Evidence of Waterborne Parasites in Mussels for Human Consumption Harvested from a Recreational and Highly Productive Bay
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Study Area
2.2. Mussel Sampling
2.3. Parasite Cyst Purification
2.4. DNA Extraction
2.4.1. Thermic Lysis
2.4.2. Enzymatic Lysis
2.4.3. Genomic DNA Purification
2.5. Parasite Gene Identification
2.6. Thermotolerant Coliform Determination
2.7. Statistical Analysis
3. Results
3.1. The Determination of Waterborne Parasites in Mussels Harvested from the Bay
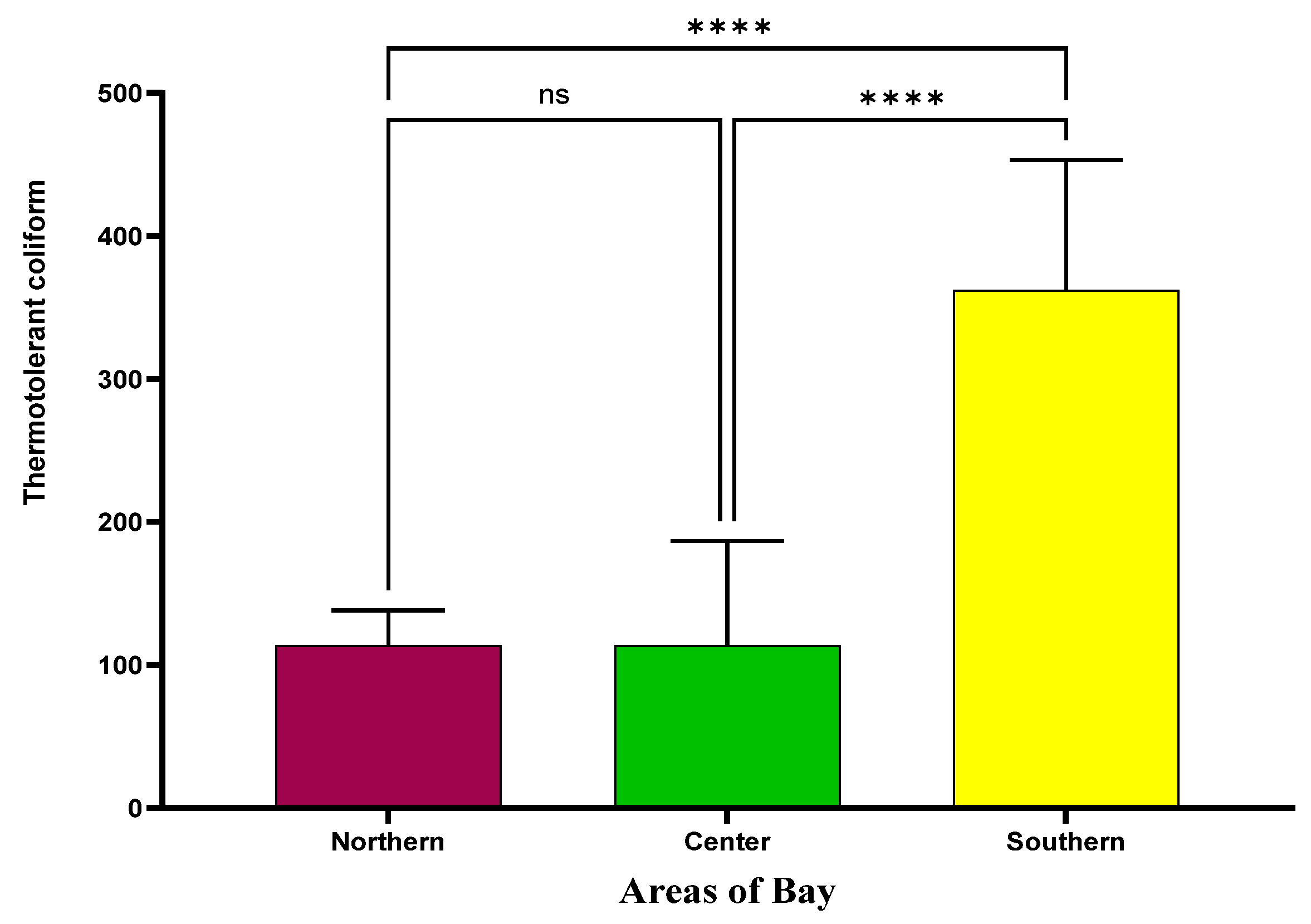
3.2. The Determination of Thermotolerant Coliforms in the Mussels Harvested from the Bay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salas, H. Submarine Outfalls: A Viable Alternative for Sewage Discharge of Coastal Cities in Latin America and the Caribbean. 2000. Available online: https://iris.paho.org/handle/10665.2/55264 (accessed on 24 May 2024).
- Mendoza, A.; Losada, M.A.; Reis, M.T.; Neves, M.G. Risk assessment in submarine outfall projects: The case of Portugal. J. Environ. Manag. 2013, 116, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.S.; Dixon, J.P.; Birch, G.F.; Besley, C.H. Deep ocean outfalls: A sustainable solution for sewage discharge for mega-coastal (Sydney, Australia): Influence on beach water quality. Mar. Pollut. Bull. 2019, 145, 691–706. [Google Scholar] [CrossRef]
- Jaubet, M.L.; Garaffo, G.V.; Cuello, G.V.; Hines, E.; Elías, R.; Llanos, E.N. Submarine outfalls and new sewage treatment plant module the response of intertidal benthic communities in a SW Atlantic area. Mar. Pollut. Bull. 2024, 199, 115946. [Google Scholar] [CrossRef]
- Bravo, J.M.; de Vicente, A. Bacterial die-off from sewage discharged through submarine outfalls. Water Sci. Technol. 1992, 25, 9–16. [Google Scholar] [CrossRef]
- Feitosa, R.C. Ocean outfalls as an alternative to minimizing risk to human and environmental health. Cienc. Saude Colet. 2017, 22, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Pinto, K.C.; de Souza, M.; Navarro, M.I.; Zanoli, M.I.; Cássi, A.; Pepe, M.T. Assessment of health risks from recreational exposure to Giardia and Cryptosporidium in coastal bathing waters. Environ. Sci. Pollut. Res. 2020, 27, 23129–23140. [Google Scholar] [CrossRef]
- Feitosa, R.C.; Rosman, P.; Carvalho, C.; Cortes, M.; Wassermman, J. Comparative study of fecal bacterial decay models for simulation of plumes of submarine sewage outfalls. Water Sci. Technol. 2013, 68, 622–631. [Google Scholar] [CrossRef]
- Masria, A.; Elejla, K.; Abualtayef, M.; Qahman, K.; Seif, A.K.; Alshammari, T.O. Modeling the dispersion of wastewater pollutans in Gaza’s coastal waters. Mar. Pollut. Bull. 2024, 208, 117071. [Google Scholar] [CrossRef]
- González-Saldias, R.R.; Pino-Maureira, N.L.; Muñoz, C.; Soto, L.; Duran, E.; Barra, M.J.; Gutiérrez, S.; Saavedra, A. Fecal pollution sources tracking and thalassogenic diseases: The temporal-spatial concordance between maximum concentration of human mitochondrial DNA in seawater and hepatitis A outbreaks among a coastal population. Sci. Total Environ. 2019, 686, 158–170. [Google Scholar] [CrossRef]
- Brandao, J.; Weiskerger, C.; Valerio, E.; Pitkanen, T.; Merilainen, P.; Avolio, L.; Heaney, C.; Sadowsky, M. Climate Change impacts on microbiota in beach sand and water: Looking ahead. Int. J. Environ. Res. Public Health 2021, 19, 1444. [Google Scholar] [CrossRef]
- Bustos-Espinoza, L.; Torres-Ramírez, P.; Figueroa, S.; González, P.S.; Pavez, M.A.; Jerez, R.; Saldías, G.S.; Espinoza, C.; Galán, A. Biochemical response of the water column of Concepción Bay, Chile, to a new regime of atmospheric and oceanographic variability. Geosciences 2024, 14, 125. [Google Scholar] [CrossRef]
- Gómez, G.; Barriga, F.; Vidal, G. Fecal Contamination on the Countries Coastline Water Research Center for Agriculture and Mining (CRHIAM), (ANID/FONDAP/1523A0001). 2023, ISSN 0718-6460 (Impress Version), ISSN 0719-3009 (Online Version). Available online: https://www.crhiam.cl/publicaciones/series-comunicacionales/ (accessed on 20 May 2025).
- Supreme Decret 90 Laws, Chile N°1475. Diario Oficial, 7 March 2000. Available online: https://www.bcn.cl/leychile/navegar?idNorma=182637 (accessed on 27 January 2025).
- Bain, R.; Cronk, R.; Wright, J.; Yang, H.; Slaymaker, T.; Bartram, J. Fecal contamination of drinking water in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001644. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, R.C.; Colonna, P.C.; Wasserman, J.C.; Bleninger, T. Microbial water quality assessment of sewage discharge through Barra da Tijuca (Rio de Janerior, Brazil) submarine outfalls. Environ. Monit. Assess. 2023, 195, 1192. [Google Scholar] [CrossRef] [PubMed]
- Ghozzi, K.; Maangi, M.; Papini, R.; Lahmar, I.; Challouf, R.; Houas, N.; Dhiab, R.B.; Normanno, G.; Babba, H.; Giagaspero, A. First report of Tunisian coastal water contamination by protozoan parasite using mollusk bivalve as biological indicators. Mar. Pollut. Bull. 2017, 177, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Nasser, A.; Benisti, N.L.; Ofer, N.; Hovers, S.; Nitzan, Y. Comparative reduction of Giardia cyst, F+ coliphages, sulphite reducing clostridia and fecal coliforms by wastewater treatment processes. J. Environ. Sci. Health Part A 2017, 52, 144–148. [Google Scholar] [CrossRef]
- Efstratiou, A.; Ongerth, J.E.; Karanis, P. Waterborne transmission of protozoan parasite: Review of worldwide outbreaks—An update 2011–2016. Water Res. 2017, 114, 14–22. [Google Scholar] [CrossRef]
- Ma, J.; Li, M.; Qi, Z.; Fu, M.; Sun, T.; Elsheikha, H.; Cong, W. Waterborne protozoan outbreaks: An update on the global, regional, and national prevalence from 2017 to 2020 and sources of contamination. Sci. Total Environ. 2022, 806, 150562. [Google Scholar] [CrossRef]
- Ryan, U.; Hijjawi, N.; Feng, Y.; Xiao, L. Giardia an reported foodborne parasite. Int. J. Parasitol. 2019, 49, 1–11. [Google Scholar] [CrossRef]
- Bourli, P.; Eslahi, A.V.; Tzoraki, O.; Karanis, P. Waterborne transmission of protozoan parasite: A review of worldwide outbreaks—An update 2017–2022. J. Water Health 2023, 23, 1421–1447. [Google Scholar] [CrossRef]
- Gajadhar, A.A.; Allen, J.R. Factors contributing to the public health and economic importance of waterborne zoonotic parasite. Vet. Parasitol. 2004, 126, 3–14. [Google Scholar] [CrossRef]
- Xiao, S.; Hu, S.; Zhang, Y.; Zhao, X.; Pan, W. Influence of sewage treatment plant effluent discharge into multipurpose river on its water quality: A quantitative health risk assessment of Cryptosporidium and Giardia. Environ. Pollut. 2018, 233, 797–805. [Google Scholar] [CrossRef]
- Leiva, A.M.; Gómez, G.; González-Rocha, G.; Piña, B.; Vidal, G. Performance of full-scale rural wastewater treatment plants in the reduction of antibiotic-resistant bacteria and antibiotic resistance genes from small-city effluents. J. Environ. Chem. Eng. 2024, 12, 112322. [Google Scholar] [CrossRef]
- Slifko, T.; Huw, S.; Joan, R. Emerging parasite zoonoses associated with water and food. Int. J. Parasitol. 2000, 30, 1379–1393. [Google Scholar] [CrossRef]
- Truchet, D.; Villagran, D.; Menone, M. Pollution biomarkers in Latin American and Caribbean marine environments: A review to identify gaps in passive biomonitoring studies. J. Hazard. Mater. Adv. 2025, 17, 100554. [Google Scholar] [CrossRef]
- Nasser, A.; Zruk, N.; Tenenbaum, L.; Netzan, Y. Comparative survival of Cryptosporidium, coxackie virus A9 and Escherichia coli in stream, brackish and sea waters. Water Sci. Technol. 2003, 47, 91–96. [Google Scholar] [CrossRef]
- Betancourt, W.Q.; Duarte, D.C.; Vásquez, R.C.; Gurian, P.L. Cryptosporidium and Giardia in tropical recreational marine waters contaminated with domestic sewage: Estimation of bathing-associated disease risk. Mar. Pollut. Bull. 2014, 85, 268–273. [Google Scholar] [CrossRef] [PubMed]
- De Abreu-Mota, M.A.; de Moaura Barboza, C.A.; Bícego, M.C.; Martins, C.C. Sedimentary biomarkers along a contamination gradient in a human-impacted sub-estuary in Southern Brazil: A multiparameter approach based on spatial and seasonal variability. Chemosphere 2014, 103, 156–163. [Google Scholar] [CrossRef]
- Kim, M.; Rueda, L.; Shapiro, K. Protozoa in bivalve shellfish: Gaps and opportunities to better understand risk to consumers. Curr. Opin. Food Sci. 2024, 55, 101104. [Google Scholar] [CrossRef]
- Ryckman, M.; Gantois, N.; Garcia, R.; Desramaut, J.; Li, L.; Even, G.; Audebert, C.; Devos, D.; Chabé, M.; Certad, G.; et al. Molecular identification and subtype analysis of Blastocystis sp. isolates from wild mussels (Mytilus edulis) in northern France. Microorganisms 2024, 12, 710. [Google Scholar] [CrossRef]
- Zhang, C.M.; Xu, P.C.; Wang, X.C. Exposure parameters and health risk of Cryptosporidium and Giardia in the recreational water activities for urban residents in China. Environ. Sci. Pollut. Res. 2022, 29, 1573–1583. [Google Scholar] [CrossRef]
- Ulrik, H.R. Inaccurate bivalve clearance rate measurements: A reply. Mar. Ecol. Prog. Ser. 2001, 221, 307–309. [Google Scholar] [CrossRef]
- Desdouits, M.; Reynaud, Y.; Philippe, C.; Le Guyader, F. A comprehensive review for the surveillance of human pathogenic microorganism in shellfish. Microorganisms 2023, 11, 2218. [Google Scholar] [CrossRef]
- Carcamo, P.; Hernandez-Miranda, E.; Veas, R.; Quiñones, R. Macrofaunal community structure in Bahía Concepción (Chile) before and after the 8.8 Mw Maule mega earthquake and tsunami. Mar. Environ. Res. 2017, 130, 233–247. [Google Scholar] [CrossRef] [PubMed]
- SERNAPESCA (National Fisheries and Aquaculture Service). Preliminary Figures for Artisanal Landing of Bivalve Resources in Coves of Bay of Concepción. Requested Information by Law 20.285, Chile. 2024. Available online: https://www.bcn.cl/leychile/navegar?idNorma=276363 (accessed on 27 January 2025).
- Suarez, P.; Vallejos-Almirall, A.; Fernández, I.; Gonzalez-Chavarria, I.; Alonso, J.L.; Vidal, G. Identification of Cryptosporidium parvum and Blastocystis hominis subtype 3 in cholga mussel and treated sewage: Preliminary evidence of fecal contamination in harvesting area. Food Waterborne Parasitol. 2024, 34, e00214. [Google Scholar] [CrossRef] [PubMed]
- Leppe, A.; Padilla, L. Penco and Tomé submarine outfalls, five years of surveillance and a global evaluation of their effects. In Proceedings of the XIII Chilean Congress of Sanitary and Environmental Engineering, Antofagasta, Chile, 21 October 1999. [Google Scholar]
- SISS (Superintendence of Sewer Services) Treatment Sewage Plants in Biobio Region, Chile. Available online: http://www.siss.gob.cl (accessed on 27 January 2025).
- Aldea, C.; Valdovinos, C. Rockyshore mollusks of the South-Central, Chile (36°–38°S) taxonomy and key identification. Gayana 2005, 69, 364–396. [Google Scholar] [CrossRef]
- Hawash, Y. DNA extraction from protozoan oocyst/cyst in feces for diagnostic PCR. Korean J. Parasitol. 2014, 52, 263–271. [Google Scholar] [CrossRef]
- Heyman, M.B.; Shigekuni, L.K.; Ammann, A. Separation of Cryptosporidium oocyst from fecal debris by density gradient centrifugation and glass bead columns. J. Clin. Microbiol. 1986, 23, 789–791. [Google Scholar] [CrossRef]
- Babei, Z.; Oormazi, H.; Rezaie, S.; Rezaian, M.; Razmjou, E. Giardia intestinalis: DNA extraction approaches to improve PCR results. Exp. Parasitol. 2011, 128, 159–162. [Google Scholar] [CrossRef]
- Böhm-Gloning, B.; Knobloch, J.; Walderich, B. Five groups of Blastocystis hominis isolates from symptomatic and asymptomatic patients reveals by restriction site analysis of PCR-amplified 16S-like rDNA. Trop. Med. Int. Health 1997, 2, 771–778. [Google Scholar] [CrossRef]
- Caccio, S.; De Giacomo, M.; Pozio, E. Sequence analysis of the β-giardin gene and development of a polymerase chain reaction-restriction fragment length polymorphism assay to genotype Giardia duodenalis cyst from human faecal samples. Int. J. Parasitol. 2002, 32, 1023–1030. [Google Scholar] [CrossRef]
- Baird, R.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Suarez, P.; Alonso, J.L.; Gómez, G.; Vidal, G. Performance of sewage treatment technologies for the removal of Cryptosporidium sp. and Giardia sp.: Toward water circularity. J. Environ. Manag. 2022, 324, 116320. [Google Scholar] [CrossRef]
- Sobarzo, M.B.; Figueroa, D.; Arcos, D.R. The influence of winds and tides in the formation of circulation layers in a bay, a case study: Concepcion Bay, Chile. Estuar. Coast. Shelf Sci. 1997, 45, 729–736. [Google Scholar] [CrossRef]
- Valle-Levinson, A.; Schneider, W.; Sobarzo, M.; Bello, M.; Bravo, L.; Castillo, M.; Duarte, L.; Fuenzalida, R.; Gallegos, J.M.; Garces-Vargas, J.; et al. Wind-induced exchange at the entrance to Concepcion Bay and equatorward facing embayment in central Chile. Deep Sea Res. Part II 2004, 51, 2371–2388. [Google Scholar] [CrossRef]
- Nana, P.A.; Mbiada, M.P.; Tchakonté, S.; Moche, K.; Mouchili, R.S.; Nola, M.; Sime-Ngando, T. Influence of season and tides on the distribution of enteric protozoa on the shores of the Atlantic Ocean in Kribi (South region of Cameroon): Health risk related to bathing. Pollutants 2023, 3, 243–254. [Google Scholar] [CrossRef]
- Faundez-Baez, P.; Morales, C.E.; Arcos, D. Spatial and temporal variability of winter hydrography in the bay system off the VIII region (central-south, Chile). Rev. Chil. Hist. Nat. 2001, 74, 817–831. [Google Scholar] [CrossRef]
- Jang, C.; Liu, C. Integrating quantitative microbiological risk assessment and disability-adjusted life years to evaluate the effects of urbanization on health risk for river recreationists. Sci. Total Environ. 2024, 932, 172667. [Google Scholar] [CrossRef]
- Decret 144/2009. Laws N°19,300 Chile. Diario Oficial, 7 April 2009. Available online: https://www.bcn.cl/leychile/navegar?idNorma=1001042 (accessed on 27 January 2025).
- Decret 977/96. Laws N°725 and N°2763 Chile. Diario Oficial, 13 May 1997. Available online: https://faolex.fao.org/docs/pdf/chi9315.pdf (accessed on 27 January 2025).
- González, Y.; Salgado, P.; Vidal, G. Disinfection behavior of a UV-treated wastewater system using constructed wetlands and the rate of reactivation of pathogenic microorganisms. Water Sci. Technol. 2020, 80, 1870–1879. [Google Scholar] [CrossRef]
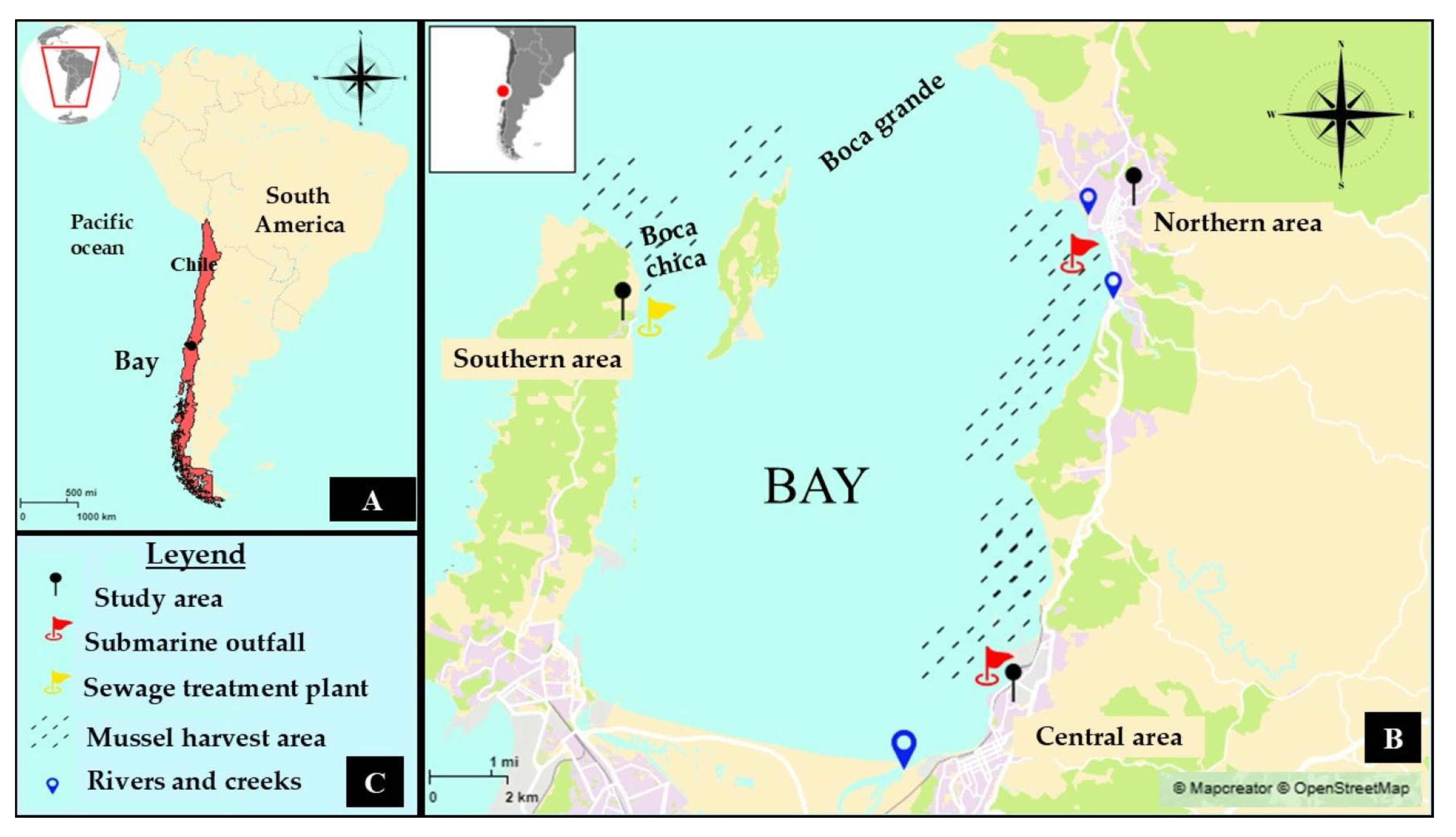
| Area | Population 1 | Sewage Discharge 2 | Maximum Daily Flow Rate (m3 day−1) | Population Served by Plant | Other Water Discharge |
|---|---|---|---|---|---|
| Northern | 54,946 | Submarine outfalls | 19,785 2 | 54,946 | Collen and Bellavista creeks |
| Center | 46,176 | Submarine outfalls | 35,554 2 | 46,176 | Lirquen Creek and Andalien river |
| Southern | 92,843 | Sewage Treatment Plant | 30,000 3 | 200 4 | Raw sewage from houses |
| Area | Total Mussel Harvested | Mussel Sample * | Giardia duodenalis | Blastocystis sp. |
|---|---|---|---|---|
| Northern | 200 | 40 | 14 (35 **) | 5 (12.5 **) |
| Center | 200 | 40 | 3 (7.5 **) | 4 (10 **) |
| Southern | 200 | 40 | 13 (32.5 **) | 13 (32.5 **) |
| Total | 600 | 120 | 30 (25 ***) | 22 (18 ***) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suarez, P.; Fernandez, I.; Alonso, J.L.; Vidal, G. Evidence of Waterborne Parasites in Mussels for Human Consumption Harvested from a Recreational and Highly Productive Bay. Microorganisms 2025, 13, 1971. https://doi.org/10.3390/microorganisms13091971
Suarez P, Fernandez I, Alonso JL, Vidal G. Evidence of Waterborne Parasites in Mussels for Human Consumption Harvested from a Recreational and Highly Productive Bay. Microorganisms. 2025; 13(9):1971. https://doi.org/10.3390/microorganisms13091971
Chicago/Turabian StyleSuarez, Pilar, Italo Fernandez, José Luís Alonso, and Gladys Vidal. 2025. "Evidence of Waterborne Parasites in Mussels for Human Consumption Harvested from a Recreational and Highly Productive Bay" Microorganisms 13, no. 9: 1971. https://doi.org/10.3390/microorganisms13091971
APA StyleSuarez, P., Fernandez, I., Alonso, J. L., & Vidal, G. (2025). Evidence of Waterborne Parasites in Mussels for Human Consumption Harvested from a Recreational and Highly Productive Bay. Microorganisms, 13(9), 1971. https://doi.org/10.3390/microorganisms13091971





