Ribosome-Associated Quality Control Mediated by Rqc2 Contributes to the Lytic Cycle and Stage Conversion of Toxoplasma gondii
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Studies and Ethical Approval
2.2. Sequences Analysis and Phylogeny
2.3. Parasite Strains and Culturing
2.4. Construction of Plasmids
2.5. Transfection and Selection of Parasites
2.6. Immunofluorescent Assay (IFA)
2.7. Plaque Assays
2.8. Invasion Assay
2.9. Replication Assay of Intracellular Parasites
2.10. Induced Egress Assay
2.11. Detection of Bradyzoite Cysts In Vivo
2.12. RNA-Seq Analysis
3. Results
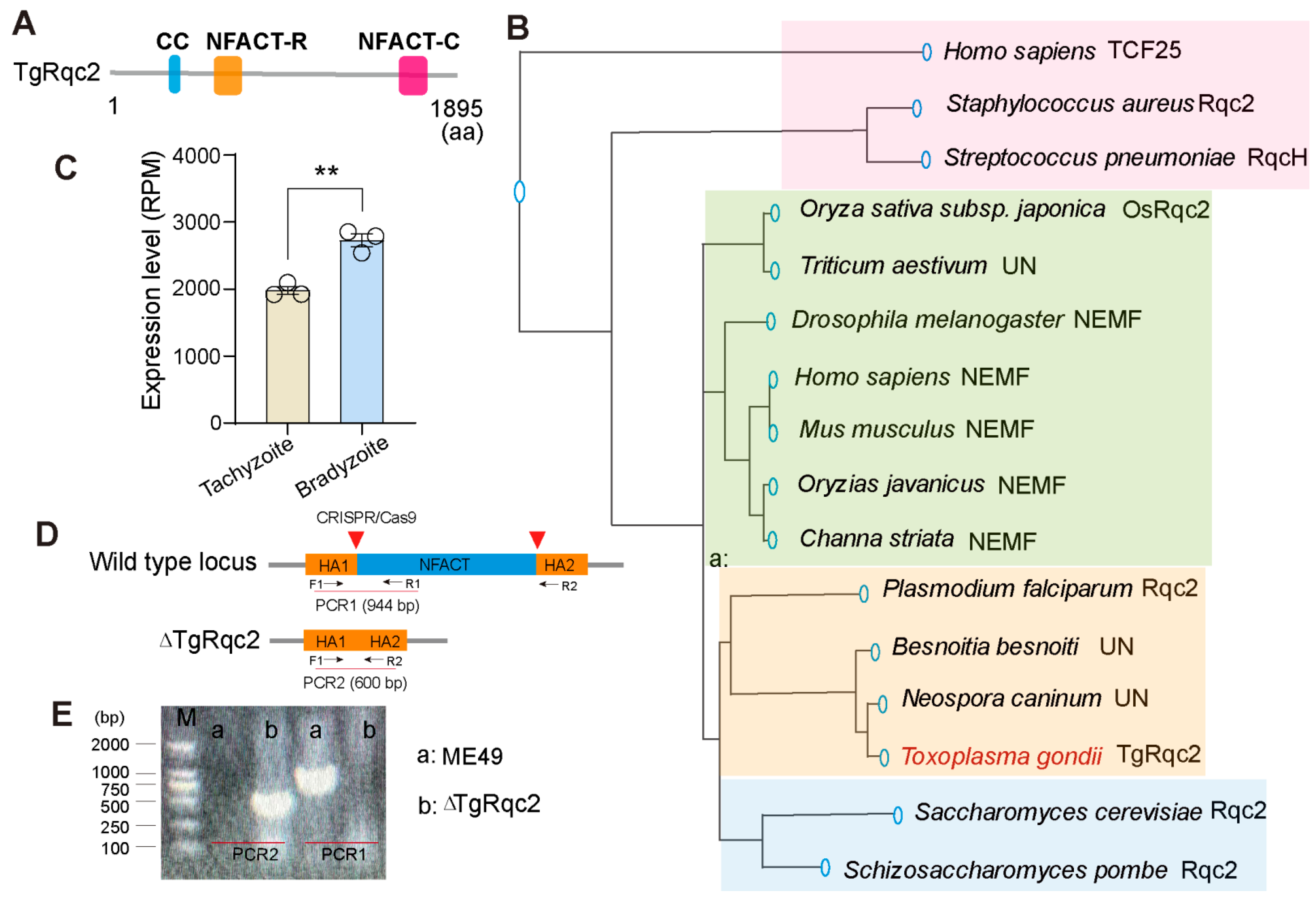
3.1. Characterization of RQC Protein TgRqc2
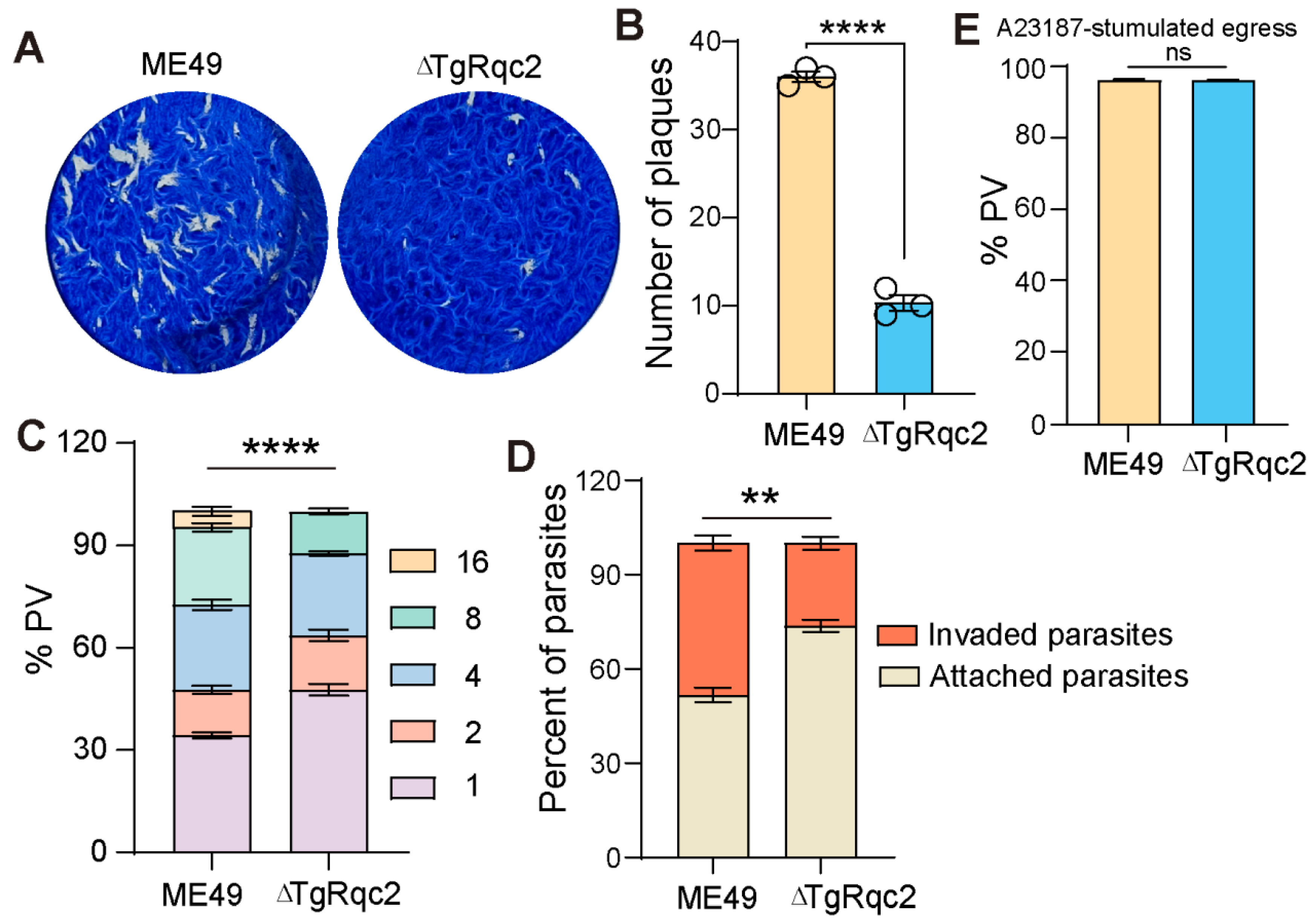
3.2. TgRqc2 Is Essential for the Lytic Cycle of T. gondii
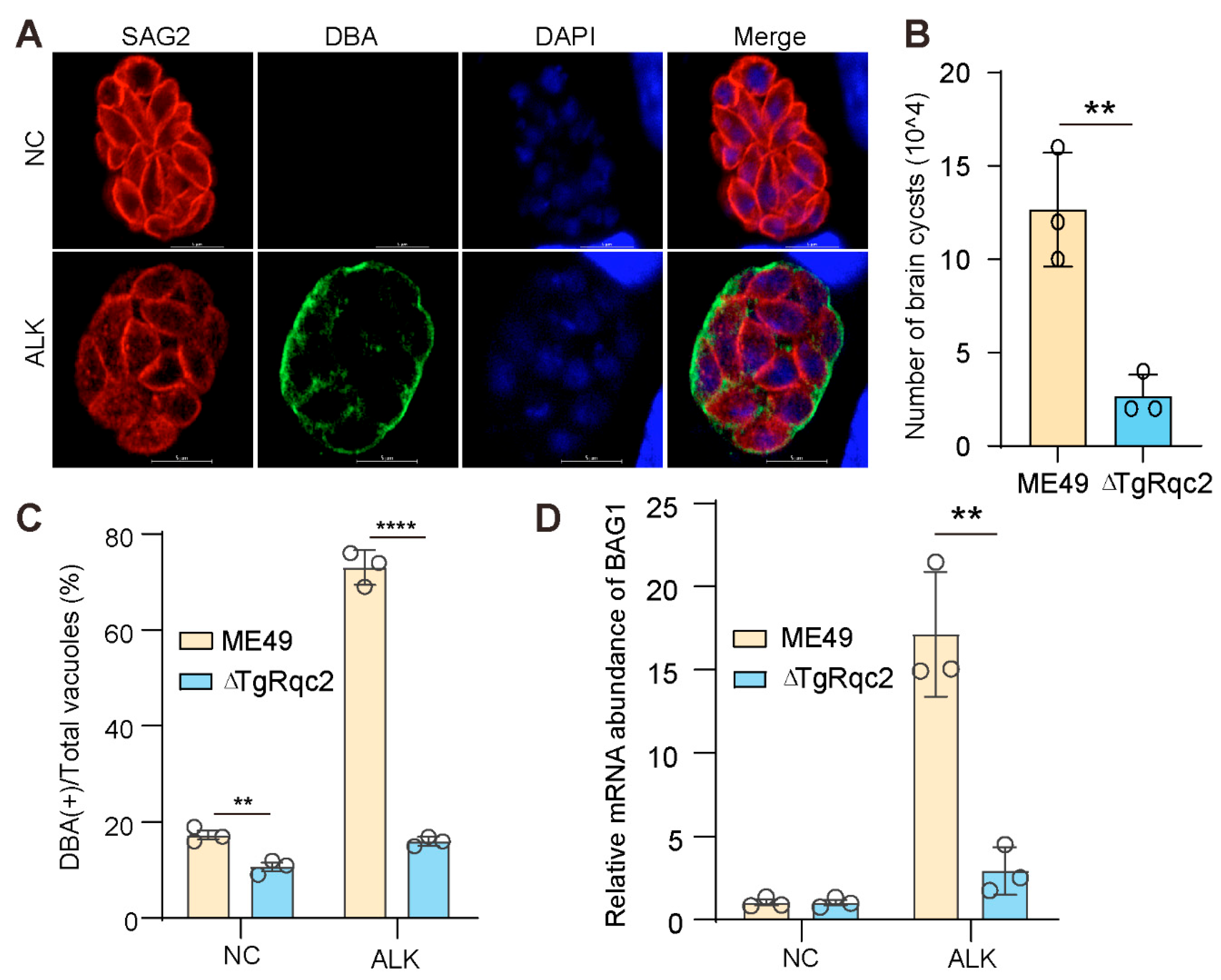
3.3. Depletion of TgRqc2 Reduces the Formation of Bradyzoites
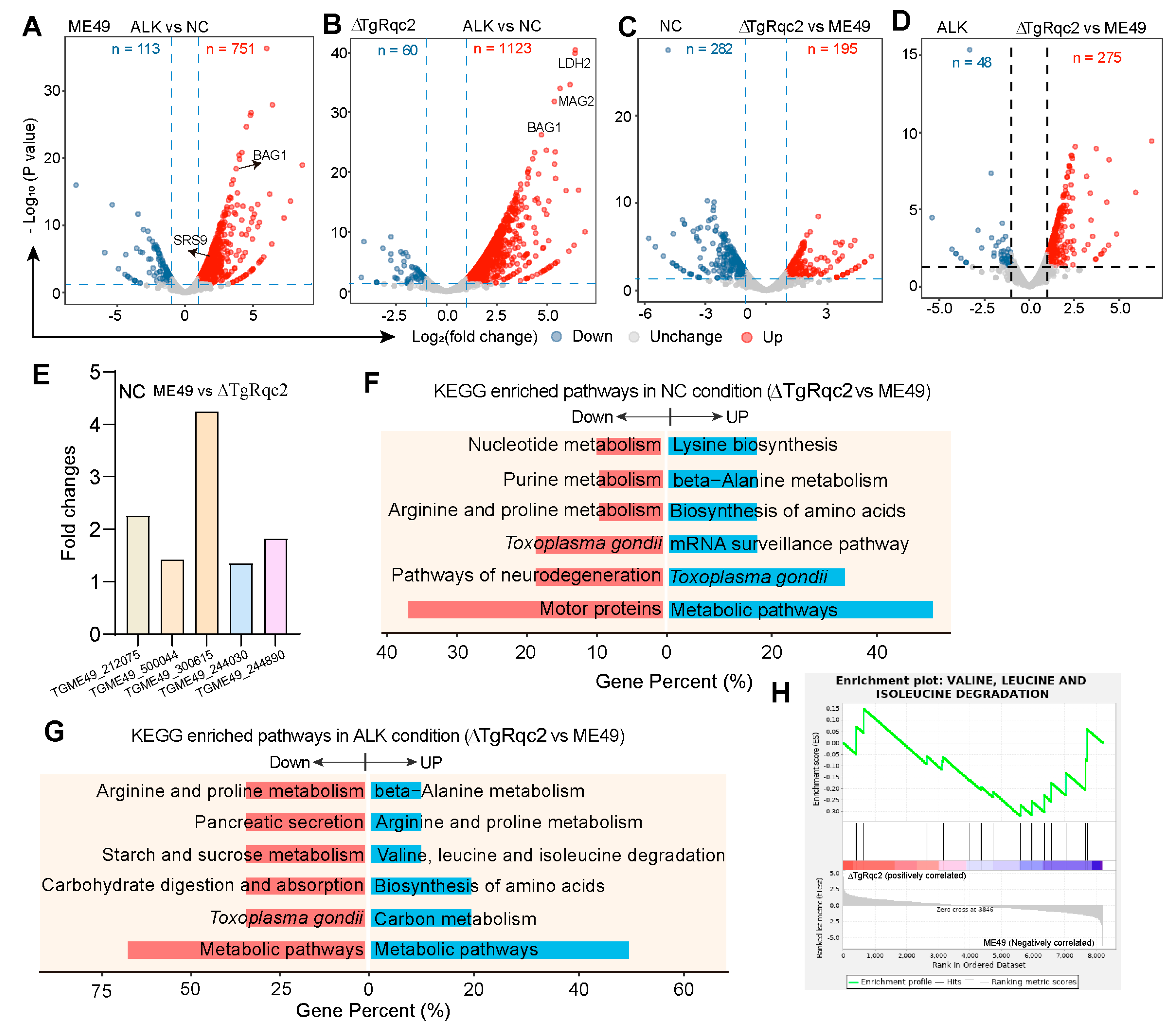
3.4. Depletion of TgRqc2 Reprograms the Transcript Profile
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P.; Lindsay, D.S.; Speer, C.A. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 1998, 11, 267–299. [Google Scholar] [CrossRef]
- Sanchez, S.G.; Bassot, E.; Cerutti, A.; Mai Nguyen, H.; Aïda, A.; Blanchard, N.; Besteiro, S. The apicoplast is important for the viability and persistence of Toxoplasma gondii bradyzoites. Proc. Natl. Acad. Sci. USA. 2023, 120, e2309043120. [Google Scholar] [CrossRef]
- Lyons, R.E.; McLeod, R.; Roberts, C.W. Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends Parasitol. 2002, 18, 198–201. [Google Scholar] [CrossRef]
- Augusto, L.; Wek, R.C.; Sullivan, W.J., Jr. Host sensing and signal transduction during Toxoplasma stage conversion. Mol. Microbiol. 2021, 115, 839–848. [Google Scholar] [CrossRef]
- Jeffers, V.; Tampaki, Z.; Kim, K.; Sullivan, W.J., Jr. A latent ability to persist: Differentiation in Toxoplasma gondii. Cell Mol. Life Sci. 2018, 75, 2355–2373. [Google Scholar] [CrossRef]
- Hershey, J.W.B.; Sonenberg, N.; Mathews, M.B. Principles of translational control. Cold Spring Harb. Perspect. Biol. 2019, 11, a032607. [Google Scholar] [CrossRef]
- Lasko, P. mRNA localization and translational control in Drosophila oogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a012294. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Sossin, W.S.; Klann, E.; Sonenberg, N. Translational control of long-lasting synaptic plasticity and memory. Neuron 2009, 61, 10–26. [Google Scholar] [CrossRef]
- Holmes, M.J.; Bastos, M.S.; Dey, V.; Severo, V.; Wek, R.C.; Sullivan, W.J., Jr. mRNA cap-binding protein eIF4E1 is a novel regulator of Toxoplasma gondii latency. mBio 2024, 15, e0295423. [Google Scholar] [CrossRef]
- Liu, X.C.; Li, C.J.; Li, X.Y.; Ehsan, M.; Lu, M.M.; Li, K.; Xu, L.X.; Yan, R.F.; Song, X.K.; Li, X.R. Proteomics analysis reveals that the proto-oncogene eIF-5A indirectly influences the growth, invasion and replication of Toxoplasma gondii tachyzoite. Parasit. Vectors 2021, 14, 283. [Google Scholar] [CrossRef]
- Treeck, M.; Sanders, J.L.; Elias, J.E.; Boothroyd, J.C. The phosphoproteomes of Plasmodium falciparum and Toxoplasma gondii reveal unusual adaptations within and beyond the parasites’ boundaries. Cell Host Microbe 2011, 10, 410–419. [Google Scholar] [CrossRef]
- Kim, K.Q.; Zaher, H.S. Canary in a coal mine: Collided ribosomes as sensors of cellular conditions. Trends Biochem. Sci. 2022, 47, 82–97. [Google Scholar] [CrossRef]
- Bengtson, M.H.; Joazeiro, C.A. Role of a ribosome-associated E3 ubiquitin ligase in protein quality control. Nature 2010, 467, 470–473. [Google Scholar] [CrossRef]
- Joazeiro, C.A.P. Mechanisms and functions of ribosome-associated protein quality control. Nat. Rev. Mol. Cell Biol. 2019, 20, 368–383. [Google Scholar] [CrossRef]
- Inada, T. Quality controls induced by aberrant translation. Nucleic Acids Res. 2020, 48, 1084–1096. [Google Scholar] [CrossRef]
- Filbeck, S.; Cerullo, F.; Pfeffer, S.; Joazeiro, C.A.P. Ribosome-associated quality-control mechanisms from bacteria to humans. Mol. Cell 2022, 82, 1451–1466. [Google Scholar] [CrossRef]
- Lv, L.; Mo, J.Y.; Qing, Y.M.; Wang, S.C.; Chen, L.J.; Mei, A.N.; Xu, R.; Huang, H.L.; Tan, J.Q.; Li, Y.F.; et al. NEMF-mediated Listerin-independent mitochondrial translational surveillance by E3 ligase Pirh2 and mitochondrial protease ClpXP. Cell Rep. 2024, 43, 113860. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Serra, F.; Bork, P. ETE 3: Reconstruction, analysis, and visualization of phylogenomic data. Mol. Biol. Evol. 2016, 33, 1635–1638. [Google Scholar] [CrossRef]
- Sokol, D.K. Beware the lies of patients. BMJ 2014, 348, g382. [Google Scholar] [CrossRef]
- Fu, J.W.; Zhao, L.; Yang, J.; Chen, H.M.; Cao, S.N.; Jia, H.L. An unconventional SNARE complex mediates exocytosis at the plasma membrane and vesicular fusion at the apical annuli in Toxoplasma gondii. PLoS Pathog. 2023, 19, e1011288. [Google Scholar] [CrossRef]
- Buchholz, K.R.; Bowyer, P.W.; Boothroyd, J.C. Bradyzoite pseudokinase 1 is crucial for efficient oral infectivity of the Toxoplasma gondii tissue cyst. Eukaryot. Cell 2013, 12, 399–410. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Alexa, A.; Rahnenführer, J.; Lengauer, T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 2006, 22, 1600–1607. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.Z.; Huang, J.J.; Ding, Y.; Wu, J.M.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L.P. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Holmes, M.J.; Padgett, L.R.; Bastos, M.S.; Sullivan, W.J., Jr. m6A RNA methylation facilitates pre-mRNA 3-end formation and is essential for viability of Toxoplasma gondii. PLoS Pathog. 2021, 17, e1009335. [Google Scholar] [CrossRef]
- Lang, W.H.; Calloni, G.; Vabulas, R.M. Polylysine is a proteostasis network-engaging structural determinant. J. Proteome Res. 2018, 17, 1967–1977. [Google Scholar] [CrossRef]
- Davis, Z.H.; Mediani, L.; Antoniani, F.; Vinet, J.; Li, S.X.; Alberti, S.; Lu, B.W.; Holehouse, A.S.; Carra, S.; Brandman, O. Protein products of nonstop mRNA disrupt nucleolar homeostasis. Cell Stress Chaperones 2021, 26, 549–561. [Google Scholar] [CrossRef]
- Frischmeyer, P.A.; van Hoof, A.; O’Donnell, K.; Guerrerio, A.L.; Parker, R.; Dietz, H.C. An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science 2002, 295, 2258–2261. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Lu, D.Q.; Tian, S.Y.; Lv, Y.; Chen, M.W.; Tian, X.; Wu, Y.T.; Luo, F.J.; Tan, F.; Mou, Y.N. Ubiquitin-activating enzyme1 (TgUAE1) acts as a key regulator of Toxoplasma gondii lytic cycle and homeostasis. Commun. Biol. 2025, 8, 728. [Google Scholar] [CrossRef]
- Lyumkis, D.; Oliveira dos Passos, D.; Tahara, E.B.; Webb, K.; Bennett, E.J.; Vinterbo, S.; Potter, C.S.; Carragher, B.; Joazeiro, C.A. Structural basis for translational surveillance by the large ribosomal subunit-associated protein quality control complex. Proc. Natl. Acad. Sci. USA. 2014, 111, 15981–15986. [Google Scholar] [CrossRef]
- Lu, J.L.; Deutsch, C. Electrostatics in the ribosomal tunnel modulate chain elongation rates. J. Mol. Biol. 2008, 384, 73–86. [Google Scholar] [CrossRef]
- Soudan, B.; Tetaert, D.; Racadot, A.; Degand, P.; Boersma, A. Decrease of testosterone level during an experimental African trypanosomiasis: Involvement of a testicular LH receptor desensitization. Acta Endocrinol. (Copenh.) 1992, 127, 86–92. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Kim, J.H.; Lee, J.; Park, H.; Lim, C. ZNF598 co-translationally titrates poly(GR) protein implicated in the pathogenesis of C9ORF72-associated ALS/FTD. Nucleic Acids Res. 2021, 49, 11294–11311. [Google Scholar] [CrossRef]
- Kriachkov, V.; Ormsby, A.R.; Kusnadi, E.P.; McWilliam, H.E.G.; Mintern, J.D.; Amarasinghe, S.L.; Ritchie, M.E.; Furic, L.; Hatters, D.M. Arginine-rich C9ORF72 ALS proteins stall ribosomes in a manner distinct from a canonical ribosome-associated quality control substrate. J. Biol. Chem. 2023, 299, 102774. [Google Scholar] [CrossRef]
- Wu, Z.H.; Tantray, I.; Lim, J.; Chen, S.J.; Li, Y.; Davis, Z.; Sitron, C.; Dong, J.; Gispert, S.; Auburger, G.; et al. MISTERMINATE mechanistically links mitochondrial dysfunction with proteostasis failure. Mol. Cell 2019, 75, 835–848.e838. [Google Scholar] [CrossRef]
- Vaillant-Beuchot, L.; Mary, A.; Pardossi-Piquard, R.; Bourgeois, A.; Lauritzen, I.; Eysert, F.; Kinoshita, P.F.; Cazareth, J.; Badot, C.; Fragaki, K.; et al. Accumulation of amyloid precursor protein C-terminal fragments triggers mitochondrial structure, function, and mitophagy defects in Alzheimer’s disease models and human brains. Acta Neuropathol. 2021, 141, 39–65. [Google Scholar] [CrossRef]
- Gao, Y.J.; Zhu, Y.X.; Sun, Q.M.; Chen, D.H. Ribosome-associated protein quality control: Implications for neurodegenerative diseases and therapeutic potential. Sci. Bull. 2024, 69, 1165–1169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Huang, K.; Jia, H.; Gao, X.; Guo, H. Ribosome-Associated Quality Control Mediated by Rqc2 Contributes to the Lytic Cycle and Stage Conversion of Toxoplasma gondii. Microorganisms 2025, 13, 2041. https://doi.org/10.3390/microorganisms13092041
Li Y, Huang K, Jia H, Gao X, Guo H. Ribosome-Associated Quality Control Mediated by Rqc2 Contributes to the Lytic Cycle and Stage Conversion of Toxoplasma gondii. Microorganisms. 2025; 13(9):2041. https://doi.org/10.3390/microorganisms13092041
Chicago/Turabian StyleLi, Yuxue, Keqin Huang, Honglin Jia, Xu Gao, and Huanping Guo. 2025. "Ribosome-Associated Quality Control Mediated by Rqc2 Contributes to the Lytic Cycle and Stage Conversion of Toxoplasma gondii" Microorganisms 13, no. 9: 2041. https://doi.org/10.3390/microorganisms13092041
APA StyleLi, Y., Huang, K., Jia, H., Gao, X., & Guo, H. (2025). Ribosome-Associated Quality Control Mediated by Rqc2 Contributes to the Lytic Cycle and Stage Conversion of Toxoplasma gondii. Microorganisms, 13(9), 2041. https://doi.org/10.3390/microorganisms13092041




