Biodegradation of Zearalenone by a Novel Bacillus Strain X13 Isolated from Volcanic Rock Soil Using the Mycotoxin as the Sole Carbon Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Regents and Media
2.2. Detection of ZEN by LC-MS/MS
2.3. Screening and Isolation of Zearalenone Degrading Strains
2.4. Identification of Strain X13
2.5. Effect of Culture Conditions on ZEN Degradation by Strain X13
2.5.1. Effect of Culture Medium on ZEN Degradation by Strain X13
2.5.2. Effect of Culture Time on ZEN Degradation by Strain X13
2.5.3. Effect of Inoculum Size on ZEN Degradation by Strain X13
2.5.4. Effect of Initial pH on ZEN Degradation by Strain X13
2.5.5. Effect of Temperature on ZEN Degradation by Strain X13
2.5.6. Effect of ZEN Concentration on ZEN Degradation by Strain X13
2.6. Localization of ZEN-Degrading Active Substances in Strain X13
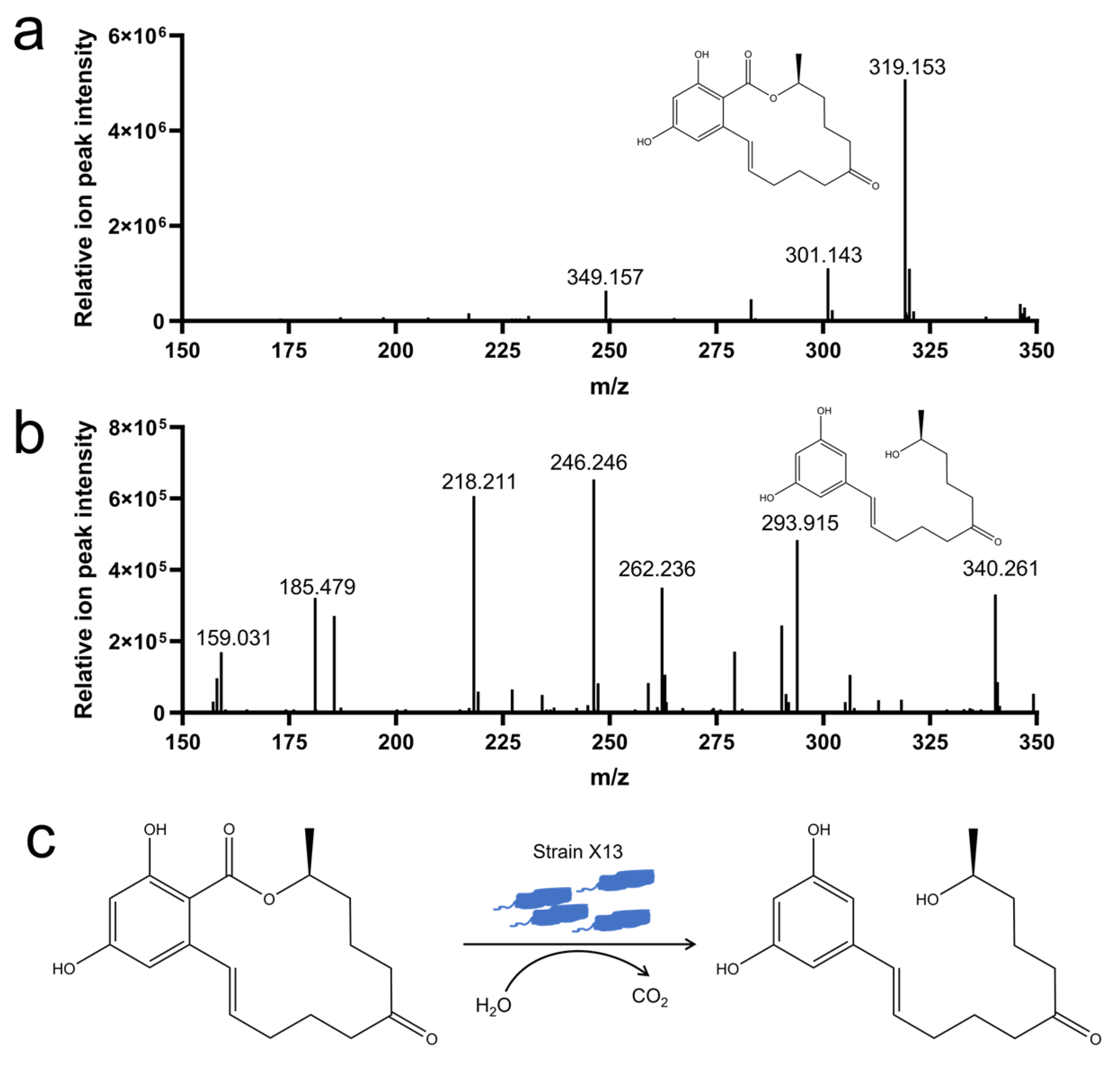
2.7. Analysis of ZEN Degradation Products by Strain X13
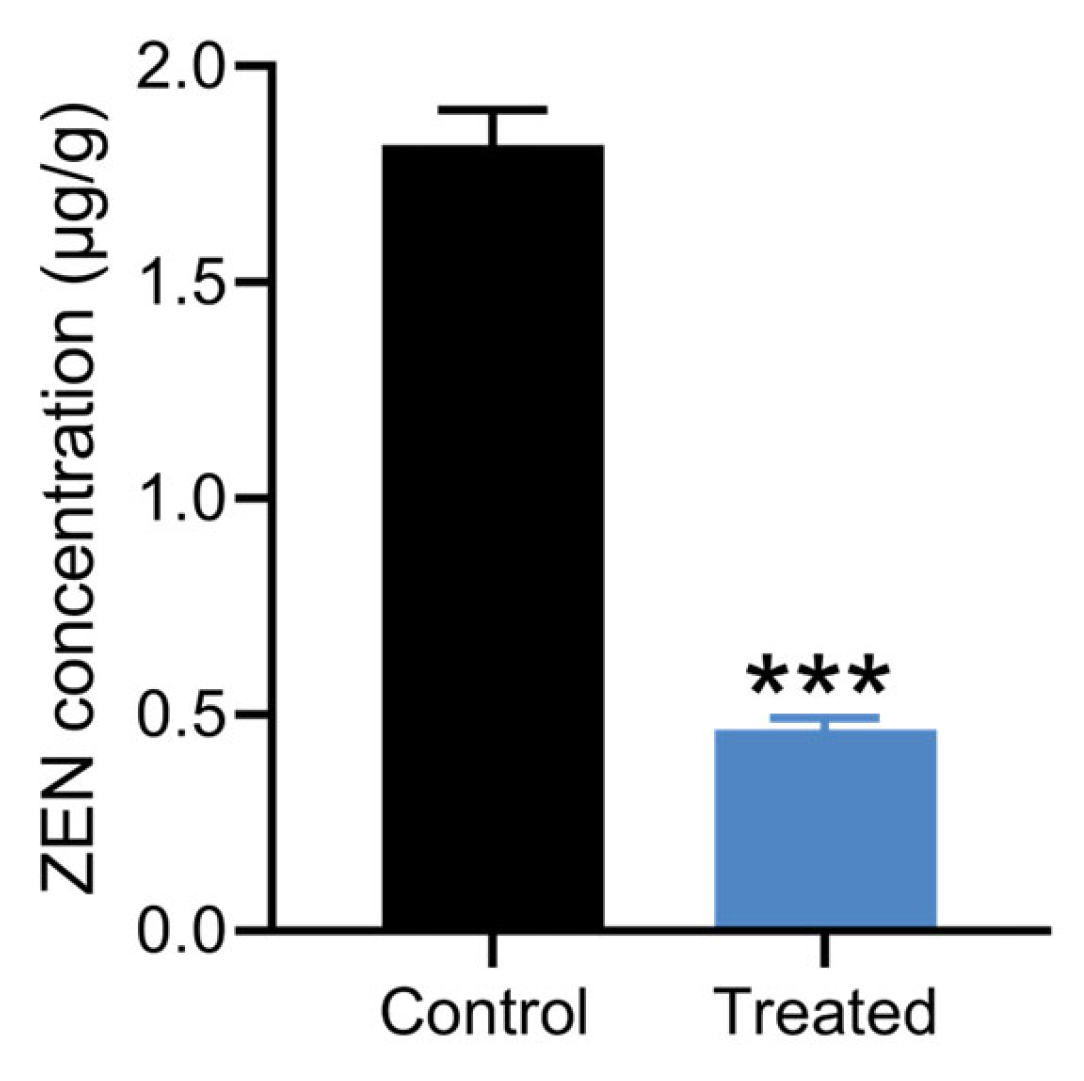
2.8. Detoxification of Moldy Maize by Strain X13
2.9. Statistical Analysis
3. Results and Discussion
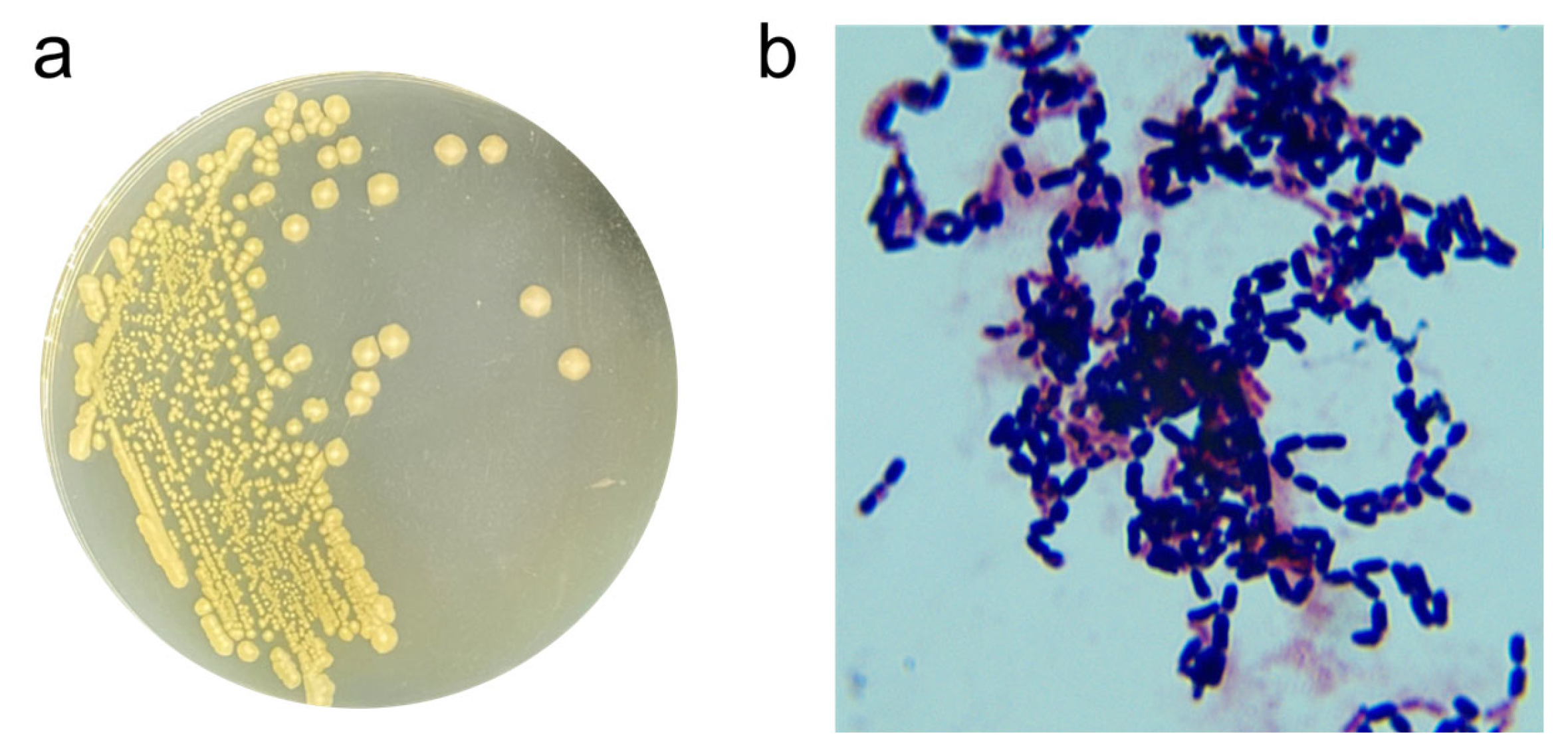
3.1. Screening and Isolation of Zearalenone Degrading Strains
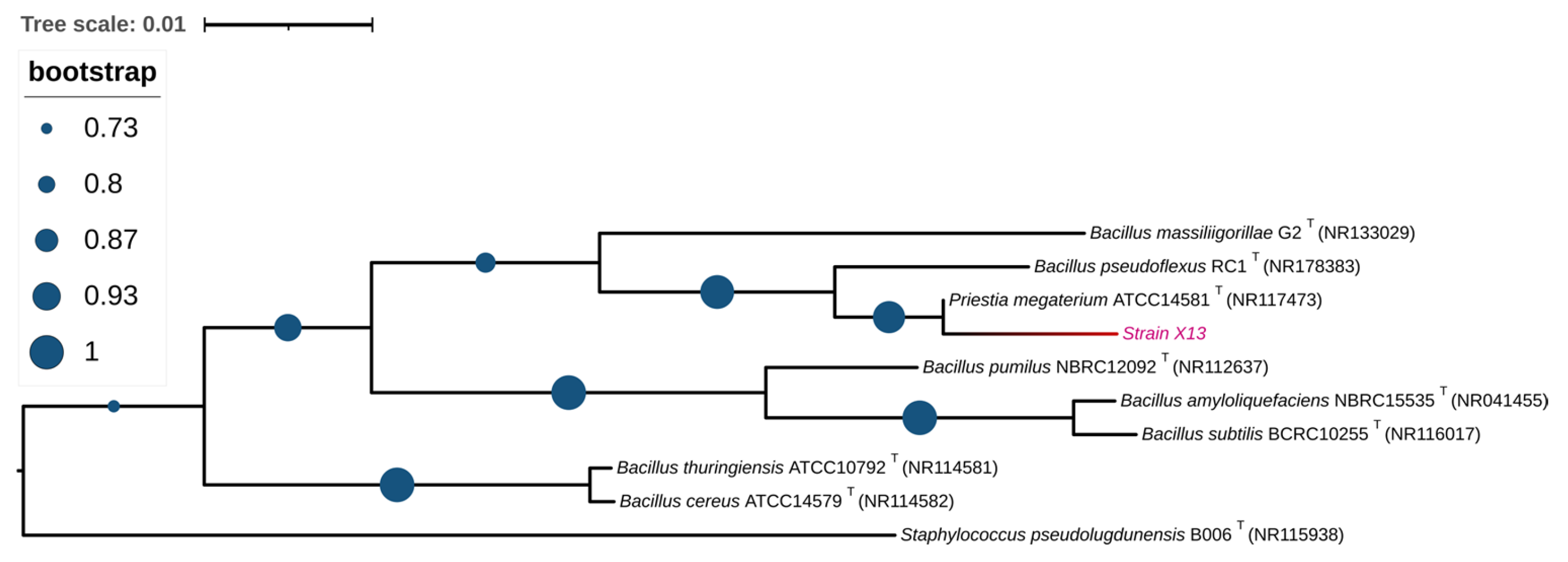
3.2. Identification of Strain X13
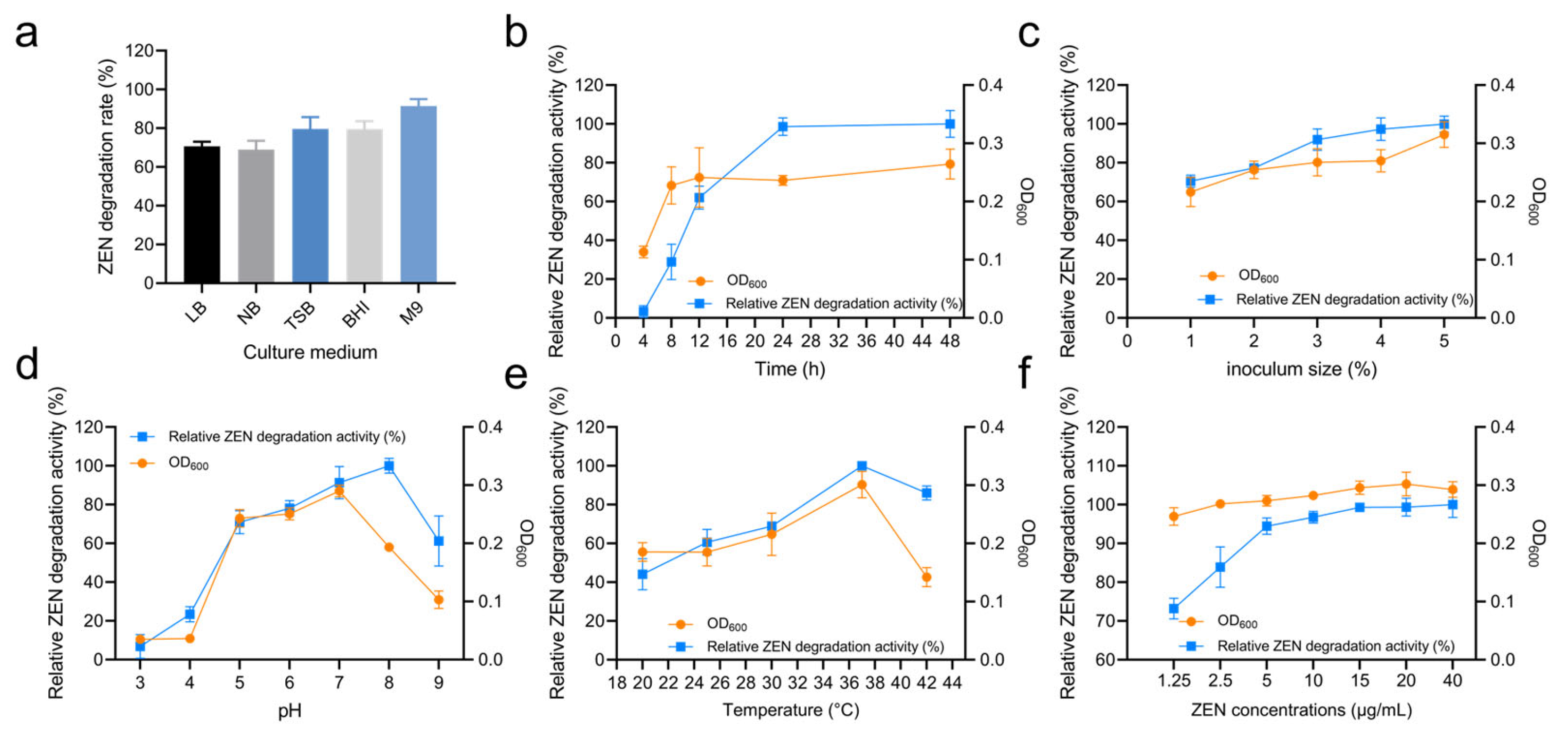
3.3. Effect of Culture Conditions on ZEN Degradation by Strain X13
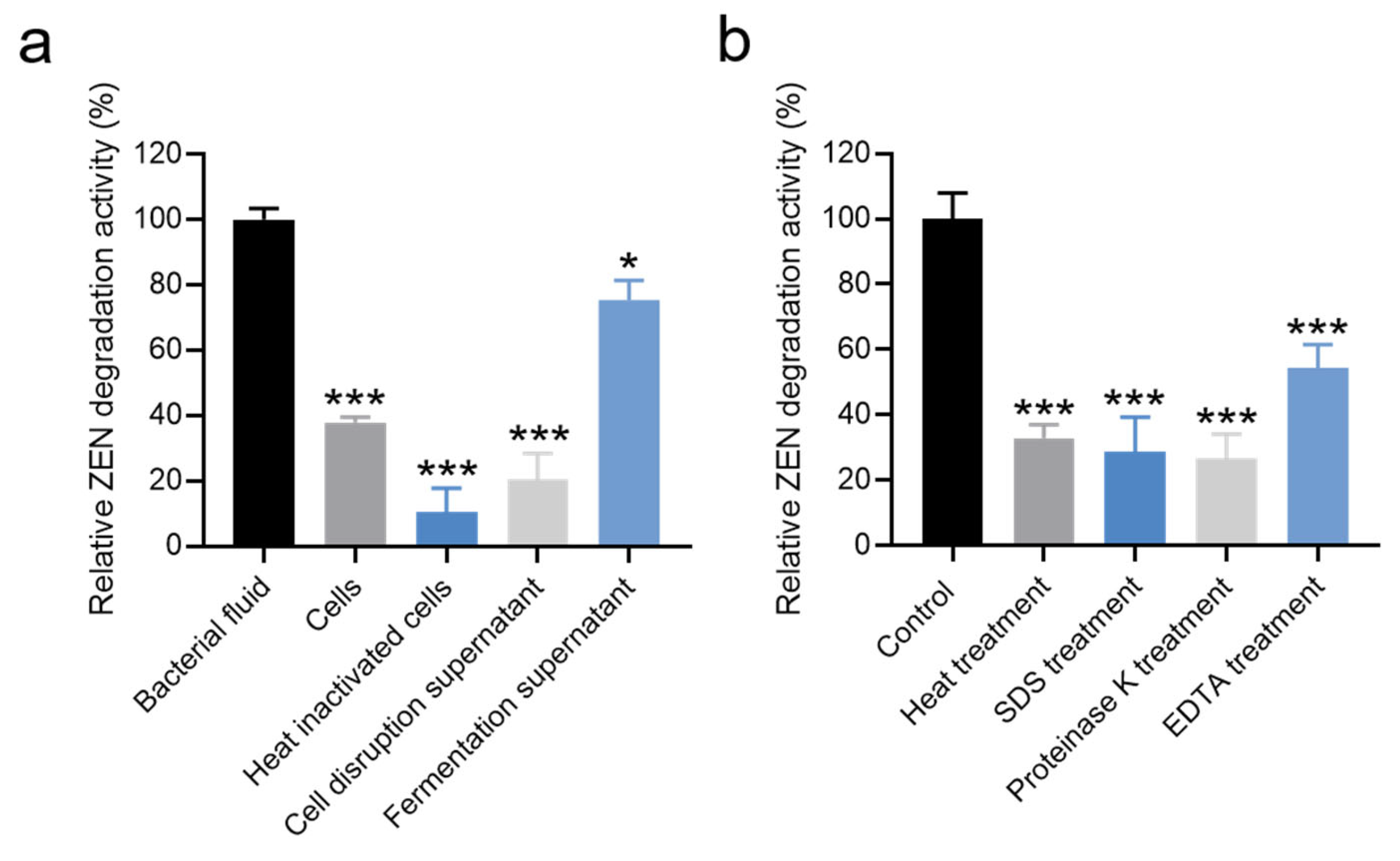
3.4. Localization of ZEN-Degrading Active Substances in Strain X13
3.5. Analysis of ZEN Degradation Products by Strain X13
3.6. Detoxification of Moldy Corn by Strain X13
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Ropejko, K.; Twarużek, M. Zearalenone and its metabolites-general overview, occurrence, and toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, M.O.; Şahin, S.; Üstündağ, Z. Detection strategies of zearalenone for food safety: A review. Crit. Rev. Anal. Chem. 2022, 52, 294–313. [Google Scholar] [CrossRef] [PubMed]
- Daković, A.; Matijasević, S.; Rottinghaus, G.E.; Dondur, V.; Pietrass, T.; Clewett, C.F. Adsorption of zearalenone by organomodified natural zeolitic tuff. J. Colloid Interface Sci. 2007, 311, 8–13. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Ji, J.; Wu, H.; Pi, F.; Zhang, Y.; Sun, X. Chemical and toxicological alterations of zearalenone under ozone treatment. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2019, 36, 163–174. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Ouyang, B.B.; Zhang, W.L.; Guang, C.E.; Xu, W.; Mu, W.M. An overview of chemical, physical and biological methods for zearalenone elimination: Recent advances and future prospective. Food Control 2023, 154, 110011. [Google Scholar] [CrossRef]
- Kriszt, R.; Krifaton, C.; Szoboszlay, S.; Cserháti, M.; Kriszt, B.; Kukolya, J.; Czéh, A.; Fehér-Tóth, S.; Török, L.; Szőke, Z.; et al. A new zearalenone biodegradation strategy using non-pathogenic Rhodococcus pyridinivorans K408 strain. PLoS ONE 2012, 7, e43608. [Google Scholar] [CrossRef]
- Kosawang, C.; Karlsson, M.; Vélëz, H.; Rasmussen, P.H.; Collinge, D.B.; Jensen, B.; Jensen, D.F. Zearalenone detoxification by zearalenone hydrolase is important for the antagonistic ability of Clonostachys rosea against mycotoxigenic Fusarium graminearum. Fungal Biol. 2014, 118, 364–373. [Google Scholar] [CrossRef]
- Batty-Smith, C.G. The detection of acetyl-methyl-carbinol in bacterial cultures: A comparative study of the methods of O’Meara and of Barritt. J. Hyg. 1941, 41, 521–529. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2002, 2, 2.3. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.-Z.; Yin, L.-J.; Ding, Z.-W.; Wang, Y.-J.; Jia, A.-Q. YtnP: One novel quorum quenching enzyme from Bacillus amyloliquefaciens W11 inhibits biofilms and spoilage of white radish by Serratia marcescens. LWT 2024, 198, 116058. [Google Scholar] [CrossRef]
- Hu, J.; Wang, G.; Hou, M.; Du, S.; Han, J.; Yu, Y.; Gao, H.; He, D.; Shi, J.; Lee, Y.-W.; et al. New hydrolase from Aeromicrobium sp. HA for the biodegradation of Zearalenone: Identification, mechanism, and application. J. Agric. Food Chem. 2023, 71, 2411–2420. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, F.; Cao, H.; Cheng, J.; Jiang, M.; Li, M.; Ni, H.; Xie, L. Comparison of microbial diversity of two typical volcanic soils in Wudalianchi, China. Microorganisms 2024, 12, 656. [Google Scholar] [CrossRef]
- Guerrero, R. Bergey’s manuals and the classification of prokaryotes. Int. Microbiol. 2001, 4, 103–109. [Google Scholar] [CrossRef]
- Kilonzi, J.M.; Otieno, S. Degradation kinetics and physiological studies of organophosphates degrading microorganisms for soil bioremediation. Stress Biol. 2024, 4, 11. [Google Scholar] [CrossRef]
- Simon, A.; Colom, J.; Mazhar, S.; Khokhlova, E.; Deaton, J.; Rea, K. Bacillus megaterium Renuspore® as a potential probiotic for gut health and detoxification of unwanted dietary contaminants. Front. Microbiol. 2023, 14, 1125616. [Google Scholar] [CrossRef]
- Xu, L.; Sun, X.; Wan, X.; Li, H.; Yan, F.; Han, R.; Li, H.; Li, Z.; Tian, Y.; Liu, X.; et al. Identification of a Bacillus amyloliquefaciens H6 thioesterase involved in Zearalenone detoxification by transcriptomic analysis. J. Agric. Food Chem. 2020, 68, 10071–10080. [Google Scholar] [CrossRef]
- Cho, K.J.; Kang, J.S.; Cho, W.T.; Lee, C.H.; Ha, J.K.; Song, K.B. In vitro degradation of zearalenone by Bacillus subtilis. Biotechnol. Lett. 2010, 32, 1921–1924. [Google Scholar] [CrossRef]
- Deng, T.; Chen, Y.; Zhang, J.; Gao, Y.; Yang, C.; Jiang, W.; Ou, X.; Wang, Y.; Guo, L.; Zhou, T.; et al. A probiotic Bacillus amyloliquefaciens D-1 strain is responsible for Zearalenone detoxifying in Coix Semen. Toxins 2023, 15, 674. [Google Scholar] [CrossRef]
- Ding, M.Z.; Tian, H.C.; Cheng, J.S.; Yuan, Y.J. Inoculum size-dependent interactive regulation of metabolism and stress response of Saccharomyces cerevisiae revealed by comparative metabolomics. J. Biotechnol. 2009, 144, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Caipo, M.L.; Duffy, S.; Zhao, L.; Schaffner, D.W. Bacillus megaterium spore germination is influenced by inoculum size. J. Appl. Microbiol. 2002, 92, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Hornbaek, T.; Jakobsen, M.; Dynesen, J.; Nielsen, A.K. Global transcription profiles and intracellular pH regulation measured in Bacillus licheniformis upon external pH upshifts. Arch. Microbiol. 2004, 182, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Buyel, J.F.; Twyman, R.M.; Fischer, R. Extraction and downstream processing of plant-derived recombinant proteins. Biotechnol. Adv. 2015, 33, 902–913. [Google Scholar] [CrossRef]
- Hsu, T.C.; Yi, P.J.; Lee, T.Y.; Liu, J.R. Probiotic characteristics and zearalenone-removal ability of a Bacillus licheniformis strain. PLoS ONE 2018, 13, e0194866. [Google Scholar] [CrossRef]
- Dey, A.; Bokka, V.; Sen, S. Dependence of bacterial growth rate on dynamic temperature changes. IET Syst. Biol. 2020, 14, 68–74. [Google Scholar] [CrossRef]
- Zhai, C.; Yu, Y.; Han, J.; Hu, J.; He, D.; Zhang, H.; Shi, J.; Mohamed, S.R.; Dawood, D.H.; Wang, G.; et al. Isolation, characterization, and application of Clostridium sporogenes F39 to degrade zearalenone under anaerobic conditions. Foods 2022, 11, 1194. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Zhang, D.; Zhao, M.; Peng, M.; Guo, P.; Cui, Z. Microbial degradation of zearalenone by a novel microbial consortium, NZDC-6, and its application on contaminated corncob by semisolid fermentation. J. Agric. Food Chem. 2020, 68, 1634–1644. [Google Scholar] [CrossRef]
- Hairong, G.; Chenxi, Z.; Yueju, Z.; Yang, L. Study on heat resistant Bacillus subtilis with high degradation efficiency of Zearalenone. J. Nucl. Agric. Sci. 2019, 33, 1399–1407. [Google Scholar] [CrossRef]
- Tinyiro, S.E.; Wokadala, C.; Xu, D.; Yao, W. Adsorption and degradation of zearalenone by bacillus strains. Folia Microbiol. 2011, 56, 321–327. [Google Scholar] [CrossRef]
- Liu, X.; Wu, N.; Zhang, M.; Xue, F.; Xu, Q. Isolation and characterization of the zearalenone-degrading strain, Bacillus spizizenii B73, inspired by esterase activity. Toxins 2023, 15, 488. [Google Scholar] [CrossRef] [PubMed]
- Seelig, J.; Seelig, A. Protein stability—Analysis of heat and cold denaturation without and with unfolding models. J. Phys. Chem. B 2023, 127, 3352–3363. [Google Scholar] [CrossRef] [PubMed]
- Winogradoff, D.; John, S.; Aksimentiev, A. Protein unfolding by SDS: The microscopic mechanisms and the properties of the SDS-protein assembly. Nanoscale 2020, 12, 5422–5434. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, S.; Sigdel, G.; Shah, S.K.; Sharma, S.K.; Grubb, J.D.; Micic, M.; Caseli, L.; Leblanc, R.M. Interfacial behavior of Proteinase K enzyme at air-saline subphase. J. Colloid Interface Sci. 2022, 616, 701–708. [Google Scholar] [CrossRef]
- Bhattacharyya, D.K.; Adak, S.; Bandyopadhyay, U.; Banerjee, R.K. Mechanism of inhibition of horseradish peroxidase-catalysed iodide oxidation by EDTA. Biochem. J. 1994, 298 Pt 2, 281–288. [Google Scholar] [CrossRef]
- Yang, W.-C.; Hsu, T.-C.; Cheng, K.-C.; Liu, J.-R. Expression of the Clonostachys rosea lactonohydrolase gene by Lactobacillus reuteri to increase its zearalenone-removing ability. Microb. Cell Factories 2017, 16, 69. [Google Scholar] [CrossRef]
- Cai, P.; Liu, S.; Tu, Y.; Shan, T. Toxicity, biodegradation, and nutritional intervention mechanism of zearalenone. Sci. Total Environ. 2024, 911, 168648. [Google Scholar] [CrossRef]
- Sun, X.; He, X.; Xue, K.s.; Li, Y.; Xu, D.; Qian, H. Biological detoxification of zearalenone by Aspergillus niger strain FS10. Food Chem. Toxicol. 2014, 72, 76–82. [Google Scholar] [CrossRef]
- COMMISSION RECOMMENDATION (EU) 2016/1319 of 29 July 2016 Amending Recommendation 2006/576/EC as Regards Deoxynivalenol, Zearalenone and Ochratoxin A in Pet Food. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016H1319 (accessed on 26 October 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, D.; Xu, K.; Liu, J.; Liao, X. Biodegradation of Zearalenone by a Novel Bacillus Strain X13 Isolated from Volcanic Rock Soil Using the Mycotoxin as the Sole Carbon Source. Microorganisms 2025, 13, 1954. https://doi.org/10.3390/microorganisms13081954
Meng D, Xu K, Liu J, Liao X. Biodegradation of Zearalenone by a Novel Bacillus Strain X13 Isolated from Volcanic Rock Soil Using the Mycotoxin as the Sole Carbon Source. Microorganisms. 2025; 13(8):1954. https://doi.org/10.3390/microorganisms13081954
Chicago/Turabian StyleMeng, Di, Kaizhong Xu, Jinbin Liu, and Xiangru Liao. 2025. "Biodegradation of Zearalenone by a Novel Bacillus Strain X13 Isolated from Volcanic Rock Soil Using the Mycotoxin as the Sole Carbon Source" Microorganisms 13, no. 8: 1954. https://doi.org/10.3390/microorganisms13081954
APA StyleMeng, D., Xu, K., Liu, J., & Liao, X. (2025). Biodegradation of Zearalenone by a Novel Bacillus Strain X13 Isolated from Volcanic Rock Soil Using the Mycotoxin as the Sole Carbon Source. Microorganisms, 13(8), 1954. https://doi.org/10.3390/microorganisms13081954






