Control of Airborne and Surface Microorganisms in Real Indoor Environments Using an Integrated System of Vaporized Free Chlorine Components and Filtration
Abstract
1. Introduction
2. Materials and Methods
2.1. Integrated System of Vaporized Free Chlorine Components and Filtration
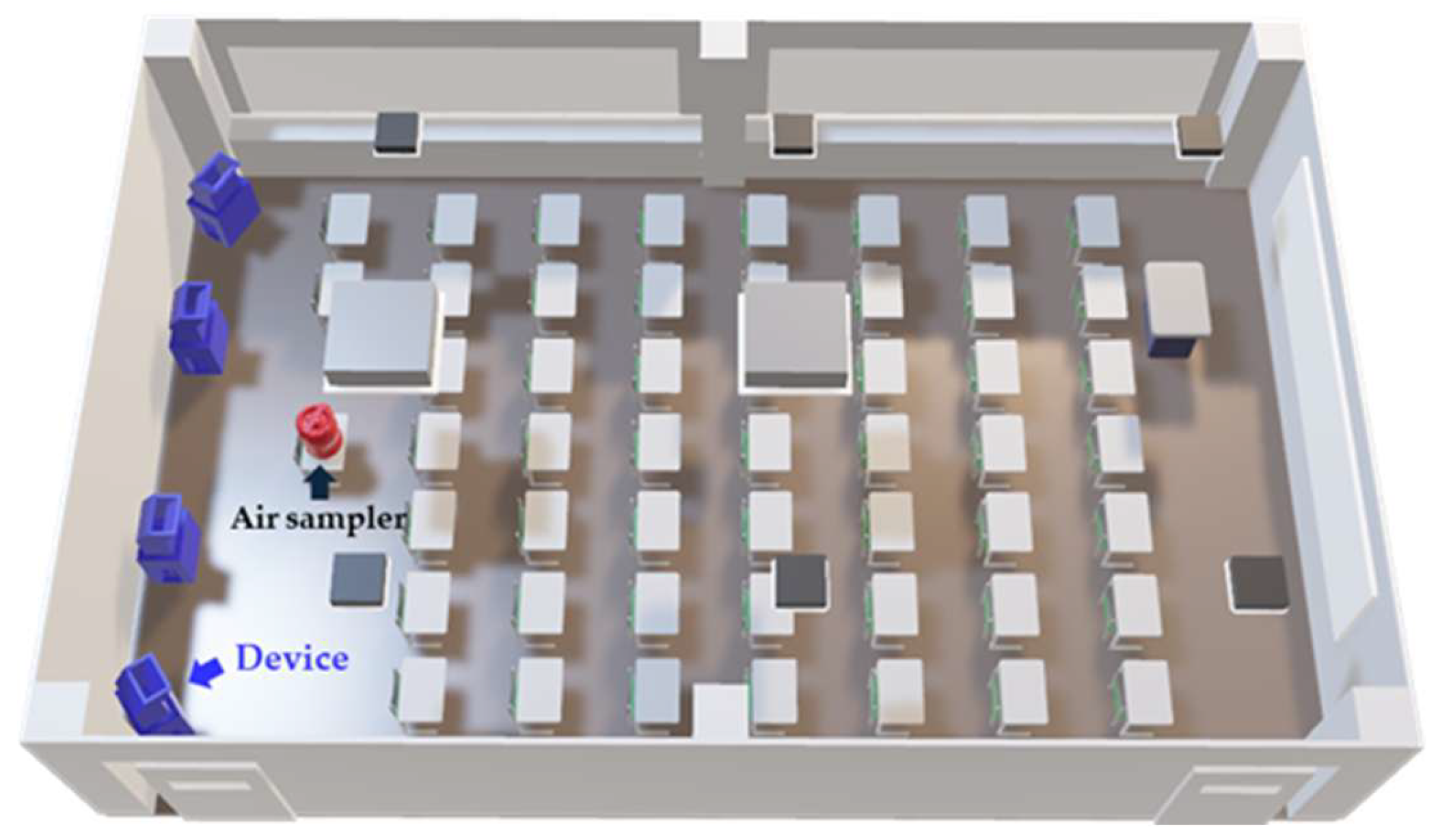
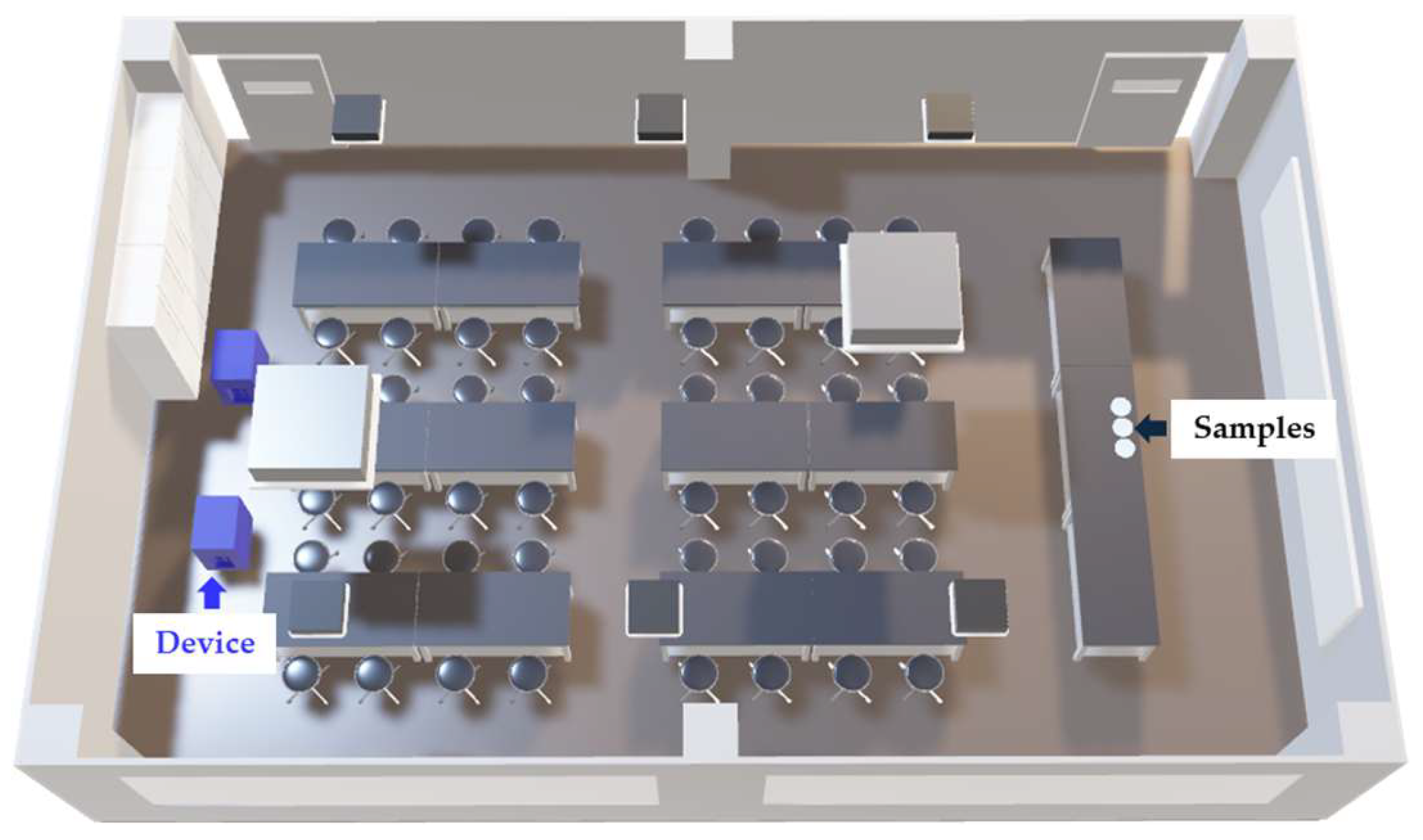
2.2. Environmental Conditions of the Examination Lecture Rooms
2.3. Airborne Bacteria Sampling Methods
2.4. Experimental Conditions for the Contact-Based Bacterial Inactivation Test with Vaporized Free Chlorine Components
2.5. Inactivation Test for Bacteria
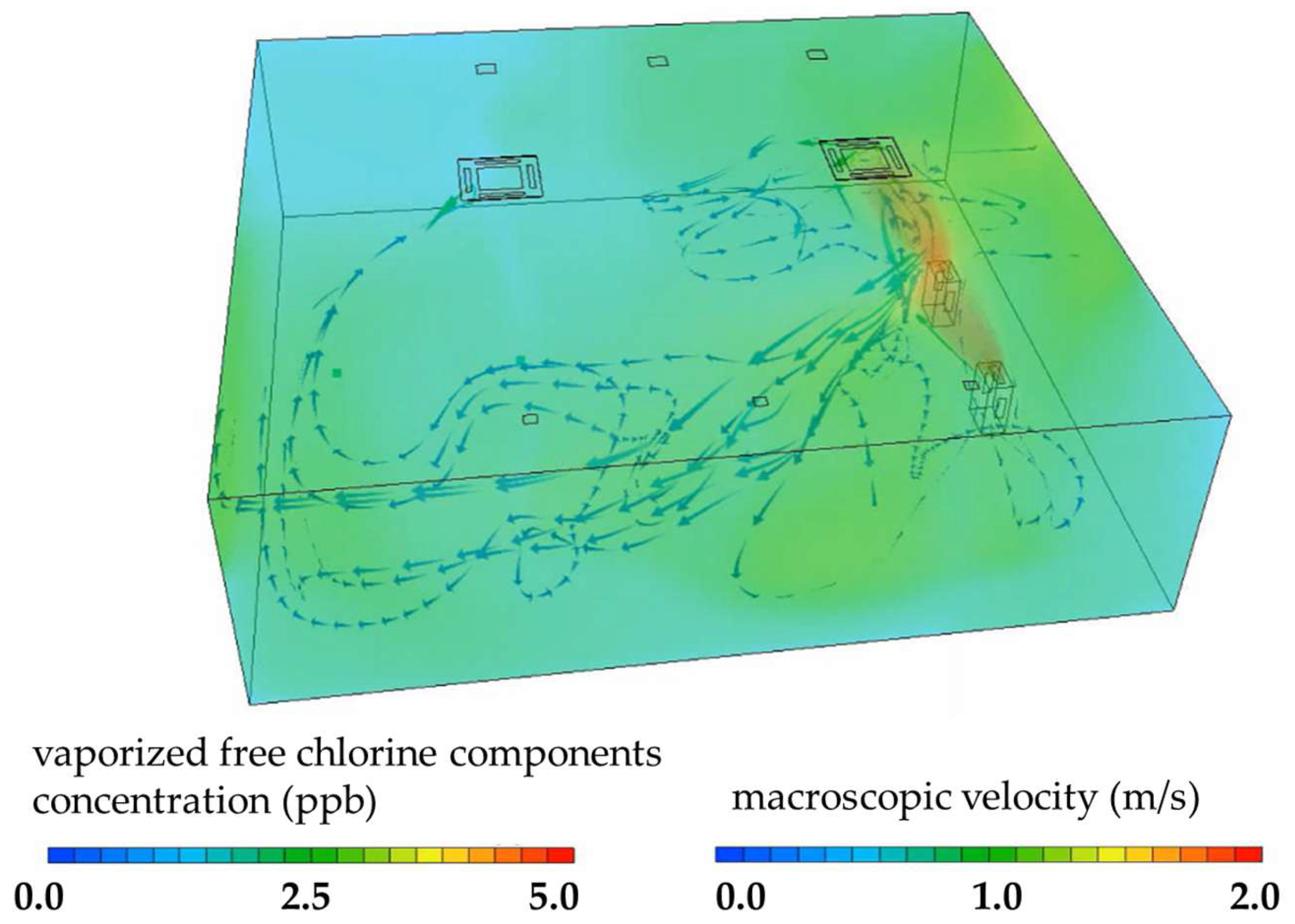
2.6. Simulation of the Concentration of Vaporized Free Chlorine Components
2.7. Statistical Analyses
3. Results
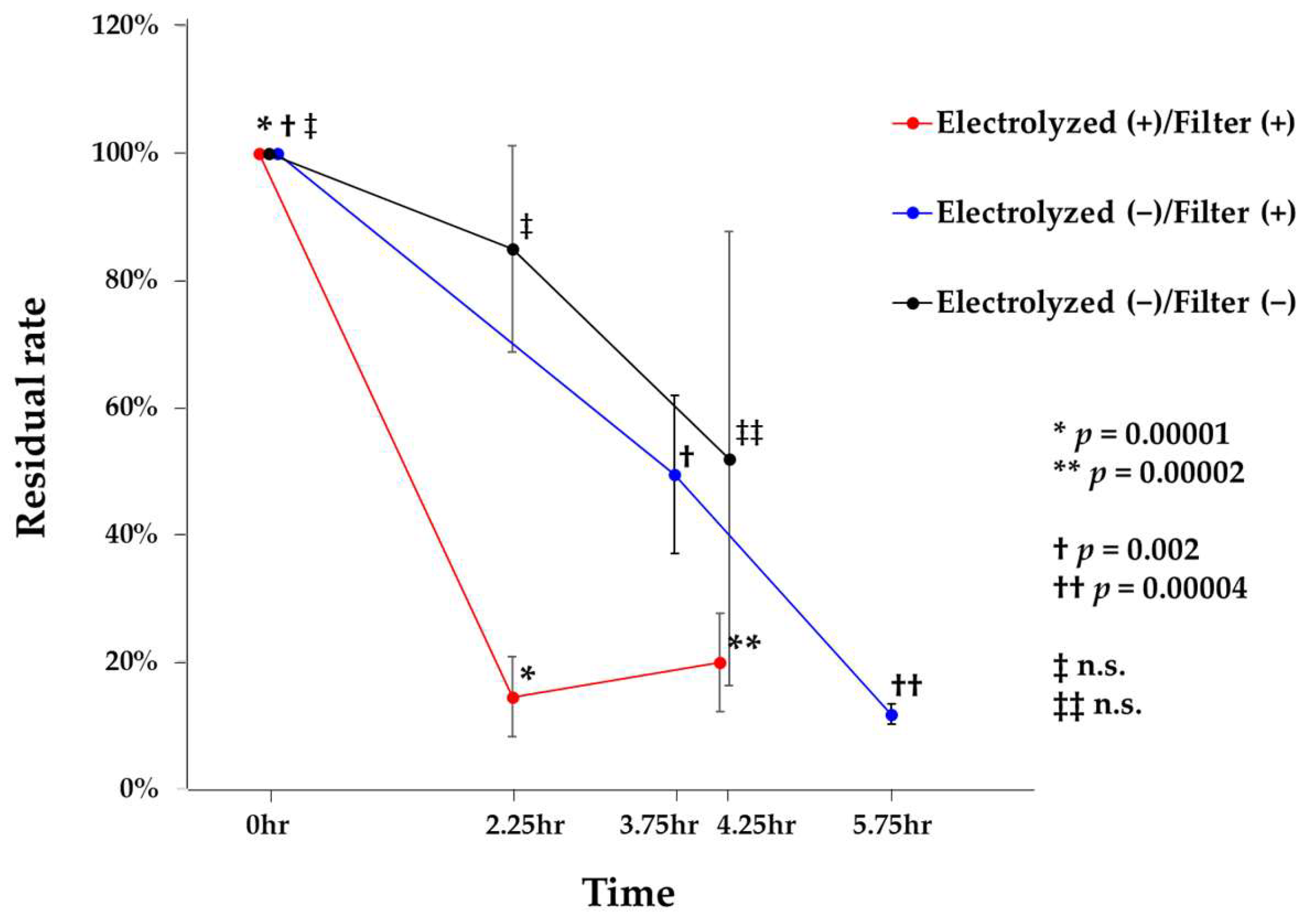
3.1. Airborne Bacteria Tests
3.2. Contact Tests for Bactericidal Inactivation
3.3. Simulation Analysis of Vaporized Free Chlorine Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsuki, M.; Mano, Y.; Furuya, N. Distribution Survey of Floating Bacteria in General Environments by Air Sampler. Jpn. J. Environ. Infect. 2019, 34, 141–146. [Google Scholar] [CrossRef]
- Terayama, K.; Taneichi, F.; Honma, H.; Kawarabayashi, T.; Yokota, M.; Aoi, Y.; Nakata, H. Studies on Bacterial Aerosol Part 5 Analysis of Airborne, Fallen and Floor Bacteria in the Room. Jpn. J. Hyg. 1980, 35, 479–485. [Google Scholar] [CrossRef]
- Naru, K.; Matsunaga, N.; Noguchi, N.; Nojo, J.; Tomizawa, T.; Ishii, T.; Namiki, U.; Yamanaka, Y.; Kumaki, U.; Suwa, J.; et al. Microbial Surveillance Conducted for Infection Control at a Hospital. Jpn. J. Pharm. Health Care Sci. 2008, 34, 441–447. [Google Scholar] [CrossRef]
- Ishimatsu, S. Recent Topics on Microbes in Indoor Environment (4) Microbial Contamination and Countermeasures in Work places. Indoor Environ. 2019, 22, 289–294.5. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). C. Air|Infection Control. Available online: https://www.cdc.gov/infection-control/hcp/environmental-control/air.html (accessed on 20 August 2025).
- Imai, H.; Endo, S.; Horiuchi, Y.; Yoshida, M.; Kaku, M. Experience with HEPA-Filtered Air Purifiers during the COVID-19 Outbreak. Jpn. J. Environ. Infect. 2024, 39, 209–213. [Google Scholar] [CrossRef]
- Shiraishi, T. Disinfectant Knowledge You Need to Know. J. Jpn. Soc. Int. Med. 2010, 99, 1916–1922. [Google Scholar] [CrossRef][Green Version]
- Yoshida, S.; Muramatsu, T.; Fukuzaki, T. Volatilization of Hypochlorous Acid from a Solution-Impregnated Fabric Filter in a Forced-Air Vaporizing System. J. Antibact. Antifung. Agents 2016, 44, 113–118. [Google Scholar][Green Version]
- Yoshida, S.; Hayashi, T.; Ibuka, S.; Horikiri, S.; Fukuzaki, T. Effect of Relative Humidity on the Bactericidal Action of Gaseous Hypochlorous Acid against Staphylococcus aureus on a Dry Solid Surface. J. Antibact. Antifung. Agents 2019, 47, 3–6. [Google Scholar][Green Version]
- Makimura, S.; Kato, R.; Yoshida, S.; Hayashi, T.; Ibuka, S.; Horikiri, S.; Fukuzaki, T. Effect of Chlorine-Consuming Organic Substances on the Bactericidal Action of Gaseous Hypochlorous Acid on a Wet Agar Plate. J. Environ. Control Tech. 2019, 37, 163–169. [Google Scholar][Green Version]
- Mizuno, Y.; Ibuka, S.; Hayashi, T.; Horikiri, S.; Fukuzaki, T. Bactericidal Action of Gaseous Hypochlorous Acid against Staphylococcus epidermidis in Aerosols in a Confined Space. J. Environ. Control Tech. 2020, 38, 152–157. [Google Scholar][Green Version]
- Nakamura, K.; Katou, R.; Hayashi, T.; Ishida, Y.; Horikiri, S.; Yoshida, S.; Fukuzaki, S. Volatility and Antimicrobial Actions of Hypochlorous Acid and Monochloramin in a Forced-Air Vaporizing System under Alkaline Conditions. J. Antibact. Antifung. Agents 2021, 49, 3–9. [Google Scholar][Green Version]
- Nakamura, K.; Hotta, H.; Hayashi, T.; Ishida, Y.; Yoshida, S.; Fukuzaki, S. Indoor Concentration and Bactericidal Action of Gaseous Hypochlorous Acid during the Operation of a Forced-Air Vaporizer Fed with Weakly Alkaline Hypochlorite Solution. J. Environ. Control Tech. 2021, 39, 300–305. [Google Scholar][Green Version]
- Boecker, D.; Breves, R.; Herth, F.; Zhang, Z.; Bulitta, C. Safe, Effective, and Cost-Efficient Air Cleaning for Populated Rooms and Entire Buildings Based on the Disinfecting Power of Vaporized Hypochlorous Acid. Int. J. Health Med. Eng. 2022, 16, 209–213. [Google Scholar][Green Version]
- Boecker, D.; Zhang, Z.; Breves, R.; Herth, F.; Kramer, A.; Bulitta, C. Antimicrobial Efficacy, Mode of Action and in vivo Use of Hypochlorous Acid (HOCl) for Prevention or Therapeutic Support of Infections. GMS Hyg. Infect. Control 2023, 18, Doc07, GBIF Occurrence Download. Available online: https://doi.org/10.3205/dgkh000433 (accessed on 1 September 2025).
- Fukuzaki, S. Uses of Gaseous Hypochlorous Acid for Controlling Microorganisms in Indoor Spaces. J. Microorg. Control 2023, 28, 165–175, GBIF Occurrence Download. Available online: https://doi.org/10.4265/jmc.28.4_165 (accessed on 1 September 2025).[Green Version]
- Hayashi, T.; Mizuno, Y.; Yoshida, S.; Fukuzaki, T. Efficacy of Gas-phase Residual Hypochlorous Acid in Disinfecting Bacteria in Aerosols under Low Humidity Conditions. J. Environ. Control Tech. 2024, 42, 85–90. [Google Scholar][Green Version]
- Ogata, N.; Sakasegawa, M.; Miura, T.; Shibata, T.; Takigawa, Y.; Taura, K.; Taguchi, K.; Matsubara, K.; Nakahara, K.; Kato, D.; et al. Inactivation of Airborne Bacteria and Viruses Using Extremely Low Concentrations of Chlorine Dioxide Gas. Pharmacology 2016, 97, 301–306, GBIF Occurrence Download. Available online: https://doi.org/10.1159/000444503 (accessed on 1 September 2025). [CrossRef]
- Kato, R.; Makimura, S.; Yoshida, S.; Muramatsu, T.; Hayashi, T.; Ibuka, S.; Fukuzaki, T. Analysis of Stripping Process of Hypochlorous Acid in a Forced-Air Vaporizing System. J. Environ. Control Tech. 2018, 36, 35–39. [Google Scholar]
- Nakato, T.; Hamatani, M.; Nikaido, M.; Morozumi, H.; Nishiki, Y.; Tsuchizaki, N.; Sudo, Y.; Kikuchi, K.; Hotta, K. Establishment of JIS B 8701 for Hypochlorous Acid Water producing Apparatus. J. Func. Water 2018, 13, 19–26, GBIF Occurrence Download. Available online: https://doi.org/10.69185/jfw.13.2_19 (accessed on 1 September 2025).
- Yoshida, S.; Hayashi, T.; Kato, R.; Kusakawa, T.; Fukuzaki, T. Simple Measurement of Gaseous Hypochlorous acid using a Chlorine Gas Detector Equipped with a Controlled Potential Electrolysis Sensor. J. Environ. Control Tech. 2017, 35, 260–266. [Google Scholar]
- Shibuya, K. Determining Airborne Biocontamination. J. Aerosol. Res. 2003, 18, 172–176. [Google Scholar]
- Yi, J.; Lee, J.; Fikri, M.A.; Sang, B.-I.; Kim, H. Application of Computational Fluid Dynamics in Chlorine-Dynamics Modeling of In-Situ Chlorination Systems for Cooling Systems. Appl. Sci. 2020, 10, 4455, GBIF Occurrence Download. Available online: https://doi.org/10.3390/app10134455 (accessed on 1 September 2025). [CrossRef]
- Kawaguchi, Y.; Oie, S.; Furukawa, H. Efficacy of Complex-Type Chlorin-Based Disinfectant Cleaner against MDRP and MDRA. Jpn. J. Environ. Infect. 2016, 31, 366–369. [Google Scholar]
- Aratani, Y. Role of Myeloperoxidase in the Host Defense against Fungal Infection. Jpn. J. Med. Mycol. 2006, 47, 195–199. [Google Scholar] [CrossRef]
- Ueda, S.; Kuwabara, Y. Air Borne Bacteria and Bacilli in a Kitchen. J. Food Sci. Technol. 1980, 27, 161–165. [Google Scholar] [CrossRef]
- Kawakami, Y. Recent Topics on Microbes in Indoor Environments (1) Introduction: Changes in Housing Patterns and Deseases Associated with Problems of Indoor Environmental Microbes. Indoor Environ. 2018, 21, 209–216. [Google Scholar] [CrossRef]
- Nakamura, S.; Kuwahara, M.; Fukuda, K.; Yamanaka, N.; Ishihara, M. Bactericidal Effect of HOCl Aqueous Solution on Microorganisms Such as Viruses and Its Application. J. Natl. Def. Med. Coll. 2017, 42, 8–14. [Google Scholar]
- Miyashita, M.; Sugawa, M.; Sone, A.; Sato, K.; Tsuruya, S.; Hirose, K.; Mori, M.; Hayashi, H.; Komoriyama, H.; Kawashima, Y. Effectiveness of Supplying Enteral Nutrition Formulas through Newly Developed Disposal Containers, the Second Report-The Investigation on Sanitary Conditions. J. Jpn. Dietet. Assoc. 2011, 54, 419–423. [Google Scholar]
- Taguchi, F.; Saito-Taki, T.; Okuda, S.; Aoki, M.; Matsuzaki, T.; Tomioka, M.; Kikuno, R.; Lee, S.M. Proposal for the Nosocomial Infection Control of Methicilin-Resistant Staphylococcus aureus (MRSA). Jpn. J. Bacteriol. 1992, 47, 767–775. [Google Scholar] [CrossRef]
- Takahashi, T.; Onoue, Y.; Mori, M. Monthly Variation of Bacterial Contamination and Incidence of Staphylococcus aureus in a Japanese Lunch “Makunouchi-Bento”. Food Hyg.Saf. 1979, 20, 204–210. [Google Scholar] [CrossRef]
- Nakamura, H.; Ogasawara, J.; Yasufuku, K.; Arikawa, K.; Ohyama, M.; Abe, N.; Hase, A. Identification and Comparison of Isolates from Several Coliform Group Detection Agar Medium. Jpn. J. Food. Microbiol. 2012, 29, 164–169. [Google Scholar] [CrossRef][Green Version]
- Clauditz, A.; Resch, A.; Wieland, K.-P.; Peschel, A.; Gotz, F. Staphyloxanthin Plays a Role in the Fitness of Staphylococcus aureus and Its Ability To Cope with Oxidative Stress. Infect. Immun. 2006, 74, 4950–4953, GBIF Occurrence Download. Available online: https://doi.org/10.1128/iai.00204-06 (accessed on 1 September 2025). [CrossRef] [PubMed]
- Linzner, N.; Loi, V.V.; Fritsch, V.N.; Antelmann, H. Thiol-Based Redox Switches in the Major Pathogen Staphylococcus aureus. Biol. Chem. 2021, 402, 333–361, GBIF Occurrence Download. Available online: https://doi.org/10.1515/hsz-2020-0272 (accessed on 1 September 2025). [CrossRef]
- Kato, I. Microbiological Knowledge Necessary for Understanding and Analyzing Data from JANIS. Jpn. J. Environ. Infect. 2019, 34, 129–134. [Google Scholar] [CrossRef]
- Chigusa, H.; Okawa, T.; Yokota, M.; Nikaido, M.; Matsumura, Y.; Iwasawa, A. Bactericidal and Corrosive Properties of Hypochlorous Acid Water at Various pH and Available Chlorine Concentrations. J. Antibact. Antifung. Agents 2017, 45, 585–593. [Google Scholar]
- Hattori, M. Advanced Technologies for the Human Gut Microbiome Analysis. Jpn. J. Clin. Immunol. 2014, 37, 412–422. [Google Scholar] [CrossRef]
- Muramatsu, T.; Kodama, K.; Yamada, T.; Yamada, A.; Fukuzaki, S. Inhalation of Gaseous Hypochlorous Acid and Its Effect on Human Respiratory Epithelial Cells in Laboratory Model Systems. J. Microorg. Control 2024, 29, 39–44, GBIF Occurrence Download. Available online: https://doi.org/10.4265/jmc.29.1_39 (accessed on 1 September 2025). [CrossRef] [PubMed]
| Bacteria | Electrolyzed (−) (CFU/mL) | Electrolyzed (+) (CFU/mL) | Reduction Rate (%) | p Value |
|---|---|---|---|---|
| E. coli | 1.3 × 104 ± 5.2 × 103 | 6.9 × 102 ± 3.0 × 102 | 94.7 ± 2.3 | 0.0286 |
| P. aeruginosa | 1.7 × 105 ± 1.6 × 105 | 5.5 × 102 ± 6.9 × 102 | 99.7 ± 0.4 | 0.0286 |
| S. aureus | 7.5 × 104 ± 2.4 × 104 | 3.1 × 104 ± 5.8 × 103 | 59.0 ± 7.7 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawahata, S.; Kondo, M.; Yamada, A.; Shimazaki, N.; Saito, M.; Takano, T.; Yamada, T.; Shimayama, Y.; Matsuoka, S.; Kimura, H. Control of Airborne and Surface Microorganisms in Real Indoor Environments Using an Integrated System of Vaporized Free Chlorine Components and Filtration. Microorganisms 2025, 13, 2053. https://doi.org/10.3390/microorganisms13092053
Kawahata S, Kondo M, Yamada A, Shimazaki N, Saito M, Takano T, Yamada T, Shimayama Y, Matsuoka S, Kimura H. Control of Airborne and Surface Microorganisms in Real Indoor Environments Using an Integrated System of Vaporized Free Chlorine Components and Filtration. Microorganisms. 2025; 13(9):2053. https://doi.org/10.3390/microorganisms13092053
Chicago/Turabian StyleKawahata, Saki, Mayumi Kondo, Atsushi Yamada, Naoya Shimazaki, Makoto Saito, Takayoshi Takano, Tetsuyoshi Yamada, Yoshinobu Shimayama, Shunsuke Matsuoka, and Hirokazu Kimura. 2025. "Control of Airborne and Surface Microorganisms in Real Indoor Environments Using an Integrated System of Vaporized Free Chlorine Components and Filtration" Microorganisms 13, no. 9: 2053. https://doi.org/10.3390/microorganisms13092053
APA StyleKawahata, S., Kondo, M., Yamada, A., Shimazaki, N., Saito, M., Takano, T., Yamada, T., Shimayama, Y., Matsuoka, S., & Kimura, H. (2025). Control of Airborne and Surface Microorganisms in Real Indoor Environments Using an Integrated System of Vaporized Free Chlorine Components and Filtration. Microorganisms, 13(9), 2053. https://doi.org/10.3390/microorganisms13092053






