Abstract
Arthrobotrys flagrans, a typical nematode-trapping fungus (NTF) that produces a three-dimensional adhesive network to capture nematodes, has excellent potential for the development of biocontrol agents against both plant and animal parasitic nematodes. Proteins containing the common fungal extracellular membrane (CFEM) domain are important for the nematodes’ trapping by A. flagrans. The loss of AfCFEM1 and AfCFEM3 resulted in a significant upregulation of proteins associated with fungal pathogenicity, forming a denser adhesive material on the trap surface and ultimately increasing nematode mortality. However, the disruption of AfCFEM2 led to the opposite result. Furthermore, the deletion of AfCFEM1-3 not only affected trap morphology, resulting in an increased proportion of irregular traps (i.e., trap cells not fused to the hyphae), but also led to a thinner cell wall of the traps. In addition, the compensatory effects among the CFEM family and other families were demonstrated. This study revealed that the AfCFEM1-3 genes in A. flagrans participated in the formation of traps, adhesive material and cell wall, and pathogenicity, providing new insights into the functions of AfCFEM in the process of nematode trapping by NTF.
1. Introduction
Plant and livestock parasitic nematodes cause serious economic losses globally every year [1,2,3]. Synthetic pesticides are common worldwide, but their excessive use is harmful to humans, plants, and agricultural ecosystems, and has led to the emergence and spread of drug resistance [1,4]. Chemical nematicides are banned or restricted, and alternatives to these are urgently needed [1]. A biocontrol agent (BCA) is either a living organism or a natural substance developed based on fungi, bacteria, and other organisms [1]. As natural enemies, nematode trapping fungi (NTF) produce various traps to capture nematodes and finally kill them, making them an ideal source for developing BCAs for managing parasitic nematodes [5]. As one of the typical NTFs, Arthrobotrys flagrans, which produces a three-dimensional network, has been proven to be efficient in controlling animal parasitic nematodes [6,7,8]. Furthermore, it was found that A. flagrans reduced the number of Xiphinema index juveniles in pot cultures of Ficus carica and was efficient in capturing Meloidogyne spp., colonizing the plant root system, and increasing phosphorus uptake, thereby promoting plant growth [9,10,11,12,13]. Thus, A. flagrans has great potential for the development of biocontrol agents against both plant and animal parasitic nematodes.
In the sequence of events involved in NTF infestation of nematodes (recognition and adhesion, penetration, digestion, and growth into host tissues), strong adhesion to the host surface is a prerequisite for penetration of the nematode stage [14,15]. Ultrastructural studies demonstrated that the NTF utilizes an adhesive layer covering the surfaces of the trap to capture nematodes; denaturation of the adhesive layer leads to a significant reduction in nematode capture efficiency [16]. A positive correlation was present between the thickness of the NTF adhesion layer and the number of up-regulated adhesion proteins [17,18,19]. Fungal extracellular membrane proteins of plant- and animal-pathogenic fungi contain the CFEM domain, which was implicated in multiple biological functions in fungi. For example, deleting PeCFEM5 and PeCFEM8 in Penicillium expansum caused reduced pathogenicity and patulin accumulation [20]. The CFEM domain of Pth11 in Magnaporthe oryzae is essential for the development of appressoria and virulence [21]. Three CFEM proteins in Candida albicans were shown to participate in hemoglobin-iron acquisition [22]. Evidence suggests that some CFEM-containing proteins are used as cell-surface receptors, signal transducers, or adhesion molecules in host–pathogen interactions, or are involved in virulence, the formation of the inner layer of the cell wall, and heme binding in fungi [20,21,22,23,24,25]. Pathogenic fungi contain more CFEM structural domain-containing proteins than non-pathogenic fungi, suggesting that the CFEM structural domain may play a potentially critical role in fungal virulence [24].
Unsurprisingly, a large number of CFEM-containing proteins (12 in Drechslerella stenobrocha and 17 in A. oligospora) are also present in NTF, and it is hypothesized that they serve an important role in nematode trapping [26]. However, the specific roles of CFEM domain-containing proteins in nematode trapping by NTF remain to be elucidated. A total of 14 CFEM domain containing genes were differentially expressed in A. flagrans, and the expression levels of AfCFEM1-8 were significantly up-regulated after 18 h of interaction with C. elegans. This study was designed to investigate the biological functions of AfCFEM1-3 in A. flagrans, which provides a basis for elucidating the role of CFEM domain-containing genes in nematode trapping by A. flagrans.
2. Materials and Methods
2.1. Organisms and Media
The fungus A. flagrans and its knockout mutants, as well as overexpression transformants, were maintained on potato dextrose agar (PDA) plates at 28 °C. Tryptone glucose (TG), tryptone yeast-extract glucose agar (TYGA), potato sucrose tryptone yeast-extract agar (PSTYA), corn meal agar (CMA), Luria–Bertani medium (LB), chlamydospore induction (CI), and WA (1.5% agarose) media were used in this research (Supplementary Information for details). Caenorhabditis elegans N2 strain was maintained at 20 °C on nematode growth medium (NGM) plates and fed with concentrated Escherichia coli OP50.
2.2. Formation of Traps in A. flagrans After Induction by C. elegans
The A. flagrans cultured on PDA plates at 28 °C for 4 days was incubated on 100 mL of CI medium in a shaker rotating at 28 °C, 180 rpm for 12 h and then at 90 rpm for 3 days to obtain chlamydospores. Approximately 104 chlamydospores were coated on cellophane-covered CMA plates (9 cm) and left to germinate until mycelium was present throughout the plate. Approximately 1000 C. elegans (L2–L4 period) were added to the CMA plates with A. flagrans, and the formation of traps was observed and recorded. The experiment was performed in three replicates.
2.3. Transcriptome Analysis of A. flagrans Interacted with C. elegans
According to 2.2, at 3, 6, 12, 18, and 24 h of nematode induction, the traps of A. flagrans developed into different morphologies. Therefore, the A. flagrans and C. elegans were collected together after adding approximately 1000 C. elegans to each plate for 3, 6, 12, 18, and 24 h, and marked as AC_3, AC_6, AC_12, AC_18, and AC_24, respectively. Samples collected immediately after adding C. elegans to A. flagrans (A. flagrans and C. elegans interacted for 0 h) were used as controls. Collected samples were immediately put into liquid nitrogen for freezing before being sent to Shanghai Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China) for sequencing, and the transcriptome data were analyzed using the Majorbio cloud platform (https://www.majorbio.com, accessed on 11 June 2023). The experiment was performed in three replicates.
2.4. Analysis of Physicochemical Properties and Domains of AfCFEM1-3
Amino acid sequences of three AfCFEM proteins, AfCFEM1 (DFL_009810), AfCFEM2 (DFL_002456), and AfCFEM3 (DFL_009809) were downloaded from the Protein database of the National Center for Biotechnology Information (NCBI). The InterProScan (https://www.ebi.ac.uk/interpro/, accessed on 11 June 2023) and ExPASy ProtParam tool (https://web.expasy.org/protparam/, accessed on 11 June 2023) were used to predict functional domains and identify the physicochemical properties of these three proteins.
2.5. Deletion and Overexpression of AfCFEM1-3
Homologous recombination was applied to delete the AfCFEM1-3. With the paired primers (Table S1), designed using CE Design (https://crm.vazyme.com/cetool/simple.html (accessed on 11 June 2023), the upstream and downstream fragments of the AfCFEM1-3 genes and the hygromycin resistance gene (Hyg) fragment were amplified using the A. flagrans genome and the pCSN44 plasmid as templates, respectively. The AfCFEM1-3 knockout vectors were constructed by ligating the amplified upstream, downstream, and Hyg resistance fragments to the pCE-Zero plasmid (Nanjing Vazyme Biotech Co., Ltd., Nanjing, China). Using AfCFEM1-3 knockout vectors as a template, the knockout fragments for AfCFEM1-3 amplified with primer AfCFEM-up-F1-3 and AfCFEM-down-R1-3 (Table S1) were used for protoplast transformation to obtain the knockout mutants as described [27]. The AfCFEM1-3 deletion mutants were verified by PCR amplification with verification paired primers (F1/R1, F1/R2, F2/R1, and F3/R3) (Table S1).
The AfGpdP (glyceraldehyde-3-phosphate dehydrogenase promoter) [27], coding sequences (CDS) of AfCFEM1-3, AfGpdT (glyceraldehyde-3-phosphate dehydrogenase terminator), and Hyg fragments were amplified with specific paired primers (Table S1) and were cloned into the pCE-Zero plasmid to obtain the overexpression vectors of AfCFEM1-3. Total RNA of A. flagrans that interacted with C. elegans for 18 h was extracted with UNlQ-10 Column Total RNA Purification Kit (Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China) and was used as a template for the reverse transcription to cDNA of AfCFEM1-3. The overexpression fragments were amplified with primer pCE-Zero-ZH-F/R for protoplast transformation. The total RNA obtained from overexpression transformants, which interacted with C. elegans for 18 h, was reverse transcribed to cDNA. The cDNA samples were used as templates to detect the transcription of AfCFEM1-3 in AfCFEM1-3 overexpression transformants by RT-qPCR assays. RT-qPCR was carried out with specific paired primers (designed by Primer 5, Table S1) and AceQ Universal SYBR qPCR Master Mix (Vazyme) using a LightCycler 480 Ⅱ (Roche Diagnostics GmbH, Mannheim, Germany), and data were analyzed using LightCycler® 480 SW 1.5.1. The 2−ΔΔCT method was used to quantify relative transcription levels of AfCFEM1-3 with the AfGpd gene as the reference. A melt curve was performed at the end of each reaction to verify PCR product specificity. The experiment was repeated in three replicates for each strain. The transformants with the highest AfCFEM1-3 gene expression were selected by RT-qPCR analysis for subsequent experiments.
2.6. Determination of the Effect of AfCFEM1-3 on Trap Formation and Pathogenicity
L4-stage C. elegans worms were prepared as described previously [28]. The WT, ΔAfCFEM1-3 mutants and OEAfCFEM1-3 transformants were incubated on WA plates (3.5 cm) at 28 °C for 3 days. The nematode mortality of each plate was calculated after 200–300 L4-stage C. elegans were added to the plates for 12, 15, 18, 24, and 48 h. The number of traps was counted at 12 h and 24 h. The proportion of regular and irregular traps was observed at 12 h, and the proportions of single-ring and multiple-ring traps were counted at 24 h. The experiment was performed in three replicates.
2.7. Cryo-Scanning Electron Microscope and Transmission Electron Microscope Observation
The WT, ΔAfCFEM1-3 mutants and OEAfCFEM1-3 transformants were cultured on WA plates (6 cm) at 28 °C for 4 days, and about 500 C. elegans were added to the plates to induce the trap formation of A. flagrans. After 15 h, the mycelium and nematodes were collected from each plate and then sent to the Kunming Institute of Botany, Chinese Academy of Sciences to complete sample pre-treatment and cryo-scanning electron microscopy (cryo-SEM) observation. For cryo-SEM, the sample was attached vertically to the sample holder using conductive adhesive. Then, the sample was freeze-fixed with a liquid nitrogen slurry, sublimated at −90 °C for 15 min, and transferred to the cold stage of the scanning electron microscope (−140 °C) under low-temperature and vacuum conditions for observation. The images were obtained using a cryo-scanning electron microscope (Zeiss Sigma 300, Oberkochen, Germany) equipped with a Quorum PP3010T cryo preparation system (East Sussex, UK), operated at 5–7 kV.
The WT and ΔAfCFEM1-3 mutants were incubated on cellophane-covered WA plates (6 cm) at 28 °C for 4 days, adding about 500 C. elegans. The mycelium and nematodes were collected when the C. elegans interacted with the mycelium for 18 h. The samples were fixed in 2.5% glutaraldehyde for 12 h and then sent to the Kunming Institute of Zoology, Chinese Academy of Sciences, to complete sample pre-treatment and transmission electron microscopy (TEM) observation. More than 30 trap cells (electron-dense microbodies are present in the cytoplasm) were randomly selected, with 5 randomly selected points per cell, and their cell wall thickness was measured using the Photoshop scale tool. For TEM, the samples were fixed overnight at 4 °C using 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH7.2). Samples were then washed three times with 0.1 M phosphate buffer (pH7.2) for 15 min. Afterward, samples were postfixed with 1% OsO4 and 1.5% K3[Fe(CN)6] for 2 h at 4 °C, then washed with ddH2O three times for 7 min, followed by serial ethanol dehydration and acetone transition for 5 min, embedding in SPI Pon 812R resin, polymerization at 60 °C for 48 h. Serial sections of uniform thickness, 800 nm for semithin sections and 60 nm for ultrathin sections, were made using a Leica EM UC7 ultramicrotome (Wetzlar, Germany). Semithin sections were prepared for light microscopy and ultrathin sections for TEM. Semithin sections were stained with toluidine blue. Ultrathin sections were then loaded onto Cu grids and double-stained with 2% uranyl acetate and lead citrate before observation using a JEM 1400 plus transmission electron microscope (JEOL, Tokyo, Japan) at 80 kV.
2.8. Determination of the Relative Transcriptional Level of AfCFEM1-8 in ΔAfCFEM1-3 Mutants
The ΔAfCFEM1-3 mutants were incubated on cellophane-covered CMA plates (9 cm) at 28 °C for 6 days. After 18 h of ΔAfCFEM1-3 mutants and C. elegans interactions, total RNA was extracted and reverse transcribed into cDNA, which was used as a template for RT-qPCR assays. The specific paired primers were designed using Primer 5 and listed in Table S2. The RT-qPCR assays were performed according to the Section 2.5.
2.9. Proteomic Analysis of ΔAfCFEM1 and ΔAfCFEM2 Mutants
The chlamydospores of WT, ΔAfCFEM1, and ΔAfCFEM2 mutants were prepared and cultured on cellophane-covered CMA plates (9 cm) at 28 °C for 3 days as described in 2.2. Approximately 1000 C. elegans were added to each plate to interact with the WT, ΔAfCFEM1, and ΔAfCFEM2 mutants for 0, 12, and 24 h. Samples of WT, ΔAfCFEM1 (AP98) and ΔAfCFEM2 (BP24) mutants that interacted with C. elegans were then collected and labeled as WTP_0, WTP_12, WTP_24, AP98_0, AP98_12, AP98_24, BP24_0, BP24_12, and BP24_24. The collected samples were washed three times with PBS buffer, followed by centrifugation at 4 °C and 5000 g for 30 min to remove the supernatant. Subsequently, the samples were immediately frozen in liquid nitrogen and sent to the Shanghai Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China) for proteome sequencing analysis. The data were analyzed using the Majorbio cloud platform (https://www.majorbio.com, accessed on 11 June 2023). The experiment was performed in three replicates.
2.10. Statistical Analysis
Statistical analysis and graphs creation were performed in GraphPad Prism 9.5.1 (GraphPad Software, San Diego, CA, USA). Significant differences were identified by analyzing the comparison between the control and treated samples using ANOVA with the Dunnett multiple comparisons test. Unless otherwise stated, error bars represent the standard error of the mean of pooled data, and statistical significance is represented by * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001
3. Results
3.1. The Process of Trap Formation of A. flagrans in Response to C. elegans Induction
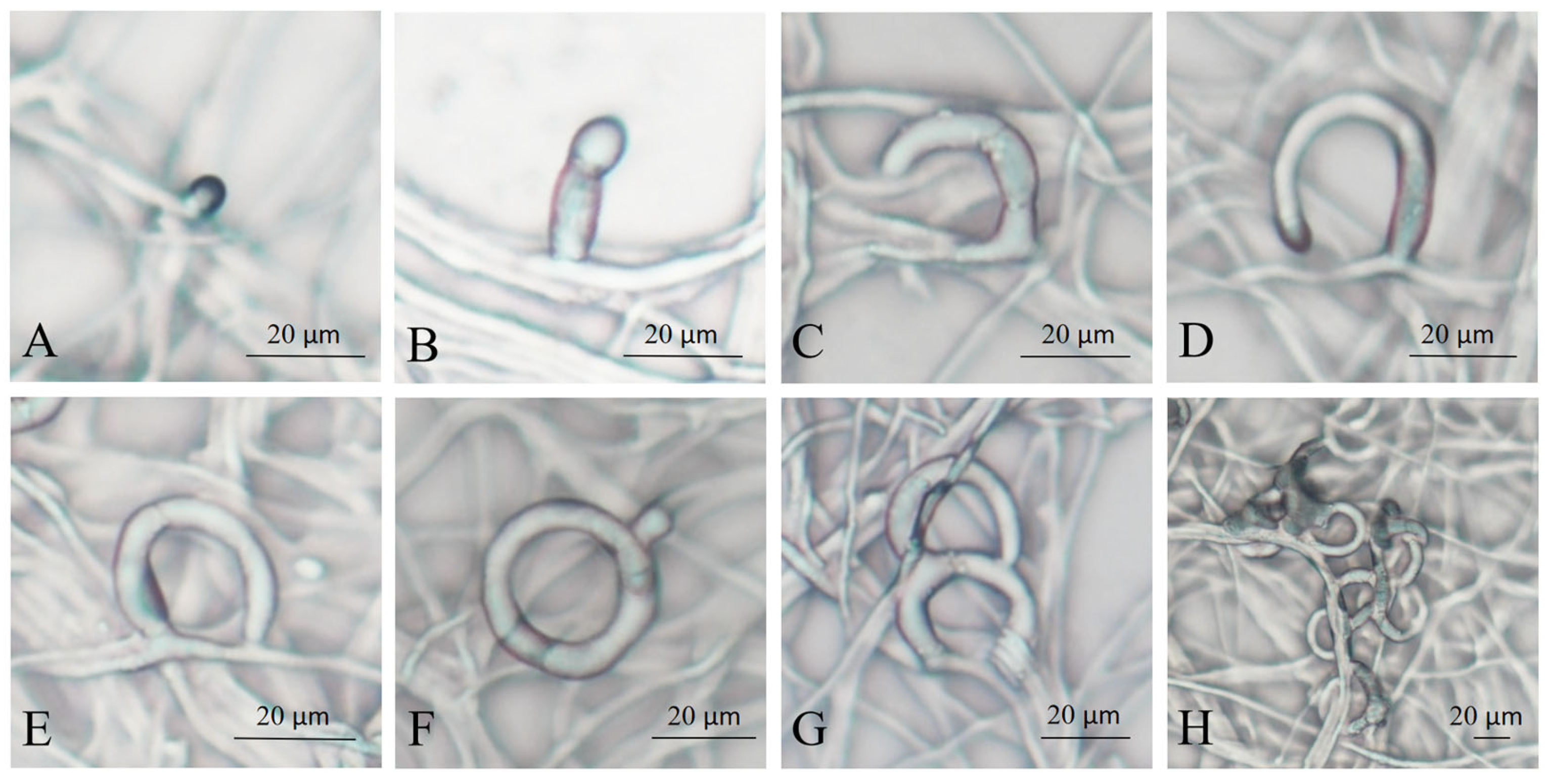
The NTF produces polyketide-derived arthrosporols to inhibit trap formation and releases volatile molecules to attract nematodes [29,30]. When A. flagrans detects the highly conserved ascarosides secreted by many soil nematodes, it causes a downregulation of arthrosporol synthesis, thereby initiating trap formation [31,32]. Commonly, traps of A. flagrans are only produced after the induction of nematodes, which were considered the most effective inducer for trap production [31,33]. To investigate the process of trap formation of A. flagrans after induction by C. elegans, regular observations were carried out after A. flagrans interaction with the nematodes. The traps of A. flagrans began to germinate at 2–3 h (Figure 1A,B) after nematode induction and grew as half rings after 6–8 h (Figure 1C,D); at 8–12 h (Figure 1E,F), most of the traps fused with the mycelium to form a complete closed regular single hyphal loop. After 12–18 h (Figure 1G) of interaction with nematodes, the traps continued to grow to form two or more rings on the base of the single ring. At 24–48 h (Figure 1H) of nematode induction, the traps develop into mature, three-dimensional networks consisting of multiple mycelial rings.
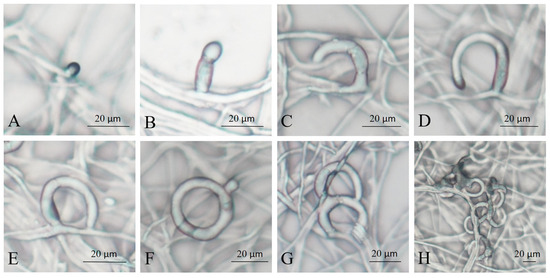
Figure 1.
Morphology of A. flagrans traps at different times of C. elegans induction. (A,B) 2–3 h; (C,D) 6–8 h; (E,F) 8–12 h; (G) 12–18 h; (H) 24–48 h.
3.2. C. elegans Induction Altered Gene Expression in A. flagrans
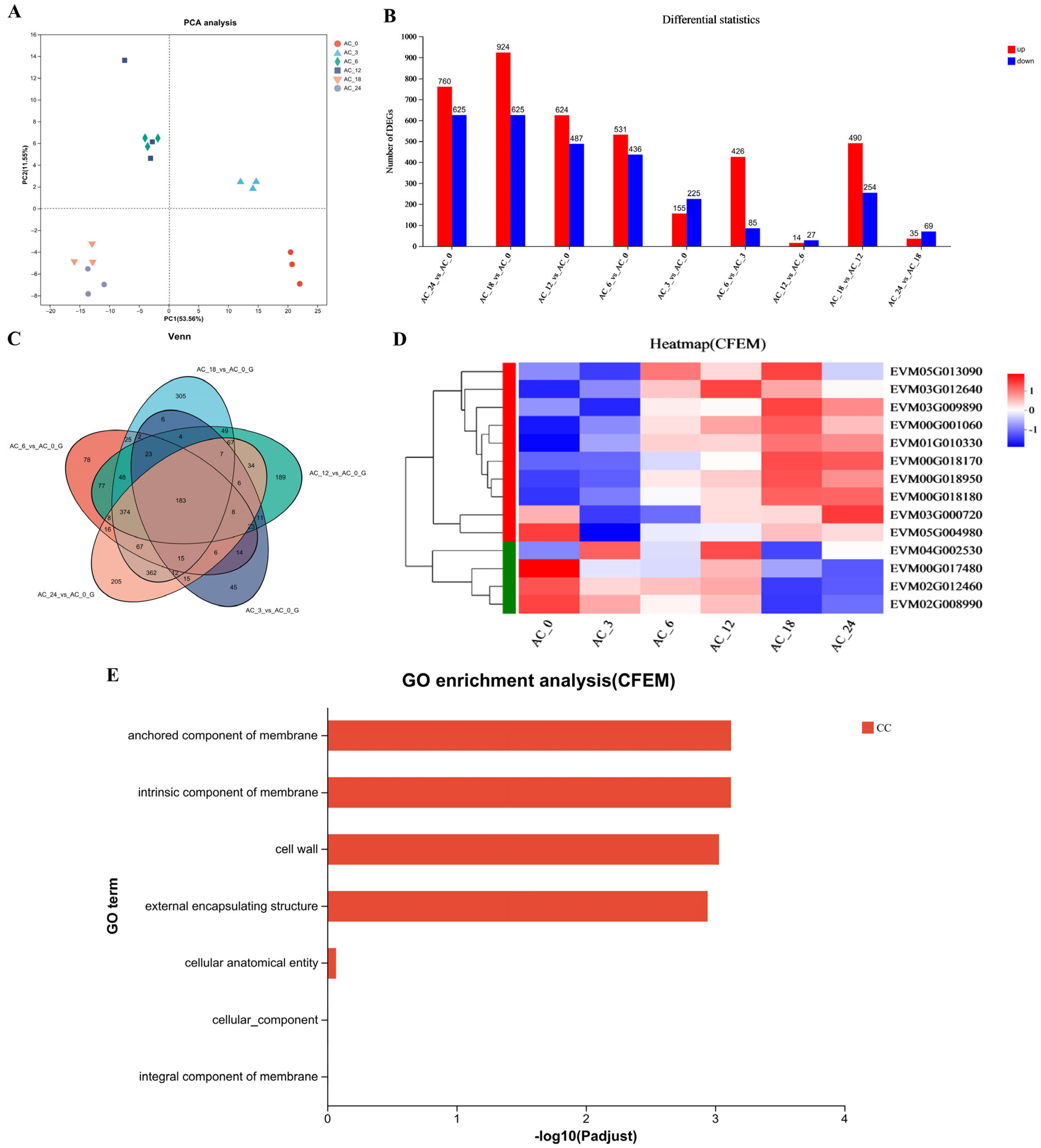
However, the formation of traps is a highly complicated biological process that may involve numerous genes and pathways [34]. To investigate the changes in gene expression of A. flagrans during trap formation after nematode induction for 3, 6, 12, 18, and 24 h compared to 0 h, the transcriptome analysis was performed. Each sample yielded 15.37–40.41 million reads; the quality of the sequence reads was evaluated by a Phred-like quality score, and the Q20, Q30, and GC contents of the clean data were determined (Table S3). More than 90% of the reads matched the reported A. flagrans genome [27] after eliminating the C. elegans transcripts. Principal component analysis (PCA) indicated high similarity among the three replicates from the same group (Figure 2A). Fold change ≥2 with p value < 0.05 was used as a screening criterion during the detection of differentially expressed genes (DEGs). Analysis of the DEGs after addition of C. elegans for 3, 6, 12, 18, and 24 h revealed that there were 380 (155 up-regulated and 225 down-regulated), 967 (531 up-regulated and 436 down-regulated), 1111 (624 up-regulated and 487 down-regulated), 1549 (924 up-regulated and 625 down-regulated) and 1385 (760 up-regulated and 625 down-regulated) DEGs were differentially expressed, respectively, compared to 0 h (Figure 2B). Venn analysis of DEGs revealed that 45, 78, 189, 305, and 205 genes were expressed at higher levels after induction of C. elegans for 3, 6, 12, 18, and 24 h, respectively. Additionally, 183 DEGs were shared across all selected time points of nematode-A. flagrans interactions (Figure 2C).
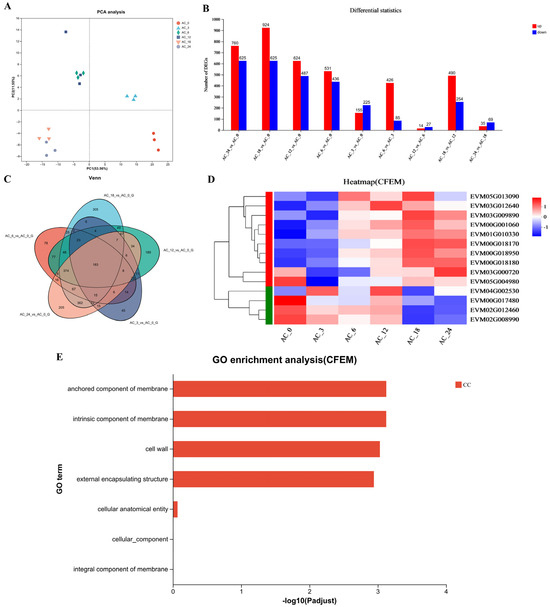
Figure 2.
Transcription analysis of A. flagrans after C. elegans addition. (A) Principal component analysis (PCA) plot for A. flagrans after induction by C. elegans for 0, 3, 6, 12, 18, and 24 h. (B) Statistics of differentially expressed genes (DEGs) in A. flagrans at 0, 3, 6, 12, 18, and 24 h of C. elegans induction. (C) Venn analysis of the DEGs in A. flagrans induced by C. elegans at 0, 3, 6, 12, 18, and 24 h. (D) Heatmap of the CFEM protein-related genes expression levels in A. flagrans after adding C. elegans for 0, 3, 6, 12, 18, and 24 h. (E) Gene ontology (GO) enrichment analysis of CFEM protein-related genes.
Furthermore, the Gene Ontology (GO) enrichment analysis and functional enrichment of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways for DEGs in A. flagrans after induction by C. elegans for 3, 6, 12, 18, and 24 h, compared with 0 h, were performed. GO enrichment analysis annotates the DEGs to molecular function (MF), cellular component (CC), and biological process (BP) involved. Among them, the MF mainly involve oxidoreductase activity, D-threo-aldose 1-dehydrogenase activity, transporter activity, structural molecule activity, catalytic activity, etc.; the CC are mostly cell wall, membranes, ribosomes, non-membrane-bounded organelle, and nucleolus components; and the BP mainly involve sulfate assimilation, amino acid transport, organic acid transport, peptide metabolic process, translation, carbohydrate metabolic process, glucose metabolic process, etc. (Figure S1). Likewise, the KEGG pathways enriched after nematode induction for more than 3 h included various metabolic processes (sulfur metabolism, tyrosine metabolism, glycolysis/gluconeogenesis, ribosome, etc.) (Figure S1).
In addition, the transcriptomic analysis revealed that the highest number of DEGs appeared 18 h after C. elegans induction; therefore, all up-regulated genes at 18 h of nematode induction were ranked according to the fold up-regulation. As a result, most of the top 20 up-regulated genes were related to the toxicity of A. flagrans to nematodes, including the Egh16 family (involved in appressorium formation and pathogenesis in pathogenic filamentous fungi), WSC (cell wall integrity and stress response component) domain related genes, and CFEM domain containing genes (Table S4).
As stated earlier, the highest number of DEGs appeared 18 h after C. elegans induction. There were 14 CFEM domain-containing genes (Figure 2D) differentially expressed in A. flagrans, of which 8 CFEM domain-containing genes, EVM00G018180 (AfCFEM1), EVM01G010330 (AfCFEM2), EVM00G018170 (AfCFEM3), EVM00G018950 (AfCFEM4), EVM03G009890 (AfCFEM5), EVM00G001060 (AfCFEM6), EVM05G013090 (AfCFEM7), and EVM03G012640 (AfCFEM8) were upregulated 11.56, 8.30, 6.83, 3.03, 2.20, 2.13, 0.98, and 0.85 folds compared to 0 h, respectively, and another 6 CFEM domain-containing genes were down-regulated (Table S5). GO enrichment analysis revealed that the CFEM domain related genes were enriched for cellular components, including cell wall and membrane components (Figure 2E). Combining GO functional annotation, Swiss-Prot annotation information, and relevant reports, we inferred that the CFEM domain-containing genes in A. flagrans might be involved in forming cell membranes, cell walls, and cell wall adhesion proteins, or encode proteins anchored to cell membranes. Therefore, three genes (AfCFEM1, AfCFEM2, AfCFEM3) with the highest up-regulation of transcript levels at 18 h of interaction with C. elegans were selected for subsequent functional studies.
The CFEM domain is unique to fungi but not present in all fungi [24,35]. The three proteins’ physicochemical properties and functional domains, AfCFEM1-3, were analyzed. The results showed that the AfCFEM1 protein contains 130 amino acids, has a molecular weight of 13.57 KDa, and an isoelectric point of pI of 8.08 (Figure S2); the AfCFEM2 protein contains 208 amino acids, has a molecular weight of 20.59 KDa, and an isoelectric point of pI of 3.76 (Figure S2); the AfCFEM3 protein contains 614 amino acids, has a molecular weight of 64.37 KDa, and an isoelectric point of pI of 3.28 (Figure S2); AfCFEM1-3 proteins all contain the CFEM domain.
3.3. AfCFEM1-3 Play a Vital Role in the Trap’s Morphology and Pathogenicity of A. flagrans
With a view to exploring the role of AfCFEM1-3 in A. flagrans, the AfCFEM1-3 were disrupted and overexpressed, and the ΔAfCFEM1-3 mutants and OEAfCFEM1-3 transformants were obtained and verified by PCR amplification (Figure S3) and RT-qPCR (Figure S4), respectively.
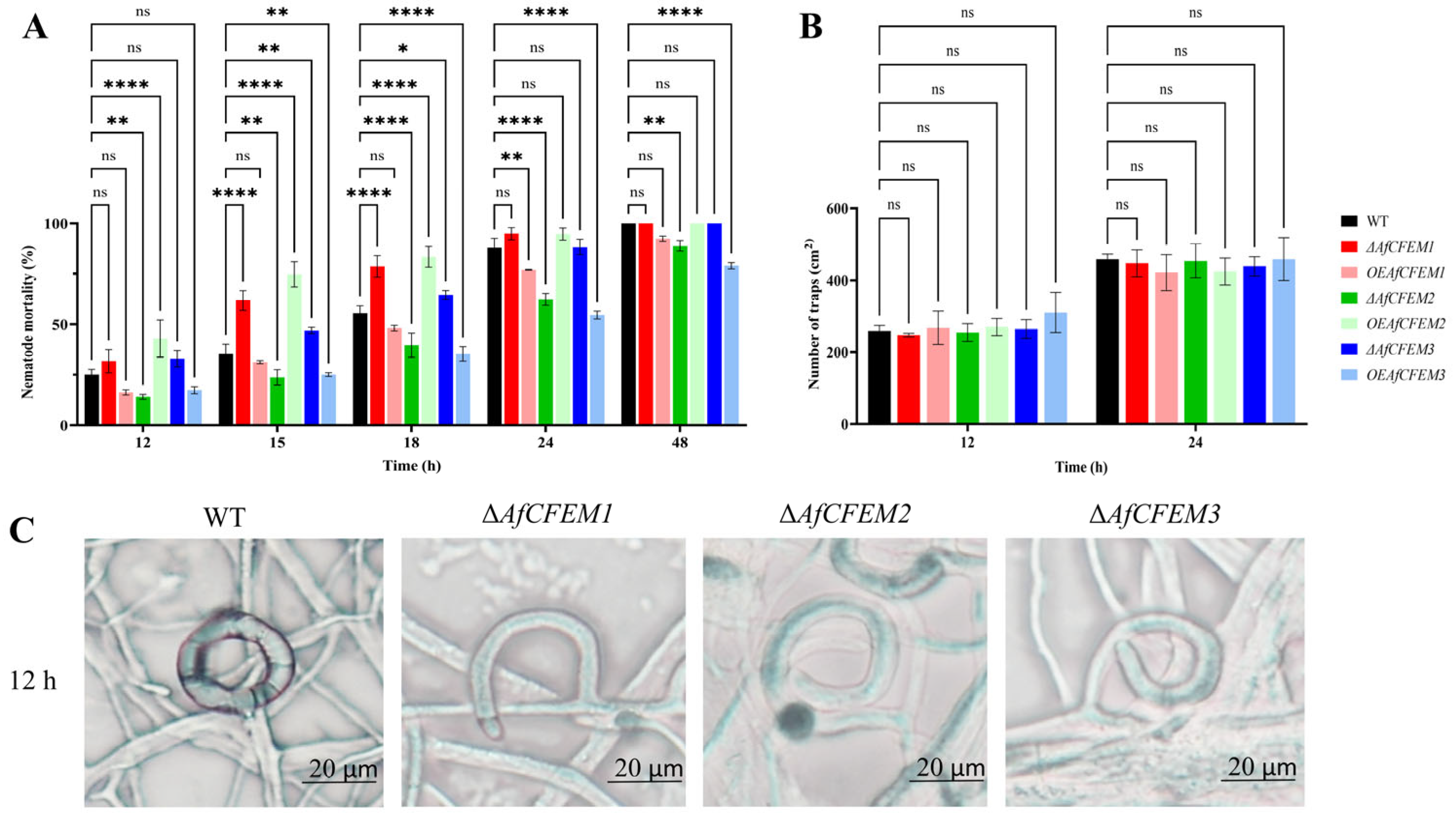
After C. elegans addition for 12, 15, 18, 24, and 48 h, the nematode mortality results of WT (25.06, 35.41, 55.57, 88.06, and 100%, respectively), ΔAfCFEM1 mutants (31.72, 61.82, 78.73, 94.87, and 100%, respectively) and OEAfCFEM1 transformants (16.28, 31.27, 48.11, 77.00 and 92.37%, respectively) were obtained (Figure 3A), the results showed that the loss of AfCFEM1 caused increased nematode mortality at 12, 15, 18 and 24 h, and was significantly different at 15 and 18 h. For AfCFEM2, the deletion of AfCFEM2 leads to a significant reduction in nematode mortality to 14.10, 23.74, 39.66, 62.38, and 88.88% at 12, 15, 18, 24, and 48 h, respectively (Figure 3A). In contrast, the nematode mortality of the OEAfCFEM2 transformant was 42.91, 74.76, 83.47, 94.68, and 100%, respectively (Figure 3A). The overexpression of AfCFEM2 caused a significant increase in nematode lethality at 12, 15, and 18 h. As for AfCFEM3, the nematode mortality of ΔAfCFEM3 mutants was 32.96, 46.96, 64.49, 88.29, and 100% after nematode addition for 12, 15, 18, 24, and 48 h, respectively, in which the nematode mortality was significantly higher than that of WT at 12, 15, and 18 h (Figure 3A). Whereas the overexpression of the AfCFEM3 gene significantly reduced nematode mortality to 17.38, 25.10, 35.35, 54.61, and 79.02% at 12, 15, 18, 24, and 48 h, respectively (Figure 3A). It can be noticed that both the knockout and overexpression of the AfCFEM1-3 resulted in changes in nematode mortality. Interestingly, there was no significant difference among the number of traps produced by WT, ΔAfCFEM1-3 mutants, and OEAfCFEM1-3 transformants at 12 and 24 h after nematode addition (Figure 3B).
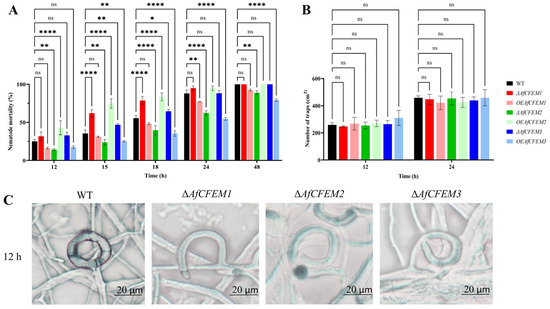
Figure 3.
The nematode mortality, number, and morphology of traps in WT, ΔAfCFEM1-3 mutants, and OEAfCFEM1-3 transformants during the interaction with C. elegans. (A) The nematode mortality of the WT, ΔAfCFEM1-3 mutants, and OEAfCFEM1-3 transformants at 12, 15, 18, 24, and 48 h after adding C. elegans. (B) The number of traps of WT, ΔAfCFEM1-3 mutants, and OEAfCFEM1-3 transformants per cm2 at 12 and 24 h after C. elegans addition. (C) The morphology of traps in WT and ΔAfCFEM1-3 mutants after adding C. elegans for 12 h. (ns p > 0.05, * p < 0.05, ** p < 0.01, and **** p < 0.0001).
It is noteworthy that AfCFEM1-3 has an important impact on trap morphology. After 12 h of nematode addition, most of the traps in the WT would fuse with the hyphae to form a closed loop (98.33%) (Figure 3C). Still, some traps in ΔAfCFEM1-3 mutants were not fused with the hyphae, resulting in traps twisted at different angles (Figure 3C). Thus, the proportion of irregular traps significantly increased to 28.12, 27.28, and 28.82%, respectively.
3.4. AfCFEM1-3 Associated with the Cell Wall Formation and Adhesive Proteins in Traps of A. flagrans
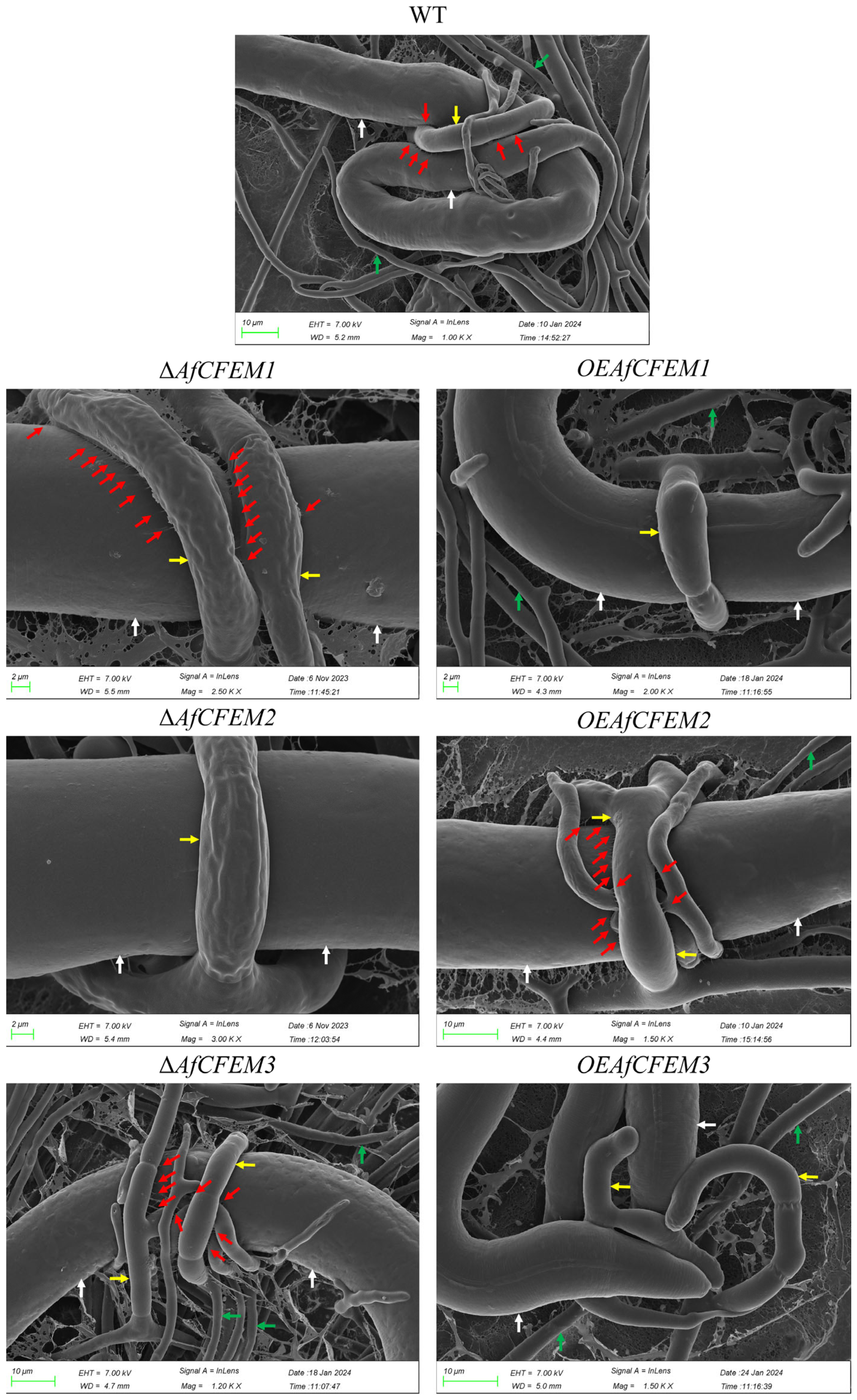
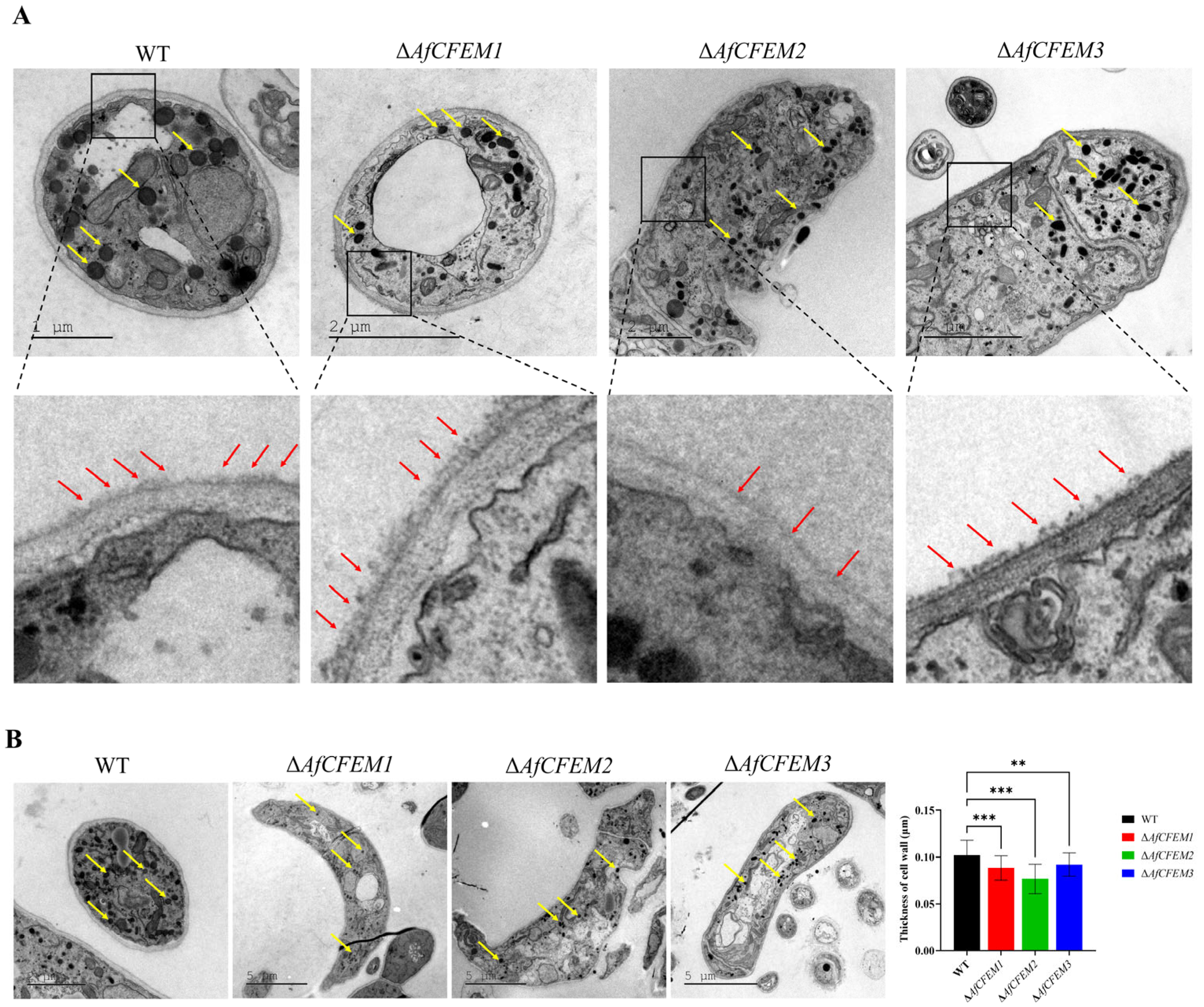
The cryo-SEM results showed that during interactions with C. elegans for 15 h, the adhesive material present on the trap–nematode contact trap surface of ΔAfCFEM1 and ΔAfCFEM3 mutants (Figure 4) was denser than that of WT. In contrast, it was barely observed in the OEAfCFEM1 and OEAfCFEM3 transformants (Figure 4). However, little adhesive material was observed on the trap surface of the ΔAfCFEM2 mutants, and there was an increasing amount of adhesive substance on the trap surface of the OEAfCFEM2 transformants (Figure 4). The electron-dense microbodies in the cytoplasm are features shared by the morphologically diverse trap cells produced by NTF, distinguishing them from hyphal cells [34]. Therefore, trap cells in TEM images were observed using electron-dense microbodies in the cytoplasm as a criterion for identification. The TEM results showed that flocculent material was present on the outer surface of the cell walls of the WT, ΔAfCFEM1, and ΔAfCFEM3 strains traps after 18 h of nematode addition, but was barely observed in the cell walls of the ΔAfCFEM2 mutants (Figure 5A). Moreover, the knockout of the AfCFEM1-3 leads to a significant reduction in the cell wall thickness of the A. flagrans traps (Figure 5B).
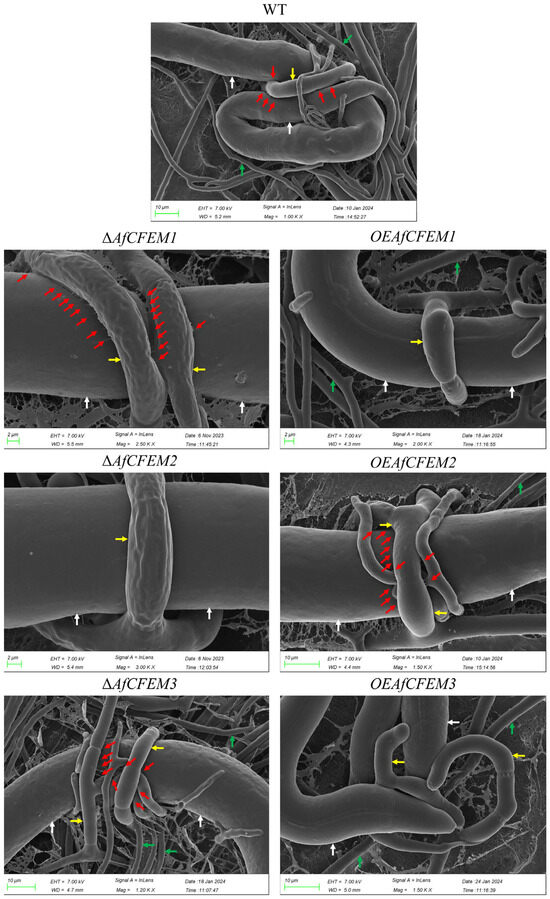
Figure 4.
Partial representative cryo-scanning electron microscope images of the adhesive material on the trap–nematode contact surface of traps in WT, ΔAfCFEM1-3 mutants, and OEAfCFEM1-3 transformants after the addition of C. elegans for 15 h. Red arrow: adhesive material; white arrow: nematode (C. elegans); green arrow: mycelium of A. flagrans; yellow arrow: trap of A. flagrans.
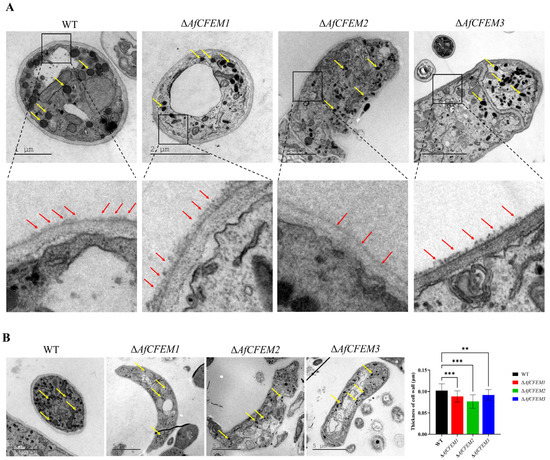
Figure 5.
The transmission electron microscope observation on traps of WT and ΔAfCFEM1-3 mutants. (A) The flocculent material (red arrow) presented on the outer surface of the cell walls of the WT and ΔAfCFEM1-3 mutants traps after 18 h of C. elegans addition. Yellow arrow: electron-dense microbodies. (B) Partial representative images and cell wall thickness of traps of WT and ΔAfCFEM1-3 mutants. (** p < 0.01, *** p < 0.001).
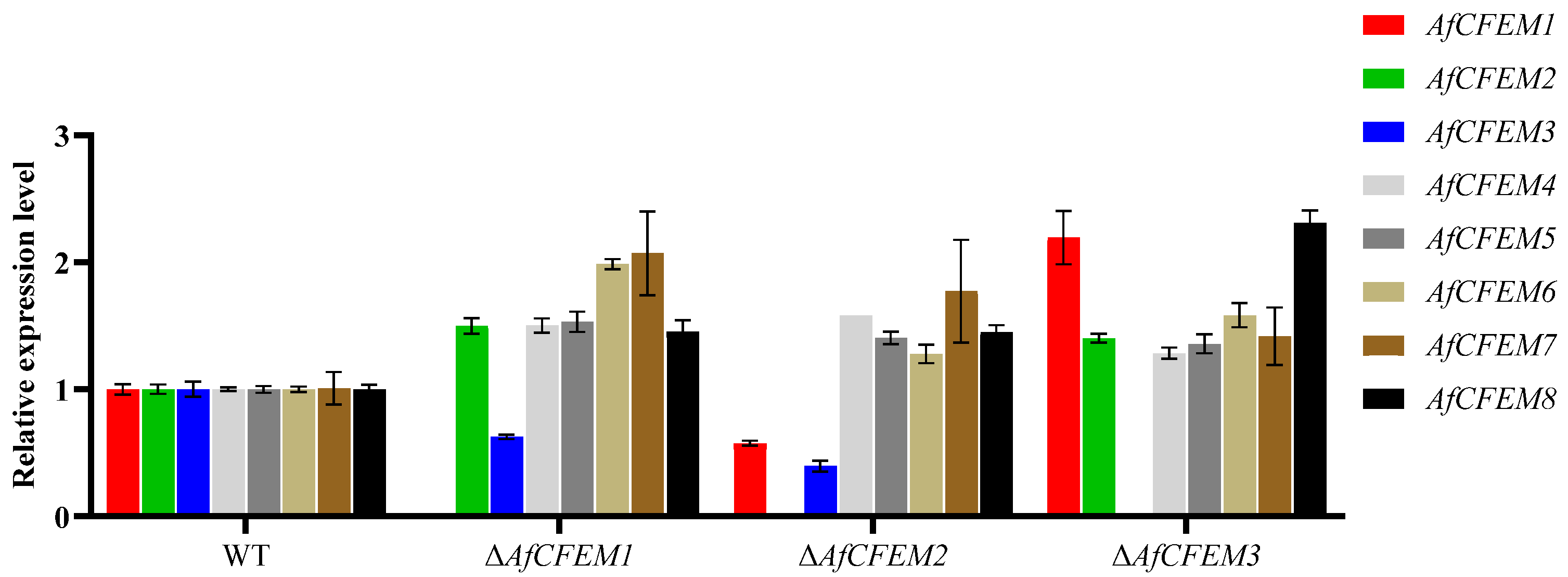
3.5. The Compensating Effects Exist Among AfCFEM1-8 of A. flagrans
In Candida albicans, the overexpression of genes coding for other proteins having adhesive properties compensates for the loss of the genes encoding CFEM domain-containing proteins [36]. The results revealed that in the ΔAfCFEM1 mutants, the relative expression levels of the other 6 AfCFEM were up-regulated compared to the WT, except for the down-regulated expression of AfCFEM3 (Figure 6). In addition, the relative expression levels of AfCFEM1 and AfCFEM3 were down-regulated, and the AfCFEM4-8 were up-regulated in ΔAfCFEM2 mutants (Figure 6). For ΔAfCFEM3 mutants, the relative expression levels of all other AfCFEM genes were up-regulated (Figure 6).
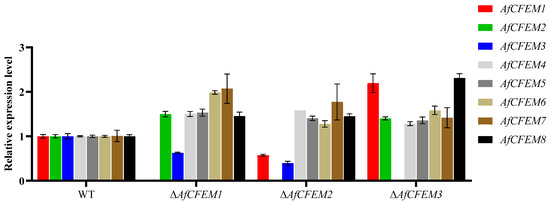
Figure 6.
The relative expression levels of AfCFEM1-8 in WT and ΔAfCFEM1-3 mutants after interacting with C. elegans for 18 h.
3.6. AfCFEM1 and AfCFEM2 Affected the Protein Expression in A. flagrans
Proteomics is one of the most important methods for investigating the function of genes. The proteomic statistics and the sample correlation heatmap (Figure S5) show that the proteomic data are of high quality and have good reproducibility between replicates, respectively. Fold change ≥ 1 with p value < 0.05 was used as a screening criterion during the detection of differentially expressed proteins (DEPs). The DEPs were statistically analyzed (Figure S6), and we focused on the adhesion-related DEPs that were up-regulated in ΔAfCFEM1 mutants and down-regulated in ΔAfCFEM2 mutants, for which the GO and KEGG annotations for the DEPs are shown in Figure S6. A further screening of virulence and adhesion proteins from the above DEPs revealed that a subtilisin-like serine protease (EVM00G001030), an aspartic protease (EVM01G001350), and a malate synthase (EVM01G004180) were up-regulated at ΔAfCFEM1 mutants at 12 h of interactions with nematodes (Table S6). Moreover, two proteins (EVM01G009790 and EVM02G001720) containing lectin domains (associated with adhesion), two subtilisin-like serine proteases (EVM00G001030 and EVM03G007260), and two aspartic acid proteases (EVM01G001350 and EVM02G016290) were up-regulated after ΔAfCFEM1 mutants interacted with C. elegans for 24 h (Table S6). In contrast, an Egh16 virulence protein (EVM02G005160) and a lipase-related protein (EVM00G010160) were down-regulated at ΔAfCFEM2 mutants that interacted with nematodes for 12 h and 24 h, respectively (Table S6). Additionally, many of the up-regulated virulence and adhesion DEPs screened in ΔAfCFEM1 mutants were annotated to physiological processes, including autophagy, MAPK signaling pathway, lysine biosynthesis, nitrogen metabolism, glycolysis/gluconeogenesis, starch and sucrose metabolism, and citrate cycle (Table S7). The downregulated virulence and adhesion DEPs of ΔAfCFEM2 mutants also annotated autophagy and MAPK signaling pathways (Table S7). In addition, AfCFEM4 protein expression was up-regulated in both ΔAfCFEM1 and ΔAfCFEM2 mutants.
4. Discussion
NTF does not spontaneously produce or produce a small number of traps. Traps can be induced in response to various inducers such as nematode extract, amino acids, small peptides, abscisic acid, ascarosides, and other substances [34]. However, living nematodes are considered the most effective inducer for trap production [33]. Here, it was observed that C. elegans induced traps of A. flagrans sprouted from hyphae and gradually developed into closed single-loop traps, eventually maturing into three-dimensional networks with multiple mycelial rings. Many genes in A. flagrans are differentially expressed during nematode-induced trap formation. The top 20 genes up-regulated after nematode induction for 18 h contained four Egh16 family genes, WSC domain-related genes, and CFEM domain-related genes, suggesting that they are critical in trap formation. It was also shown that Egh16 family genes are important for host attachment devices (traps and appressorium) development of fungi [37,38]. In contrast, WSC domain-containing proteins were found to function as cell wall sensors and were involved in fungal adhesion [39,40,41,42]. In addition, it was demonstrated that the expression level of the WSC domain was significantly higher in traps than in the mycelium of NTF [43]. Moreover, CFEM domain containing proteins were demonstrated to participate in biofilm formation and cell wall biogenesis [24,44,45]. The transcriptomes indicated that the CFEM domain containing proteins participated in the trap formation processes of NTF Drechslerella dactyloides [46]. The protein encoded by AfCFEM5 (EVM03G009890) is a homolog of the adhesive protein AoMAD1 of A. oligospora with 83.3% homology. Therefore, the CFEM containing genes in A. flagrans might be associated with the formation of cell membranes and cell wall adhesion proteins.
Transcriptomic analysis showed proteins containing the CFEM domain serve an important role in nematode trapping by A. flagrans. Pathogenic fungi contain more fungal-unique CFEM domains than non-pathogenic fungi, indicating that the amount of CFEM is positively correlated with fungal pathogenicity [24]. Target deletion of AfCFEM1-3 leads to alterations in nematode mortality, trap morphology, adhesive materials on the surface of traps, and cell wall thickness of A. flagrans. The knockout of AfCFEM1 and AfCFEM3 resulted in an increase in the adhesive material on the surface of the trap cells, contributing to the rise in nematode mortality. Whereas the loss of AfCFEM2 caused a reduction in adhesive material on the trap’s surface, which decreased the nematode mortality. Therefore, the CFEM domain-containing gene, AfCFEM1-3, of A. flagrans mediate virulence by participating in the adhesive material formation on the cell surface of the trap, which eventually leads to changes in nematode mortality.
Trap cell wall thickness was significantly thinned due to the loss of AfCFEM1-3, indicating that AfCFEM1-3 is involved in trap cell wall synthesis of A. flagrans. This result is consistent with Mrsa et al., who identified the CCW14 gene (containing the CFEM domain) as a covalently linked cell wall protein of Saccharomyces cerevisiae [24,47]. Moreover, the expression level of the CFEM domain containing gene CgCFEM1 in appressoria of Colletotrichum gloeosporioides was two hundred times more than that in mycelia and conidia [48]. Evidently, the CFEM domain is of extraordinary significance for pathogenic fungi that possess adhesion devices, such as traps and appressoria.
NTF secret extracellular enzymes, including subtilisin-like serine proteases, chitinase, and lipase, degrade the nematode cuticle and destroy the nematode epidermis, which helps digest the nematodes [49,50]. Malate synthase, a key enzyme in the glyoxylate cycle, is required for the virulence of microbial pathogens [51]. It has been reported that the malate synthase is important for trap formation and pathogenicity of A. oligospora [52]. Moreover, aspartic proteases have been reported to be implicated in the mycoparasitic process [51]. Proteome data show that subtilisin-like serine protease (EVM00G001030 and EVM03G007260) and malate synthase (EVM01G004180) were up-regulated in ΔAfCFEM1 mutants after 12 h and 24 h of interactions with nematodes. In contrast, an Egh16 virulence protein (EVM02G005160) and a lipase-related protein (EVM00G010160) were down-regulated in the ΔAfCFEM2 mutants after interacting with nematodes for 12 h and 24 h, respectively. This demonstrates that the ΔAfCFEM1 mutants are more active than the WT in the processes of nematode adhesion, penetration, and digestion, while the opposite is true for the ΔAfCFEM2 mutants. Additionally, numerous autophagy-related genes in nematode trapping fungi are involved in the positive regulation of trap formation and pathogenicity [53,54,55]. In contrast, many of the upregulated pathogenicity and adhesion DEPs identified in the ΔAfCFEM1 mutants were annotated as being associated with physiological processes, including autophagy, MAPK signaling pathway, lysine biosynthesis, nitrogen metabolism, glycolysis/gluconeogenesis, starch and sucrose metabolism, and citrate cycle. The virulence and adhesion DEPs downregulated in the ΔAfCFEM2 mutants were also annotated in the autophagy and MAPK signaling pathways. Therefore, the proteomic results partially explain the higher and lower nematode mortality of ΔAfCFEM1 and ΔAfCFEM2 mutants than that of the WT at the protein expression level, respectively. Interestingly, the deletion of AfCFEM1 caused the two proteins (EVM01G009790 and EVM02G001720) containing lectin domains (associated with adhesion) to be up-regulated. The cryo-SEM also showed more adhesive materials on the cell wall surface of traps of the ΔAfCFEM1 mutant than in WT. This result and another reported study [56] demonstrated that genetic damage caused by the deletion of CFEM family genes was compensated for by significantly increasing the expression levels of other family members. Furthermore, detecting the relative expression of other AfCEFM members in the ΔAfCFEM1-3 mutants showed that the compensatory mechanism also exists among CFEM family members in A. flagrans. This suggests that compensation mechanisms exist between CFEM families and other families and among members of CFEM families in A. flagrans.
Not only were nematode mortality and adhesive material on the surface of trap cells affected by the loss of AfCFEM1-3, but also the morphology of the traps was affected. The knockout of AfCFEM1-3 all caused an increased proportion of irregular traps that failed to form closed loops. The three-dimensional adhesive networks of A. flagrans and A. oligospora result from multiple cell fusions (anastomoses) [57]. The study of A. oligospora trap formation revealed that trap cells grow perpendicular to the parental hyphae, subsequently bend, and fuse with the small pegs that develop on the hyphae toward the trap cells, eventually forming a closed ring [58]. Intercellular communication is required for loop closure during the formation of traps in A. flagrans and A. oligospora [59]. The loss of the hyphal anastomosis gene SofT in A. flagrans disrupted the ring closure step, leading to spiral hyphae [57]. Therefore, the CFEM domain-containing genes AfCFEM1-3 are critical for cellular communication during trap formation.
5. Conclusions
The present study reports that AfCFEM1-3 were essential for producing adhesive materials, trap morphology, cell wall biogenesis, and pathogenicity of A. flagrans. The loss of AfCFEM1-3 caused changes in nematode mortality, trap surface adhesive substances, and trap cell wall thickness. Moreover, the absence of AfCFEM1-3 led to an increased proportion of irregular trap formation. By combining the results of RT-qPCR and proteomic analysis, the compensating effects among the CFEM family and between other families were demonstrated in A. flagrans. Taken together, this study elucidated functions of AfCFEM1-3, which contribute to a deeper understanding of the biological functions of adhesive proteins in nematode killing by A. flagrans.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13092001/s1. Supplementary Figure S1: Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment of DEGs in A. flagrans at 3, 6, 12, 18, and 24 h of C. elegans induction compared with 0 h. Supplementary Figure S2: Prediction of conserved domains of AfCFEM1-3 proteins. Supplementary Figure S3: Verification of the ΔAfCFEM1-3 mutants by PCR amplification using F1/R2, F2/R1, and F3/R3 primer pairs. ‘+’ and ‘−’ represent positive control and negative control, respectively. Supplementary Figure S4: Verification of the OEAfCFEM1-3 transformants by RT-qPCR analysis using specific primer pairs. Supplementary Figure S5: Basic information of protein identification. Supplementary Figure S6: Proteomic analysis of ΔAfCFEM1 and ΔAfCFEM2 mutants at 0, 12, and 24 h of C. elegans induction compared to WT. Supplementary Table S1: List of primers used in the knockout and overexpression of AfCFEM1-3. Supplementary Table S2: List of primers used in RT-qPCR to determine the relative transcriptional level of AfCFEM1-8 genes in ΔAfCFEM1-3 mutants. Supplementary Table S3: Statistics of reads, phred-like quality scores, and GC content for the A. flagrans induced by C. elegans for different time points. Supplementary Table S4: The top 20 up-regulated genes in A. flagrans at 18 h of interaction with C. elegans. Supplementary Table S5: Differential expression data of 14 CFEM protein-related genes in A. flagrans after 18 h of interaction with C. elegans compared with 0 h. Supplementary Table S6: Differentially expressed virulence and adhesion proteins of knockout strains ΔAfCFEM1 and ΔAfCFEM2. Supplementary Table S7: Pathways annotation of the virulence and adhesion-associated DEPs in ΔAfCFEM1 and ΔAfCFEM2 mutants compared to WT.
Author Contributions
Conceptualization, G.L.; methodology, X.D. and Y.Z.; software, X.D. and T.S.; validation, X.D. and T.S.; formal analysis, X.D.; investigation, G.L.; resources, G.L.; data curation, X.D., Y.Z. and T.S.; writing—original draft preparation, T.S.; writing—review and editing, G.L.; visualization, G.L.; supervision, G.L.; project administration, G.L.; funding acquisition, G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program (2023YFD1400400), the National Natural Science Foundation of China (32160012), Science and Technology Innovation Base Construction Project (202407AB110004) and Projects from the Department of Science and Technology of Yunnan Province (202501AS070058, 202401BC070010, 202301BC070017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The published paper and the associated Supplementary Files include all data generated or analyzed during this study. The RNA-seq data presented here are associated with NCBI BioProject PRJNA1307640. The mass spectrometry proteomics data have been publicly released via the iPox partner repository with the identifier PDX:067507.
Acknowledgments
We are grateful to the Microbial Library of the Germplasm Bank of Wild Species from Southwest China for preserving and providing the experimental strains. We would like to thank the Institutional Center for Shared Technologies and Facilities of Kunming Institute of Zoology (KIZ), Chinese Academy of Sciences (CAS), for our Electron Microscopy and we would be grateful to Guo Yingqi, Wu Xingcai, Fu Rongrong for their help in making the EM sample and taking EM images. We also thank Zhijia Gu (Platform for Plant Multi-dimensional Imaging and Diversity Analysis, Kunming Institute of Botany, CAS) for the Cryo-SEM technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ahmad, G.; Khan, A.; Khan, A.A.; Ali, A.; Mohhamad, H.I. Biological control: A novel strategy for the control of the plant parasitic nematodes. Antonie Van Leeuwenhoek 2021, 114, 885–912. [Google Scholar] [CrossRef]
- Ibrahim, H.; Nchiozem-Ngnitedem, V.A.; Dandurand, L.M.; Popova, I. Naturally-occurring nematicides of plant origin: Two decades of novel chemistries. Pest Manag. Sci. 2025, 81, 540–571. [Google Scholar] [CrossRef]
- Fitzpatrick, J.L. Global food security: The impact of veterinary parasites and parasitologists. Vet. Parasitol. 2013, 195, 233–248. [Google Scholar] [CrossRef]
- Ma, G.; Wang, T.; Korhonen, P.K.; Hofmann, A.; Sternberg, P.W.; Young, N.D.; Gasser, R.B. Chapter Four—Elucidating the molecular and developmental biology of parasitic nematodes: Moving to a multiomics paradigm. In Advances in Parasitology; Rollinson, D., Stothard, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 108, pp. 175–229. [Google Scholar]
- Yang, Y.; Yang, E.; An, Z.; Liu, X. Evolution of nematode-trapping cells of predatory fungi of the Orbiliaceae based on evidence from rRNA-encoding DNA and multiprotein sequences. Proc. Natl. Acad. Sci. USA 2007, 104, 8379–8384. [Google Scholar] [CrossRef] [PubMed]
- Terrill, T.H.; Larsen, M.; Samples, O.; Husted, S.; Miller, J.E.; Kaplan, R.M.; Gelaye, S. Capability of the nematode-trapping fungus Duddingtonia flagrans to reduce infective larvae of gastrointestinal nematodes in goat feces in the southeastern United States: Dose titration and dose time interval studies. Vet. Parasitol. 2004, 120, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.S.; Larsen, M.; Wolstrup, J.; Grønvold, J.; Nansen, P.; Bjørn, H. Growth rate and trapping efficacy of nematode-trapping fungi under constant and fluctuating temperatures. Parasitol. Res. 1999, 85, 661–668. [Google Scholar] [CrossRef]
- Facchini Rodrigues, J.V.; Braga, F.R.; Campos, A.K.; de Carvalho, L.M.; Araujo, J.M.; Aguiar, A.R.; Ferraz, C.M.; da Silveira, W.F.; Valadão, M.C.; de Oliveira, T. Duddingtonia flagrans formulated in rice bran in the control of Oesophagostomum spp. intestinal parasite of swine. Exp. Parasitol. 2018, 184, 11–15. [Google Scholar] [CrossRef]
- Monteiro, T.S.A.; Balbino, H.M.; Mello, I.N.K.; Coutinho, R.R.; de Araújo, J.V.; Freitas, L.G. Duddingtonia flagrans preying a plant parasitic nematode. Braz. J. Biol. 2020, 80, 197–198. [Google Scholar] [CrossRef]
- Balbino, H.M.; Monteiro, T.S.A.; Coutinho, R.R.; Pacheco, P.V.M.; Freitas, L.G.d. Association of Duddingtonia flagrans with microorganisms for management of Meloidogyne javanica and acquisition of nutrients in soybean. Biol. Control 2021, 159, 104626. [Google Scholar] [CrossRef]
- Monteiro, T.S.A.; Valadares, S.V.; de Mello, I.N.K.; Moreira, B.C.; Kasuya, M.C.M.; de Araújo, J.V.; de Freitas, L.G. Nematophagus fungi increasing phosphorus uptake and promoting plant growth. Biol. Control 2018, 123, 71–75. [Google Scholar] [CrossRef]
- Mei, X.; Wang, X.; Li, G. Pathogenicity and volatile nematicidal metabolites from Duddingtonia flagrans against Meloidogyne incognita. Microorganisms 2021, 9, 2268. [Google Scholar] [CrossRef]
- Wernet, V.; Fischer, R. Establishment of Arthrobotrys flagrans as biocontrol agent against the root pathogenic nematode Xiphinema index. Environ. Microbiol. 2023, 25, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Dijksterhuis, J.; Veenhuis, M.; Harder, W.; Nordbring-Hertz, B. Nematophagous fungi: Physiological aspects and structure-function relationships. Adv. Microb. Physiol. 1994, 36, 111–143. [Google Scholar]
- Nordbring-Hertz, B.; Stålhammar-Carlemalm, M. Capture of nematodes by Arthrobotrys oligospora, an electron microscope study. Can. J. Bot. 1978, 56, 1297–1307. [Google Scholar] [CrossRef]
- den Belder, E.; Jansen, E.; Donkers, J. Adhesive hyphae of Arthrobotrys oligospora: An ultrastructural study. Eur. J. Plant Pathol. 1996, 102, 471–478. [Google Scholar] [CrossRef]
- Ji, X.; Yu, Z.; Yang, J.; Xu, J.; Zhang, Y.; Liu, S.; Zou, C.; Li, J.; Liang, L.; Zhang, K.Q. Expansion of adhesion genes drives pathogenic adaptation of nematode-trapping fungi. iScience 2020, 23, 101057. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Ji, X.; Feng, Y.; Li, X.; Zou, C.; Xu, J.; Ren, Y.; Mi, Q.; Wu, J.; et al. Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. PLoS Pathog. 2011, 7, e1002179. [Google Scholar] [CrossRef]
- Liang, L.; Shen, R.; Mo, Y.; Yang, J.; Ji, X.; Zhang, K.-Q. A proposed adhesin AoMad1 helps nematode-trapping fungus Arthrobotrys oligospora recognizing host signals for life-style switching. Fungal Genet. Biol. 2015, 81, 172–181. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Chen, X.; Huang, Y.; Wu, Y.; Zhu, J.; Li, W. Effect of CFEM proteins on pathogenicity, patulin accumulation and host immunity of postharvest apple pathogens Penicillium expansum. Int. J. Food Microbiol. 2025, 435, 111180. [Google Scholar] [CrossRef]
- Kou, Y.; Tan, Y.H.; Ramanujam, R.; Naqvi, N.I. Structure-function analyses of the Pth11 receptor reveal an important role for CFEM motif and redox regulation in rice blast. New Phytol. 2017, 214, 330–342. [Google Scholar] [CrossRef]
- Kornitzer, D.; Roy, U. Pathways of heme utilization in fungi. Biochim. Biophys. Acta-Mol. Cell Res. 2020, 1867, 118817. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.D.; Thon, M.R.; Pan, H.; Dean, R.A. Novel G-protein-coupled receptor-like proteins in the plant pathogenic fungus Magnaporthe grisea. Genome Biol. 2005, 6, R24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.N.; Wu, Q.Y.; Zhang, G.Z.; Zhu, Y.Y.; Murphy, R.W.; Liu, Z.; Zou, C.G. Systematic analyses reveal uniqueness and origin of the CFEM domain in fungi. Sci. Rep. 2015, 5, 13032. [Google Scholar] [CrossRef]
- Mrsa, V.; Ecker, M.; Strahl-Bolsinger, S.; Nimtz, M.; Lehle, L.; Tanner, W. Deletion of new covalently linked cell wall glycoproteins alters the electrophoretic mobility of phosphorylated wall components of Saccharomyces cerevisiae. J. Bacteriol. 1999, 181, 3076–3086. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Lai, Y.; Xiang, M.; Wang, X.; Zhang, X.; Liu, X. Drechslerella stenobrocha genome illustrates the mechanism of constricting rings and the origin of nematode predation in fungi. BMC Genom. 2014, 15, 114. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Ran, Y.; Zhang, K.Q.; Li, G.H. AfLaeA, a global regulator of mycelial growth, chlamydospore production, pathogenicity, secondary metabolism, and energy metabolism in the nematode-trapping fungus Arthrobotrys flagrans. Microbiol. Spectr. 2023, 11, e0018623. [Google Scholar] [CrossRef]
- Mallick, S.; Mishra, N.; Barik, B.K.; Negi, V.D. Salmonella typhimurium fepB negatively regulates C. elegans behavioral plasticity. J. Infect. 2022, 84, 518–530. [Google Scholar] [CrossRef]
- Fischer, R.; Requena, N. Small-secreted proteins as virulence factors in nematode-trapping fungi. Trends Microbiol. 2022, 30, 615–617. [Google Scholar] [CrossRef]
- Zhang, H.X.; Tan, J.L.; Wei, L.X.; Wang, Y.L.; Zhang, C.P.; Wu, D.K.; Zhu, C.Y.; Zhang, Y.; Zhang, K.Q.; Niu, X.M. Morphology Regulatory Metabolites from Arthrobotrys oligospora. J. Nat. Prod. 2012, 75, 1419–1423. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Mahanti, P.; Schroeder, F.C.; Sternberg, P.W. Nematode-trapping fungi eavesdrop on nematode pheromones. Curr. Biol. 2013, 23, 83–86. [Google Scholar] [CrossRef]
- Yu, X.; Hu, X.; Pop, M.; Wernet, N.; Kirschhöfer, F.; Brenner-Weiß, G.; Keller, J.; Bunzel, M. Fatal attraction of Caenorhabditis elegans to predatory fungi through 6-methyl-salicylic acid. Nat. Commun. 2021, 12, 5462. [Google Scholar] [CrossRef]
- Xie, H.; Aminuzzaman, F.M.; Xu, L.; Lai, Y.; Li, F.; Liu, X. Trap induction and trapping in eight nematode-trapping fungi (Orbiliaceae) as affected by juvenile stage of Caenorhabditis elegans. Mycopathologia 2010, 169, 467–473. [Google Scholar] [CrossRef]
- Su, H.; Zhao, Y.; Zhou, J.; Feng, H.; Jiang, D.; Zhang, K.Q.; Yang, J. Trapping devices of nematode-trapping fungi: Formation, evolution, and genomic perspectives. Biol. Rev. Camb. Philos. Soc. 2017, 92, 357–368. [Google Scholar] [CrossRef]
- Kulkarni, R.D.; Kelkar, H.S.; Dean, R.A. An eight-cysteine-containing CFEM domain unique to a group of fungal membrane proteins. Trends Biochem. Sci. 2003, 28, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Ramage, G.; Blanes, R.; Murgui, A.; Casanova, M.; Martínez, J.P. Some biological features of Candida albicans mutants for genes coding fungal proteins containing the CFEM domain. FEMS Yeast Res. 2011, 11, 273–284. [Google Scholar] [CrossRef]
- Lin, H.C.; de Ulzurrun, G.V.; Chen, S.A.; Yang, C.T.; Tay, R.J.; Iizuka, T.; Huang, T.Y.; Kuo, C.Y.; Gonçalves, A.P.; Lin, S.Y.; et al. Key processes required for the different stages of fungal carnivory by a nematode-trapping fungus. PLoS Biol. 2023, 21, e3002400. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, X.; Jiao, R.; Xia, Y. The Magas1 gene is involved in pathogenesis by affecting penetration in Metarhizium acridum. J. Microbiol. Biotechnol. 2012, 22, 889–893. [Google Scholar] [CrossRef]
- Dupres, V.; Alsteens, D.; Wilk, S.; Hansen, B.; Heinisch, J.J.; Dufrêne, Y.F. The yeast Wsc1 cell surface sensor behaves like a nanospring in vivo. Nat. Chem. Biol. 2009, 5, 857–862. [Google Scholar] [CrossRef]
- Linder, T.; Gustafsson, C.M. Molecular phylogenetics of ascomycotal adhesins--a novel family of putative cell-surface adhesive proteins in fission yeasts. Fungal Genet. Biol. 2008, 45, 485–497. [Google Scholar] [CrossRef]
- Rodicio, R.; Heinisch, J.J. Together we are strong--cell wall integrity sensors in yeasts. Yeast 2010, 27, 531–540. [Google Scholar] [CrossRef]
- Maddi, A.; Dettman, A.; Fu, C.; Seiler, S.; Free, S.J. WSC-1 and HAM-7 are MAK-1 MAP kinase pathway sensors required for cell wall integrity and hyphal fusion in Neurospora crassa. PLoS ONE 2012, 7, e42374. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.M.; Meerupati, T.; Levander, F.; Friman, E.; Ahrén, D.; Tunlid, A. Proteome of the nematode-trapping cells of the fungus Monacrosporium haptotylum. Appl. Environ. Microbiol. 2013, 79, 4993–5004. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Pedrós, B.; Murgui, A.; Casanova, M.; López-Ribot, J.L.; Martínez, J.P. Biofilm formation by Candida albicans mutants for genes coding fungal proteins exhibiting the eight-cysteine-containing CFEM domain. FEMS Yeast Res. 2006, 6, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Vidanes, G.M.; Maguire, S.L.; Guida, A.; Synnott, J.M.; Andes, D.R.; Butler, G. Conserved and divergent roles of Bcr1 and CFEM proteins in Candida parapsilosis and Candida albicans. PLoS ONE 2011, 6, e28151. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, W.; Chen, Y.; Xiang, M.; Liu, X. DdaSTE12 is involved in trap formation, ring inflation, conidiation, and vegetative growth in the nematode-trapping fungus Drechslerella dactyloides. Appl. Microbiol. Biotechnol. 2021, 105, 7379–7393. [Google Scholar] [CrossRef]
- Moreno-García, J.; Coi, A.L.; Zara, G.; García-Martínez, T.; Mauricio, J.C.; Budroni, M. Study of the role of the covalently linked cell wall protein (Ccw14p) and yeast glycoprotein (Ygp1p) within biofilm formation in a flor yeast strain. FEMS Yeast Res. 2018, 18, foy005. [Google Scholar] [CrossRef]
- Feng, L.; Dong, M.; Huang, Z.; Wang, Q.; An, B.; He, C.; Wang, Q.; Luo, H. CgCFEM1 is required for the full virulence of Colletotrichum gloeosporioides. Int. J. Mol. Sci. 2024, 25, 2937. [Google Scholar] [CrossRef]
- Kanda, S.; Aimi, T.; Kano, S.; Ishihara, S.; Kitamoto, Y.; Morinaga, T. Ambient pH signaling regulates expression of the serine protease gene (spr1) in pine wilt nematode-trapping fungus, Monacrosporium megalosporum. Microbiol. Res. 2008, 163, 63–72. [Google Scholar] [CrossRef]
- Tunlid, A.; Rosén, S.; Ek, B.; Rask, L. Purification and characterization of an extracellular serine protease from the nematode-trapping fungus Arthrobotrys oligospora. Microbiology 1994, 140 Pt 7, 1687–1695. [Google Scholar] [CrossRef]
- Szabó, M.; Urbán, P.; Virányi, F.; Kredics, L.; Fekete, C. Comparative gene expression profiles of Trichoderma harzianum proteases during in vitro nematode egg-parasitism. Biol. Control 2013, 67, 337–343. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Zhao, Y.; Huang, Y.; Zhang, K.Q.; Yang, J. Malate synthase gene AoMls in the nematode-trapping fungus Arthrobotrys oligospora contributes to conidiation, trap formation, and pathogenicity. Appl. Microbiol. Biotechnol. 2014, 98, 2555–2563. [Google Scholar] [CrossRef]
- Zhou, D.; Zhu, Y.; Bai, N.; Yang, L.; Xie, M.; Yang, J.; Zhu, M.; Zhang, K.Q.; Yang, J. AoATG5 plays pleiotropic roles in vegetative growth, cell nucleus development, conidiation, and virulence in the nematode-trapping fungus Arthrobotrys oligospora. Sci. China Life Sci. 2022, 65, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, M.; Liu, Y.; Yang, L.; Yang, J. Aoatg11 and Aoatg33 are indispensable for mitophagy, and contribute to conidiation, the stress response, and pathogenicity in the nematode-trapping fungus Arthrobotrys oligospora. Microbiol. Res. 2023, 266, 127252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhu, Y.; Bai, N.; Xie, M.; Zhang, K.-Q.; Yang, J. Aolatg1 and Aolatg13 regulate autophagy and play different roles in conidiation, trap formation, and pathogenicity in the nematode-trapping fungus Arthrobotrys oligospora. Front. Cell. Infect. Microbiol. 2022, 11, 824407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Chen, B.; Peng, Y. The virulence contribution of the CFEM family genes of Beauveria bassiana is closely influenced by the external iron environment. Microbiol. Spectr. 2025, 13, e0309624. [Google Scholar] [CrossRef] [PubMed]
- Youssar, L.; Wernet, V.; Hensel, N.; Yu, X.; Hildebrand, H.G.; Schreckenberger, B.; Kriegler, M.; Hetzer, B.; Frankino, P.; Dillin, A.; et al. Intercellular communication is required for trap formation in the nematode-trapping fungus Duddingtonia flagrans. PLoS Genet. 2019, 15, e1008029. [Google Scholar] [CrossRef]
- Nordbring-Hertz, B.; Friman, E.; Veenhuis, M. Hyphal fusion during initial stages of trap formation in Arthrobotrys oligospora. Antonie Van Leeuwenhoek 1989, 55, 237–244. [Google Scholar] [CrossRef]
- Daskalov, A.; Heller, J.; Herzog, S.; Fleißner, A.; Glass, N.L. Molecular mechanisms regulating cell fusion and heterokaryon formation in filamentous fungi. Microbiol. Spectr. 2017, 5, FUNK-0015-2016. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).