Human-like Biofilm Models to Study the Activity of Antifungals Against Aspergillus fumigatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Aspergillus fumigatus Strains
2.2. Preparation of the Fungal Suspensions
2.3. Drug Preparation
2.4. Cultivation of A549 Cells
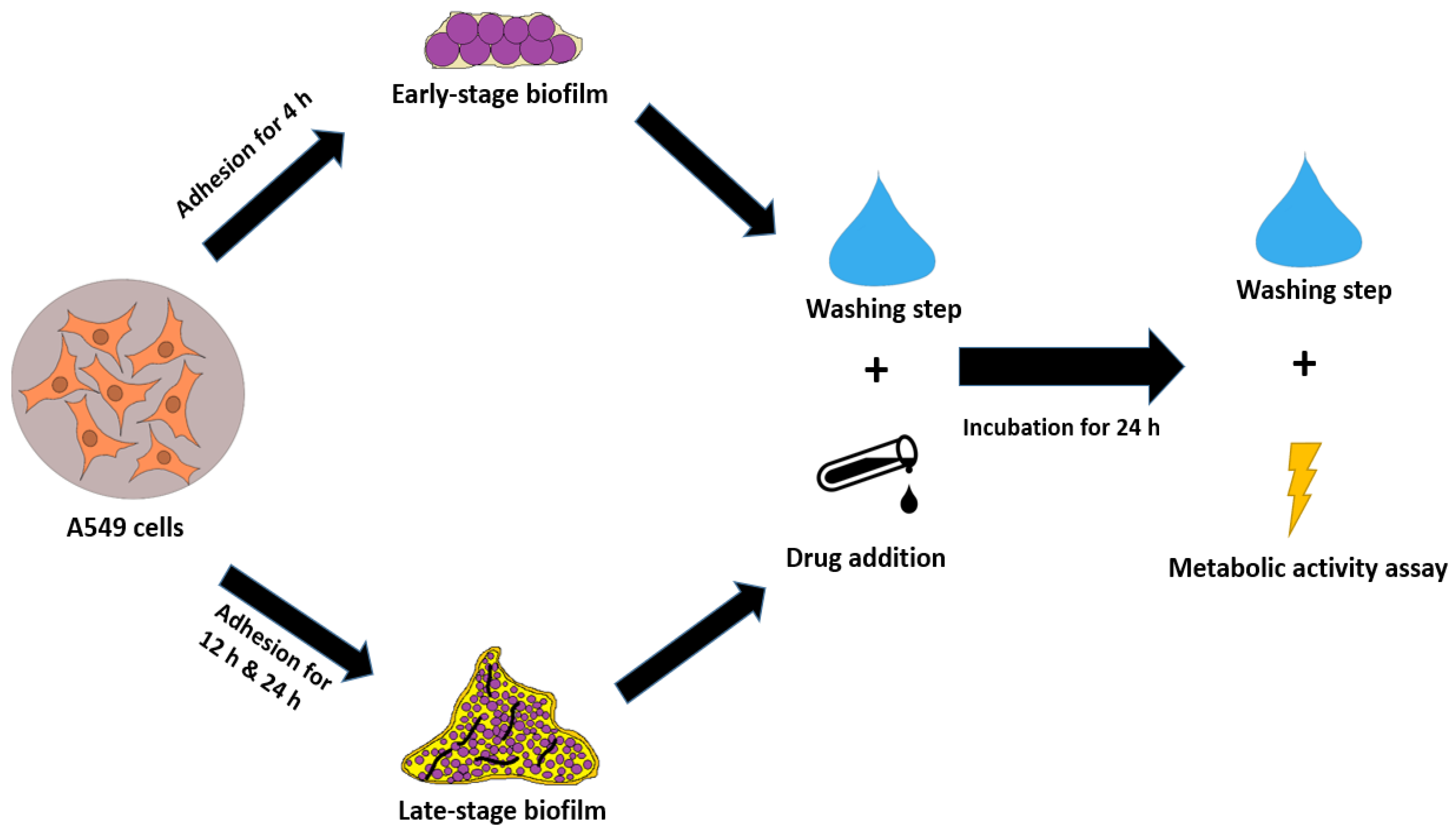
2.5. Biofilm Co-Culture with A549 Cells
2.6. Confocal Laser Scanning Microscopy
2.6.1. PKH26 Staining
2.6.2. Sample Preparation and Microscopy
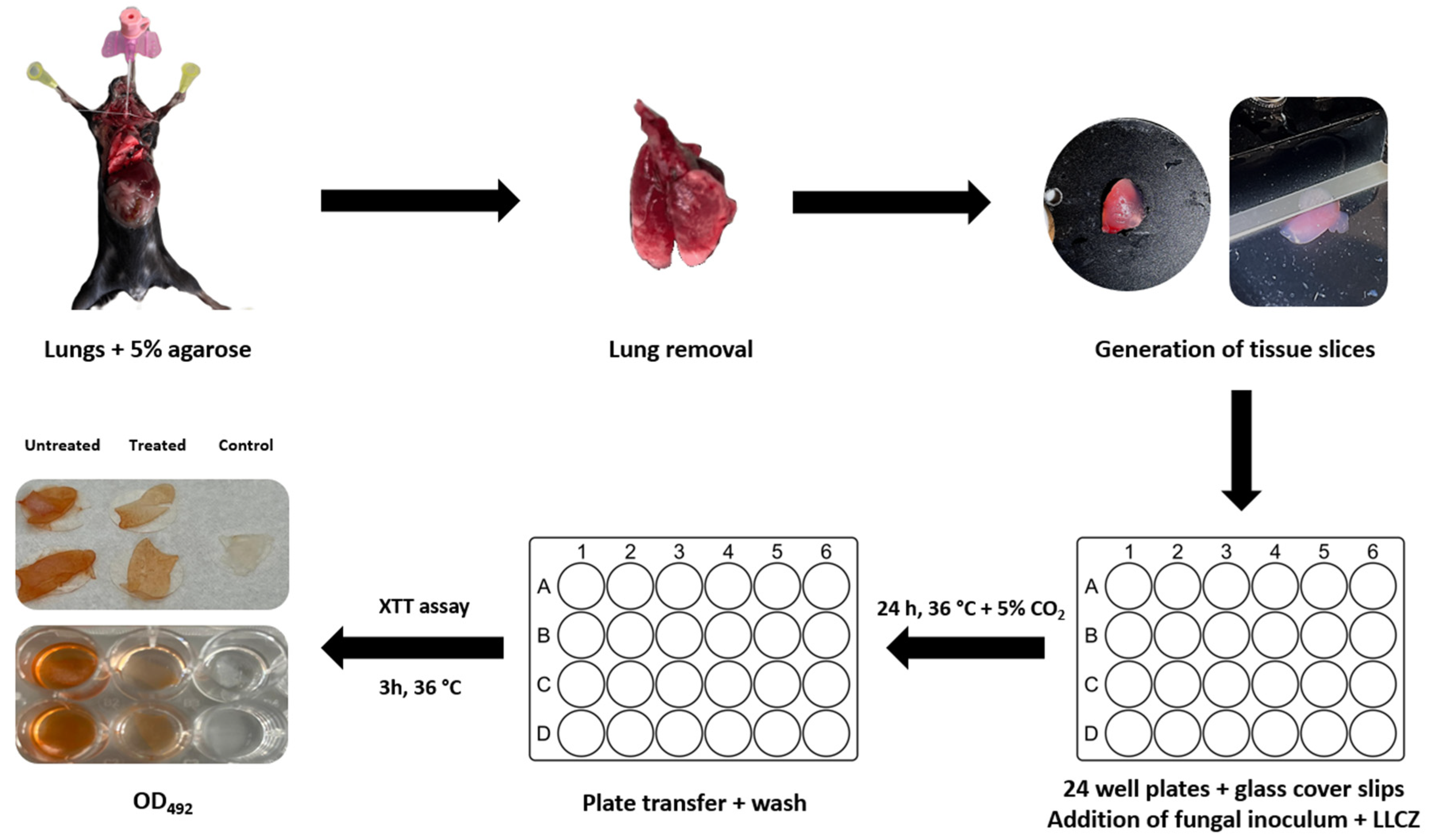
2.7. PCLS Preparation
2.8. Ex Vivo PCLS Infection and Treatment
2.9. Statistical Analysis
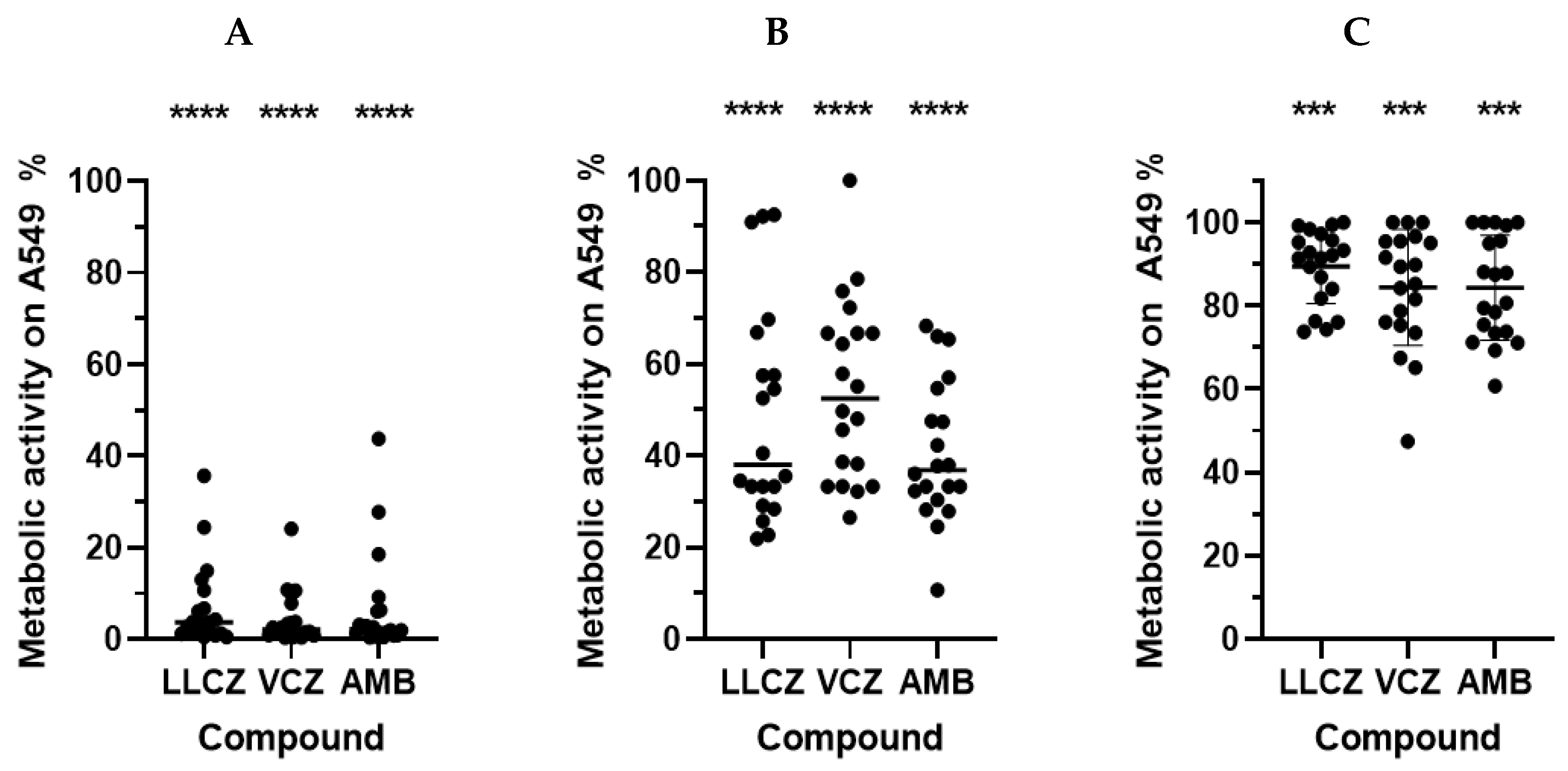
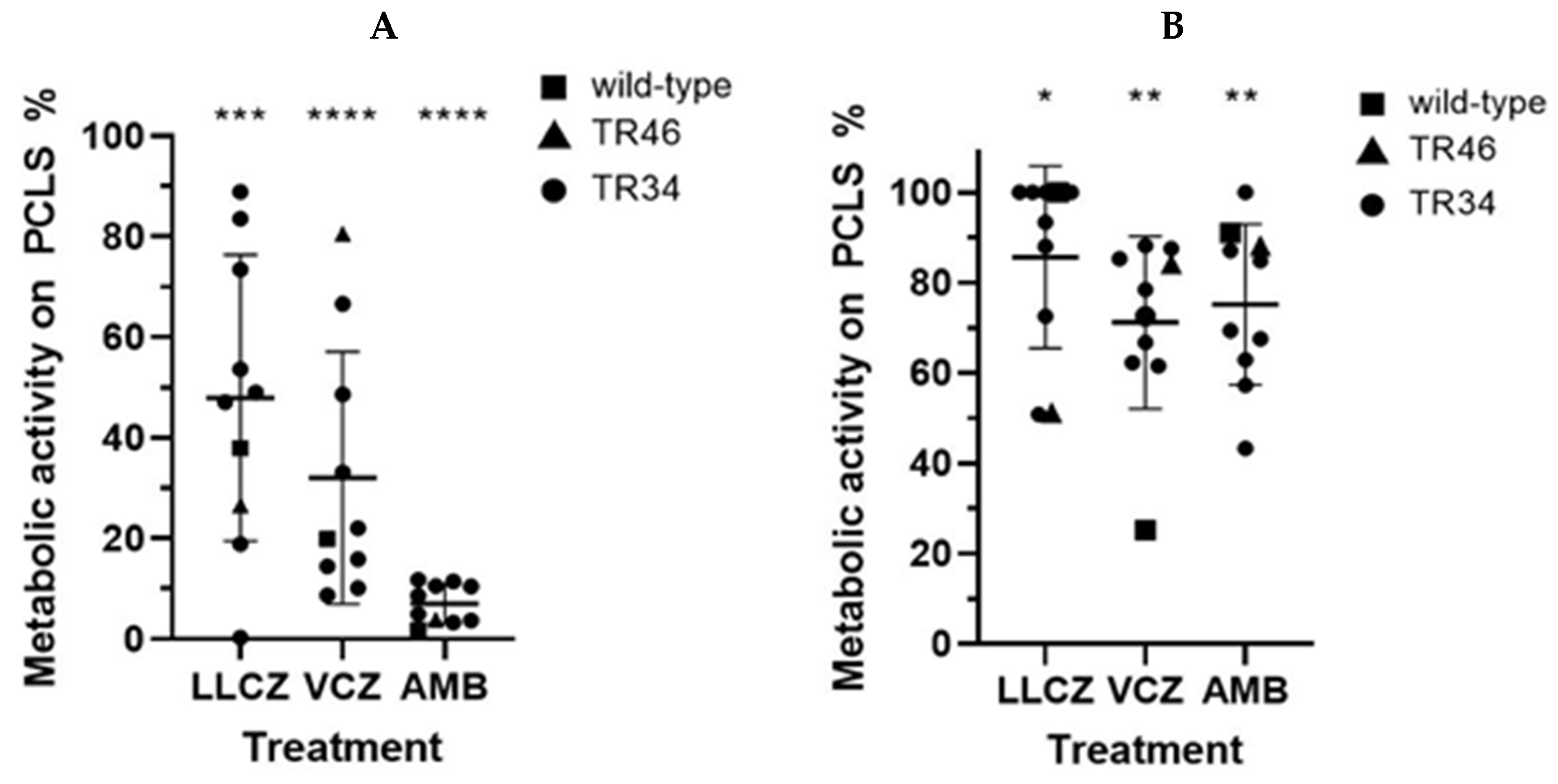
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
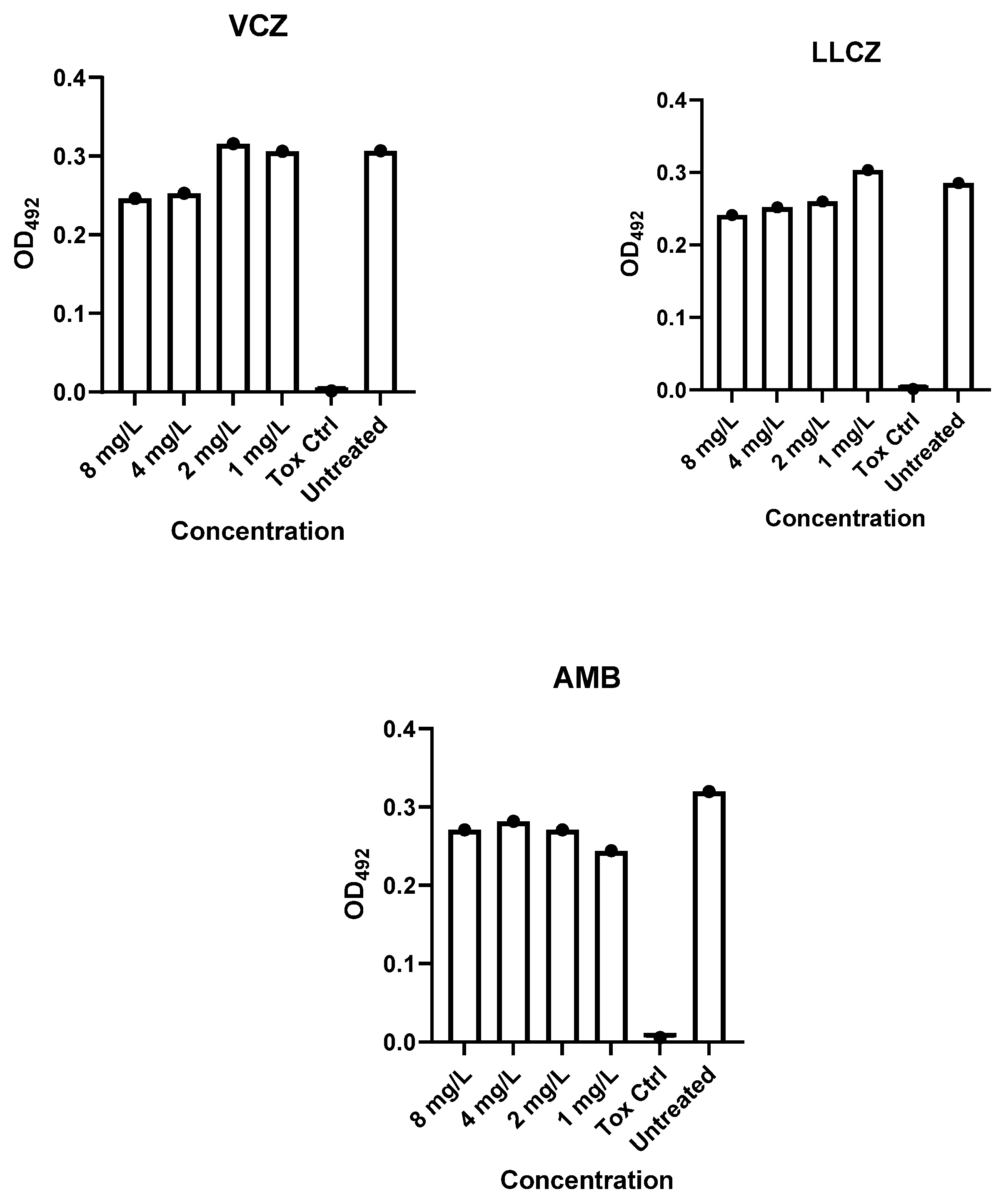
Appendix A
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 29 July 2025).
- Fisher, M.C.; Denning, D.W. The who fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023, 21, 211–212. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Gresnigt, M.S.; Romani, L.; Netea, M.G.; Latgé, J.P. Aspergillus fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 2017, 15, 661–674. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, C.M. Airborne Aspergillus fumigatus conidia: A risk factor for aspergillosis. Fungal Biol. Rev. 2011, 25, 151–157. [Google Scholar] [CrossRef]
- Balloy, V.; Chignard, M. The innate immune response to Aspergillus fumigatus. Microbes Infect. 2009, 12, 919–927. [Google Scholar] [CrossRef]
- Scharmann, U.; Verhasselt, H.L.; Kirchhoff, L.; Furnica, D.-T.; Steinmann, J.; Rath, P.-M. Microbiological non-culture-based methods for diagnosing invasive pulmonary aspergillosis in ICU patients. Diagnostics 2023, 13, 2718. [Google Scholar] [CrossRef]
- Meis, J.F.; Chowdhary, A.; Rhodes, J.L.; Fisher, M.C.; Verweij, P.E. Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Philos. Trans. R. Soc. B 2016, 371, 20150460. [Google Scholar] [CrossRef]
- Kirchhoff, L.; Dittmer, S.; Furnica, D.-T.; Buer, J.; Steinmann, E.; Rath, P.-M.; Steinmann, J. Inhibition of azole-resistant Aspergillus fumigatus biofilm at various formation stages by antifungal drugs, including olorofim. J. Antimicrob. Chemother. 2022, 77, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Furnica, D.-T.; Dittmer, S.; Sanders, M.I.; Steinmann, J.; Rath, P.-M.; Kirchhoff, L. In vitro and in vivo activity of luliconazole (nnd-502) against planktonic cells and biofilms of azole resistant Aspergillus fumigatus. J. Fungi 2022, 8, 350. [Google Scholar] [CrossRef]
- Morelli, K.A.; Kerkaert, J.D.; Cramer, R.A. Aspergillus fumigatus biofilms: Toward understanding how growth as a multicellular network increases antifungal resistance and disease progression. PLoS Pathog. 2021, 17, e1009794. [Google Scholar] [CrossRef]
- Kowalski, C.H.; Morelli, K.A.; Schultz, D.; Nadell, C.D.; Cramer, R.A. Fungal biofilm architecture produces hypoxic microenvironments that drive antifungal resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 22473–22483. [Google Scholar] [CrossRef]
- Latgé, J.P. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 2001, 9, 382–389. [Google Scholar] [CrossRef]
- Loussert, C.; Schmitt, C.; Prevost, M.-C.; Balloy, V.; Fadel, E.; Philippe, B.; Kauffmann-Lacroix, C.; Latgé, J.P.; Beauvais, A. In vivo biofilm composition of Aspergillus fumigatus. Cell Microbiol. 2010, 12, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; O’Kane, C.M.; Schroeder, G.N. Precision-cut lung slices: A powerful ex vivo model to investigate respiratory infectious diseases. Mol. Microbiol. 2022, 117, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Carranza-Rosales, P.; Carranza-Torres, I.E.; Guzmán-Delgado, N.E.; Lozano-Garza, G.; Villarreal-Treviño, L.; Molina-Torres, C.; Villarreal, J.V.; Vera-Cabrera, L.; Castro-Garza, J. Modeling tuberculosis pathogenesis through ex vivo lung tissue infection. Tuberculosis 2017, 107, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Weldearegay, Y.B.; Brogaard, L.; Rautenschlein, S.; Meens, J.; Valentin-Weigand, P.; Schaaf, D. Primary Cell Culture Systems to Investigate Host-Pathogen Interactions in Bacterial Respiratory Tract Infections of Livestock. Front. Cell. Infect. Microbiol. 2025, 12, 1565513. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Overview of Antifungal ECOFFS and Clinical Breakpoints for Yeasts, Moulds and Dermatophytes Using the EUCAST E.Def 7.4, E.Def 9.4 and E.Def 11.0 procedures. Version 4.0, 2023; EUCAST: Växjö, Sweden, 2023. [Google Scholar]
- Seidler, M.J.; Salvenmoser, S.; Müller, F.-M.C. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob. Agents Chemother. 2008, 52, 4130–4136. [Google Scholar] [CrossRef]
- Perdoni, F.; Signorelli, P.; Cirasola, D.; Caretti, A.; Galimberti, V.; Biggiogera, M.; Gasco, P.; Musicanti, C.; Morace, G.; Borghi, E. Antifungal activity of myriocin on clinically relevant Aspergillus fumigatus strains producing biofilm. BMC Microbiol. 2015, 15, 248. [Google Scholar] [CrossRef]
- Hasenberg, M.; Köhler, A.; Bonifatius, S.; Jeron, A.; Gunzer, M. Direct observation of phagocytosis and net-formation by neutrophils in infected lungs using 2-photon microscopy. J. Vis. Exp. 2011, 52, e2659. [Google Scholar]
- Niwano, Y.; Ohmi, T.; Seo, A.; Kodama, H.; Koga, H.; Sakai, A. Lanoconazole and its related optically active compound nnd-502: Novel antifungal imidazoles with a ketene dithioacetal structure. Curr. Med. Chem.-Anti-Infect. Agents. 2003, 2, 147–160. [Google Scholar] [CrossRef]
- Greer, N.D. Voriconazole: The newest triazole antifungal agent. Bayl. Univ. Med. Cent. Proc. 2003, 16, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Rosowski, E.E.; He, J.; Huisken, J.; Keller, N.P.; Huttenlocher, A. Efficacy of voriconazole against Aspergillus fumigatus infection depends on host immune function. Antimicrob. Agents Chemother. 2020, 64, e00917-19. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Antachopoulos, C.; Stergiopoulou, T.; Pournaras, S.; Roilides, E.; Walsh, T.J. Differential fungicidal activities of amphotericin b and voriconazole against Aspergillus species determined by microbroth methodology. Antimicrob. Agents Chemother. 2007, 51, 3329–3337. [Google Scholar] [CrossRef]
- Sanderson, M.J. Exploring lung physiology in health and disease with lung slices. Pulm. Pharmacol. Ther. 2011, 24, 452–465. [Google Scholar] [CrossRef]
- Hiorns, J.E.; Bidan, C.M.; Jensen, O.E.; Gosens, R.; Kistemaker, L.E.M.; Fredberg, J.J.; Butler, J.P.; Krishnan, R.; Brook, B.S. Airway and parenchymal strains during bronchoconstriction in the precision cut lung slice. Front. Physiol. 2016, 7, 309. [Google Scholar] [CrossRef]
- Stegmayr, J.; Alsafadi, H.N.; Langwiński, W.; Niroomand, A.; Lindstedt, S.; Leigh, N.D.; Wagner, D.E. Isolation of high-yield and -quality rna from human precision-cut lung slices for rna-sequencing and computational integration with larger patient cohorts. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L232–L240. [Google Scholar] [CrossRef]
- Nyuykonge, B.; Lim, W.; van Amelsvoort, L.; Bonifaz, A.; Fahal, A.; Badali, H.; Abastabar, M.; Verbon, A.; van de Sande, W. Eumycetoma causative agents are inhibited in vitro by luliconazole, lanoconazole and ravuconazole. Mycoses 2022, 65, 650–655. [Google Scholar] [CrossRef]
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue penetration of antifungal agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef]
- Beauvais, A.; Latgé, J.-P. Aspergillus Biofilm In Vitro and In Vivo. Microb. Biofilms 2015, 3, 149–161. [Google Scholar]
- Guimarães, L.L.; Takahashi, H.K. A snapshot of extracellular DNA influence on Aspergillus biofilm. Front. Microbiol. 2014, 5, 260. [Google Scholar] [CrossRef]

| Isolate | Source | Mutations |
|---|---|---|
| 1950 | BS | TR34/L98H |
| 1954 * | TS | TR34/L98H |
| 1959 | TS | TR34/L98H |
| 1962 | BAL | TR34/L98H |
| 1966 | TS | TR34/L98H |
| 1971 | Spt | TR34/L98H |
| 1972 | BS | No mutation |
| 1978 | BS | TR34/L98H |
| 1986 | Spt | TR34/L98H |
| 2047 | BAL | TR34/L98H |
| 2107 * | BAL | TR46 |
| 2119 * | Spt | TR34/L98H |
| 2133 | Spt | TR34/L98H |
| 2135 * | Spt CF | TR34/L98H |
| 2514 * | BS CF | TR34/L98H |
| 2515 * | Spt CF | TR34/L98H |
| 2607 * | Spt CF | TR34/L98H |
| 2608 * | Spt CF | TR34/L98H |
| ATCC 204305 * | ATCC | Wild-type |
| m1215 * | Spt CF | No mutation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furnica, D.-T.; Falkenstein, J.; Dittmer, S.; Steinmann, J.; Rath, P.-M.; Kirchhoff, L. Human-like Biofilm Models to Study the Activity of Antifungals Against Aspergillus fumigatus. Microorganisms 2025, 13, 2040. https://doi.org/10.3390/microorganisms13092040
Furnica D-T, Falkenstein J, Dittmer S, Steinmann J, Rath P-M, Kirchhoff L. Human-like Biofilm Models to Study the Activity of Antifungals Against Aspergillus fumigatus. Microorganisms. 2025; 13(9):2040. https://doi.org/10.3390/microorganisms13092040
Chicago/Turabian StyleFurnica, Dan-Tiberiu, Julia Falkenstein, Silke Dittmer, Joerg Steinmann, Peter-Michael Rath, and Lisa Kirchhoff. 2025. "Human-like Biofilm Models to Study the Activity of Antifungals Against Aspergillus fumigatus" Microorganisms 13, no. 9: 2040. https://doi.org/10.3390/microorganisms13092040
APA StyleFurnica, D.-T., Falkenstein, J., Dittmer, S., Steinmann, J., Rath, P.-M., & Kirchhoff, L. (2025). Human-like Biofilm Models to Study the Activity of Antifungals Against Aspergillus fumigatus. Microorganisms, 13(9), 2040. https://doi.org/10.3390/microorganisms13092040






