Poly(hexamethylene guanidine): An Effective Compound in Tackling Persistent Bacterial Subpopulations
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Construction of Persistent Bacterial Model
2.3. Antibacterial Activity Evaluation
2.4. Cytotoxicity Test
3. Results
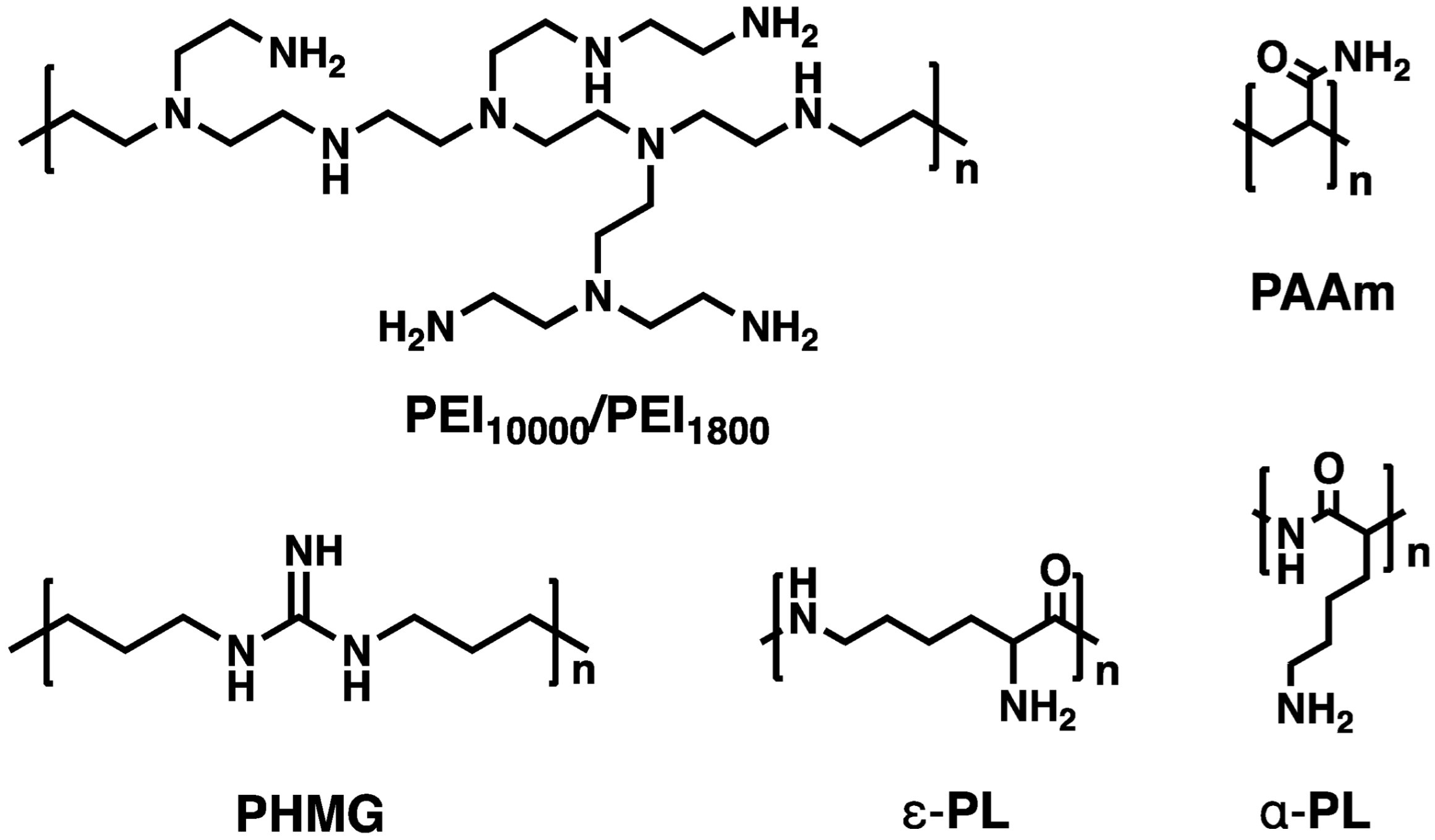
3.1. Basic Characteristics of Cationic Polymers
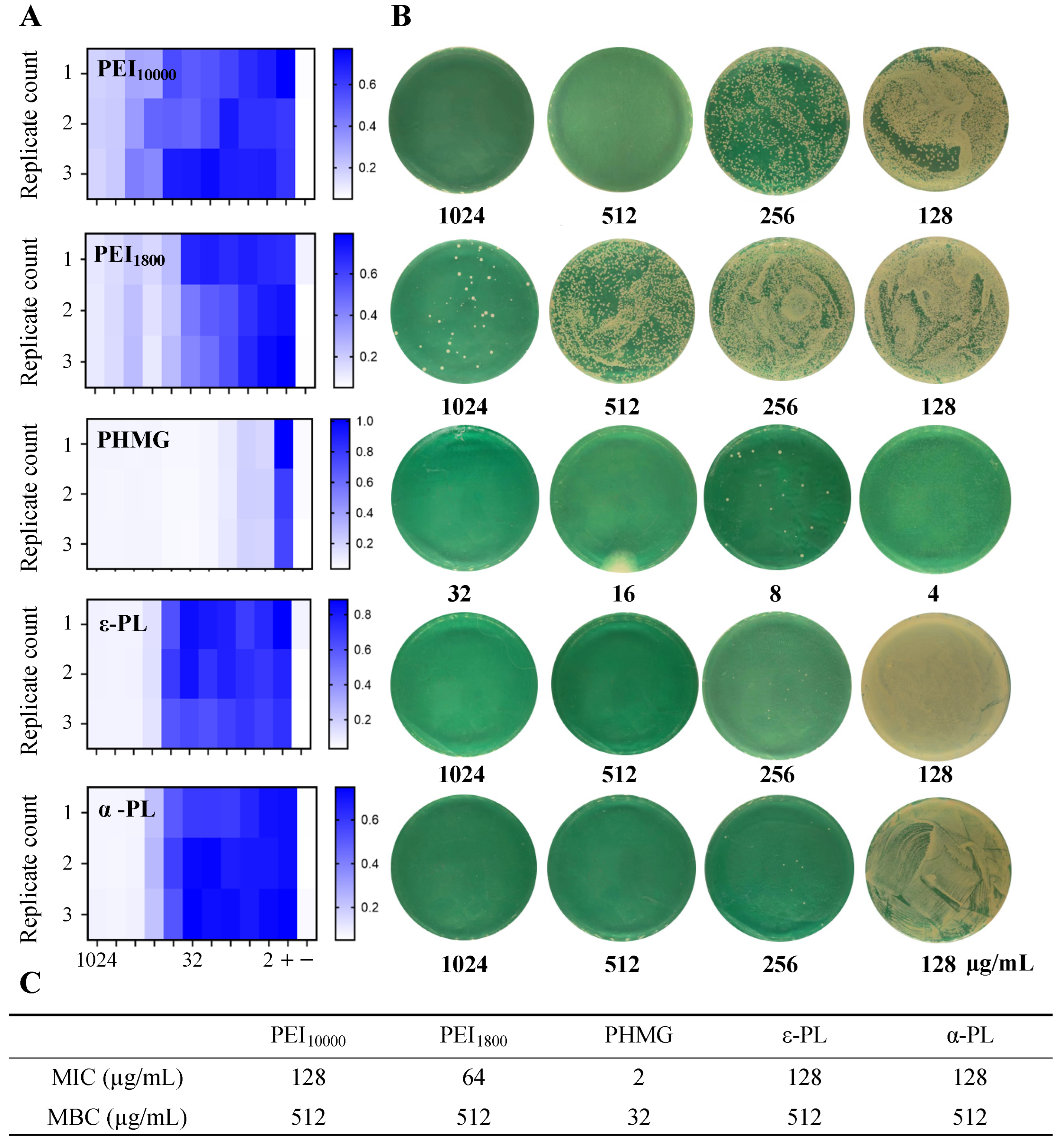
3.2. Antimicrobial Properties of Cationic Polymers Against MSSA
3.3. Antimicrobial Performance of Cationic Polymers Against MRSA
3.4. Antimicrobial Performance of Cationic Polymers Against E. coli
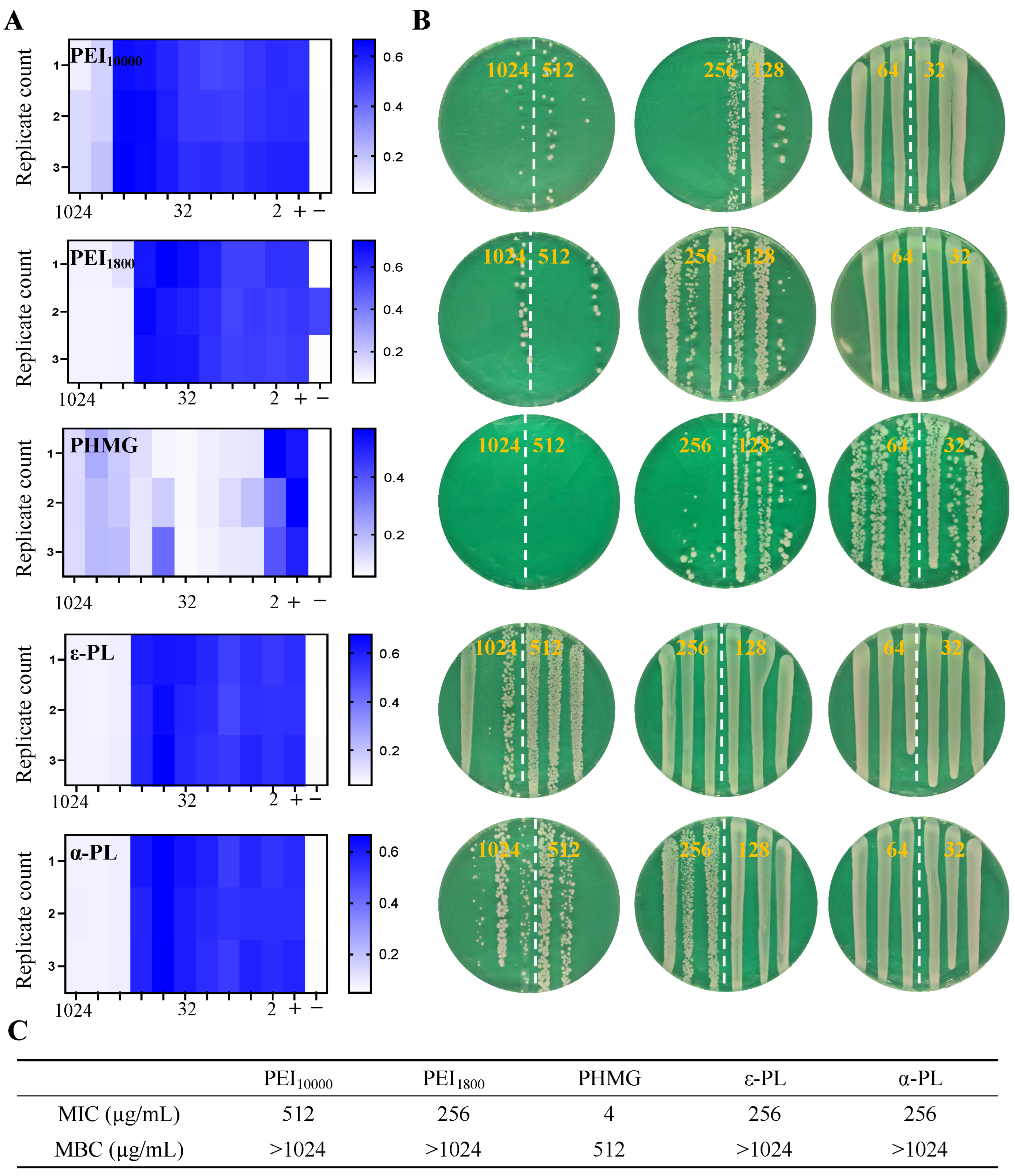
3.5. Antimicrobial Performance of Cationic Polymers Against Persistent MSSA
3.6. Antimicrobial Performance of Cationic Polymers Against Persistent MRSA
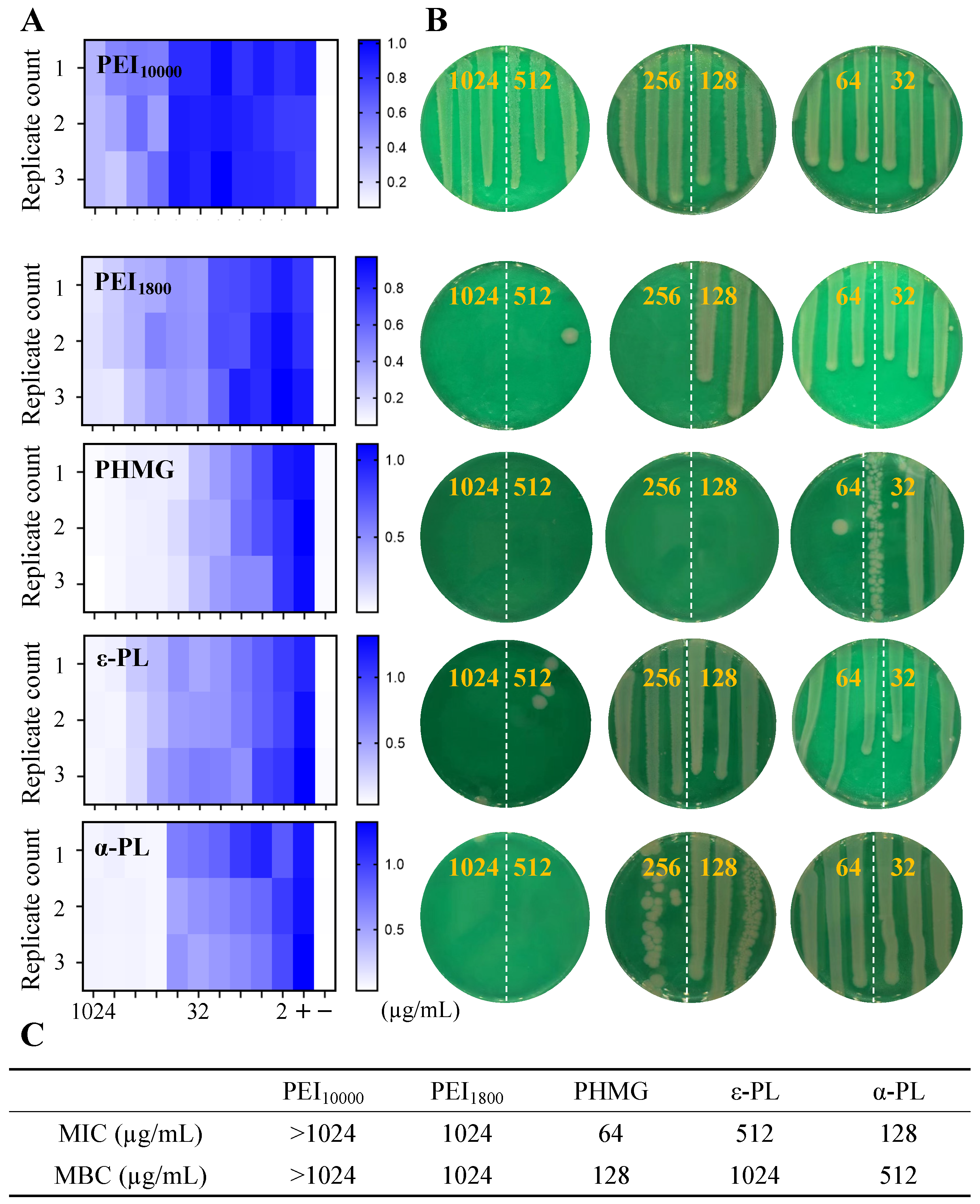
3.7. Antimicrobial Performance of Cationic Polymers Against Persistent E. coli
3.8. Cytotoxicity Evaluation of Cationic Polymers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masters, E.A.; Ricciardi, B.F.; Bentley, K.L.D.M.; Moriarty, T.F.; Schwarz, E.M.; Muthukrishnan, G. Skeletal Infections: Microbial Pathogenesis, Immunity and Clinical Management. Nat. Rev. Microbiol. 2022, 20, 385–400. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Kendall, S.; Good, L. Targeting the Hard to Reach: Challenges and Novel Strategies in the Treatment of Intracellular Bacterial Infections. Br. J. Pharmacol. 2017, 174, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. The Antibiotic Resistome: The Nexus of Chemical and Genetic Diversity. Nat. Rev. Microbiol. 2007, 5, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Nwobodo, D.C.; Ugwu, M.C.; Anie, C.O.; Al-Ouqaili, M.T.S.; Ikem, J.C.; Chigozie, U.V.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Ke, Y.; Bai, F. Active Efflux in Dormant Bacterial Cells–New Insights into Antibiotic Persistence. Drug Resist. Updat. 2017, 30, 7–14. [Google Scholar] [CrossRef]
- Pu, Y.; Zhao, Z.; Li, Y.; Zou, J.; Ma, Q.; Zhao, Y.; Ke, Y.; Zhu, Y.; Chen, H.; Baker, M.A.B.; et al. Enhanced Efflux Activity Facilitates Drug Tolerance in Dormant Bacterial Cells. Mol. Cell 2016, 62, 284–294. [Google Scholar] [CrossRef]
- Lesbats, J.; Brillac, A.; Reisz, J.A.; Mukherjee, P.; Lhuissier, C.; Fernández-Monreal, M.; Dupuy, J.-W.; Sequeira, A.; Tioli, G.; De La Calle Arregui, C.; et al. Macrophages Recycle Phagocytosed Bacteria to Fuel Immunometabolic Responses. Nature 2025, 640, 524–533. [Google Scholar] [CrossRef]
- Lewis, K. Persister Cells, Dormancy and Infectious Disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and Guidelines for Research on Antibiotic Persistence. Nat. Rev. Microbiol. 2019, 17, 441–448, Erratum in Nat. Rev. Microbiol. 2019, 17, 460. [Google Scholar] [CrossRef]
- Hurdle, J.G.; Deshpande, A. Bacterial Persister Cells Tackled. Nature 2018, 556, 40–41. [Google Scholar] [CrossRef]
- Blattman, S.B.; Jiang, W.; McGarrigle, E.R.; Liu, M.; Oikonomou, P.; Tavazoie, S. Identification and Genetic Dissection of Convergent Persister Cell States. Nature 2024, 636, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial Resistance, Mechanisms and Its Clinical Significance. Dis. Mon. 2020, 66, 100971. [Google Scholar] [CrossRef] [PubMed]
- Michiels, J.E.; Van Den Bergh, B.; Verstraeten, N.; Michiels, J. Molecular Mechanisms and Clinical Implications of Bacterial Persistence. Drug Resist. Updat. 2016, 29, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between Resistance, Tolerance and Persistence to Antibiotic Treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Merrin, J.; Chait, R.; Kowalik, L.; Leibler, S. Bacterial Persistence as a Phenotypic Switch. Science 2004, 305, 1622–1625. [Google Scholar] [CrossRef]
- Yin, Z.; Huang, D.; Kuhn, E.M.A.; Moriarty, T.F.; Li, G.; Wang, X. Unraveling Persistent Bacteria: Formation, Niches, and Eradication Strategies. Microbiol. Res. 2025, 297, 128189. [Google Scholar] [CrossRef]
- Defraine, V.; Fauvart, M.; Michiels, J. Fighting Bacterial Persistence: Current and Emerging Anti-Persister Strategies and Therapeutics. Drug Resist. Updat. 2018, 38, 12–26. [Google Scholar] [CrossRef]
- Peyrusson, F.; Varet, H.; Nguyen, T.K.; Legendre, R.; Sismeiro, O.; Coppée, J.-Y.; Wolz, C.; Tenson, T.; Van Bambeke, F. Intracellular Staphylococcus aureus Persisters upon Antibiotic Exposure. Nat. Commun. 2020, 11, 2200. [Google Scholar] [CrossRef]
- Verstraete, L.; Van Den Bergh, B.; Verstraeten, N.; Michiels, J. Ecology and Evolution of Antibiotic Persistence. Trends Microbiol. 2022, 30, 466–479. [Google Scholar] [CrossRef]
- Lobritz, M.A.; Belenky, P.; Porter, C.B.M.; Gutierrez, A.; Yang, J.H.; Schwarz, E.G.; Dwyer, D.J.; Khalil, A.S.; Collins, J.J. Antibiotic Efficacy Is Linked to Bacterial Cellular Respiration. Proc. Natl. Acad. Sci. USA 2015, 112, 8173–8180. [Google Scholar] [CrossRef]
- Van Den Bergh, B.; Fauvart, M.; Michiels, J. Formation, Physiology, Ecology, Evolution and Clinical Importance of Bacterial Persisters. FEMS Microbiol. Rev. 2017, 41, 219–251. [Google Scholar] [CrossRef]
- Niu, H.; Gu, J.; Zhang, Y. Bacterial Persisters: Molecular Mechanisms and Therapeutic Development. Signal Transduct. Target. Ther. 2024, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Gunn, N.J.; Kidd, S.P.; Solomon, L.B.; Yang, D.; Roscioli, E.; Atkins, G.J. Staphylococcus aureus Persistence in Osteocytes: Weathering the Storm of Antibiotics and Autophagy/Xenophagy. Front. Cell. Infect. Microbiol. 2024, 14, 1403289. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, J.; Hobbs, G.; Nakouti, I. Persister Cells: Formation, Resuscitation and Combative Therapies. Arch. Microbiol. 2021, 203, 5899–5906. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Su, Y.; Li, H.; Han, Y.; Guo, C.; Tian, Y.; Peng, X. Exogenous Alanine and/or Glucose plus Kanamycin Kills Antibiotic-Resistant Bacteria. Cell Metab. 2015, 21, 249–262. [Google Scholar] [CrossRef]
- Zhen, J.; Yan, S.; Li, Y.; Ruan, C.; Li, Y.; Li, X.; Zhao, X.; Lv, X.; Ge, Y.; Moure, U.A.E.; et al. L-Alanine Specifically Potentiates Fluoroquinolone Efficacy against Mycobacterium Persisters via Increased Intracellular Reactive Oxygen Species. Appl. Microbiol. Biotechnol. 2020, 104, 2137–2147. [Google Scholar] [CrossRef]
- Bojer, M.S.; Lindemose, S.; Vestergaard, M.; Ingmer, H. Quorum Sensing-Regulated Phenol-Soluble Modulins Limit Persister Cell Populations in Staphylococcus aureus. Front. Microbiol. 2018, 9, 255. [Google Scholar] [CrossRef]
- Personnic, N.; Striednig, B.; Lezan, E.; Manske, C.; Welin, A.; Schmidt, A.; Hilbi, H. Quorum Sensing Modulates the Formation of Virulent Legionella Persisters within Infected Cells. Nat. Commun. 2019, 10, 5216. [Google Scholar] [CrossRef]
- Nang, S.C.; Azad, M.A.K.; Velkov, T.; Zhou, Q.T.; Li, J. Rescuing the Last-Line Polymyxins: Achievements and Challenges. Pharmacol. Rev. 2021, 73, 679–728. [Google Scholar] [CrossRef]
- Xie, J.; Zhou, M.; Qian, Y.; Cong, Z.; Chen, S.; Zhang, W.; Jiang, W.; Dai, C.; Shao, N.; Ji, Z.; et al. Addressing MRSA Infection and Antibacterial Resistance with Peptoid Polymers. Nat. Commun. 2021, 12, 5898. [Google Scholar] [CrossRef]
- Verstraeten, N.; Knapen, W.J.; Kint, C.I.; Liebens, V.; Van den Bergh, B.; Dewachter, L.; Michiels, J.E.; Fu, Q.; David, C.C.; Fierro, A.C.; et al. Obg and Membrane Depolarization Are Part of a Microbial Bet-Hedging Strategy That Leads to Antibiotic Tolerance. Mol. Cell 2015, 59, 9–21. [Google Scholar] [CrossRef]
- Zhang, K.; Du, Y.; Si, Z.; Liu, Y.; Turvey, M.E.; Raju, C.; Keogh, D.; Ruan, L.; Jothy, S.L.; Reghu, S.; et al. Enantiomeric Glycosylated Cationic Block Co-Beta-Peptides Eradicate Staphylococcus aureus Biofilms and Antibiotic-Tolerant Persisters. Nat. Commun. 2019, 10, 4792. [Google Scholar] [CrossRef]
- Zhou, M.; Qian, Y.; Xie, J.; Zhang, W.; Jiang, W.; Xiao, X.; Chen, S.; Dai, C.; Cong, Z.; Ji, Z.; et al. Poly(2-Oxazoline)-Based Functional Peptide Mimics: Eradicating MRSA Infections and Persisters While Alleviating Antimicrobial Resistance. Angew. Chem. Int. Ed. 2020, 59, 6412–6419. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhou, M.; Chen, S.; Xie, J.; Chen, M.; Zhang, H.; Wu, Y.; Chen, X.; Liu, R. Peptide-Mimicking Poly(2-Oxazoline)s Possessing Potent Antifungal Activity and BBB Penetrating Property to Treat Invasive Infections and Meningitis. J. Am. Chem. Soc. 2023, 145, 25753–25765. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, A.M. From Nanobiotechnology, Positively Charged Biomimetic Dendrimers as Novel Antibacterial Agents: A Review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Caviglia, D. Prevention and Eradication of Biofilm by Dendrimers: A Possibility Still Little Explored. Pharmaceutics 2022, 14, 2016. [Google Scholar] [CrossRef]
- Alfei, S. Shifting from Ammonium to Phosphonium Salts: A Promising Strategy to Develop Next-Generation Weapons against Biofilms. Pharmaceutics 2024, 16, 80. [Google Scholar] [CrossRef]
- Feng, W.; Li, G.; Kang, X.; Wang, R.; Liu, F.; Zhao, D.; Li, H.; Bu, F.; Yu, Y.; Moriarty, T.F.; et al. Cascade-Targeting Poly(amino acid) Nanoparticles Eliminate Intracellular Bacteria via On-Site Antibiotic Delivery. Adv. Mater. 2022, 34, 2109789. [Google Scholar] [CrossRef]
- Conlon, B.P.; Rowe, S.E.; Gandt, A.B.; Nuxoll, A.S.; Donegan, N.P.; Zalis, E.A.; Clair, G.; Adkins, J.N.; Cheung, A.L.; Lewis, K. Persister Formation in Staphylococcus aureus Is Associated with ATP Depletion. Nat. Microbiol. 2016, 1, 16051. [Google Scholar] [CrossRef]
- Liu, S.; Huang, Y.; Jensen, S.; Laman, P.; Kramer, G.; Zaat, S.A.J.; Brul, S. Molecular Physiological Characterization of the Dynamics of Persister Formation in Staphylococcus aureus. Antimicrob. Agents Chemother. 2024, 68, e00850-23. [Google Scholar] [CrossRef]
- Yin, Z.; Huang, D.; Zhao, D.; You, Y.; Gu, J.; Xie, W.; Moriarty, F.T.; Li, G.; Wang, X. Eradication of Intracellular Staphylococcus aureus Persisters via On-Site Antibiotic Delivery by Poly(amino acid) Nanoparticles. ACS Appl. Mater. Interfaces 2025, 17, 47412–47425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Q.; Xie, J.; Cong, Z.; Cao, C.; Zhang, W.; Zhang, D.; Chen, S.; Gu, J.; Deng, S.; et al. Switching from Membrane Disrupting to Membrane Crossing, an Effective Strategy in Designing Antibacterial Polypeptide. Sci. Adv. 2023, 9, eabn0771. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Duan, S.; Hu, Y.; Ding, X.; Xu, F.-J. Fluorination of Polyethylenimines for Augmentation of Antibacterial Potency via Structural Damage and Potential Dissipation of Bacterial Membranes. ACS Appl. Mater. Interfaces 2022, 14, 44173–44182. [Google Scholar] [CrossRef] [PubMed]
- Brunot, C.; Ponsonnet, L.; Lagneau, C.; Farge, P.; Picart, C.; Grosgogeat, B. Cytotoxicity of Polyethyleneimine (PEI), Precursor Base Layer of Polyelectrolyte Multilayer Films. Biomaterials 2007, 28, 632–640. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, K.; Wang, J.; Chen, M.; Chen, Y.; She, Y.; Yan, Z.; Liu, R. Host Defense Peptide Mimicking Antimicrobial Amino Acid Polymers and beyond: Design, Synthesis and Biomedical Applications. Prog. Polym. Sci. 2023, 141, 101679. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, H.; Ji, T.; Anderson, G.; Nie, G.; Zhao, Y. Biodegradable Cationic ε-Poly-L-Lysine-Conjugated Polymeric Nanoparticles as a New Effective Antibacterial Agent. Sci. Bull. 2015, 60, 216–226. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; John, A.; Arvind Bharani, R.S.; Kavisri, M.; Moovendhan, M. Biocompatible Formulation of Cationic Antimicrobial Peptide Polylysine (PL) through Nanotechnology Principles and Its Potential Role in Food Preservation—A Review. Int. J. Biol. Macromol. 2022, 222, 1734–1746. [Google Scholar] [CrossRef]
- Sille, I.E.; Pissinis, D.E.; Fagali, N.S.; Ghilini, F.; Urrutia, M.N.; Schilardi, P.L. Antimicrobial-Loaded Polyacrylamide Hydrogels Supported on Titanium as Reservoir for Local Drug Delivery. Pathogens 2023, 12, 202. [Google Scholar] [CrossRef]
- Liu, M.; Huang, L.; Zhang, W.; Wang, X.; Geng, Y.; Zhang, Y.; Wang, L.; Zhang, W.; Zhang, Y.-J.; Xiao, S.; et al. A Transistor-like pH-Sensitive Nanodetergent for Selective Cancer Therapy. Nat. Nanotechnol. 2022, 17, 541–551, Erratum in Nat. Nanotechnol. 2022, 17, 560. [Google Scholar] [CrossRef]
- Chen, J.; Dong, Y.; Xiao, C.; Tao, Y.; Wang, X. Organocatalyzed Ring-Opening Polymerization of Cyclic Lysine Derivative: Sustainable Access to Cationic Poly(ε-Lysine) Mimics. Macromolecules 2021, 54, 2226–2231. [Google Scholar] [CrossRef]
- Rodrigues, B.; Morais, T.P.; Zaini, P.A.; Campos, C.S.; Almeida-Souza, H.O.; Dandekar, A.M.; Nascimento, R.; Goulart, L.R. Antimicrobial Activity of Epsilon-Poly-l-Lysine against Phytopathogenic Bacteria. Sci. Rep. 2020, 10, 11324. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Wang, J.; Wang, H.; Wang, H.; Gu, R.; Shen, J.; Hao, Q.; Brash, J.L.; Chen, H. Optimizing the Bacteriostatic and Cytocompatibility Properties of Poly(Hexamethylene Guanidine) Hydrochloride (PHMG) via the Guanidine/Alkane Ratio. Biomacromolecules 2022, 23, 2170–2183. [Google Scholar] [CrossRef] [PubMed]
- Jas, G.S.; Kuczera, K. Deprotonation of a Single Amino Acid Residue Induces Significant Stability in an α-Helical Heteropeptide. J. Phys. Chem. B 2018, 122, 11508–11518. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, W.; Cong, Z.; Chen, K.; She, Y.; Zhong, C.; Zhang, W.; Chen, M.; Zhou, M.; Shao, N.; et al. An Effective Strategy to Develop Potent and Selective Antifungal Agents from Cell Penetrating Peptides in Tackling Drug-Resistant Invasive Fungal Infections. J. Med. Chem. 2022, 65, 7296–7311. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; He, H.; Yi, C.; Dong, W.; Ye, S.; Zheng, D.; Tao, J.; Wu, Q.; Duan, X.; et al. Electrostatic Adsorption Behaviors of Charged Polymer-Tethered Nanoparticles on Oppositely Charged Surfaces. Macromol. Rapid Commun. 2022, 43, 2200171. [Google Scholar] [CrossRef]
- Yessine, M. Membrane-Destabilizing Polyanions: Interaction with Lipid Bilayers and Endosomal Escape of Biomacromolecules. Adv. Drug Deliv. Rev. 2004, 56, 999–1021. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Li, X.; Zhu, Y.; Ji, Y.; Lu, W.; Wang, G.; Dong, W.; Liu, M.; Wang, X.; et al. Identification of an Anti-Virulence Drug That Reverses Antibiotic Resistance in Multidrug Resistant Bacteria. Biomed. Pharmacother. 2022, 153, 113334. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-Resistant Staphylococcus aureus: An Overview of Basic and Clinical Research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Hamilton, S.M.; Alexander, J.A.N.; Choo, E.J.; Basuino, L.; Da Costa, T.M.; Severin, A.; Chung, M.; Aedo, S.; Strynadka, N.C.J.; Tomasz, A.; et al. High-Level Resistance of Staphylococcus aureus to β-Lactam Antibiotics Mediated by Penicillin-Binding Protein 4 (PBP4). Antimicrob. Agents Chemother. 2017, 61, e02727-16. [Google Scholar] [CrossRef]
- García, A.B.; Viñuela-Prieto, J.M.; López-González, L.; Candel, F.J. Correlation between Resistance Mechanisms in Staphylococcus aureus and Cell Wall and Septum Thickening. Infect. Drug Resist. 2017, 10, 353–356. [Google Scholar] [CrossRef]
- Mistretta, N.; Brossaud, M.; Telles, F.; Sanchez, V.; Talaga, P.; Rokbi, B. Glycosylation of Staphylococcus aureus Cell Wall Teichoic Acid Is Influenced by Environmental Conditions. Sci. Rep. 2019, 9, 3212. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, Z.; Zhao, Y.; Wang, C.; Shen, S.; Qiu, Z.; Cui, R.; Zhou, S.; Fang, L.; Chen, Z.; et al. Influence of Cationic Groups on the Antibacterial Behavior of Cationic Nano-Sized Hyperbranched Polymers to Enhance Bacteria-Infected Wound Healing. Nanoscale 2022, 14, 12789–12803. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Takahashi, H.; Nadres, E.T.; Mortazavian, H.; Caputo, G.A.; Younger, J.G.; Kuroda, K. A Cationic Amphiphilic Random Copolymer with pH-Responsive Activity against Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2017, 12, e0169262. [Google Scholar] [CrossRef] [PubMed]
- Delcour, A.H. Outer Membrane Permeability and Antibiotic Resistance. Biochim. Biophys. Acta. BBA Proteins Proteom. 2009, 1794, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Vad, B.S.; Stenvang, M.; Otzen, D.E.; Meyer, R.L. The Antimicrobial Mechanism of Action of Epsilon-Poly-L-Lysine. Appl. Environ. Microbiol. 2014, 80, 7758–7770. [Google Scholar] [CrossRef]
- Zhang, Y.; Hudson-Smith, N.V.; Frand, S.D.; Cahill, M.S.; Davis, L.S.; Feng, Z.V.; Haynes, C.L.; Hamers, R.J. Influence of the Spatial Distribution of Cationic Functional Groups at Nanoparticle Surfaces on Bacterial Viability and Membrane Interactions. J. Am. Chem. Soc. 2020, 142, 10814–10823. [Google Scholar] [CrossRef]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide Modification in Gram-Negative bacteria during Chronic Infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Helaine, S.; Kugelberg, E. Bacterial Persisters: Formation, Eradication, and Experimental Systems. Trends Microbiol. 2014, 22, 417–424. [Google Scholar] [CrossRef]
- Huang, D.; Kang, X.; Yin, Z.; Zhao, D.; Ning, Y.; Liu, H.; Li, F.; Xie, W.; Li, G.; Wang, X. On-Site Serine Delivery Drives Fermentation Pathway Reprogramming to Reverse Intracellular Staphylococcus aureus Persistence. ACS Nano 2025, 19, 26075–26090. [Google Scholar] [CrossRef]
- Bakkeren, E.; Diard, M.; Hardt, W.-D. Evolutionary Causes and Consequences of Bacterial Antibiotic Persistence. Nat. Rev. Microbiol. 2020, 18, 479–490. [Google Scholar] [CrossRef]
- Gray, D.A.; Wang, B.; Sidarta, M.; Cornejo, F.A.; Wijnheijmer, J.; Rani, R.; Gamba, P.; Turgay, K.; Wenzel, M.; Strahl, H.; et al. Membrane Depolarization Kills Dormant Bacillus subtilis Cells by Generating a Lethal Dose of ROS. Nat. Commun. 2024, 15, 6877. [Google Scholar] [CrossRef]
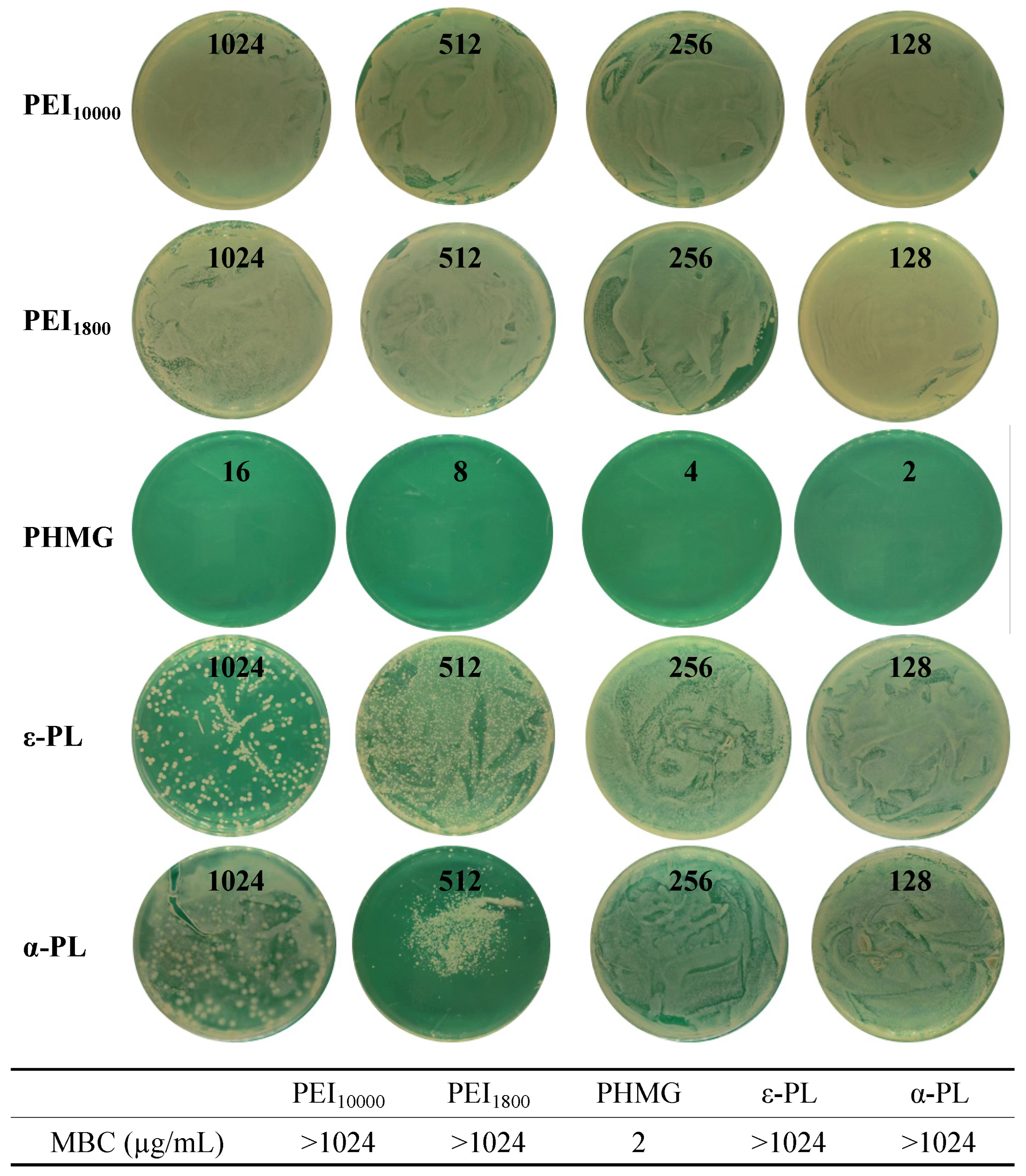
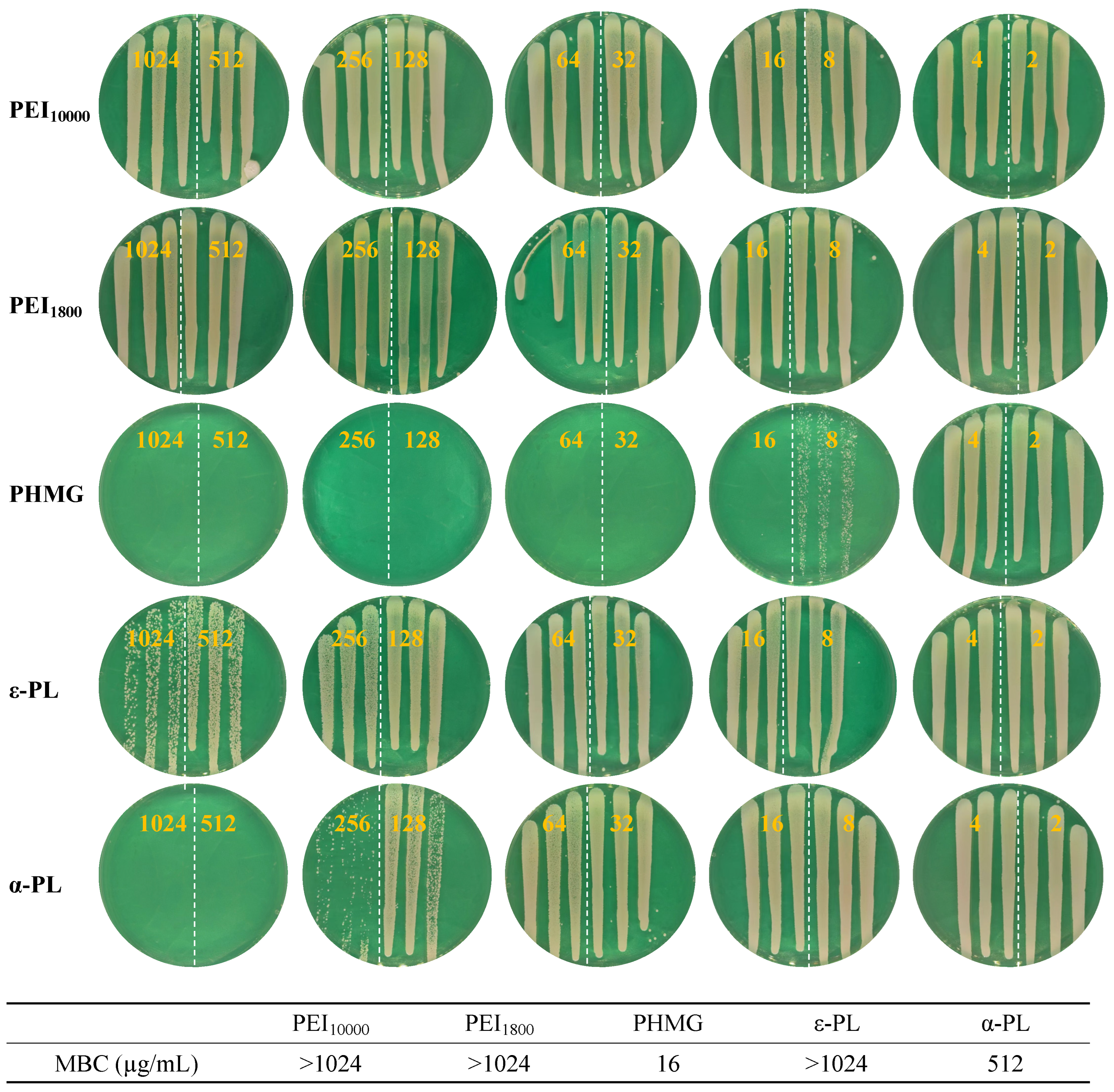
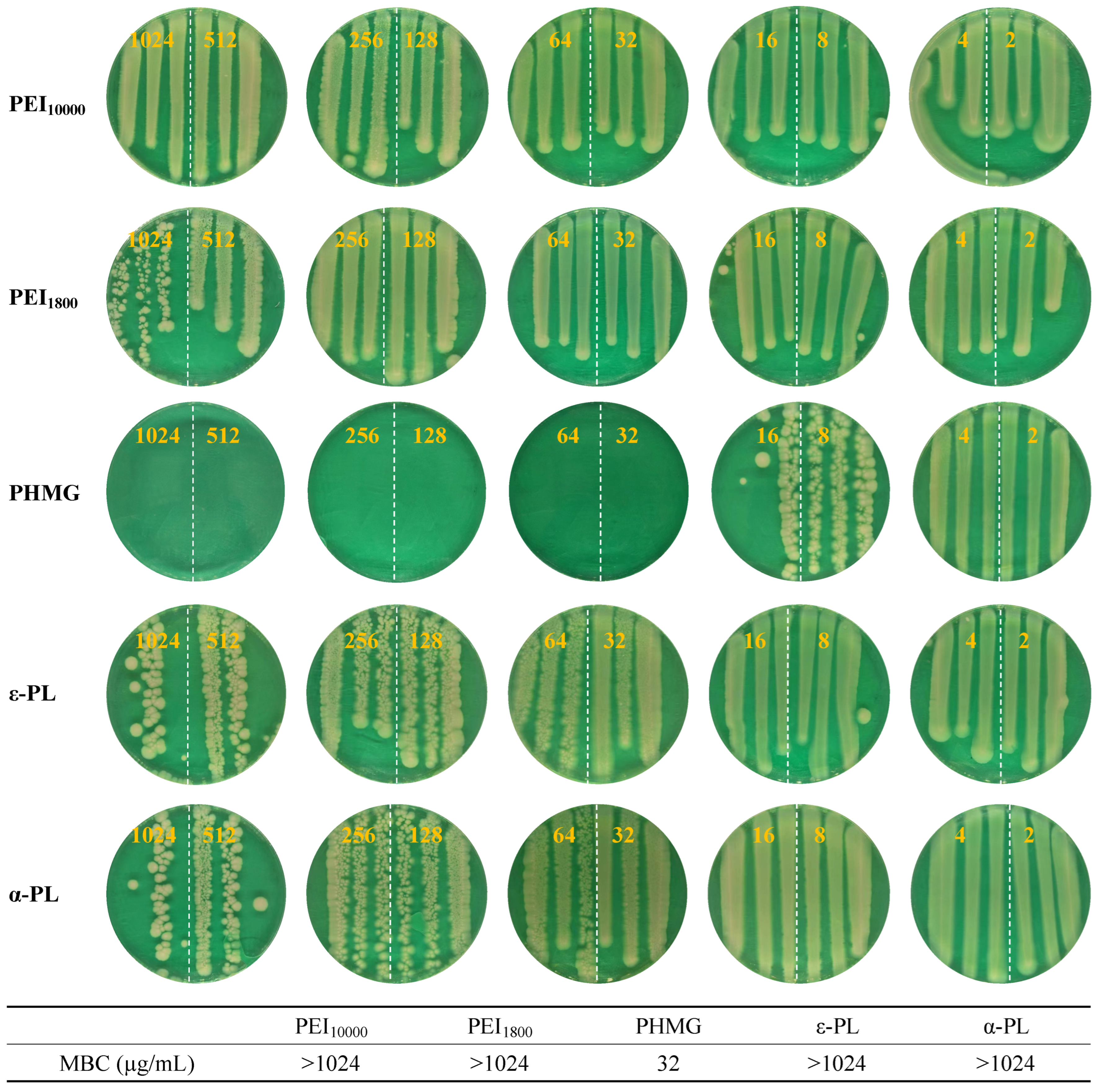

| PHMG | PEI10000 | PEI1800 | ε-PL | α-PL | |
|---|---|---|---|---|---|
| MSSA | 512 | >1024 | >1024 | >1024 | >1024 |
| Persistent MSSA | 2 | >1024 | >1024 | >1024 | >1024 |
| MRSA | 32 | 1024 | >1024 | 512 | 512 |
| Persistent MRSA | 16 | >1024 | >1024 | >1024 | 512 |
| E. coli | 128 | >1024 | 1024 | 1024 | 512 |
| Persistent E. coli | 32 | >1024 | >1024 | >1024 | >1024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhang, J.; Chen, L. Poly(hexamethylene guanidine): An Effective Compound in Tackling Persistent Bacterial Subpopulations. Microorganisms 2025, 13, 2002. https://doi.org/10.3390/microorganisms13092002
Liu W, Zhang J, Chen L. Poly(hexamethylene guanidine): An Effective Compound in Tackling Persistent Bacterial Subpopulations. Microorganisms. 2025; 13(9):2002. https://doi.org/10.3390/microorganisms13092002
Chicago/Turabian StyleLiu, Weilin, Jiang Zhang, and Liang Chen. 2025. "Poly(hexamethylene guanidine): An Effective Compound in Tackling Persistent Bacterial Subpopulations" Microorganisms 13, no. 9: 2002. https://doi.org/10.3390/microorganisms13092002
APA StyleLiu, W., Zhang, J., & Chen, L. (2025). Poly(hexamethylene guanidine): An Effective Compound in Tackling Persistent Bacterial Subpopulations. Microorganisms, 13(9), 2002. https://doi.org/10.3390/microorganisms13092002





