Ducklings Were Susceptible to Swine Acute Diarrhea Syndrome Coronavirus Under Experimental Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and SADS-CoV Preparation
2.2. Virus Titer Determination
2.3. Animal Experiment Design
2.4. SYBR Green qPCR
2.5. Detection of Serum IgG Antibodies Against SADS-CoV
2.6. Ethics Approval
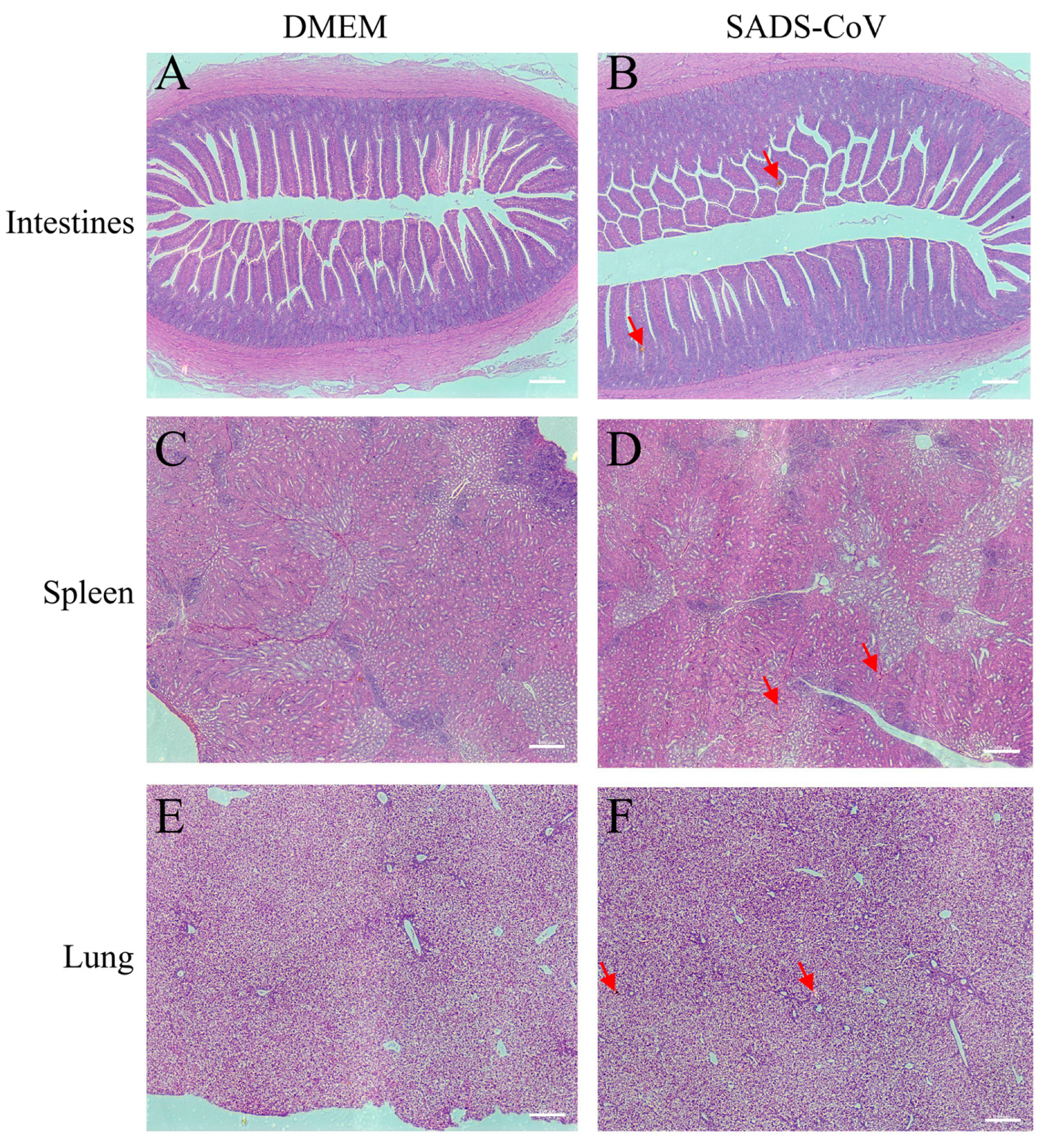
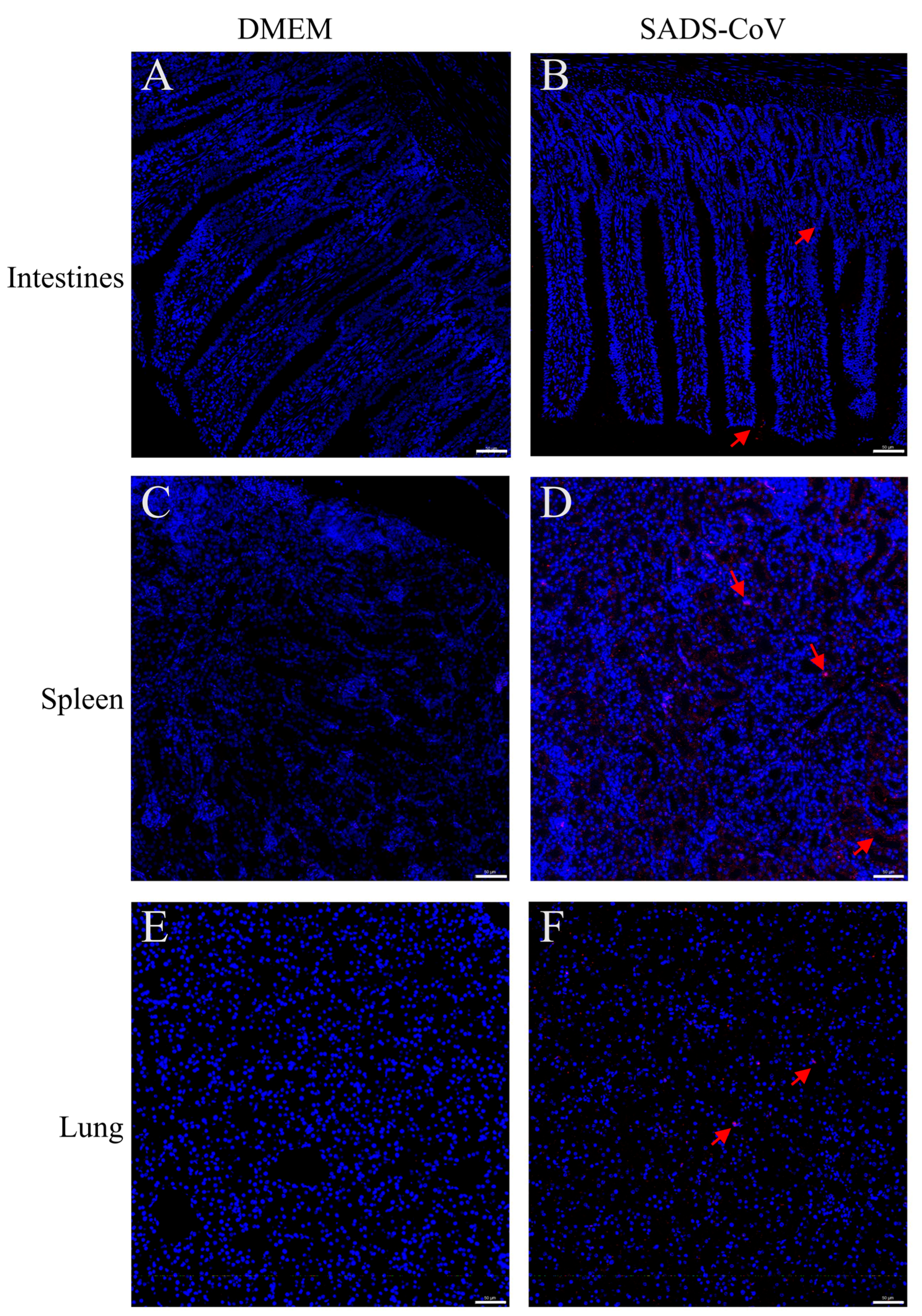
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ioannidis, J.P.A.; Pezzullo, A.M.; Cristiano, A.; Boccia, S. Global Estimates of Lives and Life-Years Saved by COVID-19 Vaccination During 2020–2024. JAMA Health Forum 2025, 6, e252223. [Google Scholar] [CrossRef]
- Velisavljev-Filipovic, G.M.; Jovanov, O. Newborn with Hypoglossia and Micrognathia with Situs Inversus Totalis Born to Mothers with SARS-CoV-2 Infection: A Case Report. Diseases 2025, 13, 192. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, K.S.; Kim, J.H.; Lim, K.B.; Jeon, M.T.; Lee, J.B.; Park, S.Y.; Song, C.S.; Lee, S.W.; Lee, D.H.; et al. Cross-Species Transmission of SARS-CoV-2 From Dogs to Hamsters and Pathological Changes in the Brain. J. Med. Virol. 2025, 97, e70496. [Google Scholar] [CrossRef]
- Attipa, C.; Warr, A.S.; Epaminondas, D.; O’Shea, M.; Hanton, A.J.; Fletcher, S.; Malbon, A.; Lyraki, M.; Hammond, R.; Hardas, A.; et al. Feline infectious peritonitis epizootic caused by a recombinant coronavirus. Nature 2025, 645, 228–234. [Google Scholar] [CrossRef]
- Rajkhowa, S.; Sonowal, J.; Pegu, S.R.; Deb, R.; Gupta, V.K. Epidemiology and Emerging Trends of Zoonotic Viral Diseases of Pigs in India. Viruses 2025, 17, 381. [Google Scholar] [CrossRef]
- Zhou, P.; Fan, H.; Lan, T.; Yang, X.L.; Shi, W.F.; Zhang, W.; Zhu, Y.; Zhang, Y.W.; Xie, Q.M.; Mani, S.; et al. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature 2018, 556, 255–258. [Google Scholar] [CrossRef]
- Zhang, T.; Yao, J.; Yang, Z.; Wang, J.; Yang, K.; Yao, L. Re-emergence of severe acute diarrhea syndrome coronavirus (SADS-CoV) in Henan, central China, 2023. Vet. Microbiol. 2024, 292, 110049. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Q.N.; Su, J.N.; Chen, G.H.; Wu, Z.X.; Luo, Y.; Wu, R.T.; Sun, Y.; Lan, T.; Ma, J.Y. The re-emerging of SADS-CoV infection in pig herds in Southern China. Transbound. Emerg. Dis. 2019, 66, 2180–2183. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Gong, L.; Huang, L.; Lin, Y.; Qin, J.; Du, Y.; Zhou, Q.; Xue, C.; Cao, Y. Isolation and characterization of a highly pathogenic strain of Porcine enteric alphacoronavirus causing watery diarrhoea and high mortality in newborn piglets. Transbound. Emerg. Dis. 2019, 66, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xing, J.; Xu, Z.Y.; Gao, H.; Xu, S.J.; Liu, J.; Zhu, D.H.; Guo, Y.F.; Yang, B.S.; Chen, X.N.; et al. Re-emergence of Severe Acute Diarrhea Syndrome Coronavirus (SADS-CoV) in Guangxi, China, 2021. J. Infect. 2022, 85, e130–e133. [Google Scholar] [CrossRef] [PubMed]
- Le, N.P.; Le, B.T.; Le, V.P.; Park, J.E. Molecular characterization of swine acute diarrhea syndrome coronavirus detected in Vietnamese pigs. Vet. Res. 2025, 56, 4. [Google Scholar] [CrossRef]
- Jang, Y.J.; Le, N.P.; Lee, E.S.; Kim, M.C.; Chang, T.K.; Park, J.E. Development of an indirect enzyme-linked immunosorbent assay based on nucleocapsid protein for the detection of swine acute diarrhea syndrome coronavirus antibody. Virol. J. 2025, 22, 201. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Qin, P.; Wang, B.; Liu, Y.; Xu, G.H.; Peng, L.; Zhou, J.; Zhu, S.J.; Huang, Y.W. Broad Cross-Species Infection of Cultured Cells by Bat HKU2-Related Swine Acute Diarrhea Syndrome Coronavirus and Identification of Its Replication in Murine Dendritic Cells In Vivo Highlight Its Potential for Diverse Interspecies Transmission. J. Virol. 2019, 93, 24. [Google Scholar] [CrossRef]
- Hassanin, A.; Tu, V.T.; Pham, P.V.; Ngon, L.Q.; Chabane, T.; Moulin, L.; Wurtzer, S. Bat Rhinacoviruses Related to Swine Acute Diarrhoea Syndrome Coronavirus Evolve under Strong Host and Geographic Constraints in China and Vietnam. Viruses 2024, 16, 1114. [Google Scholar] [CrossRef]
- Duan, Y.; Yuan, C.; Suo, X.; Li, Y.; Shi, L.; Cao, L.; Kong, X.; Zhang, Y.; Zheng, H.; Wang, Q. Bat-Origin Swine Acute Diarrhea Syndrome Coronavirus Is Lethal to Neonatal Mice. J. Virol. 2023, 97, e0019023. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, R.D.; Wang, Q.; Luo, Y.; Liu, M.Q.; Zhu, Y.; Liu, X.; He, Y.T.; Zhou, P.; Yang, X.L.; et al. Lethal Swine Acute Diarrhea Syndrome Coronavirus Infection in Suckling Mice. J. Virol. 2022, 96, e0006522. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Lam, C.S.; Lau, C.C.; Tsang, A.K.; Lau, J.H.; Bai, R.; Teng, J.L.; Tsang, C.C.; Wang, M.; et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [CrossRef]
- Mei, X.Q.; Qin, P.; Yang, Y.L.; Liao, M.; Liang, Q.Z.; Zhao, Z.; Shi, F.S.; Wang, B.; Huang, Y.W. First evidence that an emerging mammalian alphacoronavirus is able to infect an avian species. Transbound. Emerg. Dis. 2022, 69, e2006–e2019. [Google Scholar] [CrossRef]
- Zhang, T.; Yao, J.; Yang, Z.; Wang, J.; Yang, K.; Yao, L. Recombinant porcine interferon delta 8 inhibits swine acute diarrhoea syndrome coronavirus infection in vitro and in vivo. Vet. Res. 2024, 55, 92. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Meng, S.; Shuai, L.; Zhang, H.; Hu, G.; Guo, H.; Chen, J.; Shan, D.; Du, Y.; Cao, Y.; et al. Differential Susceptibility to Porcine Deltacoronavirus: Ducks Show Greater Vulnerability Than Geese. Transbound. Emerg. Dis. 2025, 2025, 2339024. [Google Scholar] [CrossRef]
- Mohamed, R.I.; Mosad, S.M.; Ali, H.S.; Albalawi, W.O.; Elsamadony, H.A.; Ramzy, N.M.; Saad, A.S.; Fallatah, D.; Abdel-Hafez, L.J.M.; Albrakati, A.; et al. A comprehensive pathological and molecular investigation of viral co-infections in ducks in Egypt. Front. Microbiol. 2025, 16, 1522669. [Google Scholar] [CrossRef]
- Dai, Y.; Cheng, X.; Liu, M.; Shen, X.; Li, J.; Yu, S.; Zou, J.; Ding, C. Experimental infection of duck origin virulent Newcastle disease virus strain in ducks. BMC Vet. Res. 2014, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hu, T.; Zhou, Z.; He, Y.; Wang, T.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Zhao, X.; et al. First establishment of a Duck Model for in vivo and in vitro studies of West nile virus (Kunjin subtype). Poult. Sci. 2025, 104, 105214. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Liu, Y.; Liu, W.; Wang, G.; Yin, L.; Wang, J.; Dai, Y.; Shen, X.; Zhao, R.; Zhan, K.; et al. Inhibitory effect of curcumin on duck tembusu virus. Poult. Sci. 2025, 104, 105381. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Li, L.; Wang, J.; Yao, J.; Xu, G.; Leng, C.; Wang, Y.; Yao, L. Ducklings Were Susceptible to Swine Acute Diarrhea Syndrome Coronavirus Under Experimental Conditions. Microorganisms 2025, 13, 2122. https://doi.org/10.3390/microorganisms13092122
Zhang T, Li L, Wang J, Yao J, Xu G, Leng C, Wang Y, Yao L. Ducklings Were Susceptible to Swine Acute Diarrhea Syndrome Coronavirus Under Experimental Conditions. Microorganisms. 2025; 13(9):2122. https://doi.org/10.3390/microorganisms13092122
Chicago/Turabian StyleZhang, Teng, Longfa Li, Jiayi Wang, Jiale Yao, Guoqing Xu, Chaoliang Leng, Yong Wang, and Lunguang Yao. 2025. "Ducklings Were Susceptible to Swine Acute Diarrhea Syndrome Coronavirus Under Experimental Conditions" Microorganisms 13, no. 9: 2122. https://doi.org/10.3390/microorganisms13092122
APA StyleZhang, T., Li, L., Wang, J., Yao, J., Xu, G., Leng, C., Wang, Y., & Yao, L. (2025). Ducklings Were Susceptible to Swine Acute Diarrhea Syndrome Coronavirus Under Experimental Conditions. Microorganisms, 13(9), 2122. https://doi.org/10.3390/microorganisms13092122




