Fecal Microbiota Transplantation in Alzheimer’s Disease: Mechanistic Insights Through the Microbiota–Gut–Brain Axis and Therapeutic Prospects
Abstract
1. Introduction
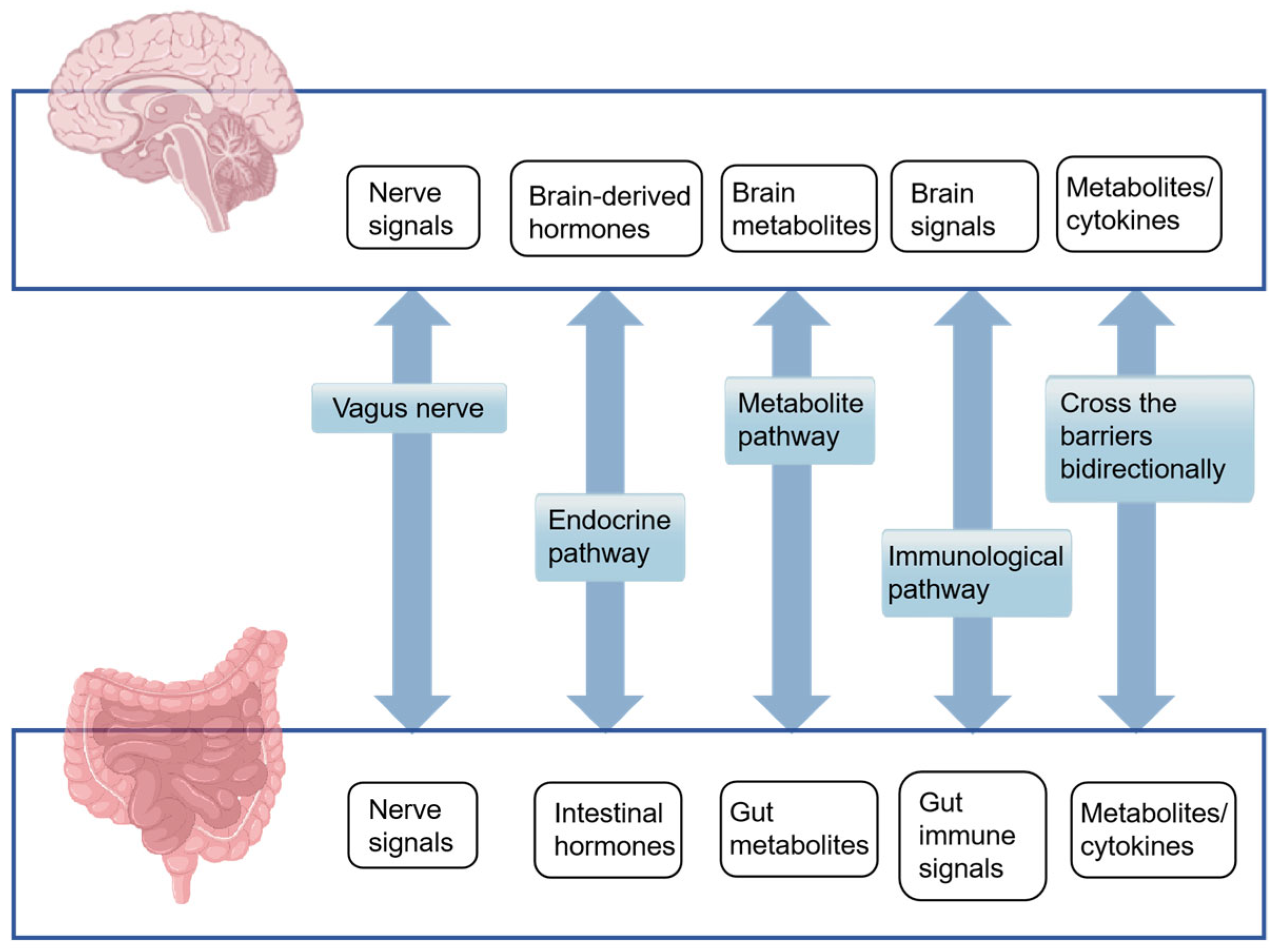
2. Gut Microbiota and Microbial–Gut–Brain Axis Mechanisms
2.1. Gut Microbiota
2.2. MGBA Mechanisms of AD
2.2.1. Neural Pathways
2.2.2. Endocrine Pathway
2.2.3. Microbial Metabolite
2.2.4. Immunological Pathway
2.2.5. Intestinal Wall Barrier and Blood–Brain Barrier
3. FMT for the Treatment of AD
3.1. FMT
3.1.1. The History and Development of FMT
3.1.2. Selection of FMT Donors
3.1.3. Fecal Microbiota Preparation
3.1.4. Antibiotic Pretreatment
3.1.5. Administration Routes of FMT
3.2. Role of FMT in Inducing and Alleviating AD in Mice
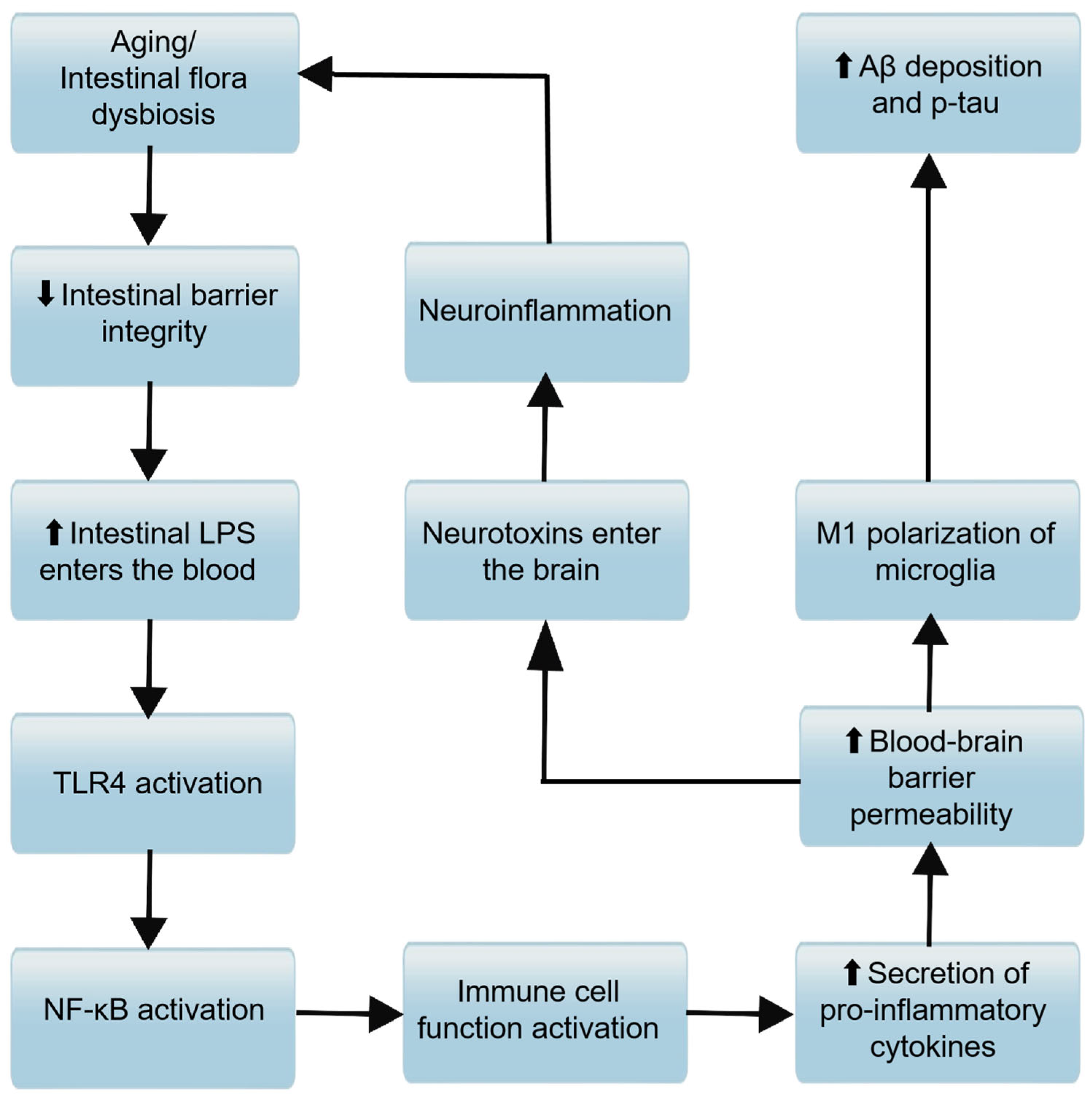
3.2.1. FMT from AD Donors Promotes Disease Progression
Increment of Brain Aβ Levels
Increment of Harmful Microbial Metabolites
Impact on Neurotransmitter Endocrinology
Activation of Inflammatory Vesicles
Abnormal Activation of Microglia
3.2.2. Healthy Donor FMT Relieves Disease Progression
Reduction Brain Aβ Level
Reduces Brain Tau Protein Phosphorylation Level
Regulation of Abnormal GM and Its Metabolites
Reducing the Inflammatory Response
Restoration of Microglia Homeostasis
3.3. The Role of FMT in Clinical Remission of AD
4. Current Limitations and Perspectives of FMT in the Treatment of AD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| GM | gut microbiota |
| MGBA | microbiota–gut–brain axis |
| FMT | fecal microbiota transplantation |
| CNS | central nervous system |
| Aβ | amyloid-β |
| NFTs | neurofibrillary tangles |
| HPA | hypothalamus–pituitary–adrenal |
| BBB | blood–brain barrier |
| SCFAs | short-chain fatty acids |
| BDNF | brain-derived neurotrophic factor |
| GIT | gastrointestinal tract |
| LPS | lipopolysaccharides |
| TLRs | toll-like receptors |
| 5-HT | 5-hydroxytryptamine |
| GABA | γ-aminobutyric acid |
| IL-1 | interleukin-1 |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor-alpha |
| iNKT | invariant natural killer T cells |
| APP | amyloid precursor protein |
| CDI | Clostridioides difficile infection |
| TMAO | Trimethylamine N-oxide |
| p-tau | tau hyperphosphorylation |
| ER | endoplasmic reticulum |
| mAChRs | Muscarinic acetylcholine receptors |
| PP2A | protein phosphatase 2A |
| CA | carnosic acid |
| DEPTACs | dephosphorylation-targeting chimera |
| BHB | β-hydroxybutyrate |
References
- Patterson, C. World alzheimer report 2018—The state of the art of dementia research: New frontiers. In NEW FRONTIERS; Alzheimer’s Disease International: London, UK, 2018. [Google Scholar]
- Jagust, W. Imaging the evolution and pathophysiology of alzheimer disease. Nat. Rev. Neurosci. 2018, 19, 687–700. [Google Scholar] [CrossRef]
- McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for dementia. Cochrane Database Syst. Rev. 2019, 3, CD003154. [Google Scholar] [CrossRef]
- Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 2018, CD001190. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in early alzheimer’s disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Jeremic, D.; Jiménez-Díaz, L.; Navarro-López, J.D. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The microbiota–gut–brain axis and alzheimer’s disease: Neuroinflammation is to blame? Nutrients 2020, 13, 37. [Google Scholar] [CrossRef]
- Zhuang, Z.-Q.; Shen, L.-L.; Li, W.-W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.-L.; et al. Gut microbiota is altered in patients with alzheimer’s disease. J. Alzheimer’s Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lukiw, W.J. Bacteroidetes neurotoxins and inflammatory neurodegeneration. Mol. Neurobiol. 2018, 55, 9100–9107. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276, Erratum in Cell 2015, 163, 258. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Humarán, L.G.; Salinas, E.; Ortiz, G.G.; Ramírez-Jirano, L.J.; Morales, J.A.; Bitzer-Quintero, O.K. From probiotics to psychobiotics: Live beneficial bacteria which act on the brain-gut axis. Nutrients 2019, 11, 890. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Varesi, A.; Pierella, E.; Romeo, M.; Piccini, G.B.; Alfano, C.; Bjørklund, G.; Oppong, A.; Ricevuti, G.; Esposito, C.; Chirumbolo, S.; et al. The potential role of gut microbiota in alzheimer’s disease: From diagnosis to treatment. Nutrients 2022, 14, 668. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, J.; Ling, Y.; Wang, F.; Gong, T.; Yang, C.; Ye, S.; Ye, K.; Wei, D.; Song, Z.; et al. Fecal microbiota transplantation alleviated alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl. Psychiatry 2019, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.-W.; et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an alzheimer’s disease animal model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, A.; Vandendriessche, C.; Hamerlinck, H.; De Looze, D.; Tate, D.J.; Vuylsteke, M.; De Commer, L.; Devolder, L.; Raes, J.; Verhasselt, B.; et al. Safety and efficacy of faecal microbiota transplantation in patients with mild to moderate Parkinson’s disease (GUT-PARFECT): A double-blind, placebo-controlled, randomised, phase 2 trial. eClinicalMedicine 2024, 71, 102563. [Google Scholar] [CrossRef]
- Hazan, S. Rapid improvement in alzheimer’s disease symptoms following fecal microbiota transplantation: A case report. J. Int. Med. Res. 2020, 48, 300060520925930. [Google Scholar] [CrossRef]
- de Jesus Rodrigues De-Paula, V.; Forlenza, A.S.; Forlenza, O.V. Relevance of gutmicrobiota in cognition, behaviour and alzheimer’s disease. Pharmacol. Res. 2018, 136, 29–34. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Haque, S.Z.; Haque, M. The ecological community of commensal, symbiotic, and pathogenic gastrointestinal microorganisms—An appraisal. Clin. Exp. Gastroenterol. 2017, 10, 91–103. [Google Scholar] [CrossRef]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.-J.; et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef]
- Mitsuoka, T. Intestinal Flora and Aging. Nutr. Rev. 2009, 50, 438–446. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and healthy aging: A mini-review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- Lau, H.C.H.; Sung, J.J.-Y.; Yu, J. Gut microbiota: Impacts on gastrointestinal cancer immunotherapy. Gut Microbes 2021, 13, 1869504. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontology 2000 2021, 87, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Adil, N.A.; Omo-Erigbe, C.; Yadav, H.; Jain, S. The oral–gut microbiome–brain axis in cognition. Microorganisms 2025, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Medel, M.; Pinto, M.P.; Goralsky, L.; Cáceres, M.; Villarroel-Espíndola, F.; Manque, P.; Pinto, A.; Garcia-Bloj, B.; de Mayo, T.; Godoy, J.A.; et al. Porphyromonas gingivalis, a bridge between oral health and immune evasion in gastric cancer. Front. Oncol. 2024, 14, 1403089. [Google Scholar] [CrossRef]
- Jia, S.; Li, X.; Du, Q. Host insulin resistance caused by Porphyromonas gingivalis-review of recent progresses. Front. Cell Infect. Microbiol. 2023, 13, 1209381. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. The vagus nerve in the neuro-immune axis: Implications in the pathology of the gastrointestinal tract. Front. Immunol. 2017, 8, 1452. [Google Scholar] [CrossRef]
- Liu, Y.; Sanderson, D.; Mian, M.F.; Neufeld, K.-A.M.; Forsythe, P. Loss of vagal integrity disrupts immune components of the microbiota-gut-brain axis and inhibits the effect of Lactobacillus rhamnosus on behavior and the corticosterone stress response. Neuropharmacology 2021, 195, 108682. [Google Scholar] [CrossRef]
- Yu, C.D.; Xu, Q.J.; Chang, R.B. Vagal sensory neurons and gut-brain signaling. Curr. Opin. Neurobiol. 2020, 62, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Goehler, L.E.; Gaykema, R.P.A.; Nguyen, K.T.; Lee, J.E.; Tilders, F.J.H.; Maier, S.F.; Watkins, L.R. Interleukin-1β in immune cells of the abdominal vagus nerve: A link between the immune and nervous systems? J. Neurosci. 1999, 19, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Kirkup, A.J.; Brunsden, A.M.; Thompson, D.G.; Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G907–G915. [Google Scholar] [CrossRef] [PubMed]
- Maestú, F.; de Haan, W.; Busche, M.A.; DeFelipe, J. Neuronal excitation/inhibition imbalance: Core element of a translational perspective on Alzheimer pathophysiology. Ageing Res. Rev. 2021, 69, 101372. [Google Scholar] [CrossRef]
- Strac, D.Š.; Pivac, N.; Mück-Šeler, D. The serotonergic system and cognitive function. Transl. Neurosci. 2016, 7, 35–49. [Google Scholar] [CrossRef]
- Armada-Moreira, A.; Gomes, J.I.; Pina, C.C.; Savchak, O.K.; Gonçalves-Ribeiro, J.; Rei, N.; Pinto, S.; Morais, T.P.; Martins, R.S.; Ribeiro, F.F.; et al. Going the extra (synaptic) mile: Excitotoxicity as the road toward neurodegenerative diseases. Front. Cell Neurosci. 2020, 14, 90. [Google Scholar] [CrossRef]
- van Bodegom, M.; Homberg, J.R.; Henckens, M.J.A.G. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front. Cell Neurosci. 2017, 11, 87. [Google Scholar] [CrossRef]
- Aziz, Q.; Doré, J.; Emmanuel, A.; Guarner, F.; Quigley, E.M.M. Gut microbiota and gastrointestinal health: Current concepts and future directions. Neurogastroenterol. Motil. 2013, 25, 4–15. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Zhan, X.; Stamova, B.; Jin, L.-W.; DeCarli, C.; Phinney, B.; Sharp, F.R. Gram-negative bacterial molecules associate with alzheimer disease pathology. Neurology 2016, 87, 2324–2332. [Google Scholar] [CrossRef]
- Wu, Y.; Shao, Y.; Shao, X.; Yu, H.; Wang, M.; Wang, J.; She, Y.; Liu, J.; Zhang, T.; Li, Z.; et al. Qingke β-glucan and lactobacillus mitigate neuroinflammation and enhance cognitive function in an alzheimer’s disease mouse model. Int. J. Biol. Macromol. 2025, 319, 145427. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Yuan, B.; Liu, L.; Zhang, H.; Zhu, M.; Chai, H.; Peng, J.; Huang, Y.; Zhou, S.; et al. Akkermansia muciniphila and its metabolite propionic acid maintains neuronal mitochondrial division and autophagy homeostasis during alzheimer’s disease pathologic process via GPR41 and GPR43. Microbiome 2025, 13, 16. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Yang, B.; Chen, K.; Kong, Y.; Fang, N.; Gong, T.; Wang, F.; Ling, Z.; Liu, J. Effect of Clostridium butyricum against microglia—Mediated neuroinflammation in alzheimer’s disease via regulating gut microbiota and metabolites butyrate. Mol. Nutr. Food Res. 2020, 64, e1900636. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liao, J.; Xia, Y.; Liu, X.; Jones, R.; Haran, J.; McCormick, B.; Sampson, T.R.; Alam, A.; Ye, K. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022, 71, 2233–2252. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.-R.; Kuang, Q.; Zhang, F.; Chen, B.; Zhong, Z.-G. Functional roles of the microbiota-gut-brain axis in alzheimer’s disease: Implications of gut microbiota-targeted therapy. Transl. Neurosci. 2021, 12, 581–600. [Google Scholar] [CrossRef] [PubMed]
- Doens, D.; Fernández, P.L. Microglia receptors and their implications in the response to amyloid β for alzheimer’s disease pathogenesis. J. Neuroinflamm. 2014, 11, 48. [Google Scholar] [CrossRef]
- Connell, E.; Le Gall, G.; Pontifex, M.G.; Sami, S.; Cryan, J.F.; Clarke, G.; Müller, M.; Vauzour, D. Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol. Neurodegener. 2022, 17, 43. [Google Scholar] [CrossRef]
- Kostiuchenko, O.; Lushnikova, I.; Skibo, G. The role of gut microbiota metabolites in the regeneration and protection of nervous tissue: A narrative review. Regen. Med. Rep. 2024, 1, 12–30. [Google Scholar] [CrossRef]
- Xia, Y.; Xiao, Y.; Wang, Z.-H.; Liu, X.; Alam, A.M.; Haran, J.P.; McCormick, B.A.; Shu, X.; Wang, X.; Ye, K. Bacteroides fragilis in the gut microbiomes of alzheimer’s disease activates microglia and triggers pathogenesis in neuronal C/EBPβ transgenic mice. Nat. Commun. 2023, 14, 5471. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 is activated in alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Tagé, B.S.S.; Gonzatti, M.B.; Vieira, R.P.; Keller, A.C.; Bortoluci, K.R.; Aimbire, F. Three main SCFAs mitigate lung inflammation and tissue remodeling Nlrp3-Dependent in murine HDM-induced neutrophilic asthma. Inflammation 2024, 47, 1386–1402. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Cui, Y.; Wan, Q. NKT Cells in neurological diseases. Front. Cell Neurosci. 2019, 13, 245. [Google Scholar] [CrossRef]
- Lucas, K.; Maes, M. Role of the toll like receptor (TLR) radical cycle in chronic inflammation: Possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2020, 11, 594150. [Google Scholar] [CrossRef]
- Kumari, S.; Dhapola, R.; Sharma, P.; Singh, S.K.; Reddy, D.H. Implicative role of cytokines in neuroinflammation mediated AD and associated signaling pathways: Current progress in molecular signaling and therapeutics. Ageing Res. Rev. 2023, 92, 102098. [Google Scholar] [CrossRef]
- Walker, K.A.; Gottesman, R.F.; Wu, A.; Knopman, D.S.; Gross, A.L.; Mosley, T.H.; Selvin, E.; Windham, B.G. Systemic inflammation during midlife and cognitive change over 20 years. Neurology 2019, 92, E1256–E1267. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, W.; Shi, Y.; Fan, Z.; Ji, G. Should we standardize the 1700-year-old fecal microbiota transplantation? Am. J. Gastroenterol. 2012, 107, 1755. [Google Scholar] [CrossRef]
- Li, S. Bencao Gangmu; Huaxia Publishing House: Jinan, China, 2011; Volume 52. [Google Scholar]
- Zhang, T.; Gao, G.; Kwok, L.Y.; Sun, Z. Gut microbiome-targeted therapies for alzheimer’s disease. Gut Microbes 2023, 15, 2271613. [Google Scholar] [CrossRef]
- Nandwana, V.; Debbarma, S. Fecal microbiota transplantation: A microbiome modulation technique for alzheimer’s disease. Cureus 2021, 13, e16503. [Google Scholar] [CrossRef]
- Society of Parenteral and Enteral Nutrition, Chinese Medical Association; Microecology Professional Committee of Shanghai Preventive Medicine Association. Chinese expert consensus on screening and management of fecal microbiota transplantation donors (2022 edition). Zhonghua Wei Chang Wai Ke Za Zhi 2022, 25, 757–765. [Google Scholar] [CrossRef]
- Saha, S.; Mara, K.; Pardi, D.S.; Khanna, S. Durability of response to fecal microbiota transplantation after exposure to risk factors for recurrence in patients with Clostridioides difficile Infection. Clin. Infect. Dis. 2020, 73, e1706–e1712. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Rossen, N.G.; van der Spek, M.J.; Hartman, J.H.A.; Huuskonen, L.; Korpela, K.; Salojärvi, J.; Aalvink, S.; de Vos, W.M.; D’hAens, G.R.; et al. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J. 2017, 11, 1877–1889. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Cochrane, K.; Schroeter, K.; Daigneault, M.C.; Yen, S.; Verdu, E.F.; Allen-Vercoe, E.; Dorrestein, P.C. Effects of antibiotic pretreatment of an ulcerative colitis-derived fecal microbial community on the integration of therapeutic bacteria in vitro. mSystems 2020, 5, e00404-19. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Song, Y.; Garg, S.; Girotra, M.; Sinha, A.; Sivaraman, A.; Phillips, L.; Dutta, S.K. Effect of aging on the composition of fecal microbiota in donors for FMT and its impact on clinical outcomes. Dig. Dis. Sci. 2017, 62, 1002–1008. [Google Scholar] [CrossRef]
- Wilson, B.C.; Vatanen, T.; Cutfield, W.S.; O’SUllivan, J.M. The super-donor phenomenon in fecal microbiota transplantation. Front. Cell Infect. Microbiol. 2019, 9, 2. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Kelly, C.R.; Mullish, B.H.; Allegretti, J.R.; Kassam, Z.; Putignani, L.; Fischer, M.; Keller, J.J.; Costello, S.P.; et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 2019, 68, 2111–2121. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, B.; He, X.; Nie, Y.; Wu, K.; Fan, D. Microbiota transplantation: Concept, methodology and strategy for its modernization. Protein Cell 2018, 9, 462–473. [Google Scholar] [CrossRef]
- Keshteli, A.H.; Millan, B.; Madsen, K.L. Pretreatment with antibiotics may enhance the efficacy of fecal microbiota transplantation in ulcerative colitis: A meta-analysis. Mucosal Immunol. 2017, 10, 565–566. [Google Scholar] [CrossRef]
- Ji, S.K.; Yan, H.; Jiang, T.; Guo, C.Y.; Liu, J.J.; Dong, S.Z.; Yang, K.L.; Wang, Y.J.; Cao, Z.J.; Li, S.L. Preparing the gut with antibiotics enhances gut microbiota reprogramming efficiency by promoting xenomicrobiota colonization. Front. Microbiol. 2017, 8, 1208. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Dozier, E.A.; Glover, M.S.; Novick, S.; Ford, M.; Morehouse, C.; Warrener, P.; Caceres, C.; Hess, S.; Sellman, B.R.; et al. Engraftment of bacteria after fecal microbiota transplantation is dependent on both frequency of dosing and duration of preparative antibiotic regimen. Microorganisms 2021, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, V.; Rajaruban, S.; Dang, J.; Kung, J.Y.; Deehan, E.C.; Madsen, K.L. Repeated fecal microbial transplantations and antibiotic pre-treatment are linked to improved clinical response and remission in inflammatory bowel disease: A Systematic Review and Pooled Proportion Meta-Analysis. J. Clin. Med. 2021, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Paaske, S.E.; Baunwall, S.M.D.; Rubak, T.; Rågård, N.; Kelsen, J.; Hansen, M.M.; Lødrup, A.B.; Erikstrup, L.T.; Mikkelsen, S.; Erikstrup, C.; et al. Clinical management of Clostridioides difficile infection with faecal microbiota transplantation: A real-world cohort study. eClinicalMedicine 2025, 85, 103302. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Tian, H.L.; Yang, B.; Lin, Z.L.; Zhao, D.; Ye, C.; Zhang, X.Y.; Qin, H.L.; Li, N. Effect of intestinal preparation on the efficacy and safety of fecal microbiota transplantation treatment. Zhonghua Wei Chang Wai Ke Za Zhi 2020, 23, 48–55. [Google Scholar] [CrossRef]
- Jandl, B.; Dighe, S.; Gasche, C.; Makristathis, A.; Muttenthaler, M.; Staley, C. Intestinal biofilms: Pathophysiological relevance, host defense, and therapeutic opportunities. Clin. Microbiol. Rev. 2024, 37, e0013323. [Google Scholar] [CrossRef]
- Prudent, V.; Demarre, G.; Vazeille, E.; Wery, M.; Quenech’dU, N.; Ravet, A.; Dauverd-Girault, J.; van Dijk, E.; Bringer, M.-A.; Descrimes, M.; et al. The crohn’s disease-related bacterial strain LF82 assembles biofilm-like communities to protect itself from phagolysosomal attack. Commun. Biol. 2021, 4, 627. [Google Scholar] [CrossRef]
- Kumar, A.; Alam, A.; Rani, M.; Ehtesham, N.Z.; Hasnain, S.E. Biofilms: Survival and defense strategy for pathogens. Int. J. Med. Microbiol. 2017, 307, 481–489. [Google Scholar] [CrossRef]
- Faccin, I.D.; de Almeida de Souza, G.H.; Vicente, J.C.P.; da Silva Damaceno, N.; de Oliveira Perez, E.V.; Martins, W.; Gales, A.C.; Simionatto, S. The potential of bacteriophages in treating multidrug-resistant ESKAPE pathogen infections. Expert Opin. Ther. Pat. 2025, 1–17. [Google Scholar] [CrossRef]
- Bokoliya, S.C.; Dorsett, Y.; Panier, H.; Zhou, Y. Procedures for fecal microbiota transplantation in murine microbiome studies. Front. Cell Infect. Microbiol. 2021, 11, 711055. [Google Scholar] [CrossRef]
- Ramai, D.; Zakhia, K.; Fields, P.J.; Ofosu, A.; Patel, G.; Shahnazarian, V.; Lai, J.K.; Dhaliwal, A.; Reddy, M.; Chang, S. Fecal microbiota transplantation (FMT) with colonoscopy is superior to enema and nasogastric tube while comparable to capsule for the treatment of recurrent clostridioides difficile infection: A systematic review and meta-analysis. Dig. Dis. Sci. 2021, 66, 369–380. [Google Scholar] [CrossRef]
- Kamath, S.; Bryant, R.V.; Costello, S.P.; Day, A.S.; Forbes, B.; Haifer, C.; Hold, G.; Kelly, C.R.; Li, A.; Pakuwal, E.; et al. Translational strategies for oral delivery of faecal microbiota transplantation. Gut 2025, gutjnl-2025-335077. [Google Scholar] [CrossRef]
- Gulati, M.; Singh, S.K.; Corrie, L.; Kaur, I.P.; Chandwani, L. Delivery routes for faecal microbiota transplants: Available, anticipated and aspired. Pharmacol. Res. 2020, 159, 104954. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.B.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Nguyen, T.T.T.; Fujimura, Y.; Kameya, N.; Nakamura, S.; Arakawa, K.; Morita, H. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with alzheimer’s disease. Biosci. Biotechnol. Biochem. 2019, 83, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Guan, Q.; Zhang, X.; Yuan, C.; Tan, Z.; Zhai, L.; Hao, Y.; Gu, Y.; Han, C. New mechanism of neuroinflammation in Alzheimer’s disease: The activation of NLRP3 inflammasome mediated by gut microbiota. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 100, 109884. [Google Scholar] [CrossRef]
- Kim, N.; Jeon, S.H.; Ju, I.G.; Gee, M.S.; Do, J.; Oh, M.S.; Lee, J.K. Transplantation of gut microbiota derived from alzheimer’s disease mouse model impairs memory function and neurogenesis in C57BL/6 mice. Brain Behav. Immun. 2021, 98, 357–365. [Google Scholar] [CrossRef]
- Wang, M.; Cao, J.; Gong, C.; Amakye, W.K.; Yao, M.; Ren, J. Exploring the microbiota—Alzheimer’s disease linkage using short-term antibiotic treatment followed by fecal microbiota transplantation. Brain Behav. Immun. 2021, 96, 227–238. [Google Scholar] [CrossRef]
- Valeri, F.; dos Santos Guilherme, M.; He, F.; Stoye, N.M.; Schwiertz, A.; Endres, K. Impact of the age of cecal material transfer donors on alzheimer’s disease pathology in 5xFAD Mice. Microorganisms 2021, 9, 2548. [Google Scholar] [CrossRef]
- Grabrucker, S.; Marizzoni, M.; Silajdžić, E.; Lopizzo, N.; Mombelli, E.; Nicolas, S.; Dohm-Hansen, S.; Scassellati, C.; Moretti, D.V.; Rosa, M.; et al. Faecal microbiota transplantation from alzheimer’s participants induces impairments in neurogenesis and cognitive behaviours in rats. bioRxiv 2022. [Google Scholar] [CrossRef]
- Wang, F.; Gu, Y.; Xu, C.; Du, K.; Zhao, C.; Zhao, Y.; Liu, X. Transplantation of fecal microbiota from APP/PS1 mice and alzheimer’s disease patients enhanced endoplasmic reticulum stress in the cerebral cortex of wild-type mice. Front. Aging Neurosci. 2022, 14, 858130. [Google Scholar] [CrossRef] [PubMed]
- Soriano, S.; Curry, K.; Wang, Q.; Chow, E.; Treangen, T.J.; Villapol, S. Fecal microbiota transplantation derived from alzheimer’s disease mice worsens brain trauma outcomes in wild-type controls. Int. J. Mol. Sci. 2022, 23, 4476. [Google Scholar] [CrossRef]
- Kim, J.-E.; Roh, Y.-J.; Choi, Y.-J.; Lee, S.-J.; Jin, Y.-J.; Song, H.-J.; Seol, A.-Y.; Son, H.-J.; Hong, J.-T.; Hwang, D.-Y. Dysbiosis of fecal microbiota in Tg2576 mice for alzheimer’s disease during pathological constipation. Int. J. Mol. Sci. 2022, 23, 14928. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Xu, Z.; Zhang, L.; Zhang, C.; Zhao, X.; Mao, Y.; Zhang, H.; Liang, X.; Wu, J.; Yang, Y.; et al. Gut-derived β-amyloid: Likely a centerpiece of the gut–brain axis contributing to alzheimer’s pathogenesis. Gut Microbes 2023, 15, 2167172. [Google Scholar] [CrossRef]
- Ling, Y.; Morgan, K.; Kalsheker, N. Amyloid precursor protein (APP) and the biology of proteolytic processing: Relevance to Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2003, 35, 1505–1535. [Google Scholar] [CrossRef]
- Viola, K.L.; Velasco, P.T.; Klein, W.L. Why alzheimer’s is a disease of memory: The attack on synapses by Aß oligomers (ADDLs). J. Nutr. Health Aging 2008, 12, S51–S57. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 2018, 17, e12768. [Google Scholar] [CrossRef]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef]
- Gründer, G.; Cumming, P. Serotonin and amyloid deposition: A link between depression and alzheimer’s disease? An editorial highlight on pimavanserin, a 5HT2A receptor inverse agonist, rapidly suppresses aβ production and related pathology in a mouse model of alzheimer’s disease on page 658. J. Neurochem. 2021, 156, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Cirrito, J.R.; Wallace, C.E.; Yan, P.; Davis, T.A.; Gardiner, W.D.; Doherty, B.M.; King, D.; Yuede, C.M.; Lee, J.-M.; Sheline, Y.I. Effect of escitalopram on Aβ levels and plaque load in an alzheimer mouse model. Neurology 2020, 95, e2666–e2674. [Google Scholar] [CrossRef] [PubMed]
- Bast, T.; Pezze, M.; McGarrity, S. Cognitive deficits caused by prefrontal cortical and hippocampal neural disinhibition. Br. J. Pharmacol. 2017, 174, 3211–3225. [Google Scholar] [CrossRef] [PubMed]
- Zhen, H.; Hu, Y.; Liu, X.; Fan, G.; Zhao, S. The protease caspase-1: Activation pathways and functions. Biochem. Biophys. Res. Commun. 2024, 717, 149978. [Google Scholar] [CrossRef]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef]
- Cowan, M.; Petri, W.A. Microglia: Immune regulators of neurodevelopment. Front. Immunol. 2018, 9, 2576. [Google Scholar] [CrossRef]
- Füger, P.; Hefendehl, J.K.; Veeraraghavalu, K.; Wendeln, A.-C.; Schlosser, C.; Obermüller, U.; Wegenast-Braun, B.M.; Neher, J.J.; Martus, P.; Kohsaka, S.; et al. Microglia turnover with aging and in an alzheimer’s model via long-term in vivo single-cell imaging. Nat. Neurosci. 2017, 20, 1371–1376. [Google Scholar] [CrossRef]
- Vandenbark, A.A.; Offner, H.; Matejuk, S.; Matejuk, A. Microglia and astrocyte involvement in neurodegeneration and brain cancer. J. Neuroinflam. 2021, 18, 298. [Google Scholar] [CrossRef]
- Kaur, D.; Sharma, V.; Deshmukh, R. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology 2019, 27, 663–677. [Google Scholar] [CrossRef]
- Zhan, G.; Yang, N.; Li, S.; Huang, N.; Fang, X.; Zhang, J.; Zhu, B.; Yang, L.; Yang, C.; Luo, A. Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging 2018, 10, 1257–1267. [Google Scholar] [CrossRef]
- Dodiya, H.B.; Kuntz, T.; Shaik, S.M.; Baufeld, C.; Leibowitz, J.; Zhang, X.; Gottel, N.; Zhang, X.; Butovsky, O.; Gilbert, J.A.; et al. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J. Exp. Med. 2019, 216, 1542–1560. [Google Scholar] [CrossRef]
- Elangovan, S.; Borody, T.J.; Holsinger, R.M.D. Fecal microbiota transplantation reduces pathology and improves cognition in a mouse model of alzheimer’s disease. Cells 2022, 12, 119. [Google Scholar] [CrossRef]
- Hang, Z.; Cai, S.; Lei, T.; Xiao, Z.; Wang, D.; Li, Y.; Bi, W.; Yang, Y.; Deng, S.; Wang, L.; et al. Transfer of tumor-bearing mice intestinal flora can ameliorate cognition in alzheimer’s disease mice. J. Alzheimer’s Dis. 2022, 86, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, H.; Shao, C.; Liu, Y.; Wen, S.; Tang, L. Application of dominant gut microbiota promises to replace fecal microbiota transplantation as a new treatment for alzheimer’s disease. Microorganisms 2023, 11, 2854. [Google Scholar] [CrossRef] [PubMed]
- Yadollahikhales, G.; Rojas, J.C. Anti-amyloid immunotherapies for alzheimer’s disease: A 2023 clinical update. Neurotherapeutics 2023, 20, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Y. Tau and neuroinflammation in Alzheimer’s disease: Interplay mechanisms and clinical translation. J. Neuroinflammation 2023, 20, 914–931. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Xia, Y.-Y.; Grundke-Iqbal, I.; Iqbal, K. Abnormal hyperphosphorylation of tau: Sites, regulation, and molecular mechanism of neurofibrillary degeneration. J. Alzheimer’s Dis. 2013, 33 (Suppl. S1), S123–S139. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Li, X.; Yang, B.; Zhang, X.; Kang, L.; Ling, Y.; Sui, P.; Tian, S.; Wang, Y. Carnosic acid inhibits tau hyperphosphorylation via PP2A signaling in an Alzheimer’s disease transgenic mouse model. Prog. Anat. Sci. 2024, 1–7. [Google Scholar]
- Congdon, E.E.; Ji, C.; Tetlow, A.M.; Jiang, Y.; Sigurdsson, E.M. Tau-targeting therapies for alzheimer disease: Current status and future directions. Nat. Rev. Neurol. 2023, 19, 715–736. [Google Scholar] [CrossRef]
- Torres, A.K.; Jara, C.; Olesen, M.A.; Tapia-Rojas, C. Pathologically phosphorylated tau at S396/404 (PHF-1) is accumulated inside of hippocampal synaptic mitochondria of aged Wild-type mice. Sci. Rep. 2021, 11, 4448. [Google Scholar] [CrossRef]
- Evans, D.B.; Rank, K.B.; Bhattacharya, K.; Thomsen, D.R.; Gurney, M.E.; Sharma, S.K. Tau phosphorylation at serine 396 and serine 404 by human recombinant tau protein kinase II inhibits tau’s ability to promote microtubule assembly. J. Biol. Chem. 2000, 275, 24977–24983. [Google Scholar] [CrossRef]
- Gu, J.; Congdon, E.E.; Sigurdsson, E.M. Two Novel Tau Antibodies Targeting the 396/404 Region Are Primarily Taken Up by Neurons and Reduce Tau Protein Pathology. J. Biol. Chem. 2013, 288, 33081–33095. [Google Scholar] [CrossRef]
- Olona, A.; Leishman, S.; Anand, P.K. The NLRP3 inflammasome: Regulation by metabolic signals. Trends Immunol. 2022, 43, 978–989. [Google Scholar] [CrossRef]
- Vinaik, R.; Barayan, D.; Auger, C.; Abdullahi, A.; Jeschke, M.G. Regulation of glycolysis and the warburg effect in wound healing. J. Clin. Investig. 2020, 5, e138949. [Google Scholar] [CrossRef] [PubMed]
- Hooftman, A.; Angiari, S.; Hester, S.; Corcoran, S.E.; Runtsch, M.C.; Ling, C.; Ruzek, M.C.; Slivka, P.F.; McGettrick, A.F.; Banahan, K.; et al. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metab. 2020, 32, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, S.-G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Chen, W.; Guo, C.; Huang, S.; Jia, Z.; Wang, J.; Zhong, J.; Ge, H.; Yuan, J.; Chen, T.; Liu, X.; et al. MitoQ attenuates brain damage by polarizing microglia towards the M2 phenotype through inhibition of the NLRP3 inflammasome after ICH. Pharmacol. Res. 2020, 161, 105122. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Li, D.; Zhang, N.; Liu, R.; Han, B.; Wei, C.; Liu, H.; Xu, X.; Hao, J. Everolimus (RAD001) ameliorates vascular cognitive impairment by regulating microglial function via the mTORC1 signaling pathway. J. Neuroimmunol. 2016, 299, 164–171. [Google Scholar] [CrossRef]
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New roles for the synaptic stripper. Neuron 2013, 77, 10–18. [Google Scholar] [CrossRef]
- Han, J.; Harris, R.A.; Zhang, X.-M. An updated assessment of microglia depletion: Current concepts and future directions. Mol. Brain 2017, 10, 25. [Google Scholar] [CrossRef]
- Rice, R.A.; Pham, J.; Lee, R.J.; Najafi, A.R.; West, B.L.; Green, K.N. Microglial repopulation resolves inflammation and promotes brain recovery after injury. Glia 2017, 65, 931–944. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, T.; Chen, Q.; Li, C.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; Chen, H.; Zhou, Z.; et al. Biomimetic dendrimer–peptide conjugates for early multi-target therapy of alzheimer’s disease by inflammatory microenvironment modulation. Adv. Mater. 2021, 33, 2100746. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.D.; Daggett, A.; Gu, X.; Jiang, L.-L.; Langfelder, P.; Li, X.; Wang, N.; Zhao, Y.; Park, C.S.; Cooper, Y.; et al. Elevated TREM2 gene dosage reprograms microglia responsivity and ameliorates pathological phenotypes in alzheimer’s disease models. Neuron 2018, 97, 1032–1048.e5. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Lee, J.H.; Shin, J.; Kim, J.-S.; Cha, B.; Lee, S.; Kwon, K.S.; Shin, Y.W.; Choi, S.H. Cognitive function improvement after fecal microbiota transplantation in Alzheimer’s dementia patient: A case report. Curr. Med. Res. Opin. 2021, 37, 1739–1744. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, J.-H.; Kim, J.-S.; Kim, T.J.; Shin, J.; Im, J.H.; Cha, B.; Lee, S.; Kwon, K.S.; Shin, Y.W.; et al. Fecal microbiota transplantation can improve cognition in patients with cognitive decline and Clostridioides difficile infection. Aging 2022, 14, 6449–6466. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Lau, R.I.; Su, Q.; Ng, S.C. Long COVID and gut microbiome: Insights into pathogenesis and therapeutics. Gut Microbes 2025, 17, 2457495. [Google Scholar] [CrossRef]
- de Almeida, V.M.; Engel, D.F.; Ricci, M.F.; Cruz, C.S.; Lopes, Í.S.; Alves, D.A.; Auriol, M.D.; Magalhães, J.; Machado, E.C.; Rocha, V.M.; et al. Gut microbiota from patients with COVID-19 cause alterations in mice that resemble post-COVID symptoms. Gut Microbes 2023, 15, 2249146. [Google Scholar] [CrossRef]
- Lau, R.I.; Su, Q.; Ching, J.Y.; Lui, R.N.; Chan, T.T.; Wong, M.T.; Lau, L.H.; Wing, Y.K.; Chan, R.N.; Kwok, H.Y.; et al. Fecal Microbiota Transplantation for Sleep Disturbance in Post-acute COVID-19 Syndrome. Clin. Gastroenterol. Hepatol. 2024, 22, 2487–2496.e6. [Google Scholar] [CrossRef]
- Bredesen, D.E.; Sharlin, K.; Jenkins, D.; Okuno, M.; Youngberg, W.; Cohen, S.H.; Stefani, A.; Brown, R.; Conger, S.; Tanio, C.; et al. Reversal of cognitive decline: 100 patients. J. Alzheimer’s Dis. Parkinsonism. 2018, 8, 2161-0460. [Google Scholar] [CrossRef]
- Baxter, M.; Colville, A. Adverse events in faecal microbiota transplant: A review of the literature. J. Hosp. Infect. 2016, 92, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Dailey, F.E.; Turse, E.P.; Daglilar, E.; Tahan, V. The dirty aspects of fecal microbiota transplantation: A review of its adverse effects and complications. Curr. Opin. Pharmacol. 2019, 49, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, M.; Wang, W.; Cao, X.; Piao, M.; Khan, S.; Yan, F.; Cao, H.; Wang, B.; Grivennikov, S. Systematic review: Adverse events of fecal microbiota transplantation. PLoS ONE 2016, 11, e0161174. [Google Scholar] [CrossRef]
- Holleran, G.; Scaldaferri, F.; Ianiro, G.; Lopetuso, L.; Mc Namara, D.; Mele, M.; Gasbarrini, A.; Cammarota, G. Fecal microbiota transplantation for the treatment of patients with ulcerative colitis and other gastrointestinal conditions beyond Clostridium difficile infection: An update. Drugs Today 2018, 54, 123–136. [Google Scholar] [CrossRef]
- Alang, N.; Kelly, C.R. Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis. 2015, 2, ofv004. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Schwartz, M.; Gluck, M.; Koon, S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am. J. Gastroenterol. 2013, 108, 1367. [Google Scholar] [CrossRef]
- Kelly, C.R.; Yen, E.F.; Grinspan, A.M.; Kahn, S.A.; Atreja, A.; Lewis, J.D.; Moore, T.A.; Rubin, D.T.; Kim, A.M.; Serra, S.; et al. Fecal microbiota transplantation is highly effective in real-world practice: Initial results from the FMT national registry. Gastroenterology 2021, 160, 183–192. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, S.; Qin, H.; Li, N.; Chen, Q. Long-term safety of faecal microbiota transplantation for gastrointestinal diseases in China. Lancet Gastroenterol. Hepatol. 2022, 7, 702–703. [Google Scholar] [CrossRef]
- Kundu, P.; Stagaman, K.; Kasschau, K.; Holden, S.; Shulzhenko, N.; Sharpton, T.J.; Raber, J. Fecal implants from AppNL–G–F and AppNL–G–F/E4 donor mice sufficient to induce behavioral phenotypes in germ-free mice. Front. Behav. Neurosci. 2022, 16, 791128. [Google Scholar] [CrossRef]
- Alaeddin, S.; Chatterjee, A.; Roberts, T.L.; Steiner-Lim, G.Z.; Jensen, S.O.; Gyengesi, E.; Muench, G.; Ho, V. Exploring the effects of faecal microbiota transplantation on cognitive function: A review of clinical trials. Brain Behav. Immun. Health 2025, 48, 101049. [Google Scholar] [CrossRef]
- Tatara, Y.; Yamazaki, H.; Katsuoka, F.; Chiba, M.; Saigusa, D.; Kasai, S.; Nakamura, T.; Inoue, J.; Aoki, Y.; Shoji, M.; et al. Multiomics and artificial intelligence enabled peripheral blood-based prediction of amnestic mild cognitive impairment. Curr. Res. Transl. Med. 2023, 71, 103367. [Google Scholar] [CrossRef]
- French, S.R.; Arias, J.C.; Zahra, S.; Ally, M.; Escareno, C.; Heitkamp, E.; Vazquez, F.; Hillis, M.; Wiskoski, H.; Ainapurapu, K.; et al. Cognitive impairment and p-tau217 are high in a vascular patient cohort. Alzheimer’s Dement. 2025, 21, e70565. [Google Scholar] [CrossRef]


| Donor | Recipient | Transplantation Technique | Results | Ref. | |
|---|---|---|---|---|---|
| 1 | 12-month-old CONVRAPPPS1 mice | 4-month-old GF-APPPS1 mice | Oral gavage of fecal contents on day 1 and day 4 | ↑ β38, β40, β42 ↓ NPE and IDE | Harach et al. [87] |
| 2 | 82-year-old male AD patients | 4-week-old germ-free C57BL/6N mice | Oral inoculation | ↓ Cognitive function ↓ OLT and ORT ↓ γ-Aminobutyrate, taurine and valine | Fujii et al. [88] |
| 3 | AD patients | APP/PS1 double transgenic mice | Oral gavage | ↑ NLRP3 and neuroinflammatory ↑ Activation of microglia | Shen et al. [89] |
| 4 | 5xFAD mice | C57BL/6 mice | Oral gavage (200 µL for 5 consecutive days) | ↑ p21, TNF-α, IL-1β, Microglia activation, ↑ Pro-inflammatory cytokines ↓ Adult hippocampal neurogenesis and BDNF | Kim et al. [90] |
| 5 | 16-month-old APPSWE/PS1∆E9 mice | 3-month-old APPSWE/PS1∆E9 mice | Antibiotic cocktails for 2 weeks and FMT for 7 days by oral gavage | ↑ Aβ plaques ↓ Astrocyte activation around Aβ plaques | Wang et al. [91] |
| 6 | 1-year-old WT mice | 4-month-old 5xFAD mice | 150 μL fecal preparation via oral gavage one time after antibiotic treatment | ↑ Serum LPS binding protein ↑ Plaques in the prefrontal cortex ↓ Firmicutes | Valeri et al. [92] |
| 7 | AD patients | 11-week-old male Sprague-Dawley rats | antibiotic treatment for 7 days, 72 h later FMT for 3 days by oral gavage, then twice per week | ↑ I L-1β, IL-10, NLRP3 ↑ Histidine, aminoadipic acid, MIF, ↓ IL-4, dendritogenesis of adult-born neurons | Grabrucker et al. [93] |
| 8 | AD patients and APP/PS1 mice | 6-week-old Male, C57BL/6 J mice | Antibiotic cocktail for 3 days then FMT by gavage for 2 weeks | ↑ TMAO in the cerebral cortex and serum | Wang et al. [94] |
| 9 | Aged 3 × TgAD donor female mice | 9–12-week-old male and female C57BL/6 mice | By oral gavage to recipient at 24 h after TBI | ↑ Bacteroidetes, neuroinflammation ↑ Microglia and Astrocytes activation ↓ Firmicutes | Soriano et al. [95] |
| 10 | Tg2576 mice | AiDM-ICR mice | By gavage for three days(10 g feces in 0.2 mL of a 1 × PBS solution) | ↓ 5-HT, mAChR M2, M3 and Gα proteins Dysregulation of the excitatory function of the ENS | Kim et al. [96] |
| 11 | 12-month-old APP/PS1 mice | Newly weaned WT mice | Gavaged with fecal supernatant (200 μL per mouse) three times a week | ↑ BACE1, Aβ42, Iba1 and iNOS ↓ Short-term spatial memory ↓ Memory for novel object recognition | Jin et al. [97] |
| Donor | Recipient | Transplantation Technique | Results | Ref. | |
|---|---|---|---|---|---|
| 1 | SAMR1 mice | Pseudo germ-free mice | 0.2 mL fecal suspension by gavage for 14 days | ↑ α diversity and β diversity ↓ Abnormal microbiota | Zhan et al. [112] |
| 2 | Age-matched APPPS1-21 | ABX-treated APPPS1-21 male | 0.2 mL fecal slurry by gastric gavage daily starting on P25 until sacrifice | ↑ Microglial physiology ↓ Aβ pathology | Dodiya et al. [113] |
| 3 | WT mice | APPswe/PS1dE9 Tg mouse model | 0.2 mL of fresh fecal solution by gastric gavage once daily for 4 weeks | ↑ SCFAs, synaptic plasticity ↓ Aβ40, Aβ42, p-tau, ↓ COX2, CD11b, neuroinflammation | Sun et al. [14] |
| 4 | WT mice | ADLPAPT transgenic mouse model | Fresh fecal matter for 4 weeks in mice pre-treated with antibiotics | ↓ Aβ plaques, NFT, inflammatory monocytes ↓ Glial reactivity, cognitive impairment | Kim et al. [15] |
| 5 | Healthy human | APP/PS1 mice transplanted with GM from AD patients | Oral gavage | ↑ Cognitive function intestinal ↓ NLRP3 and neuroinflammatory factors ↓ Activation of microglia in central hippocampus | Shen et al. [89] |
| 6 | Healthy B6SJL WT mice | Old (30–32-week-old) 5xFAD recipient mice | Oral gavage for seven days | ↑ Cognitive function, novel object recognition, spatial memory ↓ Inflammatory factors, Aβ plaques | Elangovan et al. [114] |
| 7 | WT mice | AD mice | By gavage | ↑ Bacteroidetes, Bacteroides, Sutterella ↑ Oscillospira, Odoribacter, AF12 ↑ Short-term memory level and cognitive ability ↓ Firmicutes and Prevotella | Hang et al. [115] |
| 8 | Healthy C57BL/6 J mice | APP/PS1 transgenic male mice | 0.3 mL of fresh fecal matter for five weeks intragastrically | ↑ Intestinal microbiota richness and composition ↓ IL-1β, IL-6, APP, Aβ plaques BACE1 | Li et al. [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Wang, Q.; Hong, H.; Tang, C. Fecal Microbiota Transplantation in Alzheimer’s Disease: Mechanistic Insights Through the Microbiota–Gut–Brain Axis and Therapeutic Prospects. Microorganisms 2025, 13, 1956. https://doi.org/10.3390/microorganisms13081956
Ren J, Wang Q, Hong H, Tang C. Fecal Microbiota Transplantation in Alzheimer’s Disease: Mechanistic Insights Through the Microbiota–Gut–Brain Axis and Therapeutic Prospects. Microorganisms. 2025; 13(8):1956. https://doi.org/10.3390/microorganisms13081956
Chicago/Turabian StyleRen, Jiayu, Qinwen Wang, Hang Hong, and Chunlan Tang. 2025. "Fecal Microbiota Transplantation in Alzheimer’s Disease: Mechanistic Insights Through the Microbiota–Gut–Brain Axis and Therapeutic Prospects" Microorganisms 13, no. 8: 1956. https://doi.org/10.3390/microorganisms13081956
APA StyleRen, J., Wang, Q., Hong, H., & Tang, C. (2025). Fecal Microbiota Transplantation in Alzheimer’s Disease: Mechanistic Insights Through the Microbiota–Gut–Brain Axis and Therapeutic Prospects. Microorganisms, 13(8), 1956. https://doi.org/10.3390/microorganisms13081956






