Enhancement of Mycelial Growth and Antifungal Activity by Combining Fermentation Optimization and Genetic Engineering in Streptomyces pratensis S10
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, and Culture Conditions
2.2. Preparation of Seed Fermentation Broth
2.3. Single-Factor Experiment
2.4. Response Surface Optimization Experiment
2.4.1. Screening of Medium Components of S10 by Plackett–Burman Design Assay
2.4.2. Steepest Climbing Test
2.4.3. Box–Behnken Design (BBD) Experiment
2.5. Evaluation of Biocontrol Efficacy
2.6. Construction of tetR Deletion Mutant and Complementation
2.7. Statistical Analysis
3. Results
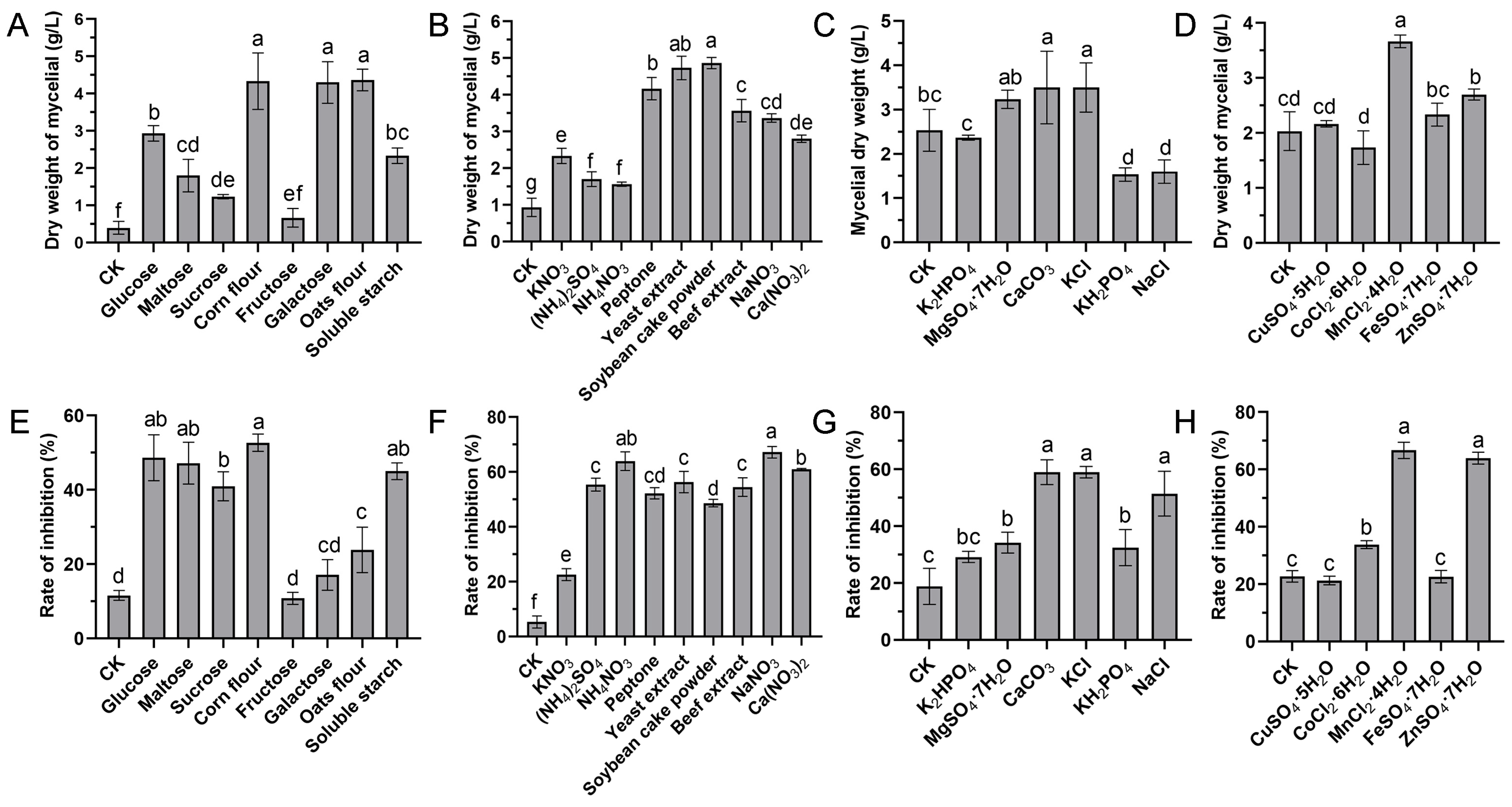
3.1. Screening of Different Medium Components
3.1.1. Screening of Carbon Sources and Concentrations
3.1.2. Screening of Nitrogen Sources and Concentrations
3.1.3. Screening of Mineral Salt Composition and Concentration
3.1.4. Trace Elements
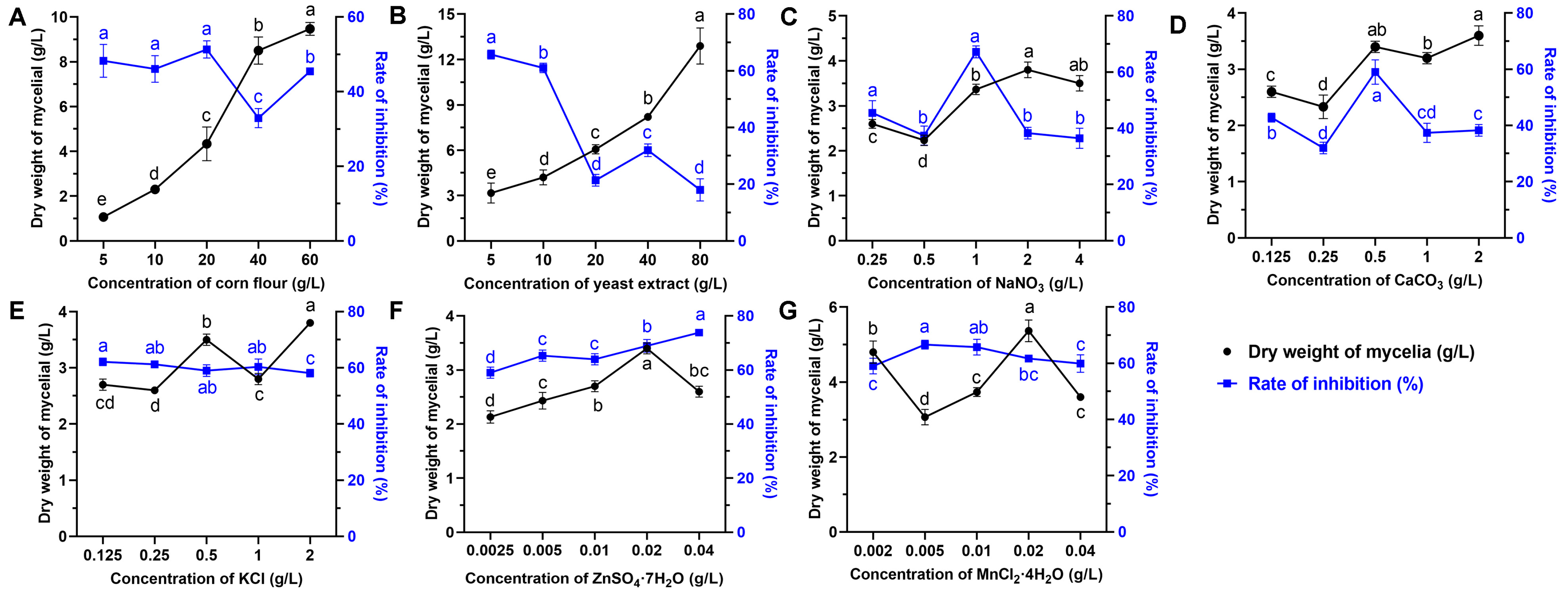
3.2. Response Surface Optimization
3.2.1. Identification of Main Variables via Plackett–Burman Design (PBD)
3.2.2. Determination of Central Point Levels by Steepest Climbing Test
3.2.3. Box–Behnken Design (BBD)
3.3. Assessment of the Model
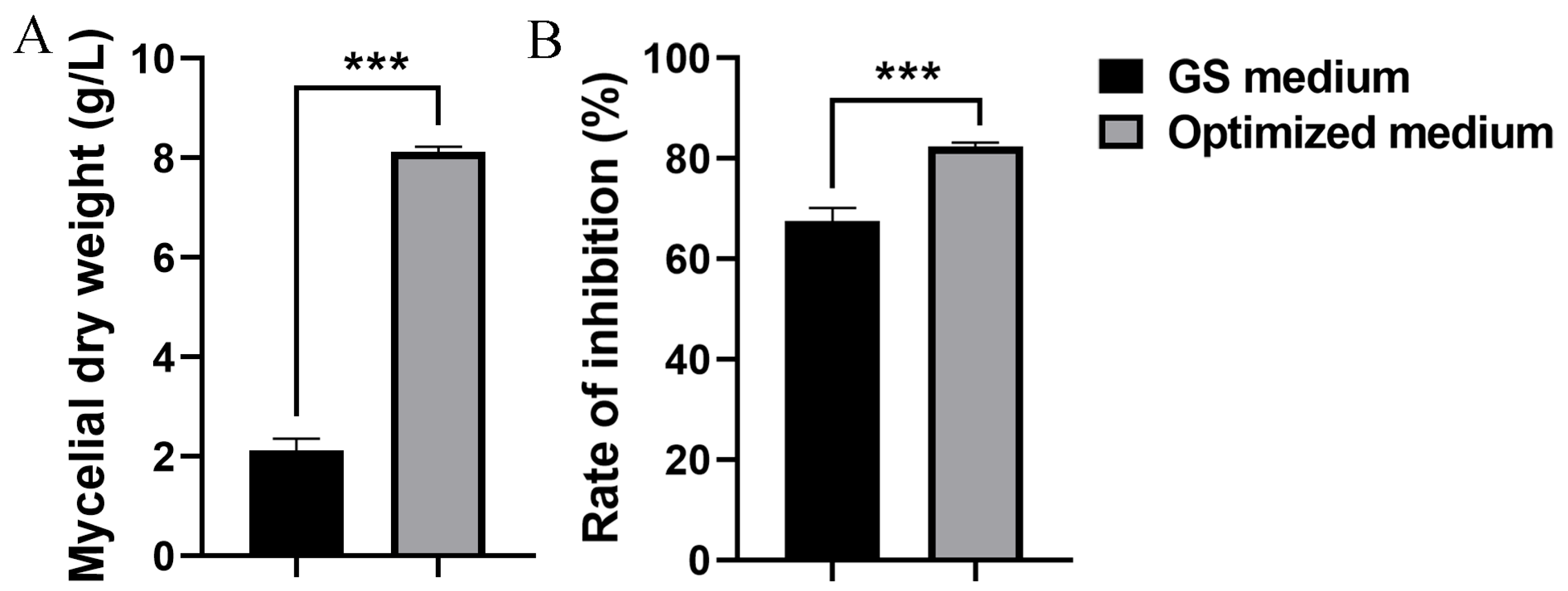
3.4. Optimization of S10 Enhanced Biocontrol Efficacy Against Fusarium Head Blight
3.5. Inactivation of tetR Enhanced Mycelial Growth and Antifungal Activity of S10
3.6. Optimized Medium Increased Antifungal Activity of ΔtetR Against F. graminearum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ristaino, J.; Anderson, P.; Bebber, D.; Brauman, K.; Cunniffe, N.; Fedoroff, N.; Finegold, C.; Garrett, K.; Gilligan, C.; Jones, C.M.; et al. The persistent threat of emerging plant disease pandemics to global food security. Proc. Natl. Acad. Sci. USA 2021, 118, e2022239118. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Grote, U.; Faße, A.; Nguyen, T.T.; Erenstein, O. Food security and the dynamics of wheat and maize value Chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases-a field perspective. Mol. Plant Path. 2017, 19, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Adamik, L.; Dou, P.S.; Philippe, G.; Blanc, R.; Vásquez-Ocmín, P.; Marti, G.; Langin, T.; Bonhomme, L. Suboptimal pre-anthesis water status mitigates wheat susceptibility to fusarium head blight and triggers specific metabolic responses. Sci. Rep. 2025, 15, 11773. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, L.; Li, D.; Wu, Y.; Bai, J.; Zhong, K.; Gao, H. Citric acid impairs type B trichothecene biosynthesis of Fusarium graminearum but enhances its growth and pigment biosynthesis: Transcriptomic and proteomic analyses. Appl. Environ. Microb. 2025, 57, 15–39. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yang, N.; Wen, Z.; Sun, X.; Chai, Y.; Ma, Z.H. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 2018, 9, 3429. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Xu, S.; Zhang, B.; Cernava, T.; Ma, Z.H.; Chen, Y. Enhancement of herbicolin A production by integrated fermentation optimization and strain engineering in Pantoea agglomerans ZJU23. Microb. Cell Fact. 2023, 22, 50. [Google Scholar] [CrossRef]
- Audenaert, K.; Callewaert, E.; Höfte, M.; Saeger, S.; Haesaert, G. Hydrogen peroxide induced by the fungicide prothioconazole triggers deoxynivalenol (DON) production by Fusarium graminearum. BMC Microbiol. 2010, 10, 112. [Google Scholar] [CrossRef]
- Amulothu, D.V.R.T.; Raghuteja, P. Advances in biological control of plant diseases. Glob. Agri. Vis. 2024, 4, 39–52. [Google Scholar]
- Zhang, J.; Chen, J.; Hu, L.F.; Jia, R.M.; Ma, Q.; Tang, J.J.; Wang, Y. Antagonistic action of Streptomyces pratensis S10 on Fusarium graminearum and its complete genome sequence. Environ. Microbiol. 2021, 23, 1925–1940. [Google Scholar] [CrossRef]
- Xie, S.; Jiang, L.; Wu, Q.; Wan, W.; Gan, Y.; Zhao, L.; Wen, J. Maize root exudates recruit Bacillus amyloliquefaciens OR2-30 to inhibit Fusarium graminearum infection. Phytopathology 2022, 112, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.M.; Kunova, A.; Pizzatti, C.; Saracchi, M.; Cortesi, P.; Pasquali, M. Selection of an endophytic Streptomyces sp. strain DEF09 from wheat roots as a biocontrol agent against Fusarium graminearum. Front. Microbiol. 2019, 10, 2356. [Google Scholar] [CrossRef] [PubMed]
- Xue, A.; Chen, Y.; Voldeng, H.; Savard, M.; Tian, X. Biological control of Fusarium head blight of wheat with Clonostachys rosea strain ACM941. Cereal Res. Commun. 2008, 36, 695–699. [Google Scholar] [CrossRef]
- Liu, H. Interactions with native microbial keystone taxa enhance the biocontrol efficiency of Streptomyces. Microbiome 2025, 13, 126. [Google Scholar] [CrossRef]
- Chen, J.; Lan, X.J.; Jia, R.M.; Hu, L.F.; Wang, Y. Response surface methodology (RSM) mediated optimization of medium components for mycelial growth and metabolites production of Streptomyces alfalfae XN-04. Microorganisms 2022, 10, 1854. [Google Scholar] [CrossRef]
- Han, C.; Yu, Z.; Zhang, Y.; Wang, Z.; Zhao, J.; Huang, S.X.; Ma, Z.; Wen, Z.; Liu, C.; Xiang, W. Discovery of frenolicin B as potential agrochemical fungicide for controlling Fusarium head blight on wheat. J. Agric. Food Chem. 2021, 69, 2108–2117. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Yang, S.H.; Zhang, L.; Suh, J.W. Effects of actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biotechnol. 2013, 97, 9621–9636. [Google Scholar] [CrossRef]
- Shi, Y.; Niu, X.; Yang, H.; Chu, M.; Wang, N.; Bao, H.; Zhan, F.; Yang, R.; Lou, K. Optimization of the fermentation media and growth conditions of Bacillus velezensis BHZ-29 using a Plackett–Burman design experiment combined with response surface methodology. Front. Microb. 2024, 15, 1355369. [Google Scholar] [CrossRef]
- Plackett, R.L.; Burman, J.P. The design of optimum multifactorial experiments. BioMetals 1946, 33, 255–276. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Xu, S.; Wang, B.; Ju, J.; Tan, H.; Li, W. Activation and enhancement of Fredericamycin A production in deepsea-derived Streptomyces somaliensis SCSIO ZH66 by using ribosome engineering and response surface methodology. Micro. Cell Fact. 2015, 14, 64. [Google Scholar] [CrossRef]
- Noman, E.A.; Al-Algheethi, A.A.; Talip, B.A.; Mohamed, R.; Kassim, A.H. Oxidative enzymes from newly local strain Aspergillus iizukae EAN605 using pumpkin peels as a production substrate; optimized production, characterization, application and techno-economic analysis. J. Hazard. Mater. 2020, 386, 121954. [Google Scholar] [CrossRef]
- Nguyen, H.P.T.; Morançais, M.; Fleurence, J.; Dumay, J. Mastocarpus stellatus as a source of R-phycoerythrin: Optimization of enzyme assisted extraction using response surface methodology. J. Appl. Phycol. 2017, 29, 1563–1570. [Google Scholar] [CrossRef]
- Kong, Y.; Zou, P.; Miao, L.; Qi, J.; Song, L.; Zhu, L.; Xu, X.X. Medium optimization for the production of anti-cyanobacterial substances by Streptomyces sp. HJC-D1 using response surface methodology. Environ. Sci. Pollut. Res. 2014, 21, 5983–5990. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, L.; Zeeshan, M.; Yang, C.; Gao, W.; Zhang, G.; Wang, C. Optimization of fermentation conditions to increase the production of antifungal metabolites from Streptomyces sp. KN37. Micro. Cell Fac. 2025, 24, 26. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, K.; Yu, Y.; Lian, X.; Jiang, L.; Meng, F.; Wang, Y.; Zhu, X.; Duan, Y. Influence of medium modifications (optimization) on high nematicidal activity of the fermentation broth of Clostridium beijerinckii. Front. Bioeng. Biotech. 2024, 11, 1283112. [Google Scholar] [CrossRef]
- Liu, B.; Wei, Q.; Yang, M.; Shi, L.; Zhang, K.C.; Ge, B.B. Effect of toyF on wuyiencin and toyocamycin production by Streptomyces albulus CK-15. World J. Microbiol. Biotechnol. 2022, 38, 65. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, Z.; Zhang, Y.; Yang, H.; Qiu, G.; Wang, D.; Lian, Y. Improvement of tacrolimus production in Streptomyces tsukubaensis by mutagenesis and optimization of fermentation medium using Plackett-Burman design combined with response surface methodology. Biotechnol. Lett. 2021, 43, 1765–1778. [Google Scholar] [CrossRef]
- Gibson, D.G.; Benders, G.A.; Axelrod, K.C.; Zaveri, J.; Algire, M.A.; Moodie, M.; Montague, M.G.; Venter, J.C.; Smith, H.O.; Hutchison, C.A. One-step assembly in yeast of 25 overlapping DNA fragments to form a complete synthetic Mycoplasma genitalium genome. Proc. Natl. Acad. Sci. USA 2008, 105, 20404–20409. [Google Scholar] [CrossRef]
- Lu, L.; Liu, N.; Fan, Z.; Liu, M.; Zhang, X.; Tian, J.; Yu, Y.; Lin, H.; Huang, Y.; Kong, Z. A novel PGPR strain, Streptomyces lasalocidi JCM 3373(T), alleviates salt stress and shapes root architecture in soybean by secreting indole-3-carboxaldehyde. Plant Cell Environ. 2024, 47, 1941–1956. [Google Scholar] [CrossRef]
- Yang, Z.; Qiao, Y.; Konakalla, N.C.; Strøbech, E.; Harris, P.; Peschel, G.; Agler-Rosenbaum, M.; Weber, T.; Andreasson, E.; Ding, L. Streptomyces alleviate abiotic stress in plant by producing pteridic acids. Nat. Commun. 2023, 14, 7398. [Google Scholar] [CrossRef]
- Yang, M.L.; Zhang, W.; Lv, Z.; Shi, L.; Zhang, K.C.; Ge, B.B. Induced defense response in soybean to Sclerotinia sclerotiorum using wuyiencin from Streptomyces albulus CK-15. Plant Dis. 2023, 107, 107–115. [Google Scholar] [CrossRef]
- Jia, R.M.; Xiao, K.Y.; Yu, L.G.; Chen, J.; Hu, L.F.; Wang, Y. A potential biocontrol agent Streptomyces tauricus XF for managing wheat stripe rust. Phytopathol. Res. 2023, 5, 14. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Ma, J.; Li, W.; Long, Y. Activity of Streptomyces globosus OPF-9 against the important pathogen Alternaria longipes and biocontrol mechanisms revealed by multi-omic analyses. Pestic. Biochem. Physiol. 2024, 204, 106094. [Google Scholar] [CrossRef]
- Xiao, L.; Niu, H.J.; Qu, T.L.; Zhang, X.F.; Du, F.Y. Streptomyces sp. FX13 inhibits fungicide-resistant Botrytis cinerea in vitro and in vivo by producing oligomycin A. Pestic. Biochem. Physiol. 2021, 175, 104834. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, L.F.; Chen, N.; Jia, R.M.; Ma, Q.; Wang, Y. The biocontrol and plant growth-promoting properties of Streptomyces alfalfae XN-04 revealed by functional and genomic analysis. Front. Microbiol. 2021, 12, 745766. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; John Martin, J.J.; Li, X.; Zhou, L.; Li, R.; Li, Q.; Zhang, J.; Fu, D.; Cao, H. Optimization of the fermentation culture conditions of Bacillus amyloliquefaciens ck-05 using response surface methodology. Front. Microbiol. 2025, 16, 1552645. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Villafán, B.; Cruz-Bautista, R.; Manzo-Ruiz, M.; Passari, A.K.; Villarreal-Gómez, K.; Rodríguez-Sanoja, R.; Sánchez, S. Carbon catabolite regulation of secondary metabolite formation, an old but not well-established regulatory system. Microb. Biotechnol. 2022, 15, 1058–1072. [Google Scholar] [CrossRef]
- Shakeel, Q.; Lyu, A.; Zhang, J.; Wu, M.D.; Chen, S.W.; Chen, W.D.; Li, G.Q.; Yang, L. Optimization of the cultural medium and conditions for production of antifungal substances by Streptomyces platensis 3–10 and evaluation of its efficacy in suppression of clubroot disease (Plasmodiophora brassicae) of oilseed rape. Biol. Control 2016, 101, 59–68. [Google Scholar] [CrossRef]
- Srivastava, A.; Singh, V.; Haque, S.; Pandey, S.; Mishra, M.; Jawed, A.; Shukla, P.K.; Singh, P.K.; Tripathi, C.K.M. Response surface methodology-genetic algorithm based medium optimization, purification, and characterization of cholesterol oxidase from Streptomyces rimosus. Sci. Rep. 2018, 8, 10913. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, Y.B.; He, H.W.; Feng, J.T.; Zhang, X.; Han, L.R. Optimization of medium compositions to improve a novel glycoprotein production by Streptomyces kanasenisi ZX01. AMB Express 2017, 7, 6. [Google Scholar] [CrossRef]
- Yu, Z.; Lv, H.; Wu, Y.; Wei, T.; Yang, S.; Ju, D.; Chen, S. Enhancement of FK520 production in Streptomyces hygroscopicus by combining traditional mutagenesis with metabolic engineering. Appl. Microbiol. Biotechnol. 2019, 103, 9593–9606. [Google Scholar] [CrossRef]
- Leal, K.; Machuca, J.; Gajardo, H.; Palma, M.; Contreras, M.J.; Nuñez-Montero, K.; Gutiérrez, Á.; Barrientos, L. Structural characterisation of TetR/AcrR regulators in Streptomyces fildesensis So13.3: An in silico CRISPR-based strategy to influence the suppression of actinomycin D production. Int. J. Mol. Sci. 2025, 26, 4839. [Google Scholar] [CrossRef] [PubMed]



| Run | Code Number | Dry Mycelial Weight (g/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | ||
| 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | 4.42 |
| 2 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 4.91 |
| 3 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | 4.25 |
| 4 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 4.54 |
| 5 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 4.31 |
| 6 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | 4.90 |
| 7 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 4.67 |
| 8 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 3.98 |
| 9 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 6.00 |
| 10 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 4.00 |
| 11 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | 4.27 |
| 12 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | 7.28 |
| Source | Sum of Squares | df | Coefficient Estimate | Mean Square | F Value | Prob > F |
|---|---|---|---|---|---|---|
| Model | 7.05 | 7 | 4.81 | 1.01 | 8.11 | 0.0305 * |
| Corn flour | 1.14 | 1 | 0.31 | 1.14 | 9.19 | 0.0387 * |
| Yeast extract | 1.40 | 1 | 0.34 | 1.40 | 11.28 | 0.0283 * |
| NaNO3 | 0.80 | 1 | −0.26 | 0.80 | 6.45 | 0.0640 |
| CaCO3 | 0.041 | 1 | −0.058 | 0.041 | 0.33 | 0.5970 |
| KCl | 0.24 | 1 | 0.14 | 0.24 | 1.94 | 0.2361 |
| ZnSO4·7H2O | 2.52 | 1 | 0.46 | 2.52 | 20.30 | 0.0108 * |
| MnCl2·4H2O | 0.91 | 1 | −0.27 | 0.91 | 7.31 | 0.0539 |
| R-Squared | 0.50 | 4 |
| Run | Corn Flour (g/L) | Yeast Extract (g/L) | ZnSO4·7H2O (g/L) | Dry Weight of Mycelium (g/L) |
|---|---|---|---|---|
| 1 | 25 | 6.25 | 0.025 | 6.42 |
| 2 | 26.5 | 7.5 | 0.0265 | 7.52 |
| 3 | 28 | 8.75 | 0.028 | 7.18 |
| 4 | 29.5 | 10.0 | 0.0295 | 13.2 |
| 5 | 31 | 11.25 | 0.031 | 8.25 |
| 6 | 32.5 | 12.5 | 0.0325 | 8.62 |
| 7 | 34 | 13.75 | 0.034 | 10.1 |
| Run | Code Number | Actual Value (g/L) | Predicted Value (g/L) | ||
|---|---|---|---|---|---|
| A | B | C | |||
| 1 | 0 | 0 | 0 | 3.03 | 3.19 |
| 2 | 1 | −1 | 0 | 6.13 | 6.38 |
| 3 | 1 | 0 | −1 | 4.34 | 4.25 |
| 4 | −1 | 0 | 0 | 2.48 | 2.84 |
| 5 | 0 | 0 | 0 | 3.13 | 3.19 |
| 6 | 0 | 1 | −1 | 4.75 | 6.08 |
| 7 | 1 | 0 | 1 | 7.98 | 8.01 |
| 8 | 0 | 0 | 0 | 3.93 | 3.19 |
| 9 | 0 | 1 | 1 | 7.42 | 9.63 |
| 10 | 0 | −1 | 1 | 5.91 | 5.67 |
| 11 | −1 | −1 | 0 | 2.05 | 2.15 |
| 12 | −1 | 0 | −1 | 2.39 | 2.27 |
| 13 | 0 | 0 | 0 | 3.00 | 3.19 |
| 14 | −1 | 1 | 0 | 6.5 | 6.16 |
| 15 | 0 | −1 | −1 | 2.24 | 2.11 |
| 16 | 0 | 0 | 0 | 3.06 | 3.19 |
| 17 | 1 | 1 | 0 | 7.51 | 7.31 |
| Source | Sum of Squares | df | Mean Square | F Value | Prob > F |
|---|---|---|---|---|---|
| Model | 65.50 | 9 | 7.28 | 41.16 | <0.0001 *** |
| A—Corn starch | 12.17 | 1 | 12.17 | 68.84 | <0.0001 *** |
| B—Yeast extract | 12.13 | 1 | 12.13 | 68.60 | <0.0001 *** |
| C—ZnSO4·7H2O | 13.08 | 1 | 13.08 | 73.99 | <0.0001 *** |
| AB | 2.36 | 1 | 2.36 | 13.33 | 0.0082 ** |
| AC | 0.27 | 1 | 0.27 | 1.54 | 0.2541 |
| BC | 0.25 | 1 | 0.25 | 1.41 | 0.2732 |
| A2 | 3.77 | 1 | 3.77 | 21.34 | 0.0024 ** |
| B2 | 6.61 | 1 | 6.61 | 37.39 | 0.0005 *** |
| C2 | 1.23 | 1 | 1.23 | 6.93 | 0.0338 * |
| Residual | 1.24 | 7 | 0.18 | – | – |
| Lack of Fit | 0.62 | 3 | 0.21 | 1.32 | 0.3845, not significant |
| Pure Error | 0.62 | 4 | 0.16 | – | – |
| Cor. Total | 66.73 | 16 | – | – | – |
| Std. Dev. | 0.45 | – | R-Squared | – | 0.9815 |
| Mean | 4.46 | – | Adj. R-Squared | – | 0.9576 |
| C.V.% | 9.42 | – | Pred. R-Squared | – | 0.7535 |
| PRESS | 16.45 | – | Adeq. Precision | – | 18.274 |
| Treatment | Disease Spike Rate (%) | Disease Index | Control Efficacy (%) |
|---|---|---|---|
| CK | 100.00 ± 00 a | 90.95 ± 6.40 a | — |
| Initial medium | 33.33 ± 4.26 b | 33.43 ± 2.81 b | 63.25 ± 3.09 a |
| Optimized medium | 20.25 ± 0.75 c | 23.01 ± 2.82 c | 74.70 ± 3.10 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Sun, Y.; Jia, R.; Dong, X.; Shen, X.; Wang, Y. Enhancement of Mycelial Growth and Antifungal Activity by Combining Fermentation Optimization and Genetic Engineering in Streptomyces pratensis S10. Microorganisms 2025, 13, 1943. https://doi.org/10.3390/microorganisms13081943
Hu L, Sun Y, Jia R, Dong X, Shen X, Wang Y. Enhancement of Mycelial Growth and Antifungal Activity by Combining Fermentation Optimization and Genetic Engineering in Streptomyces pratensis S10. Microorganisms. 2025; 13(8):1943. https://doi.org/10.3390/microorganisms13081943
Chicago/Turabian StyleHu, Lifang, Yan Sun, Ruimin Jia, Xiaomin Dong, Xihui Shen, and Yang Wang. 2025. "Enhancement of Mycelial Growth and Antifungal Activity by Combining Fermentation Optimization and Genetic Engineering in Streptomyces pratensis S10" Microorganisms 13, no. 8: 1943. https://doi.org/10.3390/microorganisms13081943
APA StyleHu, L., Sun, Y., Jia, R., Dong, X., Shen, X., & Wang, Y. (2025). Enhancement of Mycelial Growth and Antifungal Activity by Combining Fermentation Optimization and Genetic Engineering in Streptomyces pratensis S10. Microorganisms, 13(8), 1943. https://doi.org/10.3390/microorganisms13081943





