Antibiotic Resistance and Molecular Characterization of Staphylococcus aureus Strains Colonizing the Nose and Pharynx
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Identification of S. aureus
2.2. Identification of Antibiotic-Resistant Strains and MRSA
2.3. Virulence Gene Typing of MRSA
2.4. Statistical Analysis
3. Results
3.1. Sampled Population
3.2. Carriers of S. aureus
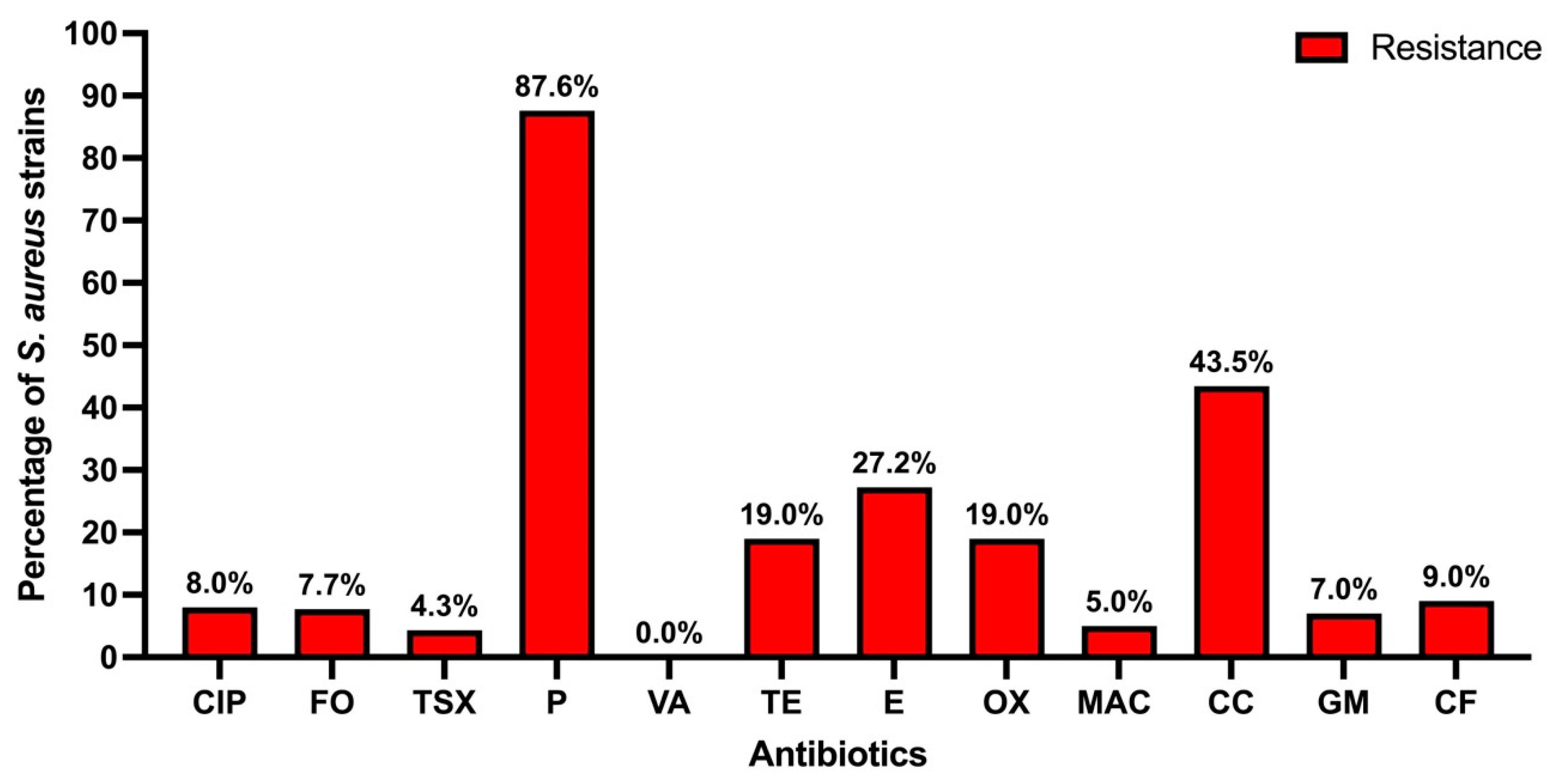
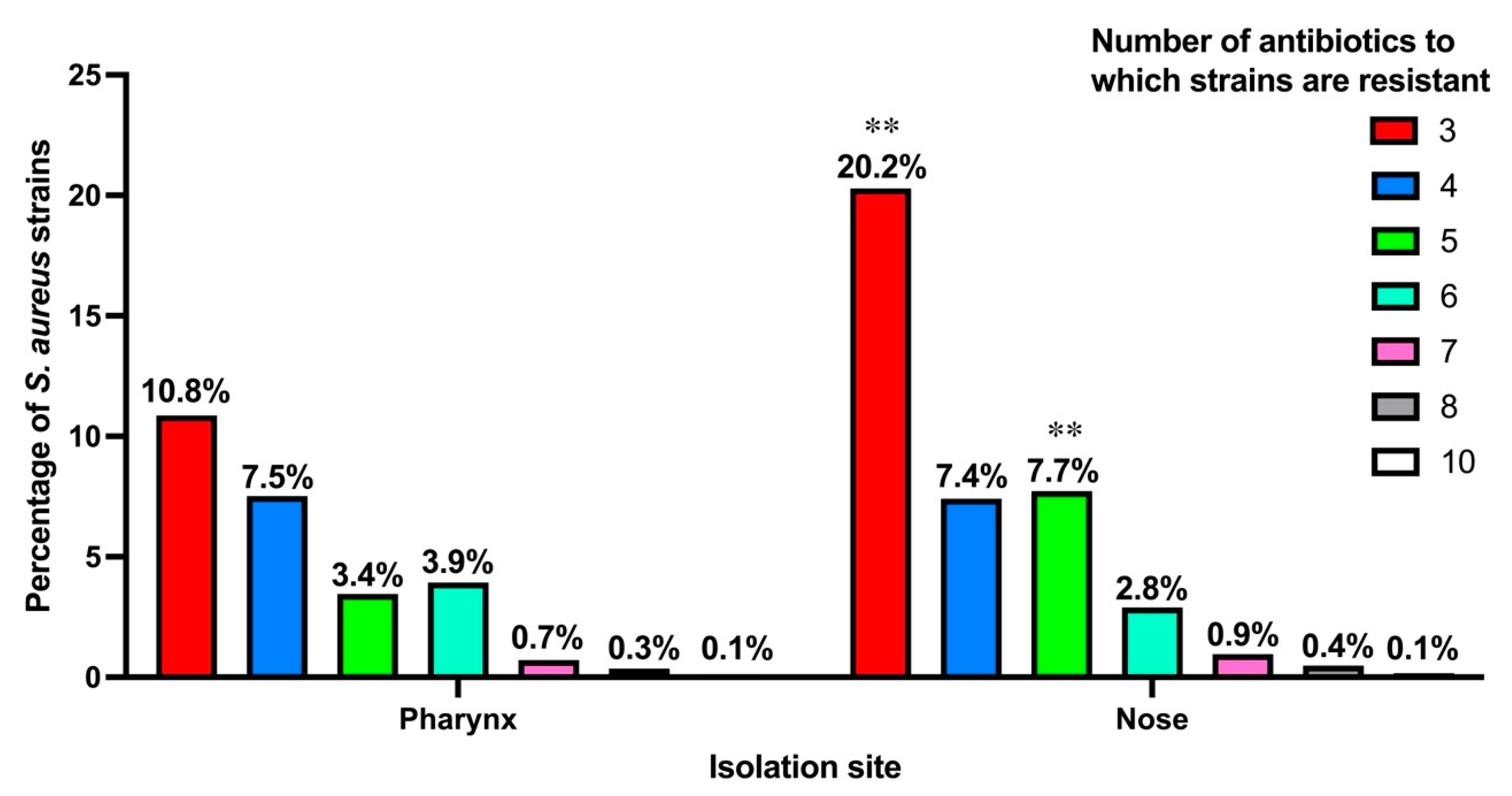
3.3. Antibiotic Resistance
3.4. Types of S. aureus Carriers by Age Group
3.5. Identification of MRSA
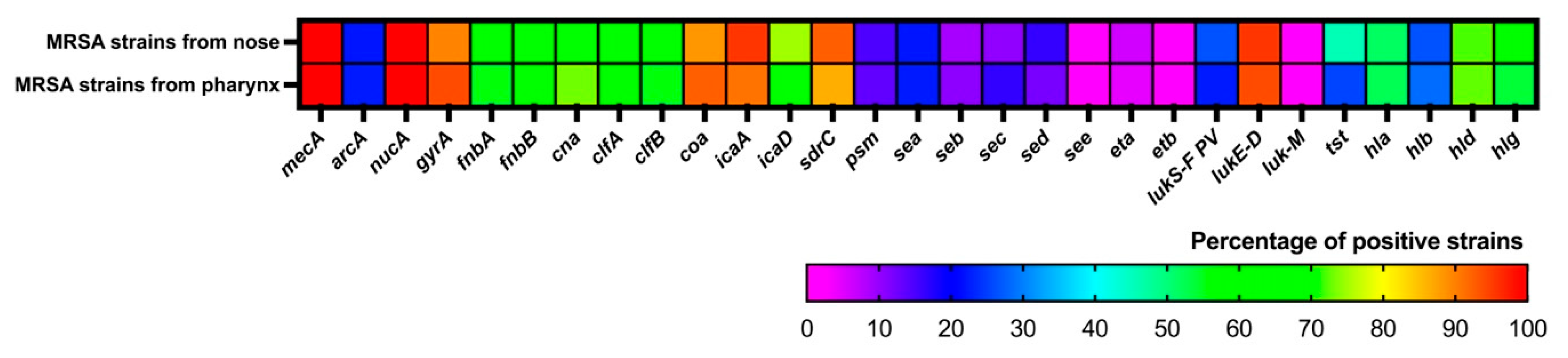
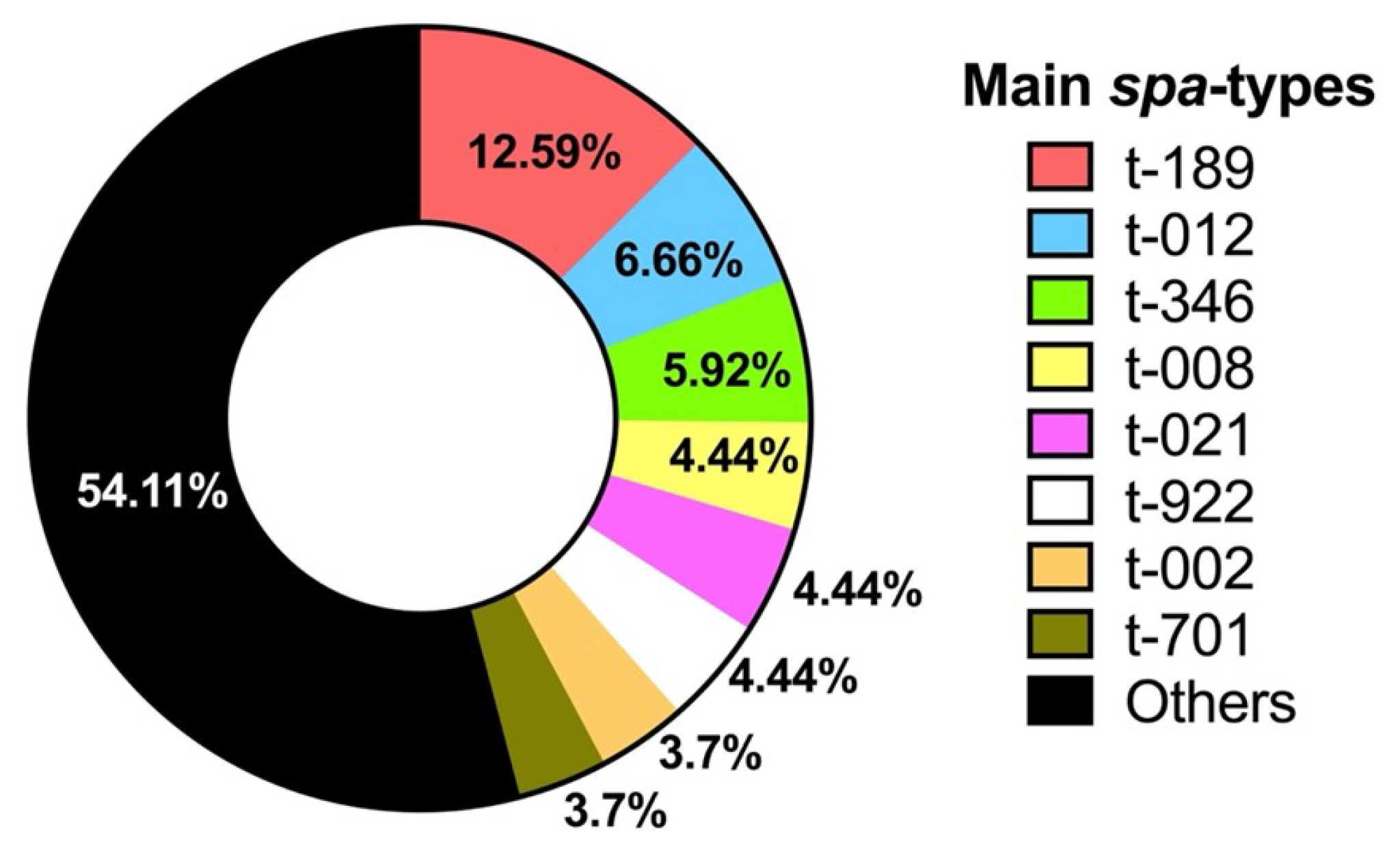
3.6. Molecular Characterization of MRSA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Touaitia, R.; Mairi, A.; Ibrahim, N.A.; Basher, N.S.; Idres, T.; Touati, A. Staphylococcus aureus: A Review of the Pathogenesis and Virulence Mechanisms. Antibiotics 2025, 14, 470. [Google Scholar] [CrossRef]
- Wertheim, H.F.L.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The Role of Nasal Carriage in Staphylococcus aureus Infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Tălăpan, D.; Sandu, A.-M.; Rafila, A. Antimicrobial Resistance of Staphylococcus aureus Isolated between 2017 and 2022 from Infections at a Tertiary Care Hospital in Romania. Antibiotics 2023, 12, 974. [Google Scholar] [CrossRef]
- Hanson, B.M.; Kates, A.E.; O’Malley, S.M.; Mills, E.; Herwaldt, L.A.; Torner, J.C.; Dawson, J.D.; Farina, S.A.; Klostermann, C.; Wu, J.Y.; et al. Staphylococcus aureus in the Nose and Throat of Iowan Families. Epidemiol. Infect. 2018, 146, 1777–1784. [Google Scholar] [CrossRef]
- Esposito, S.; Terranova, L.; Zampiero, A.; Ierardi, V.; Rios, W.P.; Pelucchi, C.; Principi, N. Oropharyngeal and Nasal Staphylococcus aureus Carriage by Healthy Children. BMC Infect. Dis. 2014, 14, 723. [Google Scholar] [CrossRef]
- Williamson, D.A.; Ritchie, S.; Keren, B.; Harrington, M.; Thomas, M.G.; Upton, A.; Lennon, D.; Leversha, A. Persistence, Discordance and Diversity of Staphylococcus aureus Nasal and Oropharyngeal Colonization in School-Aged Children. Pediatr. Infect. Dis. J. 2016, 35, 744–748. [Google Scholar] [CrossRef] [PubMed]
- González-García, S.; Hamdan-Partida, A.; Valdez-Alarcón, J.J.; Bustos-Hamdan, A.; Bustos-Martínez, J. Main Factors of Staphylococcus aureus Associated with the Interaction to the Cells for Their Colonization and Persistence. In Staphylococcal Infections-Recent Advances and Perspectives; Bustos-Martínez, J., Valdez-Alarcón, J.J., Eds.; IntechOpen: London, UK, 2023; ISBN 1-83768-206-2. [Google Scholar] [CrossRef]
- González-García, S.; Hamdan-Partida, A.; Pérez-Ramos, J.; Aguirre-Garrido, J.F.; Bustos-Hamdan, A.; Bustos-Martínez, J. Comparison of the Bacterial Microbiome in the Pharynx and Nasal Cavity of Persistent, Intermittent Carriers and Non-Carriers of Staphylococcus aureus. J. Med. Microbiol. 2024, 73, 001940. [Google Scholar] [CrossRef] [PubMed]
- Hamdan-Partida, A.; Sainz-Espuñes, T.; Bustos-Martínez, J. Characterization and Persistence of Staphylococcus aureus Strains Isolated from the Anterior Nares and Throats of Healthy Carriers in a Mexican Community. J. Clin. Microbiol. 2010, 48, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Sollid, J.U.E.; Furberg, A.S.; Hanssen, A.M.; Johannessen, M. Staphylococcus aureus: Determinants of Human Carriage. Infect. Genet. Evol. 2014, 21, 531–541. [Google Scholar] [CrossRef]
- Hamdan-Partida, A.; González-García, S.; de la Rosa García, E.; Bustos-Martínez, J. Community-Acquired Methicillin-Resistant Staphylococcus aureus Can Persist in the Throat. Int. J. Med. Microbiol. 2018, 308, 469–475. [Google Scholar] [CrossRef] [PubMed]
- González-García, S.; Hamdan-Partida, A.; Bustos-Hamdan, A.; Bustos-Martínez, J. Factors of Nasopharynx That Favor the Colonization and Persistence of Staphylococcus aureus. In Pharynx—Diagnosis and Treatment; Zhou, X., Zhang, Z., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-78985-609-5. [Google Scholar] [CrossRef]
- Galkiewicz, J.P.; Kellogg, C.A. Cross-Kingdom Amplification Using Bacteria-Specific Primers: Complications for Studies of Coral Microbial Ecology. Appl. Environ. Microbiol. 2008, 74, 7828–7831. [Google Scholar] [CrossRef] [PubMed]
- CLSI M02; Performance Standards for Antimicrobial Disk Susceptibility Test, 13th ed.; 11th ed. CLSI: Wayne, PA, USA, 2018.
- CLSI M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed. CLSI: Wayne, PA, USA, 2018.
- Oliveira, D.C.; de Lencastre, H. Multiplex PCR Strategy for Rapid Identification of Structural Types and Variants of the Mec Element in Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Boye, K.; Bartels, M.D.; Andersen, I.S.; Møller, J.A.; Westh, H. A New Multiplex PCR for Easy Screening of Methicillin-Resistant Staphylococcus aureus SCCmec Types I-V. Clin. Microbiol. Infect. 2007, 13, 725–727. [Google Scholar] [CrossRef]
- Bustos-Hamdan, A.; Hamdan-Partida, A.; González-García, S.; Guzmán Salgado, J.A.; Bustos-Martínez, J. Detection and Typing of Staphylococcus aureus Strains in Pediatric Population of Mexico City. In Advances and Perspectives of Infections Caused by Staphylococcus aureus; Bustos-Martínez, J., Valdez-Alarcón, J.J., Hamdan-Partida, A., Eds.; IntechOpen: London, UK, 2025; ISBN 978-0-85466-886-1. [Google Scholar] [CrossRef]
- Kale, P.; Dhawan, B. The Changing Face of Community-Acquired Methicillin-Resistant Staphylococcus aureus. Indian J. Med. Microbiol. 2016, 34, 275–285. [Google Scholar] [CrossRef]
- Nastaly, P.; Grinholc, M.; Bielawski, K.P. Molecular Characteristics of Community-Associated Methicillin-Resistant Staphylococcus aureus Strains for Clinical Medicine. Arch. Microbiol. 2010, 192, 603–617. [Google Scholar] [CrossRef]
- Diep, B.A.; Stone, G.G.; Basuino, L.; Graber, C.J.; Miller, A.; des Etages, S.-A.; Jones, A.; Palazzolo-Ballance, A.M.; Perdreau-Remington, F.; Sensabaugh, G.F. The Arginine Catabolic Mobile Element and Staphylococcal Chromosomal Cassette mec Linkage: Convergence of Virulence and Resistance in the USA300 Clone of Methicillin-Resistant Staphylococcus aureus. J. Infect. Dis. 2008, 197, 1523–1530. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Aasbakk, K.; Maeland, J.A. Detection of Staphylococcus aureus by Polymerase Chain Reaction Amplification of the nuc Gene. J. Clin. Microbiol. 1992, 30, 1654–1660. [Google Scholar] [CrossRef]
- Schmitz, F.-J.; Jones, M.E.; Hofmann, B.; Hansen, B.; Scheuring, S.; Lückefahr, M.; Fluit, A.; Verhoef, J.; Hadding, U.; Heinz, H.-P. Characterization of grlA, grlB, gyrA, and gyrB Mutations in 116 Unrelated Isolates of Staphylococcus aureus and Effects of Mutations on Ciprofloxacin MIC. Antimicrob. Agents Chemother. 1998, 42, 1249–1252. [Google Scholar] [CrossRef]
- da Silva, E.R.; da Silva, N. Coagulase Gene Typing of Staphylococcus aureus Isolated from Cows with Mastitis in Southeastern Brazil. Can. J. Vet. Res. 2005, 69, 260–264. [Google Scholar] [PubMed]
- Peacock, S.J.; Moore, C.E.; Justice, A.; Kantzanou, M.; Story, L.; Mackie, K.; O’Neill, G.; Day, N.P. Virulent Combinations of Adhesin and Toxin Genes in Natural Populations of Staphylococcus aureus. Infect. Immunol. 2002, 70, 4987–4996. [Google Scholar] [CrossRef]
- Nashev, D.; Toshkova, K.; Salasia, S.I.O.; Hassan, A.A.; Lämmler, C.; Zschöck, M. Distribution of Virulence Genes of Staphylococcus aureus Isolated from Stable Nasal Carriers. FEMS Microbiol. Lett. 2004, 233, 45–52. [Google Scholar] [CrossRef]
- Babra, C.; Tiwari, J.; Costantino, P.; Sunagar, R.; Isloor, S.; Hegde, N.; Mukkur, T. Human Methicillin-sensitive Staphylococcus aureus Biofilms: Potential Associations with Antibiotic Resistance Persistence and Surface Polysaccharide Antigens. J. Basic Microbiol. 2014, 54, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.J.; Deshmukh, H.S.; Nelson, C.L.; Bae, I.-G.; Stryjewski, M.E.; Federspiel, J.J.; Tonthat, G.T.; Rude, T.H.; Barriere, S.L.; Corey, R. Genotypic Characteristics of Staphylococcus aureus Isolates from a Multinational Trial of Complicated Skin and Skin Structure Infections. J. Clin. Microbiol. 2008, 46, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Mehlin, C.; Headley, C.M.; Klebanoff, S.J. An Inflammatory Polypeptide Complex from Staphylococcus epidermidis: Isolation and Characterization. J. Exp. Med. 1999, 189, 907–918. [Google Scholar] [CrossRef]
- Jarraud, S.; Mougel, C.; Thioulouse, J.; Lina, G.; Meugnier, H.; Forey, F.; Nesme, X.; Etienne, J.; Vandenesch, F. Relationships between Staphylococcus aureus Genetic Background, Virulence Factors, Agr Groups (Alleles), and Human Disease. Infect. Immun. 2002, 70, 631–641. [Google Scholar] [CrossRef]
- Choorit, W.; Kaneko, J.; Muramoto, K.; Kamio, Y. Existence of a New Protein Component with the Same Function as the LukF Component of Leukocidin or γ-Hemolysin and Its Gene in Staphylococcus aureus P83. FEBS Lett. 1995, 357, 260–264. [Google Scholar] [CrossRef]
- Shopsin, B.; Gomez, M.; Montgomery, S.O.; Smith, D.H.; Waddington, M.; Dodge, D.E.; Bost, D.A.; Riehman, M.; Naidich, S.; Kreiswirth, B.N. Evaluation of Protein A Gene Polymorphic Region DNA Sequencing for Typing of Staphylococcus aureus Strains. J. Clin. Microbiol. 1999, 37, 3556–3563. [Google Scholar] [CrossRef]
- Mertz, D.; Frei, R.; Periat, N.; Zimmerli, M.; Battegay, M.; Flückiger, U.; Widmer, A.F. Exclusive Staphylococcus aureus Throat Carriage: At-Risk Populations. Arch. Intern. Med. 2009, 169, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.; Ripa, T. Staphylococcus aureus Throat Colonization Is More Frequent than Colonization in the Anterior Nares. J. Clin. Microbiol. 2006, 44, 3334–3339. [Google Scholar] [CrossRef]
- Kuehnert, M.J.; Kruszon-Moran, D.; Hill, H.A.; McQuillan, G.; McAllister, S.K.; Fosheim, G.; McDougal, L.K.; Chaitram, J.; Jensen, B.; Fridkin, S.K. Prevalence of Staphylococcus aureus Nasal Colonization in the United States, 2001–2002. J. Infect. Dis. 2006, 193, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Locke, T.E.; Keeley, A.J.; Laundy, N.; Keil, C.; Hamilton, J.; Pandor, A.; de Silva, T.I.; Darton, T.C. Prevalence and Risk Factors for Staphylococcus aureus Colonisation among Healthy Individuals in Low-and Middle-Income Countries: A Systematic Review and Meta-Analysis. J. Infect. 2025, 90, 106462. [Google Scholar] [CrossRef]
- Flynn, M.; Dooley, J. The Microbiome of the Nasopharynx. J. Med. Microbiol. 2021, 70, 001368. [Google Scholar] [CrossRef]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance, to Guide Research, Development, and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024; ISBN 92-4-009346-X. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 15 July 2025).
- Neto, E.D.A.; Guerrero, J.; Snyder, R.E.; Pereira, R.F.A.; de Freitas, M.F.N.; Silva-Santana, G.; Riley, L.W.; Aguiar-Alves, F. Genotypic Distribution of Staphylococcus aureus Colonizing Children and Adolescents in Daycare Centers, an Outpatient Clinic, and Hospitals in a Major Brazilian Urban Setting. Diagn. Microbiol. Infect. Dis. 2020, 97, 115058. [Google Scholar] [CrossRef] [PubMed]
- Zakai, S.A. Prevalence of Methicillin-Resistant Staphylococcus aureus Nasal Colonization among Medical Students in Jeddah, Saudi Arabia. Saudi Med. J. 2015, 36, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Eslava, M.; López-Ruíz, N.; Flores-Cebada, E.M.; Rodríguez-Iglesias, M.; Galán-Sánchez, F. Staphylococcus aureus Colonization in an Institutionalized Elderly Population in the Bay of Cadiz Area, Spain: Prevalence and Associated Risk Factors. Med. Clínica (Engl. Ed.) 2019, 152, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, I.; Sabri, Z.; Illangasinghe, A.; Isurindi, A.; Jayakodi, R.; Jayasekara, W.; Jayarathna, K.; Jayasinghe, N.; Ishani, M.; Jayasekara, I. Brief Report: Nasal Colonization with Staphylococcus aureus and Methicillin Resistant Staphylococcus aureus among Community-Dwelling Older Adults with Comorbidities Seeking Follow-up Medical Care in Central Sri Lanka. Access Microbiol. 2024, 6, 000724-v3. [Google Scholar] [CrossRef]
- Almeida, S.T.; Paulo, A.C.; Babo, J.; Borralho, J.; Figueiredo, C.; Gonçalves, B.; Lança, J.; Louro, M.; Morais, H.; Queiroz, J. Absence of Methicillin-Resistant Staphylococcus aureus Colonization among Immunocompetent Healthy Adults: Insights from a Longitudinal Study. PLoS ONE 2021, 16, e0253739. [Google Scholar] [CrossRef]
- Takahashi, T.; Kim, H.; Kim, H.-S.; Kim, H.S.; Song, W.; Kim, J.-S. Comparative Genomic Analysis of Staphylococcal Cassette Chromosome mec Type V Staphylococcus aureus Strains and Estimation of the Emergence of SCCmec V Clinical Isolates in Korea. Ann. Lab. Med. 2024, 44, 47–55. [Google Scholar] [CrossRef]
- Thakar, V.H.; Kumar, M.; Modak, M.; Mehrotra, N.; Devhare, D.; Babu, A.; Dalal, B.; Paul, S.; Yadav, L.; Sawant, S. Prevalence and Outcome of Infections Caused by Staphylococcus aureus Strains Harboring the Panton-Valentine Leukocidin Gene. Cureus 2025, 17, e81687. [Google Scholar] [CrossRef]
- Wong, J.W.; Ip, M.; Tang, A.; Wei, V.W.; Wong, S.Y.; Riley, S.; Read, J.M.; Kwok, K.O. Prevalence and Risk Factors of Community-Associated Methicillin-Resistant Staphylococcus aureus Carriage in Asia-Pacific Region from 2000 to 2016: A Systematic Review and Meta-Analysis. Clin. Epidemiol. 2018, 10, 1489–1501. [Google Scholar] [CrossRef]
- Cheung, G.Y.; Bae, J.S.; Otto, M. Pathogenicity and Virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Kot, B.; Piechota, M.; Jakubczak, A.; Gryzińska, M.; Witeska, M.; Grużewska, A.; Baran, K.; Denkiewicz, P. The Prevalence of Virulence Determinants in Methicillin-Resistant Staphylococcus aureus Isolated from Different Infections in Hospitalized Patients in Poland. Sci. Rep. 2022, 12, 5477. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.; Zhao, H.; Wang, X.; Rao, L.; Guo, Y.; Yi, X.; Hu, L.; Chen, S.; Han, L.; et al. Methicillin-Resistant Staphylococcus aureus in China: A Multicentre Longitudinal Study and Whole-Genome Sequencing. Emerg. Microbes Infect. 2022, 11, 532–542. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Nelson, J.D.; Kilgore, S.H.; Radoshevich, L.; Klingelhutz, A.J.; Leung, D.Y.M. Purification, Characterization, and Cloning of a Novel pro-Inflammatory Secreted Protein from Staphylococcus aureus. Microbiol. Spectr. 2023, 11, e02898-23. [Google Scholar] [CrossRef]
- Bergmann, B.; Jirholt, P.; Henning, P.; Lindholm, C.; Ohlsson, C.; McInnes, I.B.; Lerner, U.H.; Gjertsson, I. Antibiotics with Interleukin-15 Inhibition Reduce Joint Inflammation and Bone Erosions but Not Cartilage Destruction in Staphylococcus aureus-Induced Arthritis. Infect. Immun. 2018, 86, e00960-17. [Google Scholar] [CrossRef]
- Rosales-González, N.C.; González-Martín, M.; Abdullahi, I.N.; Tejedor-Junco, M.T.; Latorre-Fernández, J.; Torres, C. Prevalence, Antimicrobial Resistance, and Genetic Lineages of Nasal Staphylococcus aureus among Medical Students at a Spanish University: Detection of the MSSA-CC398-IEC-Type-C Subclade. Res. Microbiol. 2024, 175, 104176. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Karow, M.E.; Brady, J.M.; Stemper, M.E.; Kislow, J.; Moore, N.; Wroblewski, K.; Chyou, P.-H.; Warshauer, D.M.; Reed, K.D. Virulence Genes and Genotypic Associations in Nasal Carriage, Community-Associated Methicillin-Susceptible and Methicillin-Resistant USA400 Staphylococcus aureus Isolates. J. Clin. Microbiol. 2010, 48, 3582–3592. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xu, S.; Zhao, H.; Hu, F.; Xu, X.; Jin, S.; Yang, H.; Gong, F.; Liu, Q. Leukotoxin and Pyrogenic Toxin Superantigen Gene Backgrounds in Bloodstream and Wound Staphylococcus aureus Isolates from Eastern Region of China. BMC Infect. Dis. 2018, 18, 395. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Thomsen, I.P. Epidemiological and Clinical Evidence for the Role of Toxins in S. aureus Human Disease. Toxins 2020, 12, 408. [Google Scholar] [CrossRef]
- Kananizadeh, P.; Ohadian Moghadam, S.; Sadeghi, Y.; Rahimi Foroushani, A.; Adibi, H.; Pourmand, M.R. Molecular Characteristics of Methicillin-Resistant Staphylococcus aureus (MRSA) Isolated from Diabetic Foot Infection. Iran. J. Pathol. 2019, 14, 329–337. [Google Scholar] [CrossRef]
- Yu, S.; Jiang, B.; Jia, C.; Wu, H.; Shen, J.; Hu, X.; Xie, Z. Investigation of Biofilm Production and Its Association with Genetic and Phenotypic Characteristics of OM (Osteomyelitis) and Non-OM Orthopedic Staphylococcus aureus. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 10. [Google Scholar] [CrossRef]
- Motamedi, H.; Asghari, B.; Tahmasebi, H.; Arabestani, M.R. Identification of Hemolysine Genes and Their Association with Antimicrobial Resistance Pattern among Clinical Isolates of Staphylococcus aureus in West of Iran. Adv. Biomed. Res. 2018, 7, 153. [Google Scholar] [CrossRef]
- Dehnad, A.; Agdam, M.H.G.; Rahbarnia, L.; Naghili, B.; Saffarian, P. Detection of Hemolysine Genes in Methicillin-Resistant S. aureus Isolates Obtained from a Healthy Population in North-West of Iran. Gene Rep. 2020, 21, 100874. [Google Scholar] [CrossRef]
- Azmi, K.; Qrei, W.; Abdeen, Z. Screening of Genes Encoding Adhesion Factors and Biofilm Production in Methicillin Resistant Strains of Staphylococcus aureus Isolated from Palestinian Patients. BMC Genom. 2019, 20, 578. [Google Scholar] [CrossRef] [PubMed]
- Gowrishankar, S.; Kamaladevi, A.; Balamurugan, K.; Pandian, S.K. In Vitro and In Vivo Biofilm Characterization of Methicillin-Resistant Staphylococcus aureus from Patients Associated with Pharyngitis Infection. BioMed Res. Int. 2016, 2016, 1289157. [Google Scholar] [CrossRef] [PubMed]
- Sakr, A.; Brégeon, F.; Mège, J.-L.; Rolain, J.-M.; Blin, O. Staphylococcus aureus Nasal Colonization: An Update on Mechanisms, Epidemiology, Risk Factors, and Subsequent Infections. Front. Microbiol. 2018, 9, 2419. [Google Scholar] [CrossRef]
- Zhao, N.; Cheng, D.; Jian, Y.; Liu, Y.; Liu, J.; Huang, Q.; He, L.; Wang, H.; Miao, F.; Li, M. Molecular Characteristics of Staphylococcus aureus Isolates Colonizing Human Nares and Skin. Med. Microecol. 2021, 7, 100031. [Google Scholar] [CrossRef]
- Park, S.; Jung, D.; O’Brien, B.; Ruffini, J.; Dussault, F.; Dube-Duquette, A.; Demontier, É.; Lucier, J.-F.; Malouin, F.; Dufour, S. Comparative Genomic Analysis of Staphylococcus aureus Isolates Associated with Either Bovine Intramammary Infections or Human Infections Demonstrates the Importance of Restriction-Modification Systems in Host Adaptation. Microb. Genom. 2022, 8, 000779. [Google Scholar] [CrossRef]
- Asadollahi, P.; Farahani, N.N.; Mirzaii, M.; Khoramrooz, S.S.; van Belkum, A.; Asadollahi, K.; Dadashi, M.; Darban-Sarokhalil, D. Distribution of the Most Prevalent Spa Types among Clinical Isolates of Methicillin-Resistant and-Susceptible Staphylococcus aureus around the World: A Review. Front. Microbiol. 2018, 9, 163. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Duo, L.; Xiong, J.; Gong, Y.; Yang, J.; Wang, Z.; Wu, X.; Lu, Z.; Meng, X.; et al. Characterization of Staphylococcus aureus from Distinct Geographic Locations in China: An Increasing Prevalence of Spa-T030 and SCC Mec Type III. PLoS ONE 2014, 9, e96255, Correction in PLoS ONE 2014, 9, e112002. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, T.; Xu, K.; Li, C.; Li, Y. Molecular Characteristics and Virulence Gene Profiles of Staphylococcus aureus Isolates in Hainan, China. BMC Infect. Dis. 2019, 19, 873. [Google Scholar] [CrossRef]
- Thapaliya, D.; Kadariya, J.; Capuano, M.; Rush, H.; Yee, C.; Oet, M.; Lohani, S.; Smith, T.C. Prevalence and Molecular Characterization of Staphylococcus aureus and Methicillin-Resistant S. aureus on Children’s Playgrounds. Pediatr. Infect. Dis. J. 2019, 38, e43–e47. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wu, S.; Huang, J.; Wu, Q.; Zhang, F.; Zhang, J.; Wang, J.; Ding, Y.; Zhang, S.; Yang, X.; et al. Prevalence and Characterization of Staphylococcus aureus Isolated From Pasteurized Milk in China. Front. Microbiol. 2019, 10, 641. [Google Scholar] [CrossRef] [PubMed]
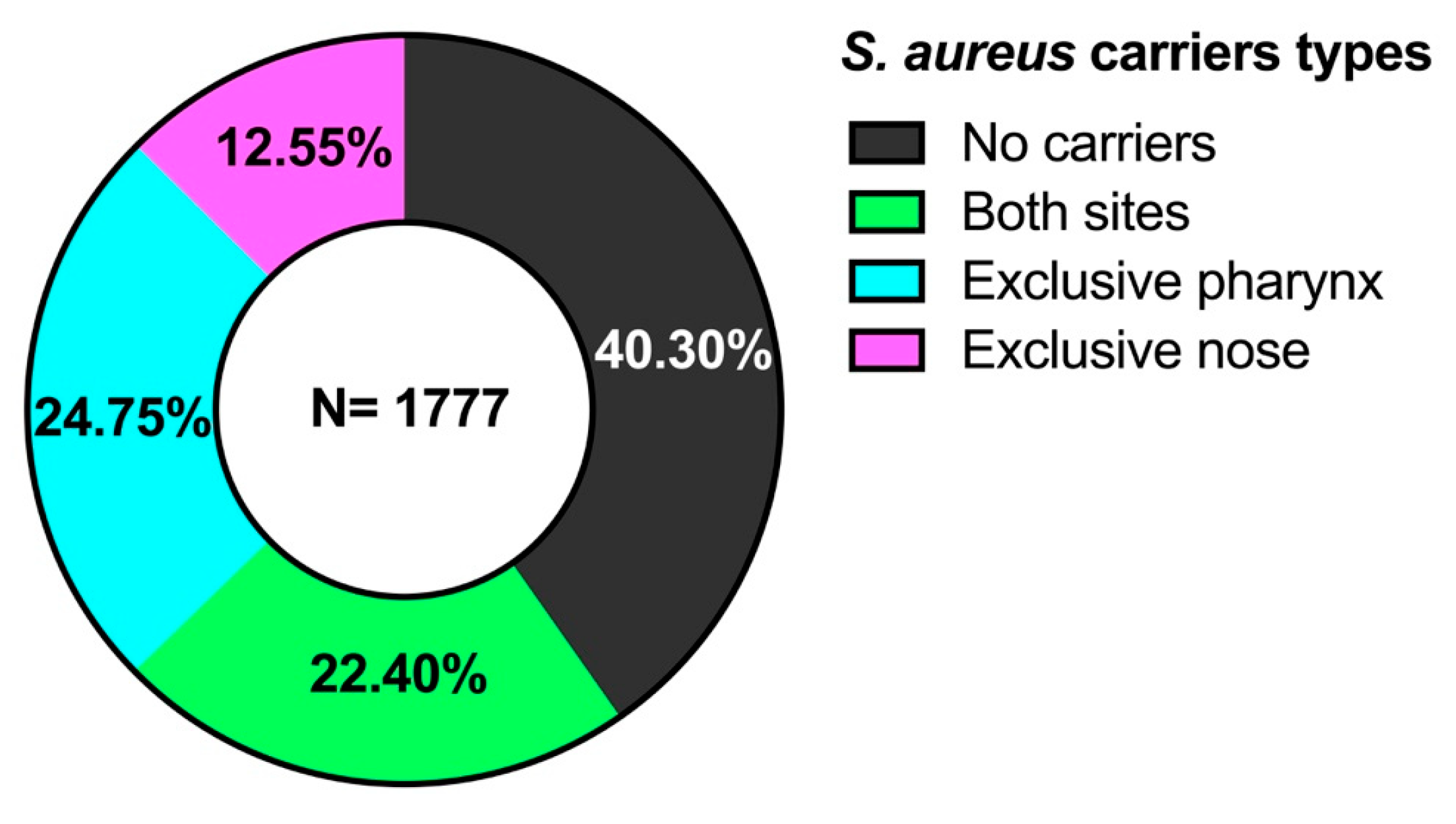

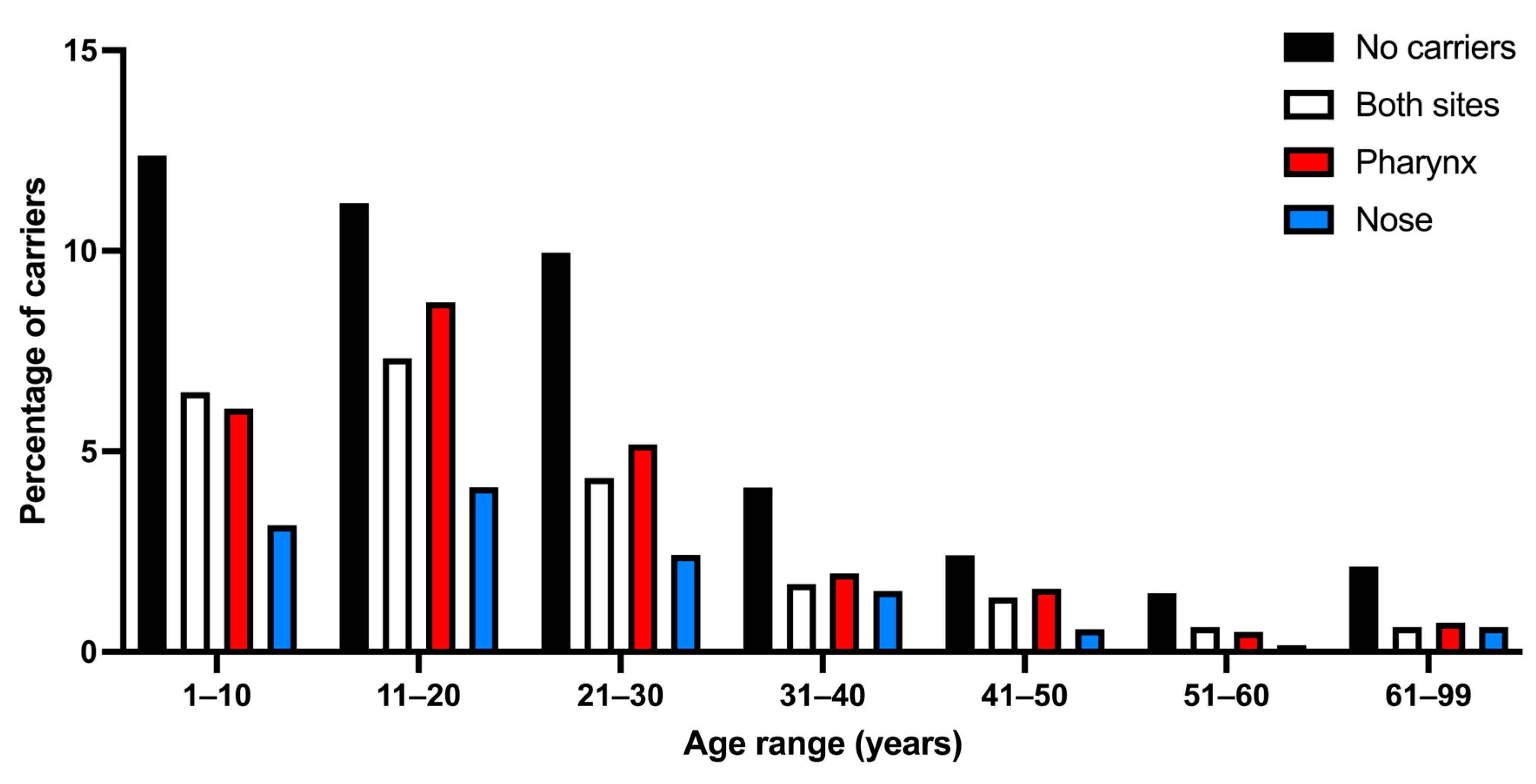
| Age Groups (Years) (n = 1777) | No Carriers (n = 716) (40.30%) | Both Sites (n = 398) (22.40%) | Exclusive Pharynx (n = 440) (24.75%) *,b | Exclusive Nose (n = 223) (12.55%) | Total Carriers (n = 1061) (59.70%) |
|---|---|---|---|---|---|
| 1–10 (n = 499) (28.08%) | 220 (44.08%) | 115 (23.04%) | 108 (21.64%) *,b | 56 (11.22%) | 279 (55.92%) |
| 11–20 (n = 557) (31.34%) | 199 (35.72%) | 130 (23.33%) | 155 (27.82%) *,b | 73 (13.10%) | 358 (64.28%) *,a |
| 21–30 (n = 329) (18.51%) | 117 (35.56%) | 77 (23.40%) | 92 (27.96%) *,b | 43 (13.06%) | 212 (64.44%) *,a |
| 31–40 (n = 165) (9.28%) | 73 (44.24%) | 30 (18.18%) | 35 (21.21%) *,b | 27 (16.36%) | 92 (55.76%) |
| 41–50 (n = 105) (5.90%) | 43 (40.95%) | 24 (22.85%) | 28 (26.66%) *,b | 10 (9.52%) | 62 (59.05%) *,a |
| 51–60 (n = 49) (2.75%) | 26 (53.06%) | 11 (22.44%) | 9 (18.36%) *,b | 3 (6.12%) | 23 (46.94%) |
| 61–99 (n = 73) (4.10%) | 38 (52.05%) | 11 (15.06%) | 13 (17.80%) | 11 (15.06%) | 35 (47.95%) |
| Age Group | MRSA Pharynx | MRSA Nose | MRSA Total (n = 135) |
|---|---|---|---|
| 1–10 (n = 394 strains) | 18 | 16 | 34 (8.62%) |
| 11–20 (n = 488 strains) | 22 | 24 | 46 (9.42%) |
| 21–30 (n = 289 strains) | 11 | 17 | 28 (9.68%) |
| 31–40 (n = 122 strains) | 7 | 4 | 11 (9.01%) |
| 41–50 (n = 86 strains) | 4 | 3 | 7 (8.13%) |
| 51–60 (n =34 strains) | 2 | 2 | 4 (11.76%) |
| 61–99 (n = 46 strains) | 2 | 3 | 5 (10.86%) |
| SCCmec Type | Pharynx Strains (n = 67) | Nose Strains (n = 68) | Total (N = 135) |
|---|---|---|---|
| II | 19 (28.35%) | 13 (19.11%) | 32 (23.70%) |
| III | 0 | 1 (1.47%) | 1 (0.74%) |
| IV | 36 (53.73%) | 35 (51.47%) | 71 (52.59%) |
| IVa | 11 (16.41%) | 17 (25%) | 28 (20.74%) |
| V | 1 (1.49%) | 2 (2.94%) | 3 (2.22%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-García, S.; Bustos-Hamdan, A.; Hamdan-Partida, A.; Bustos-Martínez, J. Antibiotic Resistance and Molecular Characterization of Staphylococcus aureus Strains Colonizing the Nose and Pharynx. Microorganisms 2025, 13, 1978. https://doi.org/10.3390/microorganisms13091978
González-García S, Bustos-Hamdan A, Hamdan-Partida A, Bustos-Martínez J. Antibiotic Resistance and Molecular Characterization of Staphylococcus aureus Strains Colonizing the Nose and Pharynx. Microorganisms. 2025; 13(9):1978. https://doi.org/10.3390/microorganisms13091978
Chicago/Turabian StyleGonzález-García, Samuel, Anaíd Bustos-Hamdan, Aída Hamdan-Partida, and Jaime Bustos-Martínez. 2025. "Antibiotic Resistance and Molecular Characterization of Staphylococcus aureus Strains Colonizing the Nose and Pharynx" Microorganisms 13, no. 9: 1978. https://doi.org/10.3390/microorganisms13091978
APA StyleGonzález-García, S., Bustos-Hamdan, A., Hamdan-Partida, A., & Bustos-Martínez, J. (2025). Antibiotic Resistance and Molecular Characterization of Staphylococcus aureus Strains Colonizing the Nose and Pharynx. Microorganisms, 13(9), 1978. https://doi.org/10.3390/microorganisms13091978






