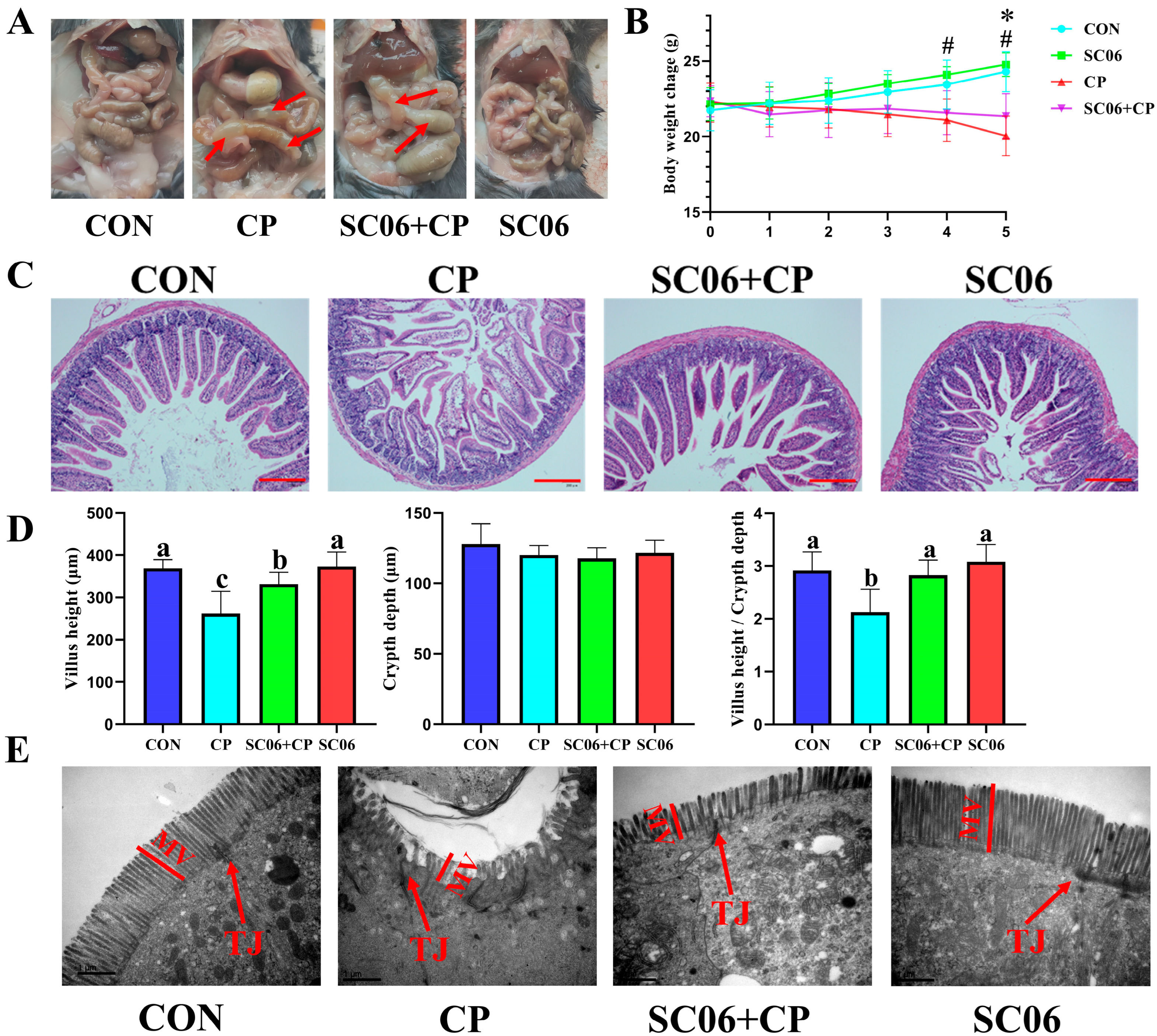
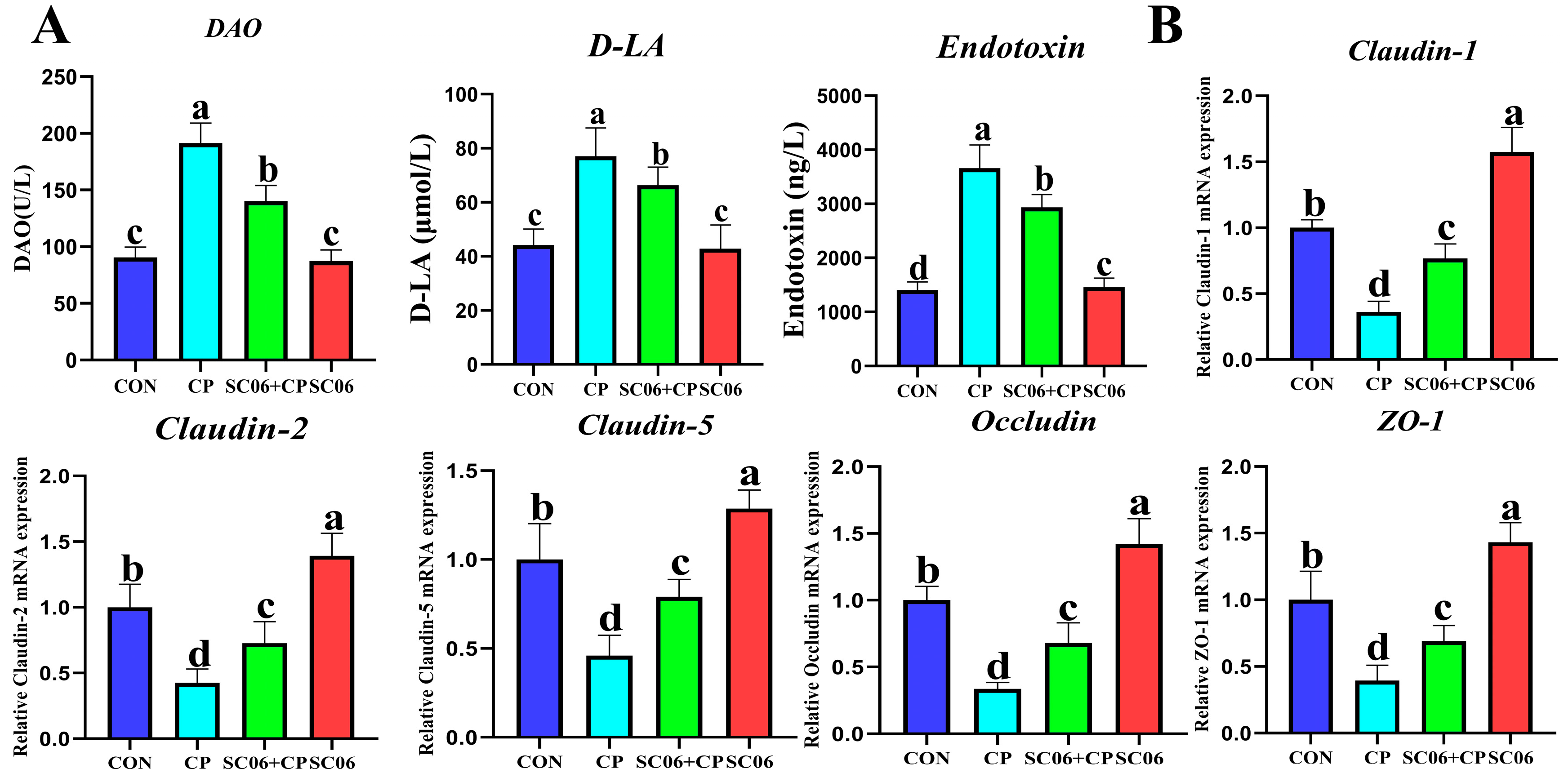
3.1. B. amyloliquefaciens SC06 Improved the Growth Performance and Intestinal Integrity of C. perfringens-Challenged Mouse
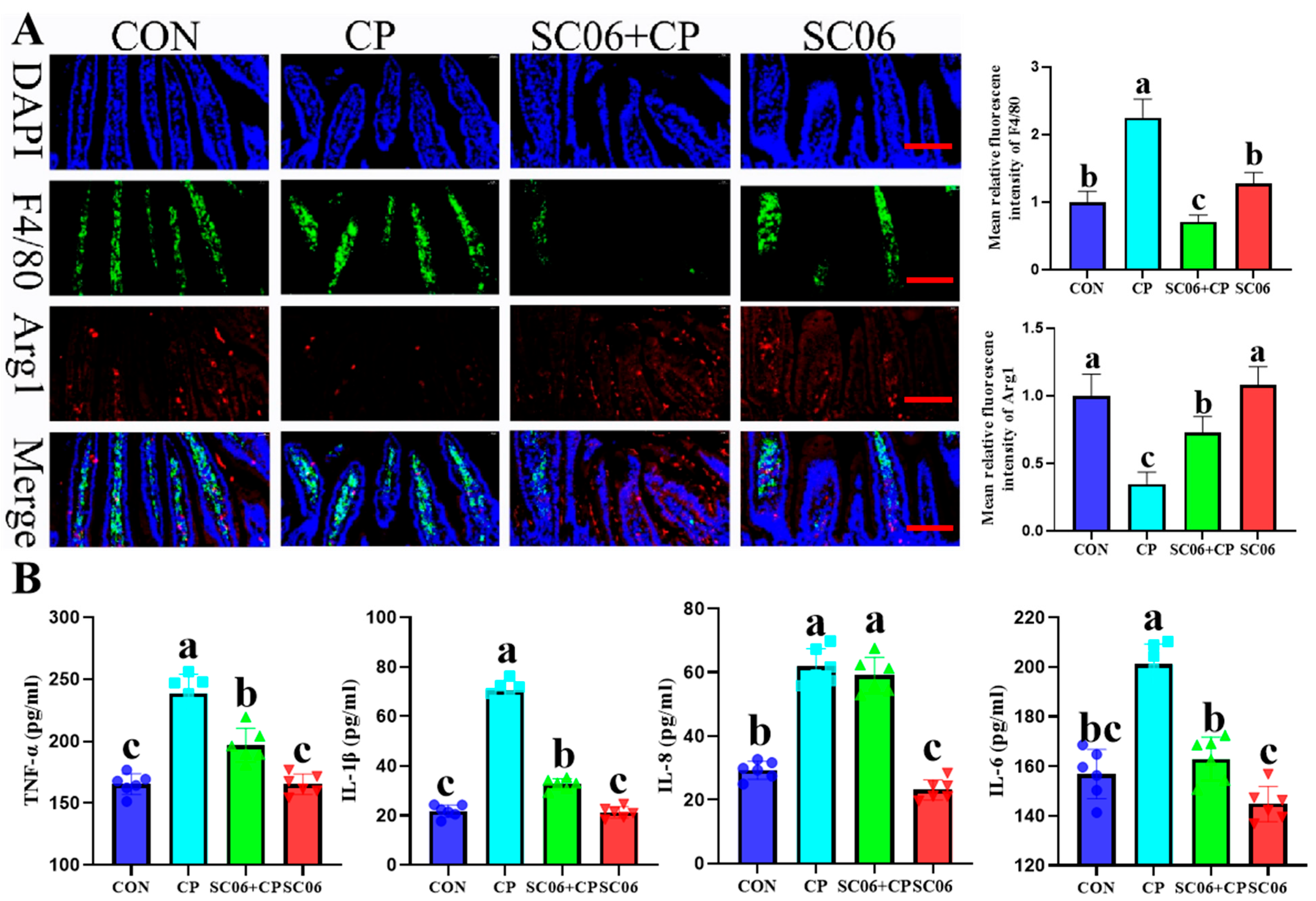
3.2. B. amyloliquefaciens SC06 Suppressed the Levels of Inflammatory Cytokines and Oxidative Stress in C. perfringens-Challenged Mouse
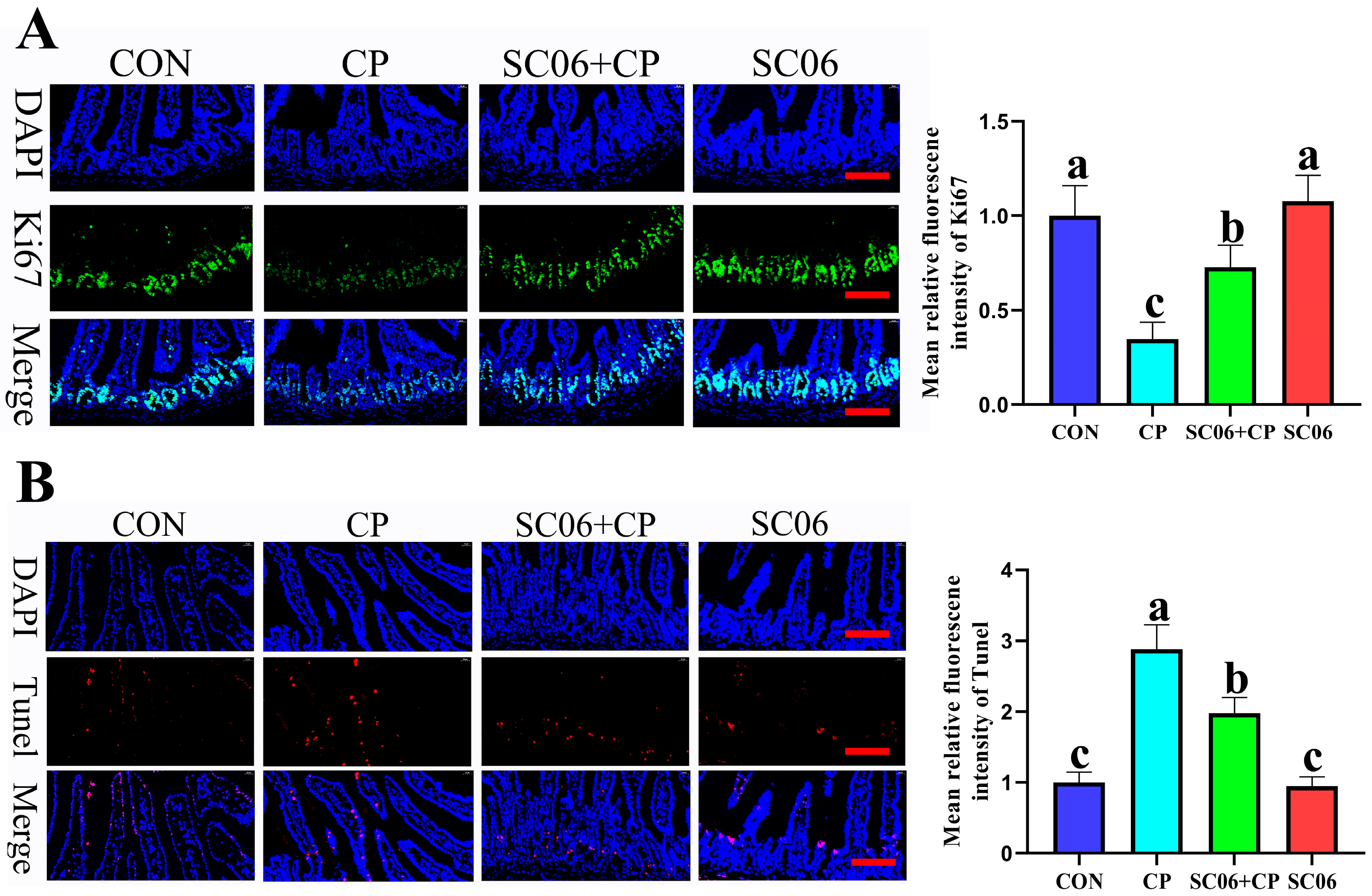
3.3. B. amyloliquefaciens SC06 Decreased the Intestinal Cell Apoptosis Rates of Cells in C. perfringens-Challenged Mice
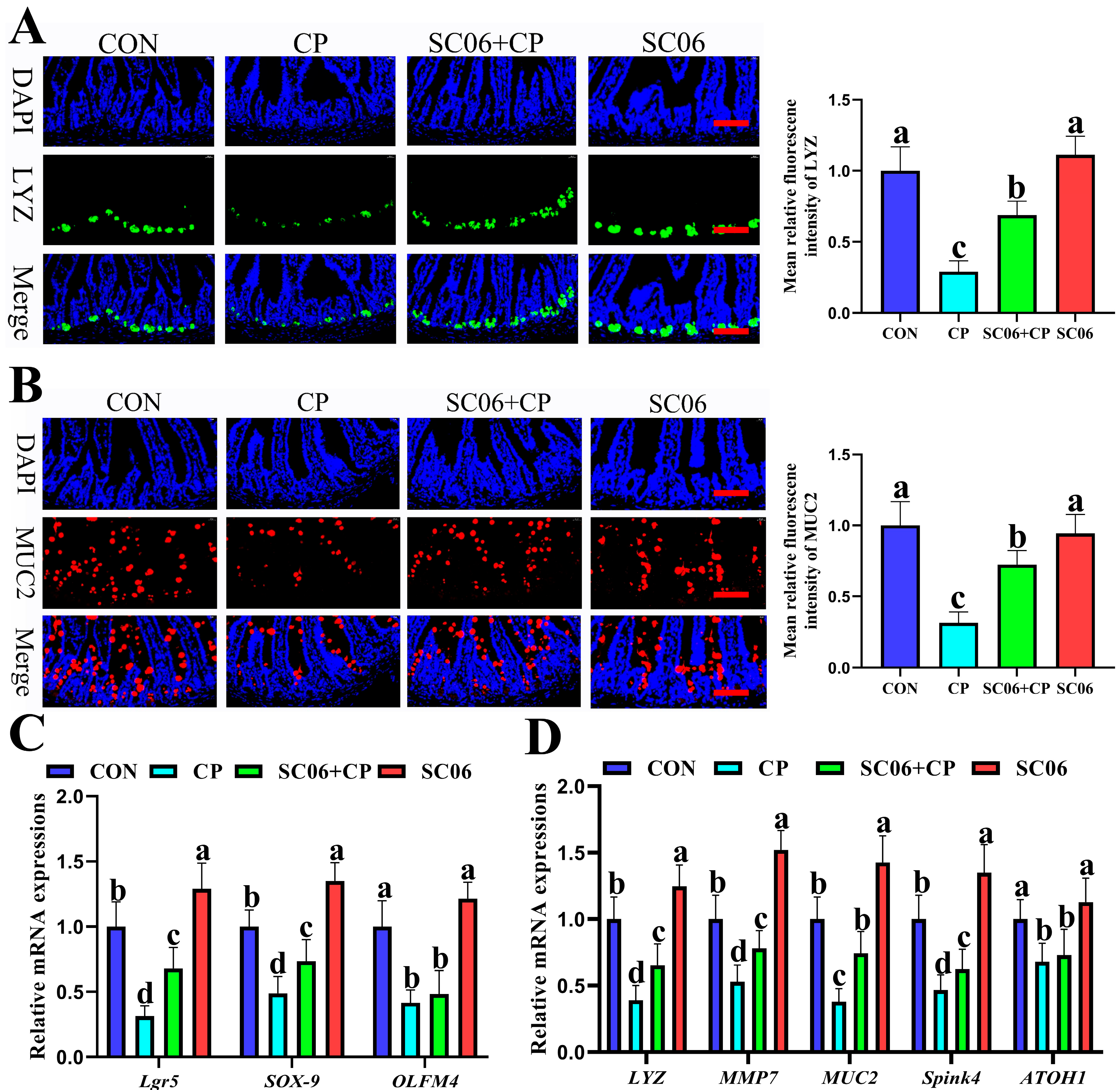
3.4. B. amyloliquefaciens SC06 Promoted ISC Proliferation and Differentiation in C. perfringens-Challenged Mice
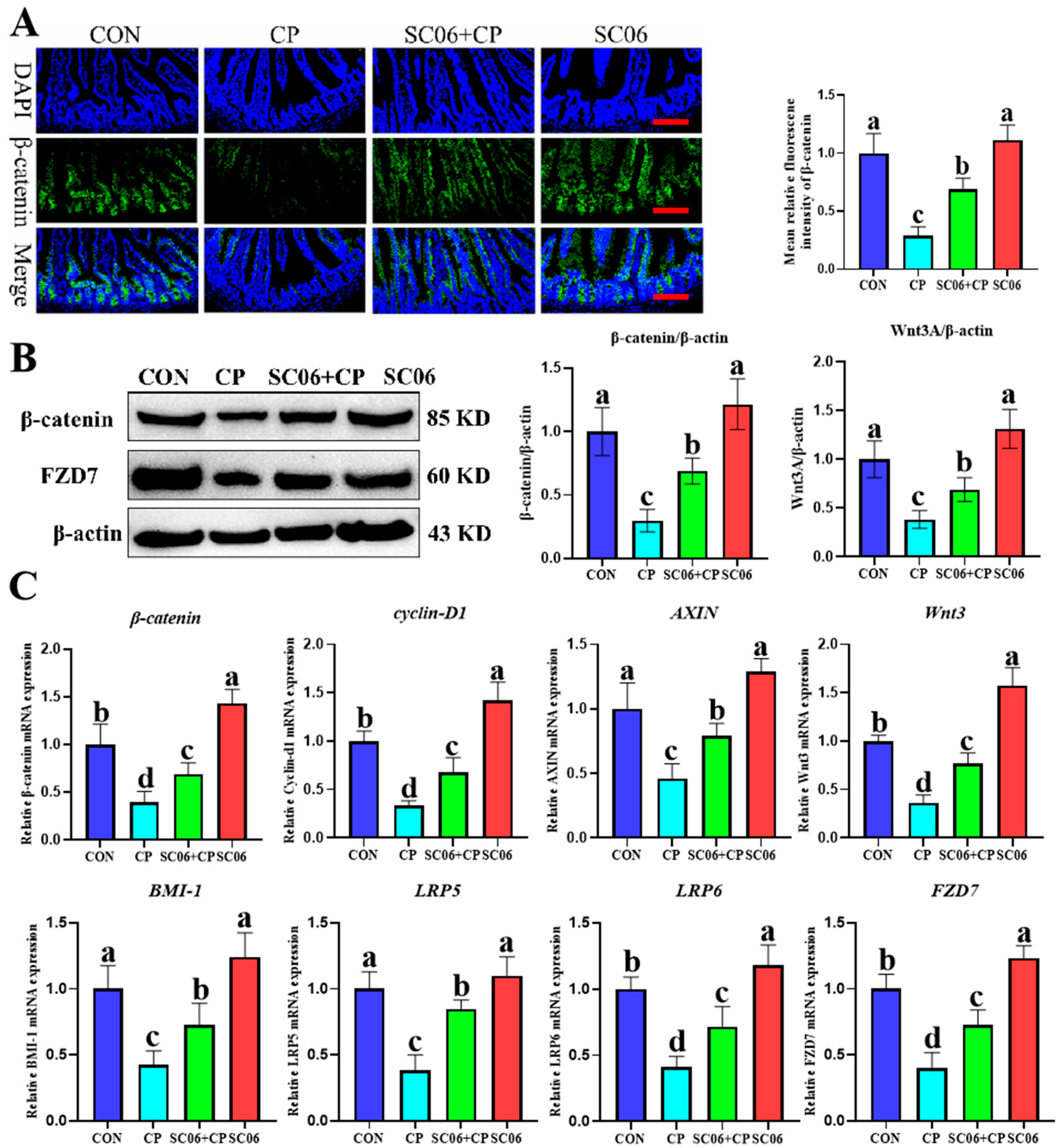
3.5. B. amyloliquefaciens SC06 Activated the Wnt/β-Catenin Signaling Pathway in C. perfringens-Challenged Mice
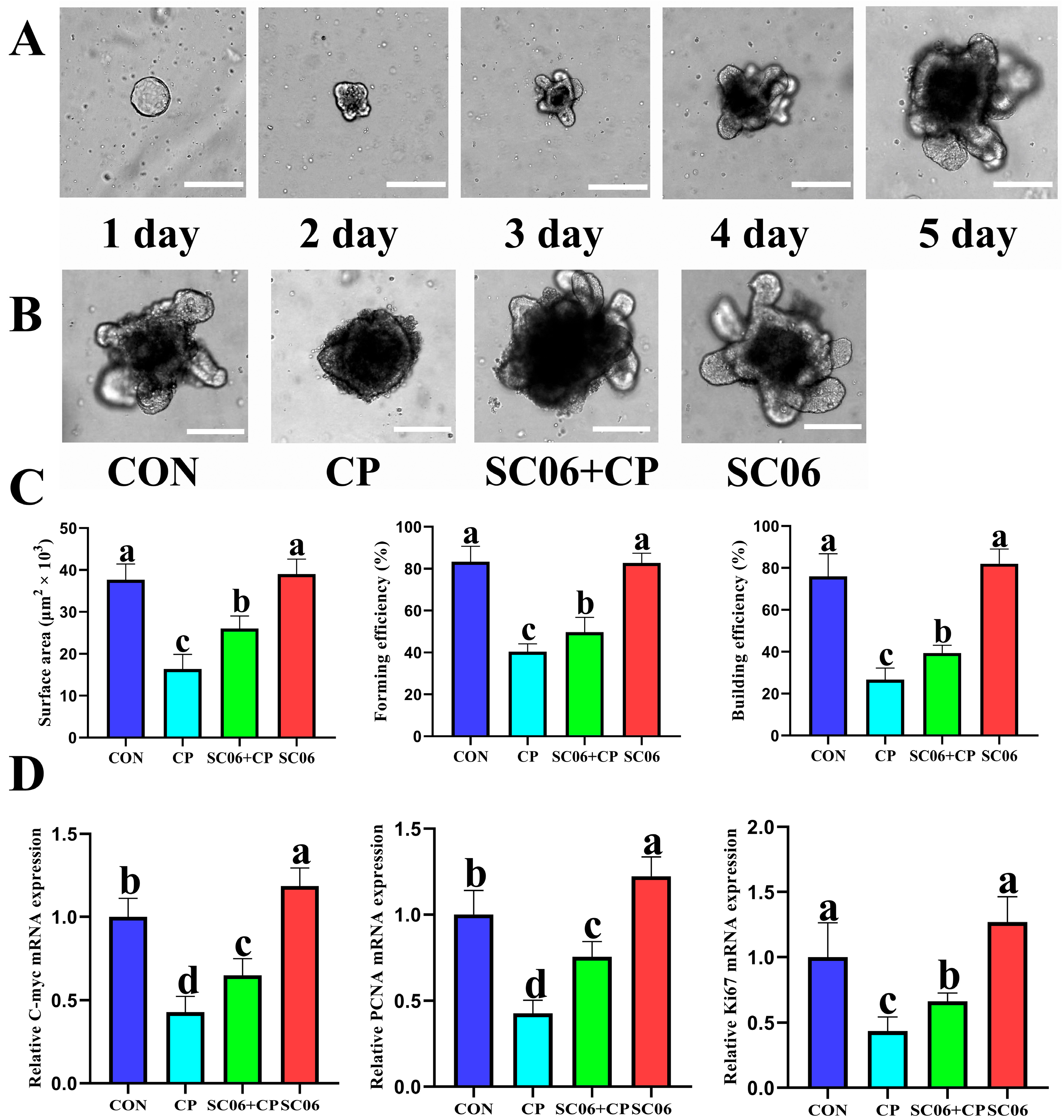
3.6. B. amyloliquefaciens SC06 Accelerates ISC Proliferation in Jejunum Organoids of Mouse
3.7. B. amyloliquefaciens SC06 Accelerated ISC Proliferation and Differentiation in C. perfringens-Challenged Organoid
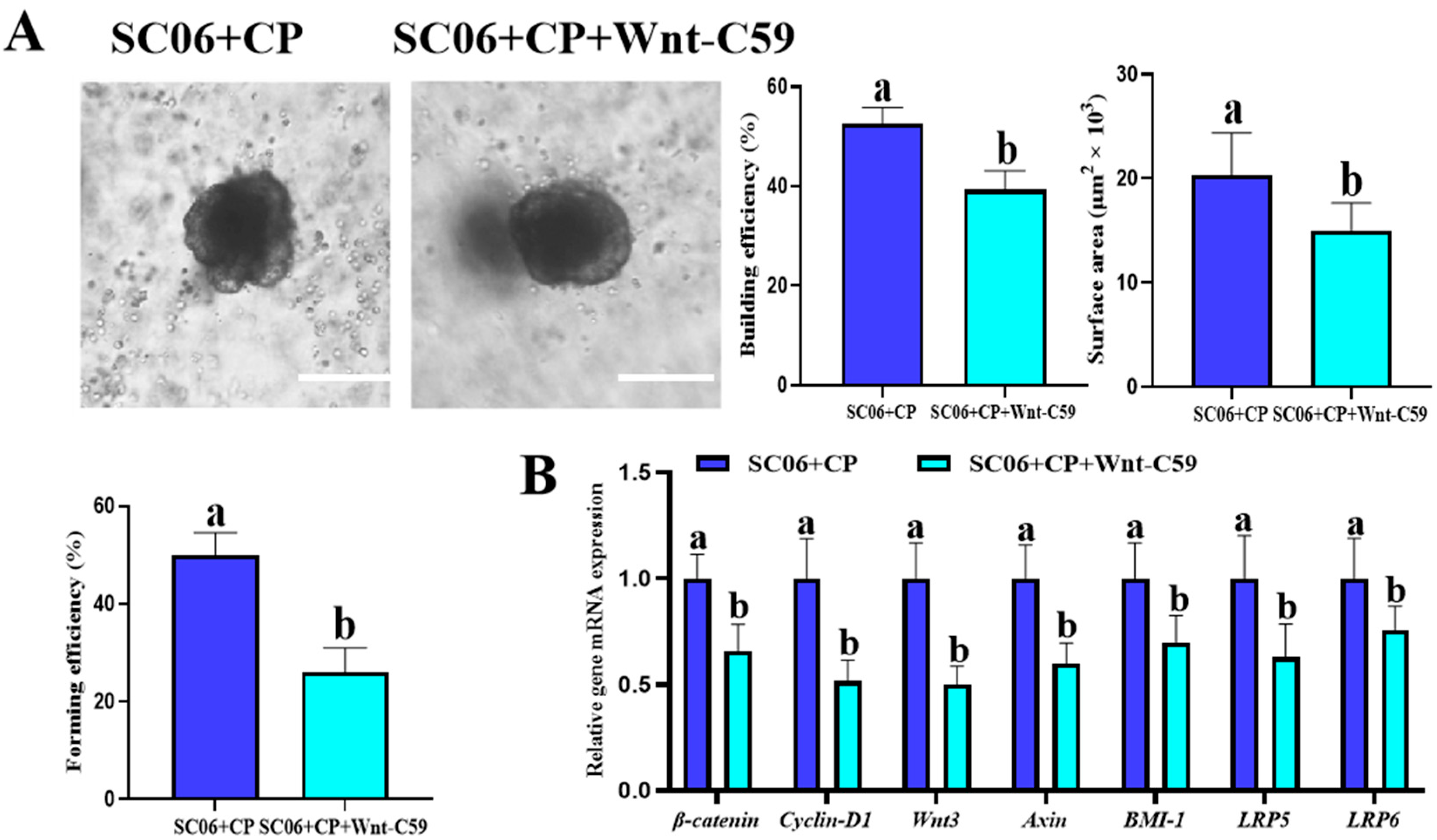
3.8. Wnt Inhibition Attenuated the Protective Effect of B. amyloliquefaciens SC06 on C. perfringens–Challenged Jejunum Organoids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehdizadeh Gohari, I.; Kropinski, A.M.; Weese, S.J.; Parreira, V.R.; Whitehead, A.E.; Boerlin, P.; Prescott, J.F. Plasmid Characterization and Chromosome Analysis of Two netF plus Clostridium perfringens Isolates Associated with Foal and Canine Necrotizing Enteritis. PLoS ONE 2016, 11, e0148344. [Google Scholar] [CrossRef]
- Hassan, K.A.; Elbourne, L.D.; Tetu, S.G.; Melville, S.B.; Rood, J.I.; Paulsen, I.T. Genomic analyses of Clostridium perfringens iso-lates from five toxinotypes. Res. Microbiol. 2015, 166, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.Y.; Liang, B.S.; Zhang, X.Y.; Chen, H.; Ma, N.; Lan, J.L.; Li, D.Y.; Zhou, Z.W.; Yang, M. Molecular characterization of Clostridium perfringens isolates from a tertiary children’s hospital in Guangzhou, China, establishing an association between bacterial colonization and food allergies in infants. Gut Pathog. 2023, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, S.; Zakaria, I.; Moustafa, A.; AboSakaya, R.; Selim, A. Molecular epidemiology and genetic characterization of Clostridium perfringens infections in lambs. Microb. Pathog. 2022, 173, 105822. [Google Scholar] [CrossRef]
- Bendary, M.M.; Abd El-Hamid, M.I.; El-Tarabili, R.M.; Hefny, A.A.; Algendy, R.M.; Elzohairy, N.A.; Ghoneim, M.M.; Al-Sanea, M.M.; Nahari, M.H.; Moustafa, W.H. Clostridium perfringens Associated with Foodborne Infections of Animal Origins: Insights into Prevalence, Antimicrobial Resistance, Toxin Genes Profiles, and Toxinotypes. Biology 2022, 11, 551. [Google Scholar] [CrossRef]
- Hessain, A.M.; Alhazmi, M.I.; Moussa, I.M. Clostridium perfringens in Chicken: Relevant to Public Health from Egypt. J. Pure Appl. Microbiol. 2013, 7, 1877–1884. [Google Scholar]
- Lyhs, U.; Perko-Makela, P.; Kallio, H.; Brockmann, A.; Heinikainen, S.; Tuuri, H.; Pedersen, K. Characterization of Clostridium perfringens isolates from healthy turkeys and from turkeys with necrotic enteritis. Poult. Sci. 2013, 92, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Marlow, M.A.; Luna-Gierke, R.E.; Griffin, P.M.; Vieira, A.R. Foodborne Disease Outbreaks in Correctional Institutions-United States, 1998–2014. Am. J. Public Health 2017, 107, 1150–1156. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Mahon, B.E.; Jones, T.F.; Griffin, P.M. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol. Infect. 2015, 143, 2795–2804. [Google Scholar] [CrossRef]
- Colombel, J.-F.; D’Haens, G.; Lee, W.-J.; Petersson, J.; Panaccione, R. Outcomes and Strategies to Support a Treat-to-target Approach in Inflammatory Bowel Disease: A Systematic Review. J. Crohns Colitis 2020, 14, 254–266. [Google Scholar] [CrossRef]
- Liu, C.Y.; Cham, C.M.; Chang, E.B. Epithelial wound healing in inflammatory bowel diseases: The next therapeutic frontier. Transl. Res. 2021, 236, 35–51. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, Y.-G. Efficient Culture of Intestinal Organoids with Blebbistatin. In Organoids: Stem Cells, Structure, and Function; Turksen, K., Ed.; Methods in Molecular Biology; Springer Science + Business Media: New York, NY, USA, 2019; Volume 1576, pp. 113–121. [Google Scholar]
- Clevers, H. The Intestinal Crypt, A Prototype Stem Cell Compartment. Cell 2013, 154, 274–284. [Google Scholar] [CrossRef]
- Hatzis, P.; Snippert, H.J.G. Long noncoding RNAs in gut stem cells. Nat. Cell Biol. 2018, 20, 1106–1107. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Yuan, S.; Meng, H.; Hou, X.-T.; Li, J.; Xue, J.-C.; Li, Y.; Wang, Q.; Nan, J.-X.; Jin, X.-J.; et al. Stem Cell-Based Therapies for Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 23, 8494. [Google Scholar] [CrossRef]
- Kuang, J.-H.; Huang, Y.-Y.; Hu, J.-S.; Yu, J.-J.; Zhou, Q.-Y.; Liu, D.-M. Exopolysaccharides from Bacillus amyloliquefaciens DMBA-K4 ameliorate dextran sodium sulfate-induced colitis via gut microbiota modulation. J. Funct. Foods 2020, 75, 104212. [Google Scholar] [CrossRef]
- Kang, M.; Choi, H.J.; Yun, B.; Lee, J.; Yoo, J.; Yang, H.-J.; Jeong, D.-Y.; Kim, Y.; Oh, S. Bacillus amyloliquefaciens SCGB1 Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice Through Immune Regulation. J. Med. Food 2021, 24, 709–719. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, H.; Lu, Y.; Meng, F.; Lu, Z.; Lu, Y. Surfactin Mitigates Dextran Sodium Sulfate-Induced Colitis and Behavioral Disorders in Mice by Mediating Gut-Brain-Axis Balance. J. Agric. Food Chem. 2023, 71, 1577–1592. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.P.; Wang, B.K.; Xu, H.; Mei, X.Q.; Xu, X.G.; Zhang, X.P.; Ni, J.J.; Li, W.F. Bacillus amyloliquefaciens SC06 Protects Mice Against High-Fat Diet-Induced Obesity and Liver Injury via Regulating Host Metabolism and Gut Microbiota. Front. Microbiol. 2019, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Mahe, M.M.; Aihara, E.; Schumacher, M.A.; Zavros, Y.; Montrose, M.H.; Helmrath, M.A.; Sato, T.; Shroyer, N.F. Establishment of Gastrointestinal Epithelial Organoids. Curr. Protoc. Mouse Biol. 2013, 3, 217–240. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.R.; Kumar, S.; Jain, S.K.; Raj, R.C. Relative mRNA expression of CRBP IV gene and its association with layer economic traits in Rhode Island Red chicken. Indian J. Anim. Sci. 2019, 89, 1345–1348. [Google Scholar] [CrossRef]
- Han, Y.; Tang, C.; Zhao, Q.; Fan, S.; Yang, P.; Zhang, J. Butyrate Mitigates Lipopolysaccharide-Induced Intestinal Morphological Changes in Weanling Piglets by Regulating the Microbiota and Energy Metabolism, and Alleviating Inflammation and Apoptosis. Microorganisms 2022, 10, 2001. [Google Scholar] [CrossRef]
- Deng, Z.; Duarte, M.E.; Kim, S.Y.; Hwang, Y.; Kim, S.W. Comparative effects of soy protein concentrate, enzyme-treated soybean meal, and fermented soybean meal replacing animal protein supplements in feeds on growth performance and intestinal health of nursery pigs. J. Anim. Sci. Biotechnol. 2023, 14, 89. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, H.; Guo, Q.; Duan, Y.; Chen, S.; Han, M.; Li, F.; Jin, M.; Wang, Y. Engineered Bacillus subtilis alleviates intestinal oxidative injury through Nrf2-Keap1 pathway in enterotoxigenic Escherichia coli (ETEC) K88-infected piglet. J. Zhejiang Univ.-Sci. B 2023, 24, 496–509. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, X.; Dong, Y.; Li, R.; Shen, M.; Yi, D.; Wu, T.; Wang, L.; Zhao, D.; Hou, Y. Bacillus coagulans prevents the decline in average daily feed intake in young piglets infected with enterotoxigenic Escherichia coli K88 by reducing intestinal injury and regulating the gut microbiota. Front. Cell. Infect. Microbiol. 2023, 13, 1284166. [Google Scholar] [CrossRef]
- Mehdizadeh Gohari, I.; Navarro, M.A.; Li, J.; Shrestha, A.; Uzal, F.; McClane, B.A. Pathogenicity and virulence of Clostridium perfringens. Virulence 2021, 12, 723–753. [Google Scholar] [CrossRef]
- Camargo, A.; Ramírez, J.D.; Kiu, R.; Hall, L.J.; Muñoz, M. Unveiling the pathogenic mechanisms of Clostridium perfringens toxins and virulence factors. Emerg. Microbes Infect. 2024, 13, 2341968. [Google Scholar] [CrossRef]
- Lee, K.; Lillehoj, H. Role of Clostridium perfringens necrotic enteritis B-like toxin in disease pathogenesis. Vaccines 2021, 10, 61. [Google Scholar] [CrossRef]
- Goossens, E.; Valgaeren, B.R.; Pardon, B.; Haesebrouck, F.; Ducatelle, R.; Deprez, P.R.; Van Immerseel, F. Rethinking the role of alpha toxin in Clostridium perfringens-associated enteric diseases: A review on bovine necro-haemorrhagic enteritis. Vet. Res. 2017, 48, 9. [Google Scholar] [CrossRef]
- Moe, P.C.; Heuck, A.P. Phospholipid hydrolysis caused by Clostridium perfringens α-toxin facilitates the targeting of perfringolysin O to membrane bilayers. Biochemistry 2010, 49, 9498–9507. [Google Scholar] [CrossRef]
- Yamamura, K.; Ashida, H.; Okano, T.; Kinoshita-Daitoku, R.; Suzuki, S.; Ohtani, K.; Hamagaki, M.; Ikeda, T.; Suzuki, T. Inflammasome activation induced by Perfringolysin O of Clostridium perfringens and its involvement in the progression of gas gangrene. Front. Microbiol. 2019, 10, 2406. [Google Scholar] [CrossRef]
- Li, B.; Lee, C.; Chuslip, S.; Lee, D.; Biouss, G.; Wu, R.; Koike, Y.; Miyake, H.; Ip, W.; Gonska, T.; et al. Intestinal epithelial tight junctions and permeability can be rescued through the regulation of endoplasmic reticulum stress by amniotic fluid stem cells during necrotizing enterocolitis. FASEB J. 2021, 35, e21265. [Google Scholar] [CrossRef]
- Celi, P.; Cowieson, A.J.; Fru-Nji, F.; Steinert, R.E.; Kluenter, A.M.; Verlhac, V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed. Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, S.; Ma, N.; Johnston, L.J.; Wu, C.; Ma, X. Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: A therapeutic target to control intestinal inflammation. Med. Res. Rev. 2021, 41, 1061–1088. [Google Scholar] [CrossRef]
- Jiang, Z.; Su, W.; Wen, C.; Li, W.; Zhang, Y.; Gong, T.; Du, S.; Wang, X.; Lu, Z.; Jin, M.; et al. Effect of Porcine Clostridium perfringens on Intestinal Barrier, Immunity, and Quantitative Analysis of Intestinal Bacterial Communities in Mice. Front. Vet. Sci. 2022, 9, 881878. [Google Scholar] [CrossRef]
- Fernandez-Miyakawa, M.E.; Sayeed, S.; Fisher, D.J.; Poon, R.; Adams, V.; Rood, J.I.; McClane, B.A.; Saputo, J.; Uzal, F.A. Development and application of an oral challenge mouse model for studying Clostridium perfringens type D infection. Infect. Immun. 2007, 75, 4282–4288. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, J.; Jin, Z.; Bai, Y. Analysis of the action pattern of sequential a-amylases from B. stearothermophilus and B. amyloliquefaciens on highly concentrated soluble starch. Carbohydr. Polym. 2023, 320, 121190. [Google Scholar] [CrossRef]
- Bomba, L.; Minuti, A.; Moisa, S.J.; Trevisi, E.; Eufemi, E.; Lizier, M.; Chegdani, F.; Lucchini, F.; Rzepus, M.; Prandini, A.; et al. Gut response induced by weaning in piglet features marked changes in immune and inflammatory response. Funct. Integr. Genom. 2014, 14, 657–671. [Google Scholar] [CrossRef]
- Uzal, F.A.; McClane, B.A. Animal models to study the pathogenesis of enterotoxigenic Clostridium perfringens infections. Microbes Infect. 2012, 14, 1009–1016. [Google Scholar] [CrossRef]
- Saladrigas-Garcia, M.; Sola-Oriol, D.; Lopez-Verge, S.; D’Angelo, M.; Collado, M.C.; Nielsen, B.; Faldyna, M.; Perez, J.F.; Martin-Orue, S.M. Potential effect of two Bacillus probiotic strains on performance and fecal microbiota of breeding sows and their piglets. J. Anim. Sci. 2022, 100, skac163. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, W.; Su, W.; Wen, C.; Gong, T.; Zhang, Y.; Wang, Y.; Jin, M.; Lu, Z. Protective Effects of Bacillus amyloliquefaciens 40 Against Clostridium perfringens Infection in Mice. Front. Nutr. 2021, 8, 733591. [Google Scholar] [CrossRef]
- Feng, J.; Cai, Z.; Chen, Y.; Zhu, H.; Chang, X.; Wang, X.; Liu, Z.; Zhang, J.; Nie, G. Effects of an exopolysaccharide from Lactococcus lactis Z-2 on innate immune response, antioxidant activity, and disease resistance against Aeromonas hydrophila in Cyprinus carpio L. Fish Shellfish Immunol. 2020, 98, 324–333. [Google Scholar] [CrossRef]
- Zhou, Q.; Shen, B.; Huang, R.; Liu, H.; Zhang, W.; Song, M.; Liu, K.; Lin, X.; Chen, S.; Liu, Y.; et al. Bacteroides fragilis strain ZY-312 promotes intestinal barrier integrity via upregulating the STAT3 pathway in a radiation-induced intestinal injury mouse model. Front. Nutr. 2022, 9, 1063699. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Wang, T.C.; Li, P.; Chen, J.; Nepovimova, E.; Long, M.; Wu, W.D.; Kuca, K. Bacillus amyloliquefaciens B10 can alleviate aflatoxin B1-induced kidney oxidative stress and apoptosis in mice. Ecotoxicol. Environ. Saf. 2021, 218, 112286. [Google Scholar] [CrossRef]
- Rumah, K.R.; Linden, J.; Fischetti, V.A.; Vartanian, T. Isolation of Clostridium perfringens Type B in an Individual at First Clinical Presentation of Multiple Sclerosis Provides Clues for Environmental Triggers of the Disease. PLoS ONE 2013, 8, e76359. [Google Scholar] [CrossRef]
- Posthaus, H.; Kittl, S.; Tarek, B.; Bruggisser, J. Clostridium perfringens type C necrotic enteritis in pigs: Diagnosis, pathogenesis, and prevention. J. Vet. Diagn. Investig. 2020, 32, 203–212. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Zhou, Y.; Wang, L.; Wen, Y.; Ding, K.; Zou, L.; Liu, X.; Li, A.; Wang, Y.; et al. Jwa participates the maintenance of intestinal epithelial homeostasis via ERK/FBXW7-mediated NOTCH1/PPARγ/STAT5 axis and acts as a novel putative aging related gene. Int. J. Biol. Sci. 2022, 18, 5503–5521. [Google Scholar] [CrossRef]
- Duan, C.; Wu, J.; Wang, Z.; Tan, C.; Hou, L.; Qian, W.; Han, C.; Hou, X. Fucose promotes intestinal stem cell-mediated intestinal epithelial development through promoting Akkermansia-related propanoate metabolism. Gut Microbes 2023, 15, 2233149. [Google Scholar] [CrossRef]
- Wang, W.M.; Geng, M.; Zhu, C.X.; Huang, L.; Zhang, Y.; Zhang, T.X.; Zhao, C.J.; Zhang, T.C.; Du, X.J.; Wang, N. Protective Effects and Mechanism of a Novel Probiotic Strain Ligilactobacillus salivarius YL20 against Cronobacter sakazakii-Induced Necrotizing Enterocolitis In Vitro and In Vivo. Nutrients 2022, 14, 3827. [Google Scholar] [CrossRef]
- Yan, M.; Su, A.; Pavasutthipaisit, S.; Spriewald, R.; GraSsl, G.A.; Beineke, A.; Hoeltig, D.; Herrler, G.; Becher, P. Infection of porcine enteroids and 2D differentiated intestinal epithelial cells with rotavirus A to study cell tropism and polarized immune response. Emerg. Microbes Infect. 2023, 12, 2239937. [Google Scholar] [CrossRef]
- Liu, Y.; Reyes, E.; Castillo-Azofeifa, D.; Klein, O.D.; Nystul, T.; Barber, D.L. Intracellular pH dynamics regulates intestinal stem cell lineage specification. Nat. Commun. 2023, 14, 3745. [Google Scholar] [CrossRef]
- Lunnemann, H.M.; Shealy, N.G.; Reyzer, M.L.; Shupe, J.A.; Green, E.H.; Siddiqi, U.; Lacy, D.B.; Byndloss, M.X.; Markham, N.O. Cecum axis (CecAx) preservation reveals physiological and pathological gradients in mouse gastrointestinal epithelium. Gut Microbes 2023, 15, 2185029. [Google Scholar] [CrossRef]
- Calibasi-Kocal, G.; Mashinchian, O.; Basbinar, Y.; Ellidokuz, E.; Cheng, C.-W.; Yilmaz, O.H. Nutritional Control of Intestinal Stem Cells in Homeostasis and Tumorigenesis. Trends Endocrinol. Metab. 2021, 32, 20–35. [Google Scholar] [CrossRef]
- Yan, Y.; Cheng, Y.-Y.; Li, Y.-R.; Jiao, X.-W.; Liu, Y.-M.; Cai, H.-Y.; Ding, Y.-X. Inhibitor of Wnt receptor 1 suppresses the effects of Wnt1, Wnt3a and beta-catenin on the proliferation and migration of C6 GSCs induced by low-dose radiation. Oncol. Rep. 2024, 51, 22. [Google Scholar] [CrossRef]
- Mohapatra, P.; Madhulika, S.; Behera, S.; Singh, P.; Sa, P.; Prasad, P.; Swain, R.K.; Sahoo, S.K. Nimbolide-based nanomedicine inhibits breast cancer stem-like cells by epigenetic reprogramming of DNMTs-SFRP1-Wnt/fβ-catenin signaling axis. Mol. Ther.-Nucleic Acids 2023, 34, 102031. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Zhao, G.; Deng, L.; Yin, X. Leveraging Temporal Wnt Signal for Efficient Differentiation of Intestinal Stem Cells in an Organoid Model. Stem Cells Dev. 2023, 33, 11–26. [Google Scholar] [CrossRef]
- Li, X.-G.; Zhu, M.; Chen, M.-X.; Fan, H.-B.; Fu, H.-L.; Zhou, J.-Y.; Zhai, Z.-Y.; Gao, C.-Q.; Yan, H.-C.; Wang, X.-Q. Acute exposure to deoxynivalenol inhibits porcine enteroid activity via suppression of the Wnt/β-catenin pathway. Toxicol. Lett. 2019, 305, 19–31. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Huang, D.-G.; Gao, C.-Q.; Yan, H.-C.; Zou, S.-G.; Wang, X.-Q. Heat-stable enterotoxin inhibits intestinal stem cell expansion to disrupt the intestinal integrity by downregulating the Wnt/β-catenin pathway. Stem Cells 2021, 39, 482–496. [Google Scholar] [CrossRef]
- Flanagan, D.J.; Phesse, T.J.; Barker, N.; Schwab, R.H.M.; Amin, N.; Malaterre, J.; Stange, D.E.; Nowell, C.J.; Currie, S.A.; Saw, J.T.S.; et al. Frizzled7 Functions as a Wnt Receptor in Intestinal Epithelial Lgr5+ Stem Cells. Stem Cell Rep. 2015, 4, 759–767. [Google Scholar] [CrossRef]
- Du, Y.; de Jong, I.E.M.; Gupta, K.; Waisbourd-Zinman, O.; Har-Zahav, A.; Soroka, C.J.; Boyer, J.L.; Llewellyn, J.; Liu, C.Y.; Naji, A.; et al. Human vascularized bile duct-on-a chip: A multi-cellular micro-physiological system for studying cholestatic liver disease. Biofabrication 2024, 16, 015004. [Google Scholar] [CrossRef]
- Nishimura, R.; Shirasaki, T.; Tsuchiya, K.; Miyake, Y.; Watanabe, Y.; Hibiya, S.; Watanabe, S.; Nakamura, T.; Watanabe, M. Establishment of a system to evaluate the therapeutic effect and the dynamics of an investigational drug on ulcerative colitis using human colonic organoids. J. Gastroenterol. 2019, 54, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.; Peng, C.; Gui, Q.; Li, J.; Xu, Z.; Yang, Y. Lactobacillus rhamnosus GG Promotes Recovery of the Colon Barrier in Septic Mice through Accelerating ISCs Regeneration. Nutrients 2023, 15, 672. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Q.; Xie, S.; Miao, J.F.; Li, Y.C.; Wang, Z.H.; Wang, M.J.; Yu, Q.H. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes 2020, 11, 997–1014. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Accession Numbers | Primer Sequence (5′-3′) |
|---|---|---|
| Ki67 | NM_001081117.2 | F: TCATTGACCGCTCCTTTAGGTATG |
| R: TTGGTATCTTGACCTTCCCCA | ||
| C-myc | NM_010849.4 | F: CGTTGGAAACCCCGCAGA |
| R: TACGGAGTCGTAGTCGAGGT | ||
| PCNA | NM_011045.2 | F: GGGTTGGTAGTTGTCGCTGT |
| R: GATTCACGATGCAGAAGCGG | ||
| Claudin-1 | NM_016674.4 | F: CAACCCGAGCCTTGATGGTA |
| R: TCATGCCAATGGTGGACACA | ||
| Claudin-2 | XM_006528486.4 | F: ACCTAGTCCTTGTCCCCAGAA |
| R: GAAGGCATCTAGAAAACGACCAG | ||
| Claudin-5 | NM_013805.4 | F: CCCAGTTAAGGCACGGGTAG |
| R: GGCACCGTCGGATCATAGAA | ||
| β-catenin | NM_001165902.2 | F: CTCGTGTCCTGTGAAGCCC |
| R: CAGGTCAGCTTGAGTAGCCA | ||
| Wnt3A | NM_009522.3 | F: TCCTGTCTGGGATACGGGTT |
| R: TGTCGGGTCAAGAGAGGAGT | ||
| Cyclin-D1 | NM_001379248.1 | F: CAACTTCCTCTCCTGCTACCG |
| R: TGGAGGGGGTCCTTGTTTAG | ||
| LRP5 | NM_008513.3 | F: TGGACTGGATGGGCAAGAAC |
| R: CCTGGGGTTGTCAAGGTCTC | ||
| LRP6 | NM_008514.4 | F: CTGCGGTGGACTTTGTGTTT |
| R: CTCCAAGCCAATCACATGCC | ||
| FZD7 | NM_008057.3 | F: ACCCTACTGCTCCCTACCTG |
| R: AGAAGGGGAAAGACAAGCGG | ||
| BMI-1 | NM_001416911.1 | F: TGCTGGAGAGCTGGAAAGTG |
| R: GTGAGGGAACTGTGGGTGAG | ||
| AXIN | NM_001159598.2 | F: CTTCAGGGCTCTGGGCTC |
| R: AAAACCGGCTGCTCACTCTC | ||
| Lgr5 | NM_010195.2 | F: AATGCCTCTCCACACTTCGG |
| R: ACATCGAACACCTGCGTGAA | ||
| SOX9 | NM_011448.4 | F: AGCACAAGAAAGACCACCCC |
| R: CTCCGCTTGTCCGTTCTTCA | ||
| OLFM4 | NM_001351947.1 | F: GGCACGATGAGTTACAGCCT |
| R: TTCTGCCCAAGAAGTGCCTC | ||
| MUC2 | NM_023566.4 | F: GAAGCCAGATCCCGAAACCA |
| R: GAATCGGTAGACATCGCCGT | ||
| ATOH1 | NM_007500.5 | F: CCCGTCAAAGTACGGGAACA |
| R: CTCGTCCACTACAACCCCAC | ||
| Spink4 | NM_011463.2 | F: CTTGGCATGGACAGGGAACT |
| R: TGGTTTTCATCCGGGTCAGG | ||
| LYZ | NM_013590.4 | F: GACTAGTGAGCTGTGCCTGT |
| R: TGCTCCTGTGGTTATTGGCTG | ||
| MMP7 | NM_001319986.2 | F: TTGGGCAGAATGTTCCTGGTT |
| R: TTTTCCAGTCATGGGCAGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Cheng, S.; Fan, J.; Hao, H.; Fang, D.; Li, W.; Wang, Q. Bacillus amyloliquefaciens SC06 Ameliorated Intestinal Mucosal Injury by Regulated Intestinal Stem Cells Proliferation and Differentiation via Activating Wnt/β-Catenin Signal Pathway in Clostridium perfringens-Challenged Mouse. Microorganisms 2025, 13, 2136. https://doi.org/10.3390/microorganisms13092136
Deng H, Cheng S, Fan J, Hao H, Fang D, Li W, Wang Q. Bacillus amyloliquefaciens SC06 Ameliorated Intestinal Mucosal Injury by Regulated Intestinal Stem Cells Proliferation and Differentiation via Activating Wnt/β-Catenin Signal Pathway in Clostridium perfringens-Challenged Mouse. Microorganisms. 2025; 13(9):2136. https://doi.org/10.3390/microorganisms13092136
Chicago/Turabian StyleDeng, Hongbin, Si Cheng, Jiemei Fan, Haibin Hao, Dandong Fang, Weiqin Li, and Qi Wang. 2025. "Bacillus amyloliquefaciens SC06 Ameliorated Intestinal Mucosal Injury by Regulated Intestinal Stem Cells Proliferation and Differentiation via Activating Wnt/β-Catenin Signal Pathway in Clostridium perfringens-Challenged Mouse" Microorganisms 13, no. 9: 2136. https://doi.org/10.3390/microorganisms13092136
APA StyleDeng, H., Cheng, S., Fan, J., Hao, H., Fang, D., Li, W., & Wang, Q. (2025). Bacillus amyloliquefaciens SC06 Ameliorated Intestinal Mucosal Injury by Regulated Intestinal Stem Cells Proliferation and Differentiation via Activating Wnt/β-Catenin Signal Pathway in Clostridium perfringens-Challenged Mouse. Microorganisms, 13(9), 2136. https://doi.org/10.3390/microorganisms13092136






