Clostridium butyricum Supplementation Reduces Diarrhea in Preweaning Calves by Modulating Fecal Short-Chain Fatty Acids and Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Animals and Diet
2.3. Sample Collection
2.4. Analysis of Growth Performance and the Incidence of Diarrhea
2.5. Serum Analysis
2.6. 16 S rRNA Gene Sequencing and Analysis
2.7. Targeted SCFA Metabolomics
2.7.1. Sample Preparation
2.7.2. GC-MS Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth Performance and Diarrhea Incidence
3.2. Plasma Immunoglobulin, Cytokine, and Antioxidant Index Levels
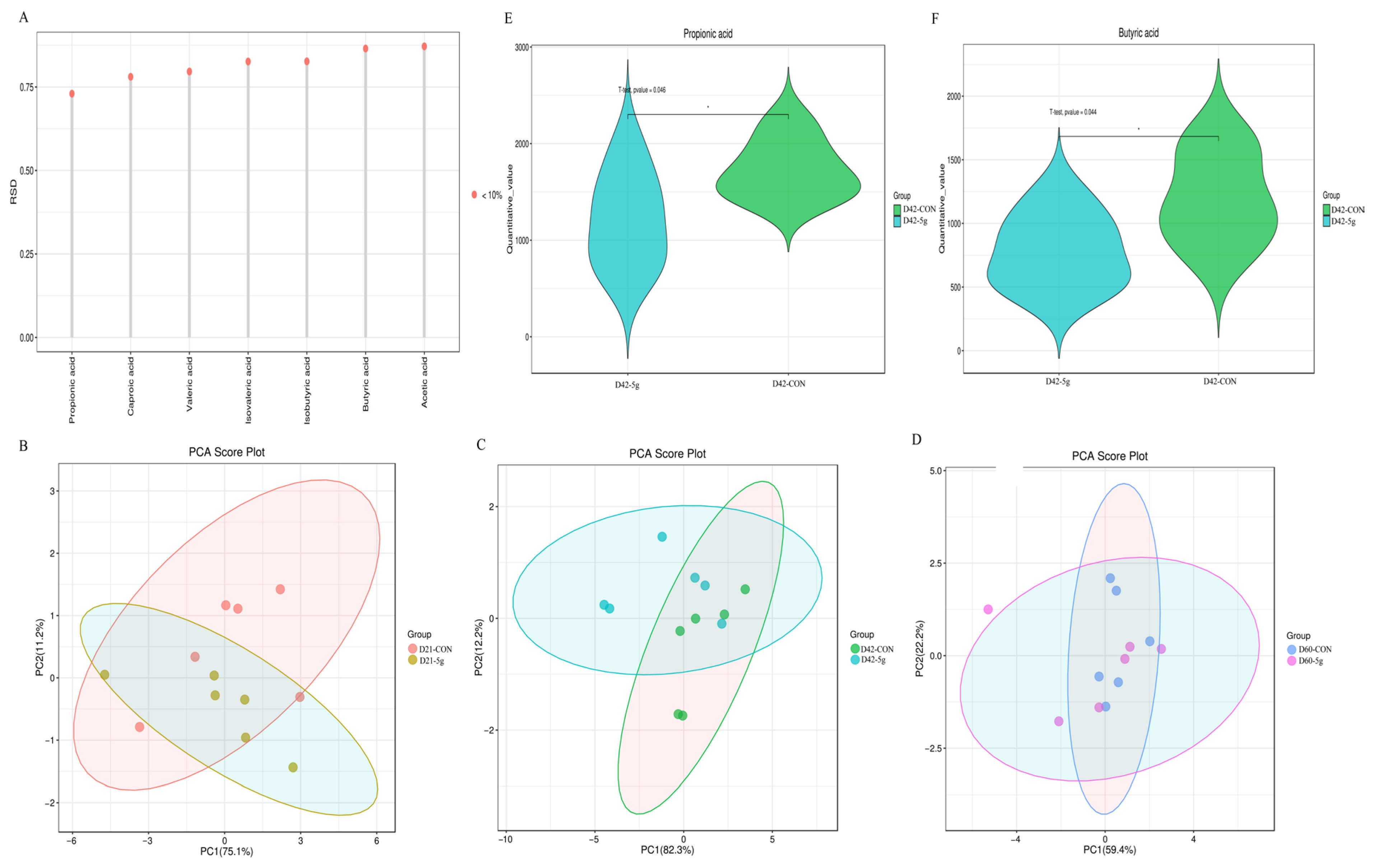
3.3. Comparative Analysis of Short-Chain Fatty Acids
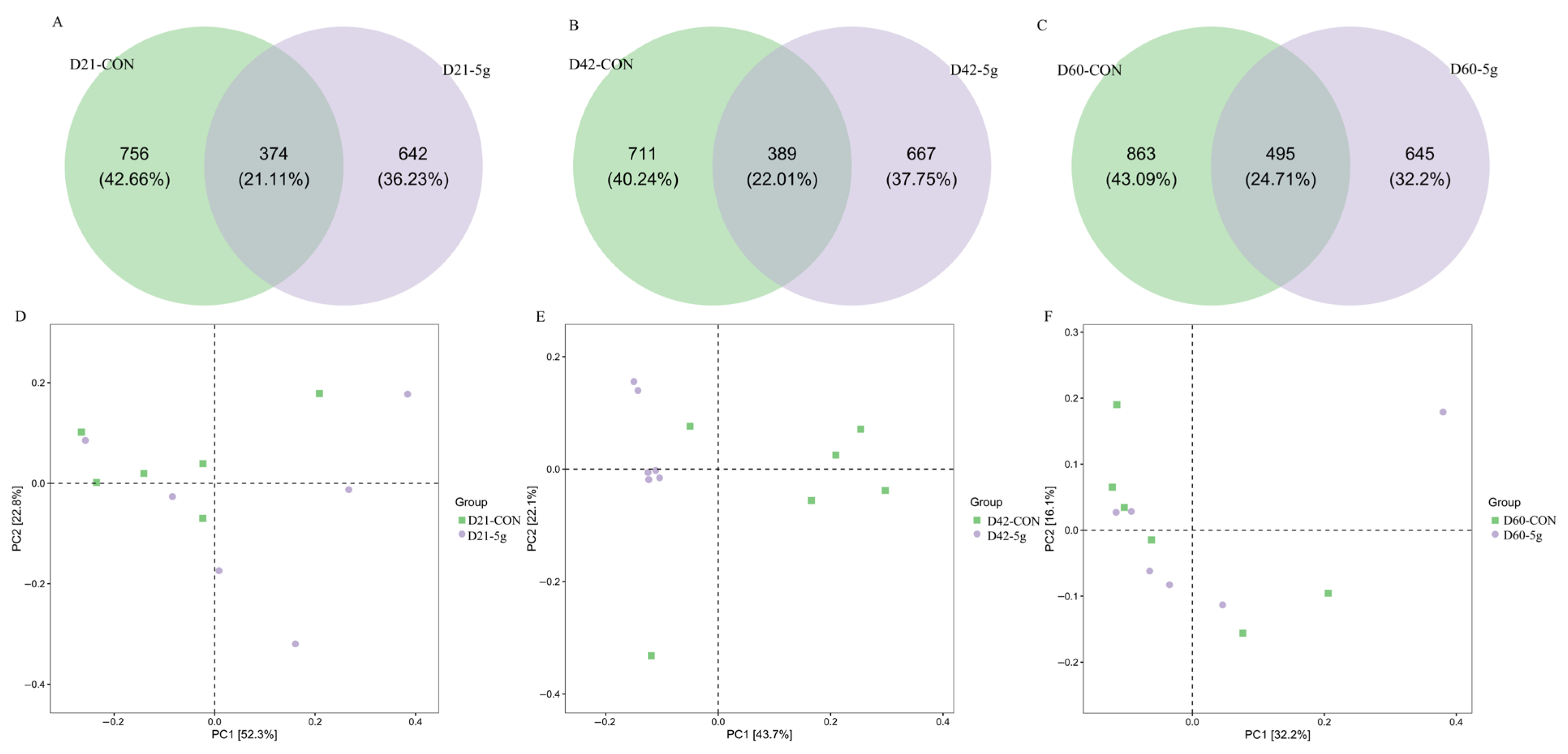
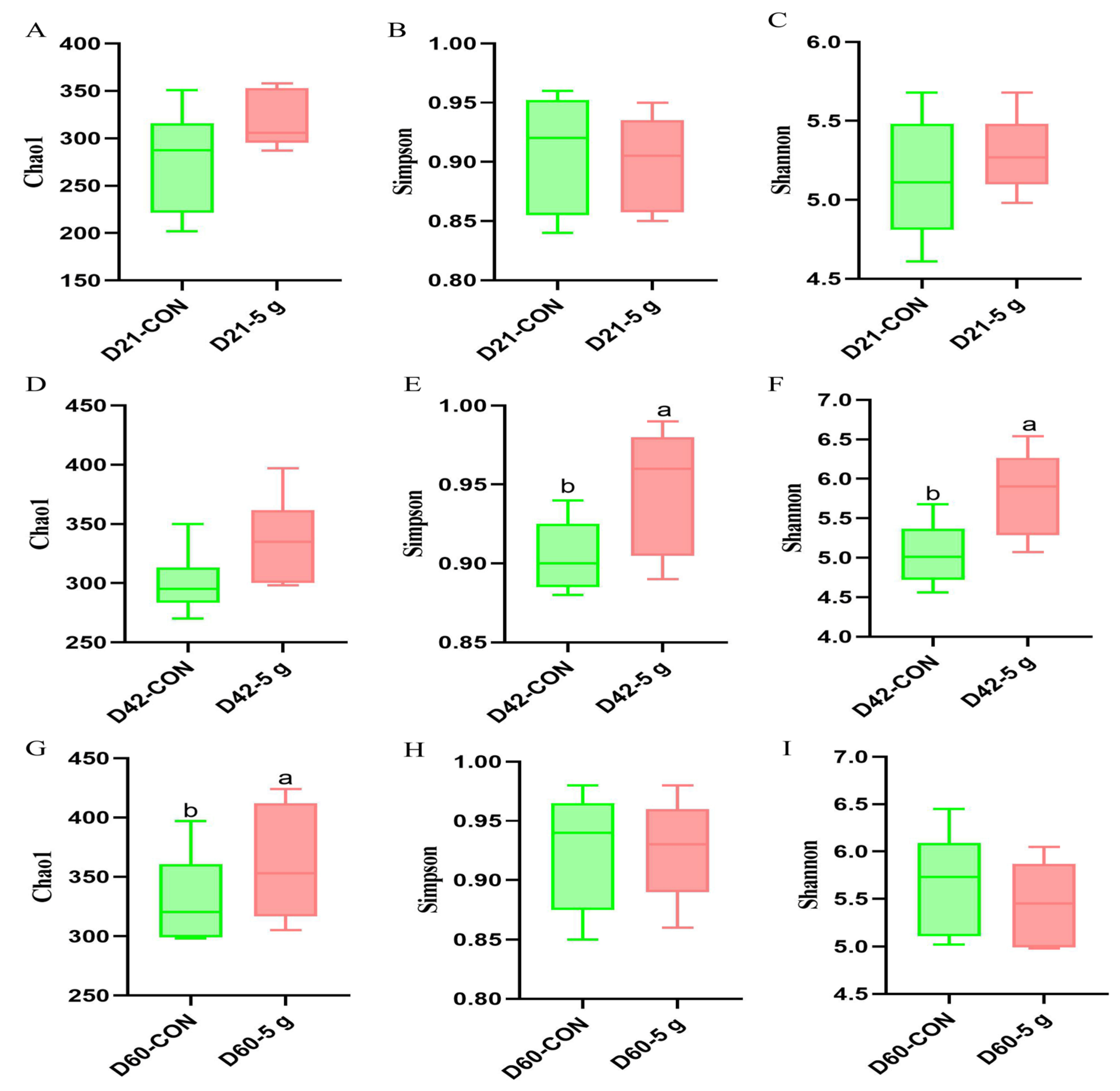
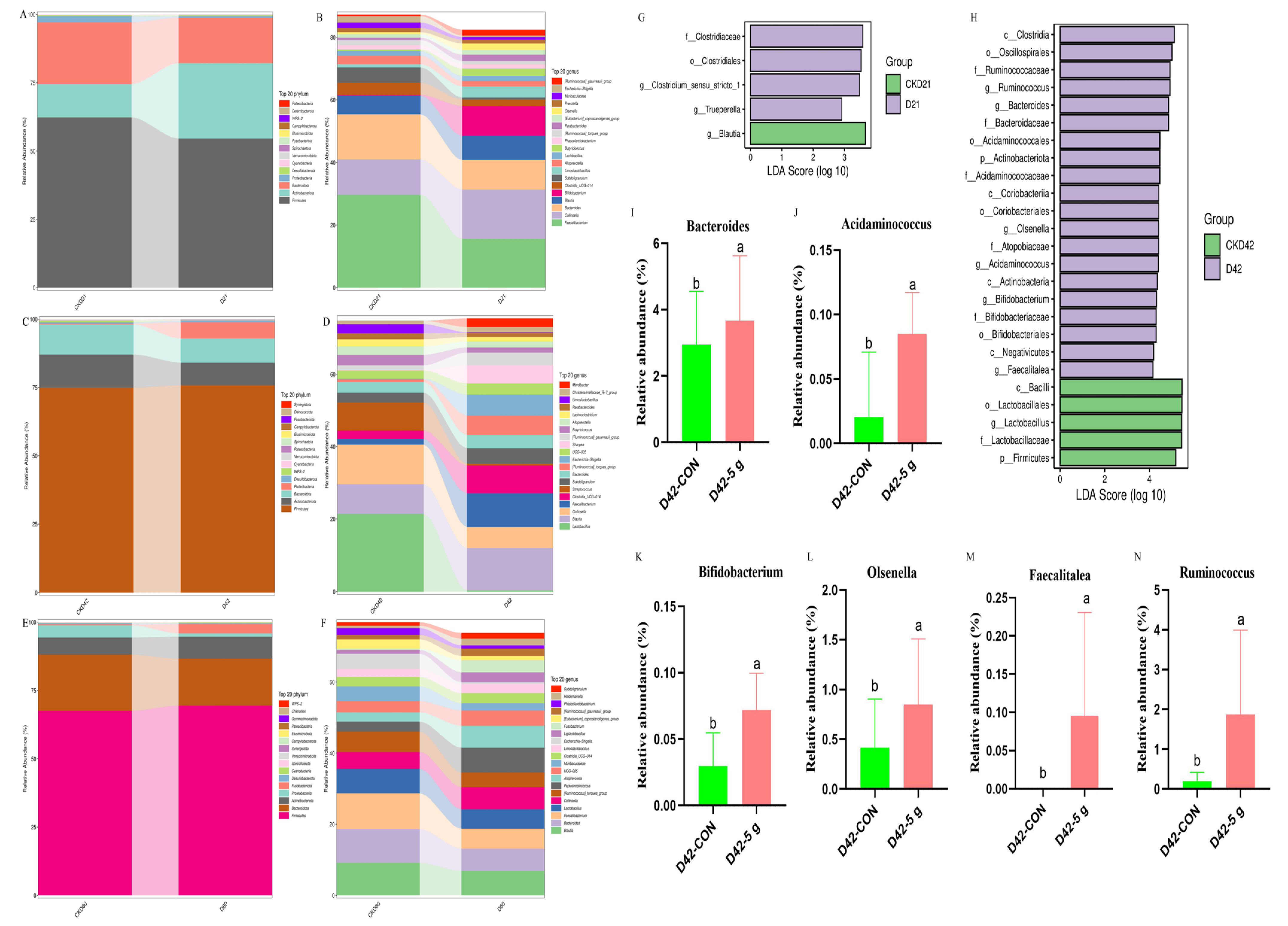
3.4. Rectal Microbial Changes
4. Discussion
4.1. Effects of Clostridium butyricum on Growth Performance in Preweaning Calves
4.2. Effects of Clostridium butyricum on Diarrhea Reduction in Preweaning Calves
4.3. Effects of Clostridium butyricum on Serum Immune and Antioxidant Functions in Preweaning Calves
4.4. Effects of Clostridium butyricum on Intestinal SCFA Production in Preweaning Calves
4.5. Effects of Clostridium butyricum on the Gut Microbiota’s Structure and Microbial Abundance in Preweaning Calves
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Why did Europe choose to ban antibiotics in animal nutrition: Feed science. AFMA Matrix 2015, 24, 35–37.
- Aghamohammad, S.; Rohani, M. Antibiotic resistance and the alternatives to conventional antibiotics: The role of probiotics and microbiota in combating antimicrobial resistance. Microbiol. Res. 2023, 267, 127275. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, T.; Xiao, X.; Cheng, Y.; Wang, F.; Jin, M.; Wang, Y.; Zong, X. Clostridium butyricum ZJU-F1 Benefits the Intestinal Barrier Function and Immune Response Associated with Its Modulation of Gut Microbiota in Weaned Piglets. Cells 2021, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Heczko, P.B.; Giemza, M.; Ponikiewska, W.; Strus, M. Importance of Lactobacilli for Human Health. Microorganisms 2024, 12, 2382. [Google Scholar] [CrossRef]
- Xie, H.; Yu, T.; Zhou, Q.; Na, K.; Lu, S.; Zhang, L.; Guo, X. Comparative evaluation of spores and vegetative forms of Bacillus subtilis and bacillus licheniformis on probiotic functionality In vitro and In vivo. Probiotics Antimicrob. Proteins 2024, 1–19. [Google Scholar] [CrossRef]
- Samal, L.; Behura, N.C. Prebiotics: An Emerging Nutritional Approach for Improving Gut Health of Livestock and Poultry. Asian J. Anim. Vet. Adv. 2015, 10, 724–739. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Liu, P.; Zhao, J.; Sun, J.; Guan, W.; Johnston, L.J.; Levesque, C.L.; Fan, P.; He, T. Dietary Clostridium butyricum Induces a Phased Shift in Fecal Microbiota Structure and Increases the Acetic Acid-Producing Bacteria in a Weaned Piglet Model. J. Agric. Food Chem. 2018, 66, 5157–5166. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Yang, Y.; Zhan, T.; Bu, D.; Ma, L. Early-life Clostridium butyricum supplementation improved rumen development and immune by promoting the maturation of intestinal microbiota. J. Agric. Food Res. 2024, 18, 101517. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Huasai, S.; Han, A.; Zhang, J.; He, L.; Aorigele, C. Compound probiotics improve the diarrhea rate and intestinal microbiota of newborn calves. Animals 2022, 12, 322. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- AOAC International. Official Methods of Analysis, 18th ed.; AOAC International: Washington, DC, USA, 2005. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccha rides in relation to animal nutrition. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Lee, H.J.; Khan, M.A.; Lee, W.S.; Yang, S.H.; Kim, S.B.; Ki, K.S.; Choi, Y.J. Influence of equalizing the gross composition of milk replacer to that of whole milk on the performance of Holstein calves. J. Anim. Sci. 2009, 87, 1129–1137. [Google Scholar] [CrossRef]
- Van De Stroet, D.L.; Díaz, J.C.; Stalder, K.J.; Heinrichs, A.J.; Dechow, C.D. Association of calf growth traits with production characteristics in dairy cattle. J. Dairy Sci. 2016, 99, 8347–8355. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Cheng, J.; Zhang, D.; Huang, K.; Zhang, Y.; Zhang, X. Relationship between sheep feces scores and gastrointestinal microorganisms and their effects on growth traits and blood indicators. Front. Microbiol. 2024, 15, 1348873. [Google Scholar] [CrossRef]
- Zhao, X.; Zhuang, J.; Zhang, F.; Li, H.; Yu, J.; Wang, C.; Zhang, J. Effects of compatibility of Clostridium butyricum and Bacillus subtilis on growth performance, lipid metabolism, antioxidant status and cecal microflora of broilers during the starter phase. Anim. Biosci. 2024, 37, 1933. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Du, M.; Zhang, J.; Ahmad, B.; Cheng, Q.; Wang, X.; Si, D. Effects of Clostridium butyricum as an Antibiotic Alternative on Growth Performance, Intestinal Morphology, Serum Biochemical Response, and Immunity of Broilers. Antibiotics 2023, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Wang, D.; Zhang, F.; Cai, L. Prophylactic Feeding of Clostridium butyricum and Saccharomyces cerevisiae Were Advantageous in Resisting the Adverse Effects of Heat Stress on Rumen Fermentation and Growth Performance in Goats. Animals 2022, 12, 2455. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, M.; Zhou, S.; Xu, Q. The Mixture of Saccharomyces cerevisiae and Clostridium butyricum Could Promote Rumen Fermentation and Improve the Growth Performance of Goats in Hot Summer. Metabolites 2023, 13, 104. [Google Scholar] [CrossRef]
- Dang, D.X.; Zou, Q.; Xu, Y.; Cui, Y.; Li, X.; Xiao, Y.; Li, D. Feeding Broiler Chicks with Bacillus subtilis, Clostridium butyricum, and Enterococcus faecalis Mixture Improves Growth Performance and Regulates Cecal Microbiota. Probiotics Antimicrob. Proteins 2022, 16, 113–124. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Zhan, X.A.; Zeng, X.; Zhou, L.; Cao, G.; Yang, C. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechnol. 2016, 7, 107–115. [Google Scholar] [CrossRef]
- Liang, J.; Kou, S.; Chen, C.; Raza, S.H.A.; Wang, S.; Ma, X.; Nie, C. Effects of Clostridium butyricum on growth performance, metabonomics and intestinal microbial differences of weaned piglets. BMC Microbiol. 2021, 21, 85. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Guo, Y.; Long, F. Effects of dietary lipids and Clostridium butyricum on the performance and the digestive tract of broiler chickens. Arch. Anim. Nutr. 2011, 65, 329–339. [Google Scholar] [CrossRef]
- Furman, O.; Shenhav, L.; Sasson, G.; Kokou, F.; Honig, H.; Jacoby, S.; Mizrahi, I. Stochasticity constrained by deterministic effects of diet and age drive rumen microbiome assembly dynamics. Nat. Commun. 2020, 11, 1904. [Google Scholar] [CrossRef]
- Timmerman, H.M.; Koning, C.J.; Mulder, L.; Rombouts, F.M.; Beynen, A.C. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Frizzo, L.S.; Zbrun, M.V.; Soto, L.P.; Signorini, M.L. Effects of probiotics on growth performance in young calves: A meta-analysis of randomized controlled trials. Anim. Feed. Sci. Technol. 2011, 169, 147–156. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.; Zheng, J.; Li, W.; Jiang, X.; Zhao, X.; Wu, D. Effects of dietary Clostridium butyricum supplementation on growth performance, intestinal development, and immune response of weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. Biotechnol. 2018, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, J.; Lin, Z.; Liu, C.; Zhang, Y.; Zhang, S.; Ma, X. Clostridium butyricum alleviates weaned stress of piglets by improving intestinal immune function and gut microbiota. Food Chem. 2023, 405, 135014. [Google Scholar] [CrossRef]
- Jung, Y.; Ku, J.Y.; Kim, B.; Kim, Y.; Park, K.M.; Baek, J.; Park, J. Determining lactate concentrations in Korean indigenous calves and evaluating its role as a predictor for acidemia in calf diarrhea. BMC Vet. Res. 2024, 20, 373. [Google Scholar] [CrossRef]
- Wang, T.; Fu, J.; Xiao, X.; Lu, Z.; Wang, F.; Jin, M.; Zong, X. CBP22, a Novel Bacteriocin Isolated from Clostridium butyricum ZJU-F1, Protects against LPS-Induced Intestinal Injury through Maintaining the Tight Junction Complex. Mediat. Inflamm. 2021, 2021, 8032125. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, G.T.; Zeng, X.F.; Zhou, L.; Ferket, P.R.; Xiao, Y.P.; Chen, A.G.; Yang, C.M. Effects of Clostridium butyricum on Growth Performance, Immune Function, and Cecal Microflora in Broiler Chickens Challenged with Escherichia coli K88 Parkinson’s Disease. Microb. Pathog. 2021, 160, 105187–105194. [Google Scholar] [CrossRef]
- Masek, T.; Mikulec, Ž.; Valpotic, H.; Antunac, N.; Mikulec, N.; Stojevic, Z.; Filipovic, N.; Pahovic, S.; Valpotic, H.; Antunac, N.; et al. Influence of live yeast culture (Saccharomyces cerevisiae) on milk production and composition, and blood biochemistry of grazing dairy ewes during the milking period. Acta Vet. Brno 2008, 77, 547–554. [Google Scholar] [CrossRef]
- Ramjiganesh, T.; Roy, S.; Freake, H.C.; Fernandez, M.L.; McIntyre, J.C. Corn fiber oil lowers plasma cholesterol by altering hepatic cholesterol metabolism and up-regulating LDL receptors in guinea pigs. J. Nutr. 2002, 132, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, H.; Zhao, Z.; Cao, D.; Zhang, L. Protective effects of Clostridium butyricum against oxidative stress induced by food processing and lipid-derived aldehydes in caco-2 cells. Appl. Microbiol. Biotechnol. 2020, 104, 9343–9361. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Raza, S.H.A.; Kou, S.; Chen, C.; Yao, M.; Wu, Y.; Wang, S.H.; Ma, X.; Zhang, W.J.; Nie, C.X. Effect of Clostridium butyricum on Plasma Immune Function, Antioxidant Activity and Metabolomics of Weaned Piglets. Livest. Sci. 2020, 241, 104267. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2024, 12, 28. [Google Scholar] [CrossRef]
- Bartoszek, A.; Von Moo, E.; Binienda, A.; Fabisiak, A.; Krajewska, J.B.; Mosińska, P.; Fichna, J. Free Fatty Acid Receptors as new potential therapeutic target in inflammatory bowel diseases. Pharmacol. Res. 2020, 152, 104604. [Google Scholar] [CrossRef]
- Patra, S.; Chelikani, P.K. Microencapsulated Propionate and Butyrate Improved Energy Balance and Gut Microbiota Composition in Diet-Induced Obese Rats. Nutrients 2025, 17, 2180. [Google Scholar] [CrossRef]
- Wolever, T.M.; Spadafora, P.; Eshuis, H. Interaction between colonic acetate and propionate in humans. Am. J. Clin. Nutr. 1991, 53, 681–687. [Google Scholar] [CrossRef]
- Saleem, A.S.; Abdelnour, S.; Bassiony, S.M.; Abdel-Monem, U.M.; Elaref, M.Y.; Al-Marakby, K.M. Probiotic supplementation in sustainable sheep production: Impacts on health, performance, and methane mitigation. Trop. Anim. Health Prod. 2025, 57, 206. [Google Scholar] [CrossRef]
- Li, Y.; Ali, I.; Lei, Z.; Li, Y.; Yang, M.; Yang, C.; Li, L. Effect of a Multistrain Probiotic on Feline Gut Health through the Fecal Microbiota and Its Metabolite SCFAs. Metabolites 2023, 13, 228. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, W.L.; Chen, G.M.; Qian, M.; Han, J.Z.; Lv, X.C.; Ni, L. Pediococcus acidilactici FZU106 alleviates high-fat diet-induced lipid metabolism disorder in association with the modulation of intestinal microbiota in hyperlipidemic rats. Curr. Res. Food Sci. 2022, 5, 775–788. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, H.J.; Cui, Z.Q.; Zhang, Y.G. Effects of supplementation with Lactobacillus plantarum 299v on the performance, blood metabolites, rumen fermentation and bacterial communities of preweaning calves. Livest. Sci. 2020, 239, 104120. [Google Scholar] [CrossRef]
- Celi, P.; Cowieson, A.J.; Fru-Nji, F.; Steinert, R.E.; Kluenter, A.M.; Verlhac, V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed. Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Griebel, P.J.; Guan, L.L. The Gut Microbiome and Its Potential Role in the Development and Function of Newborn Calf Gastrointestinal Tract. Front. Vet. Sci. 2015, 2, 36. [Google Scholar] [CrossRef]
- Cai, X.; Yi, P.; Chen, X.; Wu, J.; Lan, G.; Li, S.; Shen, P. Intake of compound probiotics accelerates the construction of immune function and gut microbiome in Holstein calves. Microbiol. Spectr. 2024, 12, e0190923. [Google Scholar] [CrossRef]
- Amat, S.; Holman, D.B.; Schmidt, K.; Menezes, A.C.B.; Baumgaertner, F.; Winders, T.; Dahlen, C.R. The Nasopharyngeal, Ruminal, and Vaginal Microbiota and the Core Taxa Shared across These Microbiomes in Virgin Yearling Heifers Exposed to Divergent In Utero Nutrition during Their First Trimester of Gestation and in Pregnant Beef Heifers in Response to Mineral Supplementation. Microorganisms 2021, 9, 2011. [Google Scholar] [CrossRef]
- Hinsu, A.T.; Tulsani, N.J.; Panchal, K.J.; Pandit, R.J.; Jyotsana, B.; Dafale, N.A.; Jakhesara, S.J. Characterizing rumen microbiota and CAZyme profile of Indian dromedary camel (Camelus dromedarius) in response to different roughages. Sci. Rep. 2021, 11, 9400. [Google Scholar] [CrossRef]
- Virginio Junior, G.F.; Silva, A.P.D.; Toledo, A.F.D.; Poczynek, M.; Cezar, A.M.; Montenegro, H.; Bittar, C.M.M. Ruminal and Fecal Bacteriome of Dairy Calves Fed Different Levels and Sources of NDF. Animals 2021, 11, 2705. [Google Scholar] [CrossRef]
- Maslen, B.N.; Hine, B.C.; Duff, C.; Alexandre, P.A.; Clark, S.A.; van der Werf, J.; Pant, S.D. Faecal microbial profiles of Angus beef cattle with divergent immune responses. Livest. Sci. 2025, 297, 105719. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Qiao, S.; Wang, T.; Sun, J.; Han, J.; Dai, H.; Du, M.; Liu, H. Cross-feeding-based rational design of a probiotic combination of Bacterides xylanisolvens and Clostridium butyricum therapy for metabolic diseases. Gut Microbes 2025, 25, 17. [Google Scholar] [CrossRef] [PubMed]
- Rogosa, M. Acidaminococcus gen. n., Acidaminococcus fermentans sp. n., Anaerobic Gram-negative Diplococci Using Amino Acids as the Sole Energy Source for Growth. J. Bacteriol. 1969, 98, 756–766. [Google Scholar] [CrossRef]
- Xu, S.Y.; Feng, X.R.; Zhao, W.; Bi, Y.L.; Diao, Q.Y.; Tu, Y. Rumen and hindgut microbiome regulate average daily gain of preweaning Holstein heifer calves in different ways. Microbiome 2024, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, L.; Yu, C.; Zhou, Q.; Li, H.; Zhang, R.; Che, L. Dietary supplementation with Bacillus subtilis PB6 alleviates diarrhea and improves growth performance and immune function in weaned piglets fed a high-protein diet. Front. Vet. Sci. 2025, 12, 1525354. [Google Scholar] [CrossRef]
- Cook, G.M.; Wells, J.E.; Russell, J.B. Ability of Acidaminococcus fermentans to oxidize trans-aconitate and decrease the accumulation of tricarballylate, a toxic end product of ruminal fermentation. Appl. Environ. Microbiol. 1994, 60, 2533–2537. [Google Scholar] [CrossRef] [PubMed]
- Hartel, U.; Buckel, W. Sodium ion-dependent hydrogen production in Acidaminococcus fermentans. Arch. Microbiol. 1996, 166, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, G.; Teixeira, A.G.V.; Foditsch, C.; Bicalho, M.L.; Machado, V.S.; Bicalho, R.C. Fecal microbial diversity in pre-weaned dairy calves as described by pyrosequencing of metagenomic 16S rDNA. Associations of Faecalibacterium species with health and growth. PLoS ONE 2017, 8, e63157. [Google Scholar] [CrossRef]
- Hirata, M.; Matsuoka, M.; Hashimoto, T.; Oura, T.; Ohnuki, Y.; Yoshida, C.; Morimatsu, F. Supplemental Clostridium butyricum MIYAIRI 588 affects intestinal bacterial composition of finishing pigs. Microbes Environ. 2022, 37, ME22011. [Google Scholar] [CrossRef]
- Wu, F.; Wuri, G.; Fang, B.; Shi, M.; Zhang, M.; Zhao, L. Alleviative mechanism and effect of Bifidobacterium animalis A6 on dextran sodium sulfate-induced ulcerative colitis in mice. Food Sci. Nutr. 2023, 11, 892–902. [Google Scholar] [CrossRef]
- Menard, O.; Butel, M.J.; Gaboriau-Routhiau, V.; Waligora-Dupriet, A.J. Gnotobiotic Mouse Immune Response Induced by Bifidobacterium sp. Strains Isolated from Infants. Appl. Environ. Microbiol. 2008, 74, 660–666. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, S.; Cai, Q.; Li, D.; Li, H.; Yang, W. In Vitro Interactions between Okadaic Acid and Rat Gut Microbiome. Mar. Drugs 2022, 20, 556. [Google Scholar] [CrossRef]
- Ye, X.X.; Li, K.Y.; Li, Y.F.; Lu, J.N.; Guo, P.T.; Liu, H.Y.; Gan, Q.F. The effects of Clostridium butyricum on Ira rabbit growth performance, cecal microbiota and plasma metabolome. Front. Microbiol. 2022, 13, 974337. [Google Scholar] [CrossRef]
- Rôças, I.N.; Siqueira, J.F., Jr. Species-directed 16S rRNA gene nested PCR detection of Olsenella species in association with endodontic diseases. Lett. Appl. Microbiol. 2005, 41, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.; Brown, K.; Ramay, H.; McCoy, K.D. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, S.; Weng, H. Effects of Clostridium butyricum and inulin supplementation on intestinal microbial composition in high-fat diet fed mice. Food Funct. 2024, 15, 10870–10884. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Zhou, S.; Luo, R.; Gesang, Z.; Suolang, S. Metagenomic insights into the diversity of carbohydrate-degrading enzymes in the yak fecal microbial community. BMC Microbiol. 2020, 20, 302. [Google Scholar] [CrossRef]
- He, X.; Hu, M.; Xu, Y.; Xia, F.; Tan, Y.; Wang, Y.; Zhou, H. The gut–brain axis underlying hepatic encephalopathy in liver cirrhosis. Nat. Med. 2025, 31, 627–638. [Google Scholar] [CrossRef]
- Solans-Lopez, M.C.; Sanchez-Somolinos, M.; Igualada-Blazquez, C.; Quevedo-Narciso, T.; Vicente-Herrera, E.; Riquelme-García, O.; Esparragoza-Cabrera, L. Ruminococcus gnavus, an unusual cause of surgical site infection following vertebral posterior instrumentation: A case report. J. Spine Surg. 2023, 9, 102. [Google Scholar] [CrossRef]
| Items | Starter | Alfalfa |
|---|---|---|
| Ingredients, % | ||
| Corn | 55.10 | - |
| Soybean meal | 18.60 | - |
| Corn gluten meal | 10.00 | - |
| DGGS | 13.00 | - |
| Limestone | 1.70 | - |
| NaCl | 0.60 | - |
| Premix (1) | 1.00 | - |
| Nutrient composition, % | ||
| Dry matter | 87.33 | 91.33 |
| Crude protein | 19.72 | 16.72 |
| Ether extract | 4.64 | 1.56 |
| Ash | 5.38 | 8.17 |
| Neutral detergent fiber | 16.53 | 49.44 |
| Acid detergent fiber | 6.02 | 33.93 |
| Ca | 1.15 | 1.33 |
| P | 0.58 | 0.25 |
| Items | Treatment | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | 1 g | 3 g | 5 g | |||
| Initial BW, kg | 40.67 | 40.44 | 40.71 | 40.56 | 2.654 | 0.273 |
| Final BW, kg | 82.77 | 85.01 | 85.51 | 88.84 | 2.812 | 0.161 |
| ADG, g/d | 701.67 b | 742.83 ab | 746.67 ab | 804.67 a | 28.44 | 0.040 |
| Starter intake, g of DM/d | 23.10 | 28.47 | 27.98 | 30.41 | 5.016 | 0.523 |
| Total feed intake, g of DM/d | 1231.16 | 1281.04 | 1288.77 | 1302.60 | 8.121 | 0.523 |
| Feed efficiency, kg of DMI/kg of gain | 1.75 | 1.72 | 1.73 | 1.62 | 0.023 | 0.833 |
| Incidence of diarrhea (Days 1 to 21), % | 20.91 | 17.69 | 15.48 | 12.33 | 0.079 | 0.075 |
| Incidence of diarrhea (Days 22 to 42), % | 5.24 a | 3.29 a | 3.69 a | 1.59 b | 0.016 | 0.039 |
| Incidence of diarrhea (Days 43 to 60), % | 1.12 | 1.13 | - | - | 0.009 | 0.084 |
| Items | Treatment | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| Con | 1 g | 3 g | 5 g | |||
| IgA, μg/mL | ||||||
| 21 d | 190.556 | 191.437 | 197.874 | 218.361 | 9.55 | 0.359 |
| 42 d | 193.604 | 203.705 | 211.753 | 216.104 | 11.94 | 0.483 |
| 60 d | 195.187 | 187.155 | 201.536 | 213.853 | 10.2 | 0.288 |
| IgG, μg/mL | ||||||
| 21 d | 1904.875 | 2108.836 | 2162.57 | 2178.854 | 94.56 | 0.159 |
| 42 d | 1959.556 | 2103.27 | 2248.133 | 2275.524 | 110.32 | 0.254 |
| 60 d | 2086.701 | 2032.398 | 2182.325 | 2150.534 | 72.38 | 0.383 |
| IgM, μg/mL | ||||||
| 21 d | 131.158 | 136.441 | 143.427 | 141.858 | 5.41 | 0.598 |
| 42 d | 137.292 b | 145.163 ab | 141.593 ab | 148.927 a | 3.21 | 0.032 |
| 60 d | 139.627 | 134.684 | 142.073 | 140.109 | 6.32 | 0.149 |
| IL-1β, ng/L | ||||||
| 21 d | 49.097 | 48.928 | 48.448 | 47.363 | 2.57 | 0.269 |
| 42 d | 52.534 a | 51.676 ab | 48.828 ab | 46.385 b | 3.96 | 0.036 |
| 60 d | 52.535 | 49.076 | 48.846 | 48.946 | 2.15 | 0.365 |
| IL-2, ng/L | ||||||
| 21 d | 387.501 | 382.243 | 396.41 | 403.77 | 19.45 | 0.613 |
| 42 d | 403.425 | 396.398 | 409.63 | 423.397 | 32.28 | 0.357 |
| 60 d | 390.366 | 371.807 | 398.174 | 409.329 | 21.22 | 0.288 |
| TNF-α, ng/L | ||||||
| 21 d | 300.834 | 299.167 | 293.484 | 298.382 | 15.05 | 0.873 |
| 42 d | 314.88 | 315.263 | 303.297 | 299.74 | 13.1 | 0.091 |
| 60 d | 314.863 | 298.86 | 293.086 | 288.66 | 19.42 | 0.343 |
| MDA, nmol/mL | ||||||
| 21 d | 3.127 a | 2.995 a | 2.856 ab | 2.62 b | 0.25 | 0.033 |
| 42 d | 3.176 a | 3.122 a | 2.992 ab | 2.825 b | 0.23 | 0.018 |
| 60 d | 3.038 | 3.06 | 2.822 | 2.823 | 0.24 | 0.133 |
| T-AOC, μmol/mL | ||||||
| 21 d | 65.79 | 64.723 | 70.461 | 71.638 | 3.53 | 0.318 |
| 42 d | 59.498 b | 65.377 ab | 68.988 a | 72.290 a | 2.94 | 0.003 |
| 60 d | 68.711 b | 69.477 ab | 68.636 ab | 75.575 a | 2.38 | 0.033 |
| Items | Treatment | SEM | p-Value | |
|---|---|---|---|---|
| CON | 5 g | |||
| Acetate, μg/g | 3001.94 | 2658.38 | 179.703 | 0.368 |
| Propionic acid, μg/g | 1307.89 | 1513.08 | 132.627 | 0.466 |
| Isobutyric acid, μg/g | 203.45 | 314.14 | 56.076 | 0.348 |
| Butyrate, μg/g | 962.71 | 1179.29 | 121.704 | 0.399 |
| Isovaleric acid, μg/g | 186.02 | 304.58 | 57.388 | 0.324 |
| Valeric acid, μg/g | 143.10 | 98.02 | 43.556 | 0.628 |
| Caproic acid, μg/g | 4.97 | 1.92 | 1.277 | 0.25 |
| Total SCFA, μg/mL | 5810.08 | 5792.21 | 514.052 | 0.987 |
| Items | Treatment | SEM | p-Value | |
|---|---|---|---|---|
| CON | 5 g | |||
| Acetate, μg/g | 5810.08 | 5792.21 | 514.052 | 0.078 |
| Propionic acid, μg/g | 1211.59 b | 1732.94 a | 136.799 | 0.046 |
| Isobutyric acid, μg/g | 1211.59 | 1691.47 | 138.301 | 0.081 |
| Butyrate, μg/g | 782.2 b | 1197.94 a | 108.358 | 0.04 |
| Isovaleric acid, μg/g | 289.91 | 448.56 | 65.551 | 0.244 |
| Valeric acid, μg/g | 273.45 | 340.30 | 76.251 | 0.682 |
| Caproic acid, μg/g | 2.70 | 3.13 | 0.696 | 0.775 |
| Total SCFA, μg/mL | 5760.36 | 7885.69 | 611.363 | 0.080 |
| Items | Treatment | SEM | p-Value | |
|---|---|---|---|---|
| CON | 5 g | |||
| Acetate, μg/g | 2015.03 | 2401.64 | 207.547 | 0.377 |
| Propionic acid, μg/g | 896.76 | 1023.94 | 123.294 | 0.629 |
| Isobutyric acid, μg/g | 159.61 | 161.54 | 28.744 | 0.975 |
| Butyrate, μg/g | 580.64 | 585.92 | 75.729 | 0.947 |
| Isovaleric acid, μg/g | 151.31 | 132.54 | 32.030 | 0.785 |
| Valeric acid, μg/g | 135.82 | 108.03 | 33.584 | 0.699 |
| Caproic acid, μg/g | 3.97 | 1.24 | 1.112 | 0.239 |
| Total SCFA, μg/mL | 3943.15 | 4377.55 | 445.772 | 0.652 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Pang, S.; Wang, Q.; Tang, Y.; Li, Q.; Zhang, W.; Nie, C.; Ma, X.; Niu, J. Clostridium butyricum Supplementation Reduces Diarrhea in Preweaning Calves by Modulating Fecal Short-Chain Fatty Acids and Gut Microbiota. Microorganisms 2025, 13, 1993. https://doi.org/10.3390/microorganisms13091993
Gao P, Pang S, Wang Q, Tang Y, Li Q, Zhang W, Nie C, Ma X, Niu J. Clostridium butyricum Supplementation Reduces Diarrhea in Preweaning Calves by Modulating Fecal Short-Chain Fatty Acids and Gut Microbiota. Microorganisms. 2025; 13(9):1993. https://doi.org/10.3390/microorganisms13091993
Chicago/Turabian StyleGao, Peiyun, Shaoyang Pang, Qianqian Wang, Yaqin Tang, Qiuyan Li, Wenju Zhang, Cunxi Nie, Xiaoling Ma, and Junli Niu. 2025. "Clostridium butyricum Supplementation Reduces Diarrhea in Preweaning Calves by Modulating Fecal Short-Chain Fatty Acids and Gut Microbiota" Microorganisms 13, no. 9: 1993. https://doi.org/10.3390/microorganisms13091993
APA StyleGao, P., Pang, S., Wang, Q., Tang, Y., Li, Q., Zhang, W., Nie, C., Ma, X., & Niu, J. (2025). Clostridium butyricum Supplementation Reduces Diarrhea in Preweaning Calves by Modulating Fecal Short-Chain Fatty Acids and Gut Microbiota. Microorganisms, 13(9), 1993. https://doi.org/10.3390/microorganisms13091993






