Insights into the Genomic Architecture and Improvement of the Capabilities of Acinetobacter calcoaceticus for the Biodegradation of Petroleum Hydrocarbons
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Medium
2.2. Whole-Genome Sequencing Analysis of A. calcoaceticus 21#
2.3. Degradation Efficiency Assay
2.4. Construction of Expression Vectors
2.5. Expression of av-almA Gene in E. coli BL21(DE3) and Purification of Recombinant Protein av-almA
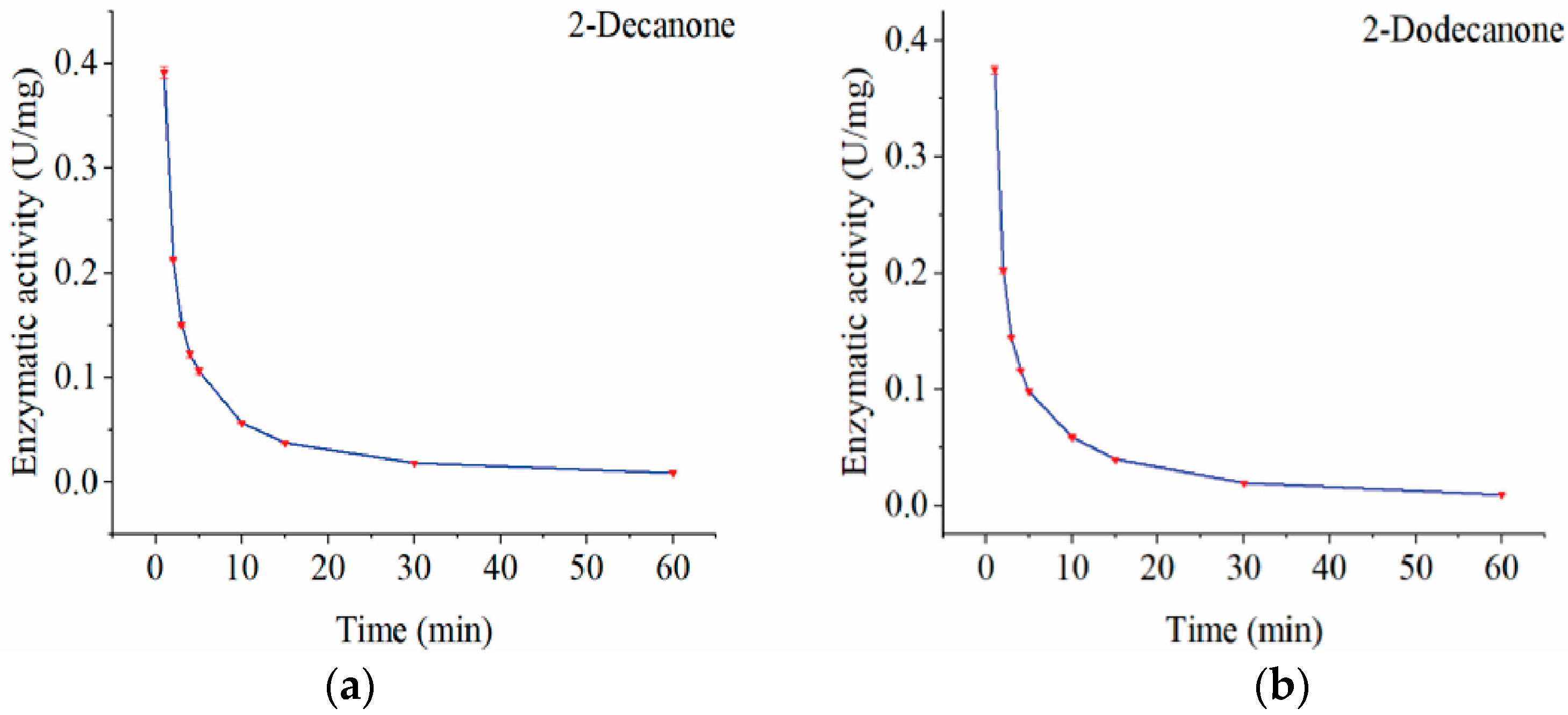
2.6. Activity Assay of av-almA Protein
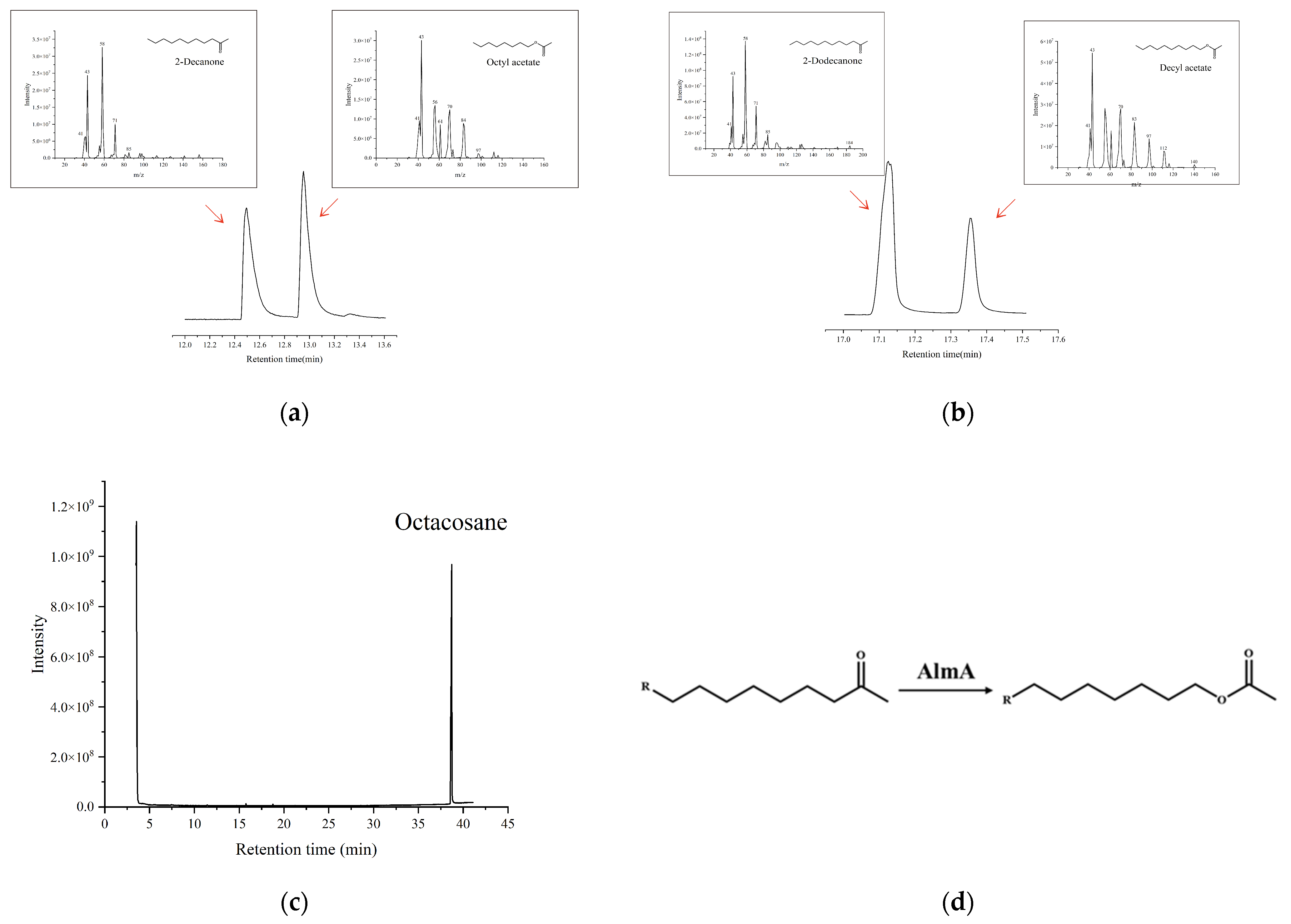
2.7. Metabolite Profiling of Recombinant AlmA Protein
3. Results and Discussion
3.1. Genomic Insights into A. calcoaceticus 21#
3.1.1. Alkane Degradation-Related Genes
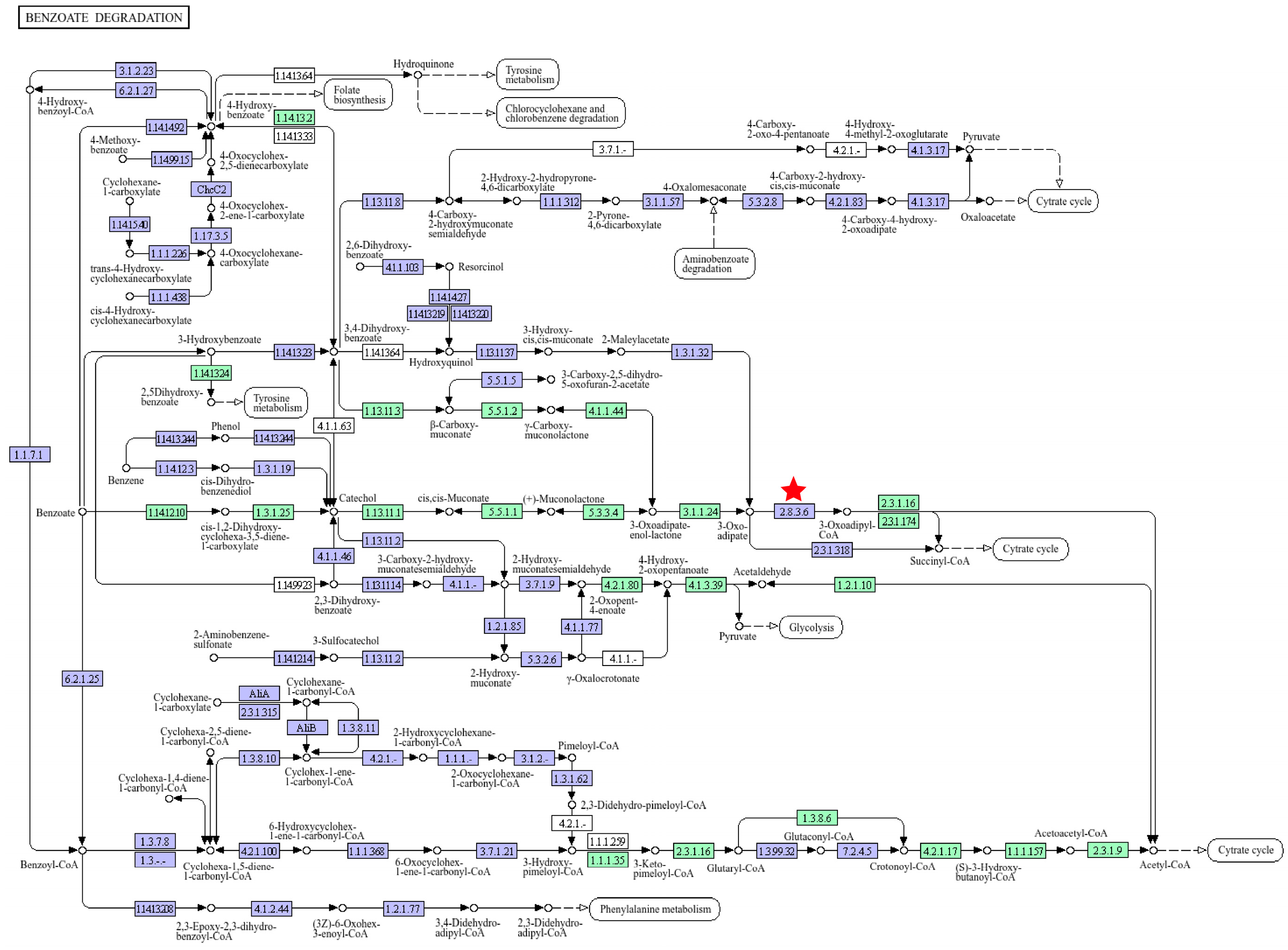
3.1.2. Genes Associated with Aromatic Hydrocarbon Degradation
3.2. Hydrocarbon Degradation Capacities
3.3. Construction of Prokaryotic Expression Vectors pET-28a(+)-av-almA-BH
3.4. Functional Identification of the Recombinant AlmA Proteins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falih, K.T.; Mohd Razali, S.F.; Abdul Maulud, K.N.; Abd Rahman, N.; Abba, S.I.; Yaseen, Z.M. Assessment of Petroleum Contamination in Soil, Water, and Atmosphere: A Comprehensive Review. Int. J. Environ. Sci. Technol. 2024, 21, 8803–8832. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Chebbi, A.; Formicola, F.; Prasad, S.; Gomez, F.H.; Franzetti, A.; Vaccari, M. Remediation of Soil Polluted with Petroleum Hydrocarbons and Its Reuse for Agriculture: Recent Progress, Challenges, and Perspectives. Chemosphere 2022, 293, 133572. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Saeed, T.; Jupp, B.; Faizuddin, M. Petroleum Hydrocarbon Pollution in Sediments from the Gulf and Omani Waters: Status and Review. Mar. Pollut. Bull. 2021, 173, 112913. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Geng, P.; Zeng, Y.; Hu, F.; Sun, P.; Zhuang, G.; Ma, A. Advancing Biodegradation of Petroleum Contaminants by Indigenous Microbial Consortia through Assembly Strategy Innovations. Chem. Eng. J. 2023, 475, 146142. [Google Scholar] [CrossRef]
- Pritchard, P.H.; Mueller, J.G.; Rogers, J.C.; Kremer, F.V.; Glaser, J.A. Oil Spill Bioremediation: Experiences, Lessons and Results from the Exxon Valdez Oil Spill in Alaska. Biodegradation 1992, 3, 315–335. [Google Scholar] [CrossRef]
- Alaidaroos, B.A. Advancing Eco-Sustainable Bioremediation for Hydrocarbon Contaminants: Challenges and Solutions. Processes 2023, 11, 3036. [Google Scholar] [CrossRef]
- Varjani, S.; Upasani, V.N.; Pandey, A. Bioremediation of Oily Sludge Polluted Soil Employing a Novel Strain of Pseudomonas aeruginosa and Phytotoxicity of Petroleum Hydrocarbons for Seed Germination. Sci. Total Environ. 2020, 737, 139766. [Google Scholar] [CrossRef]
- Chettri, B.; Singha, N.A.; Singh, A.K. Efficiency and Kinetics of Assam Crude Oil Degradation by Pseudomonas aeruginosa AKS1 and Bacillus sp. Arch. Microbiol. 2021, 203, 5793–5803. [Google Scholar]
- Siddiqui, Z.; Grohmann, E.; Malik, A. Degradation of Alkane Hydrocarbons by Priestia Megaterium ZS16 and Sediments Consortia with Special Reference to Toxicity and Oxidative Stress Induced by the Sediments in the Vicinity of an Oil Refinery. Chemosphere 2023, 317, 137886. [Google Scholar] [CrossRef]
- Hu, X.; Qiao, Y.; Chen, L.-Q.; Du, J.-F.; Fu, Y.-Y.; Wu, S.; Huang, L. Enhancement of Solubilization and Biodegradation of Petroleum by Biosurfactant from Rhodococcus Erythropolis HX-2. Geomicrobiol. J. 2020, 37, 159–169. [Google Scholar] [CrossRef]
- Andreolli, M.; Villanova, V.; Zanzoni, S.; D’Onofrio, M.; Vallini, G.; Secchi, N.; Lampis, S. Characterization of Trehalolipid Biosurfactant Produced by the Novel Marine Strain Rhodococcus sp. SP1d and Its Potential for Environmental Applications. Microb. Cell Fact. 2023, 22, 126. [Google Scholar] [CrossRef]
- Luckarift, H.R.; Sizemore, S.R.; Farrington, K.E.; Fulmer, P.A.; Biffinger, J.C.; Nadeau, L.J.; Johnson, G.R. Biodegradation of Medium Chain Hydrocarbons by Acinetobacter Venetianus 2AW Immobilized to Hair-Based Adsorbent Mats. Biotechnol. Prog. 2011, 27, 1580–1587. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Shah, S.B.; Zanaroli, G.; Xu, P.; Tang, H. Phenol Biodegradation by Acinetobacter Radioresistens APH1 and Its Application in Soil Bioremediation. Appl. Microbiol. Biotechnol. 2020, 104, 427–437. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, F.; Wang, J.; Zhao, Q.; Zheng, L.; Wang, Z.; Zhang, X.; Gao, Y.; Chen, G.; Huang, Y. Complete Genome Sequence Analysis of a Novel Alkane-Degrading Bacterial Strain, Acinetobacter Vivianii KJ-1, and Its Diesel Degradation Ability. Front. Environ. Sci. 2022, 10, 1044754. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Coil, D.; Jospin, G.; Darling, A.E. A5-Miseq: An Updated Pipeline to Assemble Microbial Genomes from Illumina MiSeq Data. Bioinformatics 2014, 31, 587–589. [Google Scholar] [CrossRef]
- Besemer, J.; Lomsadze, A.; Borodovsky, M. GeneMarkS: A Self-Training Method for Prediction of Gene Starts in Microbial Genomes. Implications for Finding Sequence Motifs in Regulatory Regions. Nucleic Acids Res. 2001, 29, 2607–2618. [Google Scholar] [CrossRef]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An Automatic Genome Annotation and Pathway Reconstruction Server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef]
- HJ 478-2009; China. Ministry of Environmental Protection. Water Quality—Determination of Polycyclic Aromatic Hydrocarbons—Liquid-Liquid Extraction and Solid-Phase Extraction Followed by High Performance Liquid Chromatographic Method. China Environmental Science Press: Beijing, China, 2009.
- Soussan, L.; Pen, N.; Belleville, M.-P.; Marcano, J.S.; Paolucci-Jeanjean, D. Alkane Biohydroxylation: Interests, Constraints and Future Developments. J. Biotechnol. 2016, 222, 117–142. [Google Scholar] [CrossRef]
- Rojo, F. Degradation of Alkanes by Bacteria. Environ. Microbiol. 2009, 11, 2477–2490. [Google Scholar] [CrossRef]
- van Beilen, J.B.; Panke, S.; Lucchini, S.; Franchini, A.G.; Röthlisberger, M.; Witholt, B. Analysis of Pseudomonas Putida Alkane-Degradation Gene Clusters and Flanking Insertion Sequences: Evolution and Regulation of the Alk genes The EMBL Accession Numbers for the Sequences Reported in This Paper Are AJ245436 [P. Putida (Oleovorans) GPo1 Alk Gene Clusters and Flanking DNA], AJ233397 (P. Putida P1 Alk Gene Clusters and Flanking DNA), AJ249793 (P. Putida P1 nahKJ Genes), AJ249825 [P. Putida (Oleovorans) GPo1 16S RNA Gene] and AJ271219 (P. Putida P1 16S RNA Gene). Microbiology 2001, 147, 1621–1630. [Google Scholar] [CrossRef]
- Malik, P.K. Use of Activated Carbons Prepared from Sawdust and Rice-Husk for Adsorption of Acid Dyes: A Case Study of Acid Yellow 36. Dye. Pigment. 2003, 56, 239–249. [Google Scholar] [CrossRef]
- Throne-Holst, M.; Wentzel, A.; Ellingsen, T.E.; Kotlar, H.-K.; Zotchev, S.B. Identification of Novel Genes Involved in Long-Chain n-Alkane Degradation by Acinetobacter sp. Strain DSM 17874. Appl. Environ. Microbiol. 2007, 73, 3327–3332. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, Y.; Liao, J.; Liu, X.; Peng, J.; Wang, J.-H.; Shao, Z. Broad-Spectrum Hydrocarbon-Degrading Microbes in the Global Ocean Metagenomes. Sci. Total Environ. 2024, 926, 171746. [Google Scholar] [CrossRef]
- Yin, C.-F.; Nie, Y.; Li, T.; Zhou, N.-Y. AlmA Involved in the Long-Chain n-Alkane Degradation Pathway in Acinetobacter Baylyi ADP1 Is a Baeyer–Villiger Monooxygenase. Appl. Environ. Microbiol. 2024, 90, e01625-23. [Google Scholar] [CrossRef] [PubMed]
- Fraaije, M.W.; Kamerbeek, N.M.; van Berkel, W.J.H.; Janssen, D.B. Identification of a Baeyer–Villiger Monooxygenase Sequence Motif. FEBS Lett. 2002, 518, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Bunyat-zada, A.R.; Ducharme, S.E.; Cleveland, M.E.; Hoffman, E.R.; Howe, G.W. Genome Mining Leads to the Identification of a Stable and Promiscuous Baeyer-Villiger Monooxygenase from a Thermophilic Microorganism. ChemBioChem 2024, 25, e202400443. [Google Scholar] [CrossRef]
- Wang, W.; Shao, Z. The Long-Chain Alkane Metabolism Network of Alcanivorax Dieselolei. Nat. Commun. 2014, 5, 5755. [Google Scholar] [CrossRef]
- Lin, M.; Ning, X.; An, T.; Zhang, J.; Chen, C.; Ke, Y.; Wang, Y.; Zhang, Y.; Sun, J.; Liu, J. Degradation of Polycyclic Aromatic Hydrocarbons (PAHs) in Textile Dyeing Sludge with Ultrasound and Fenton Processes: Effect of System Parameters and Synergistic Effect Study. J. Hazard. Mater. 2016, 307, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Wirth, N.T.; Nikel, P.I. Combinatorial Pathway Balancing Provides Biosynthetic Access to 2-Fluoro-Cis, Cis-Muconate in Engineered Pseudomonas Putida. Chem Catal. 2021, 1, 1234–1259. [Google Scholar] [CrossRef]
- Veselý, M.; Knoppová, M.; Nešvera, J.; Pátek, M. Analysis of catRABC Operon for Catechol Degradation from Phenol-Degrading Rhodococcus Erythropolis. Appl. Microbiol. Biotechnol. 2007, 76, 159–168. [Google Scholar] [CrossRef]
- Bains, J.; Kaufman, L.; Farnell, B.; Boulanger, M.J. A Product Analog Bound Form of 3-Oxoadipate-Enol-Lactonase (PcaD) Reveals a Multifunctional Role for the Divergent Cap Domain. J. Mol. Biol. 2011, 406, 649–658. [Google Scholar] [CrossRef]
- Tan, Z.; Clomburg, J.M.; Cheong, S.; Qian, S.; Gonzalez, R. A Polyketoacyl-CoA Thiolase-Dependent Pathway for the Synthesis of Polyketide Backbones. Nat. Catal. 2020, 3, 593–603. [Google Scholar] [CrossRef]
- Ru, J.; Chen, H.; Chen, S.; Yan, Z.; Liu, Y.; Qin, C.; Zhang, T. Genomic and Transcriptional Analysis of Genes Involved in New Isolated Hexadecane and Naphthalene Utilization Acinetobacter calcoaceticus Aca13 Strain. Environ. Pollut. Bioavailab. 2024, 36, 2376008. [Google Scholar] [CrossRef]
- Rao, Q.; Lu, J.; Liu, S.; Chen, M.; Ma, Y. Biodegradation of C18 N-Alkane by Biosurfactant-Producing Pseudomonas aeruginosa TJM4 and Its Genomic Analysis. J. Environ. Chem. Eng. 2025, 13, 115590. [Google Scholar] [CrossRef]
- Van Den Heuvel, R.H.H.; Tahallah, N.; Kamerbeek, N.M.; Fraaije, M.W.; van Berkel, W.J.H.; Janssen, D.B.; Heck, A.J.R. Coenzyme Binding during Catalysis Is Beneficial for the Stability of 4-Hydroxyacetophenone Monooxygenase. J. Biol. Chem. 2005, 280, 32115–32121. [Google Scholar] [CrossRef]
- Sheng, D.; Ballou, D.P.; Massey, V. Mechanistic Studies of Cyclohexanone Monooxygenase: Chemical Properties of Intermediates Involved in Catalysis. Biochemistry 2001, 40, 11156–11167. [Google Scholar] [CrossRef] [PubMed]
- Minerdi, D.; Zgrablic, I.; Sadeghi, S.J.; Gilardi, G. Identification of a Novel Baeyer-Villiger Monooxygenase from Acinetobacter Radioresistens: Close Relationship to the Mycobacterium Tuberculosis Prodrug Activator EtaA. Microb. Biotechnol. 2012, 5, 700–716. [Google Scholar] [CrossRef] [PubMed]





| Strains or Plasmids | Characteristics | Source |
|---|---|---|
| A. calcoaceticus 21# | Wild-type, with petroleum degradation capability (CGMCC NO.31080) (GenBank accession: CP196955) | Lab store |
| A. calcoaceticus 21#-A3 | Genetically engineered bacterium, with the almA gene from KJ-1 | Lab store |
| A. vivianii KJ-1 | Wild-type, with diesel degradation capability (GenBank accession: CP085083) | Lab store |
| E. coli DH5α | Suitable for efficient gene cloning, ensure stable inheritance of high copy plasmids | Vazyme-China |
| pLB-vector | Ampr, containing lethal gene at MCS | TianGen-China |
| pLB-av-almA-BH | Ampr, pLB vector containing gene-av-almA-BH | This study |
| E. coli DH5α-av-almA-BH | Ampr, containing vector pLB-av-almA-BH | This study |
| pET-28a(+) | Kanr, containing T7 promoter and 6×His Tag | Lab store |
| pET-28a(+)-av-almA-BH | Kanr, pET-28a(+) vector containing gene-av-almA-BH | This study |
| E. coli BL21(DE3)-av-almA-BH | Kanr, containing vector pET-28a(+)-av-almA-BH | This study |
| KEGG ID | Gene Name | Annotation |
|---|---|---|
| K00496 | alkB1_1 | Alkane 1-monooxygenase |
| K00496 | alkB1_2 | Alkane 1-monooxygenase |
| — | rubA | Rubredoxin |
| — | rubB | Rubredoxin reductase RubB |
| K13980 | ADH4 | Alcohol dehydrogenase |
| K13954 | yiaY | Alcohol dehydrogenase |
| K00121 | frmA | S-(hydroxymethyl) glutathione dehydrogenase/alcohol dehydrogenase |
| K00138 | ald1 | Long-chain-aldehyde dehydrogenase |
| K00140 | ALDH6A1 | Methylmalonate-semialdehyde dehydrogenase |
| K01897 | fadD | Long-chain-fatty-acid-CoA ligase |
| K00249 | AFT10-1 | Acyl-CoA dehydrogenase |
| K00255 | ACADL | Acyl-Coa dehydrogenase |
| K06445 | fade | Acyl-coenzyme A dehydrogenase |
| K01825 | fadB | Fatty acid oxidation complex subunit alpha |
| KEGG ID | Gene Name | Annotation |
|---|---|---|
| K03381 | catA | Catechol 1,2-dioxygenase |
| K03464 | catC | Muconolactone D-isomerase |
| K01856 | catB | Muconate cycloisomerase |
| K13954 | yiaY | Alcohol dehydrogenase |
| K05549 | benA | Benzoate/toluate 1,2-dioxygenase subunit alpha |
| K05550 | benB | Benzoate/toluate 1,2-dioxygenase subunit beta |
| K05784 | benC | Benzoate/toluate 1,2-dioxygenase reductase component |
| K05783 | benD | Dihydroxycyclohexadiene carboxylate dehydrogenase |
| K01055 | pcaD | 3-oxoadipate enol-lactonase |
| K07823 | pcaF | 3-oxoadipyl-CoA thiolase |
| K00449 | pcaH | Protocatechuate 3,4-dioxygenase, beta subunit |
| K0048 | pcaG | Protocatechuate 3,4-dioxygenase, alpha subunit |
| K00121 | frmA | S-(hydroxymethyl)glutathione dehydrogenase/alcohol dehydrogenase |
| - | nagAb | Naphthalene 1,2-dioxygenase/salicylate 5-hydroxylase systems |
| K05710 | ndoA | Naphthalene 1,2-dioxygenase system, ferredoxin component |
| - | tcpC | 6-chlorohydroxyquinol 1,2-dioxygenase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Wang, M.; Chang, X.; Wang, L.; Fu, X.; Huang, Y.; Song, F.; Ji, L.; Wang, J. Insights into the Genomic Architecture and Improvement of the Capabilities of Acinetobacter calcoaceticus for the Biodegradation of Petroleum Hydrocarbons. Microorganisms 2025, 13, 1953. https://doi.org/10.3390/microorganisms13081953
Zeng Y, Wang M, Chang X, Wang L, Fu X, Huang Y, Song F, Ji L, Wang J. Insights into the Genomic Architecture and Improvement of the Capabilities of Acinetobacter calcoaceticus for the Biodegradation of Petroleum Hydrocarbons. Microorganisms. 2025; 13(8):1953. https://doi.org/10.3390/microorganisms13081953
Chicago/Turabian StyleZeng, Yaning, Mutian Wang, Xiaoyu Chang, Leilei Wang, Xiaowen Fu, Yujie Huang, Fanyong Song, Lei Ji, and Jianing Wang. 2025. "Insights into the Genomic Architecture and Improvement of the Capabilities of Acinetobacter calcoaceticus for the Biodegradation of Petroleum Hydrocarbons" Microorganisms 13, no. 8: 1953. https://doi.org/10.3390/microorganisms13081953
APA StyleZeng, Y., Wang, M., Chang, X., Wang, L., Fu, X., Huang, Y., Song, F., Ji, L., & Wang, J. (2025). Insights into the Genomic Architecture and Improvement of the Capabilities of Acinetobacter calcoaceticus for the Biodegradation of Petroleum Hydrocarbons. Microorganisms, 13(8), 1953. https://doi.org/10.3390/microorganisms13081953






