Abstract
Some bacterial species within the Enterobacteriaceae family possess different types of fimbrial (pili) adhesins that promote adherence to cells and colonization of host tissues. One of the well-characterized fimbrial systems is the pap operon, which encodes P fimbriae, a key virulence factor in urinary and systemic infections. One of the key regulators of P fimbriae is the transcriptional regulator PapB which plays a pivotal role as a master switch, not only by directing phase-variable expression of its own operon but also by influencing expression of heterologous fimbrial systems. This review explores the structural organization, biogenesis, and multi-tiered regulatory control of P fimbriae, with emphasis on PapB and homologous regulatory proteins such as SfaB, FocB, PixB, and PefB. Comparative genomics and phylogenetic analyses reveal that regulators belonging to the PapB family are evolutionarily conserved across π-fimbrial systems and also regulate other types of fimbriae. These regulators respond to epigenetic changes, host-derived signals, and global transcriptional cues to control levels of production of specific fimbriae in a bacterial population to dynamically modulate bacterial adhesion in different environmental niches. Optimally, understanding these mechanisms could lead to novel approaches to perturb PapB-family proteins and abrogate production of some types of fimbriae as a targeted strategy to prevent bacterial infections dependent on adherence mediated by PapB family regulators.
1. Introduction
Escherichia coli is a facultative anaerobic bacterium that naturally colonizes the gastrointestinal tract of mammals and birds as part of the commensal microbiota. However, a subset of strains has evolved to acquire virulence traits, giving rise to various pathogenic E. coli pathotypes, including extraintestinal pathogenic E. coli (ExPEC), enterotoxigenic E. coli (ETEC), and diffusely adherent E. coli (DAEC) [1,2,3]. These strains are responsible for a broad spectrum of diseases such as enteric infections, urinary tract infections (UTIs), neonatal meningitis, septicemia in humans, and colibacillosis in poultry—an economically significant condition marked by high morbidity and mortality [4,5]. Uropathogenic E. coli (UPEC), a major ExPEC pathotype, causes both lower and upper UTIs, while avian pathogenic E. coli (APEC) contributes to systemic and reproductive tract infections in birds, including peritonitis and salpingitis [2,6,7]. The virulence capability of such strains is governed by a combination of different key elements known as virulence factors [8].
Fimbrial adhesins can contribute to virulence by facilitating bacterial attachment to host cells, promoting colonization, and biofilm formation. Pathogenic E. coli often encode multiple types of fimbrial adhesins, allowing them to adapt to different host tissues, environmental conditions, and evade host immune responses, as the dynamic switching between adhesins can help circumvent immune detection and clearance [2,6,8,9]. Extensive research on Escherichia coli fimbrial adhesins has provided critical insights into their roles in host–pathogen interactions and bacterial virulence. Among these, type 1 and P fimbriae are particularly well characterized, regarding the structural organization, biogenesis, and pathogenic functions of these adhesins [8]. Type 1 fimbriae, which mediate adhesion through mannose-binding receptors, are important for the virulence of extraintestinal pathogenic E. coli (ExPEC), facilitating colonization and persistence within the host [10]. P fimbriae, first identified in uropathogenic E. coli (UPEC), exhibit a strong affinity for P blood group oligosaccharides, promoting adhesion to host epithelial surfaces. Due to their frequent association with E. coli strains isolated from pyelonephritis cases, they are also referred to as pyelonephritis-associated pili (Pap). In addition to association with UPEC, P fimbriae have been identified in some other extraintestinal pathogenic E. coli (ExPEC), including avian pathogenic E. coli (APEC) and strains linked to systemic infections in swine, highlighting their broader significance in bacterial virulence [10,11,12,13].
A critical feature of fimbrial expression across various pathogenic Enterobacteriaceae is its tight regulation, which enables bacterial populations to adaptively respond to changing environments during infection. This regulation involves mechanisms such as phase variation, transcriptional control, and environmental sensing, allowing bacteria to switch production of fimbriae on or off depending on the stage of infection or the specific host niche environment. Such plasticity is crucial for maximizing colonization potential, avoiding immune detection, and conserving energy. For instance, the ability to toggle between different fimbrial types or suppress fimbrial expression altogether enables bacteria to transition from adhesion to motility or from mucosal colonization to systemic dissemination [8,14]. Beyond ExPEC, the ability to regulate fimbrial expression is a fundamental strategy employed by diverse members of the Enterobacteriaceae family to navigate dynamic host environments. Tight regulation and phase-variable expression of multiple fimbrial systems—including but not limited to P fimbriae—enable bacteria to fine-tune adhesion, immune evasion, and tissue-specific colonization. For example, K88 (F4) fimbriae expressed by enterotoxigenic E. coli (ETEC) are activated in a highly controlled manner during infection of the porcine small intestine [14,15,16,17]. These regulatory systems confer phenotypic plasticity and serve as bet-hedging mechanisms, ensuring that subpopulations are primed for survival amid host immune pressures or environmental shifts. This broader perspective underscores the evolutionary significance of fimbrial regulation as a central determinant of pathogenic versatility across different bacterial species and niches [14]. This diversity of fimbrial systems reflects the evolutionary pressure on enteric pathogens to fine-tune adhesin expression for optimal fitness.
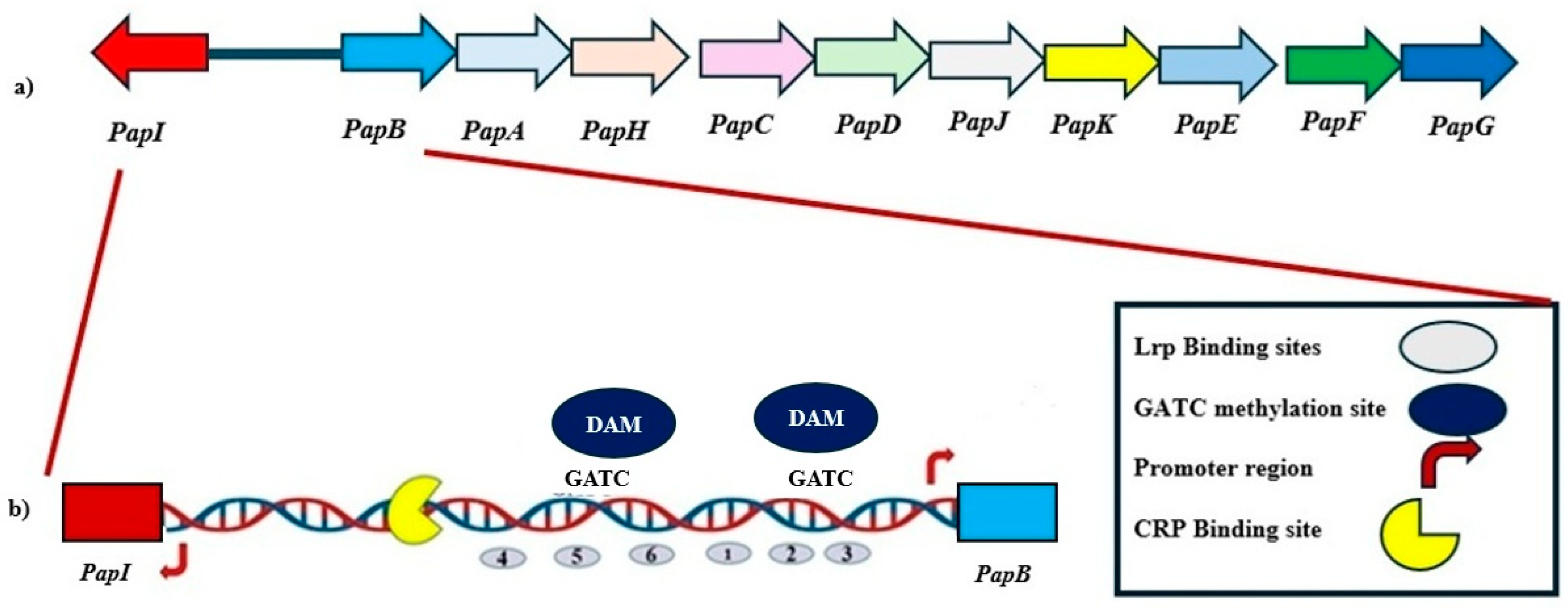
P fimbriae are defining members of the π fimbrial family [18]. The π fimbrial family encompasses a range of adhesive structures that can contribute to colonization and persistence of pathogenic E. coli and related enteric bacteria. P fimbriae are the prototypical representative of the π fimbrial family, alongside structurally and functionally related systems such as Pix and PL fimbriae in uropathogenic E. coli (UPEC), Sfp fimbriae encoded on plasmids in enterohemorrhagic E. coli (EHEC), and Pef fimbriae present in Salmonella enterica. These fimbrial systems facilitate adhesion to host tissues and are tightly regulated to adapt to changing environmental conditions and host niches [2,18,19,20,21,22]. P fimbriae are encoded by the pap operon, which comprises 11 genes encoding structural subunits, secretion machinery, and regulatory proteins (Figure 1). Among these, the transcriptional regulators PapB and PapI govern phase-variable expression of the pap genes. Notably, PapB acts as a master regulator, influencing not only its own operon but also modulating the expression of heterologous fimbrial loci.
Figure 1.
Genetic Organization and Regulatory Architecture of the pap Operon. (a) The pap gene cluster in Escherichia coli encodes multiple genes responsible for the assembly and regulation of P fimbriae. The gene cluster is divergently transcribed, with the regulatory genes papI and papB located upstream of structural genes (papA through papG). (b) The intergenic region between papI and papB contains multiple regulatory elements that coordinate phase-variable expression of P fimbriae. This includes Leucine-responsive Regulatory Protein (Lrp) binding sites (gray ovals), GATC motifs (blue circles) that serve as methylation targets for DNA adenine methyltransferase (Dam), and promoter regions for papI and papB. Methylation of specific GATC sites influences Lrp binding, thereby modulating promoter accessibility and gene expression. A cAMP receptor protein (CRP) binding site (yellow crescent) further integrates environmental signals into the regulatory output. This configuration supports a reversible ON-OFF switch controlled by epigenetic modifications and regulatory proteins, enabling dynamic adaptation of fimbrial expression during infection.
PapB also represents a broader family of fimbrial regulators, present in different E. coli and Salmonella enterica strains [10,19,23]. Fimbrial gene expression in E. coli is governed by multilayered regulation operating at genetic, transcriptional, and post-transcriptional levels [24,25,26]. These mechanisms include phase variation, DNA methylation, global transcriptional regulators, and small RNAs, all of which enable reversible switching between active and repressed states. Such regulatory plasticity allows bacteria to modulate fimbrial production according to environmental conditions, such as temperature, osmolarity, nutrient status, and host signals—thereby optimizing energy expenditure and enhancing bacterial survival [24,27,28,29]. The differential regulation of multiple fimbrial types further facilitates tissue-specific adhesion and broadens the ecological adaptability of pathogenic strains [18].
This review provides an overview of the regulatory functions of PapB and PapB-like proteins, with an emphasis on their role in controlling the expression of fimbrial adhesins. This review also explores the interplay between PapB and global regulators, positioning PapB as a central player within the fimbrial regulatory network. Understanding these mechanisms not only sheds light on bacterial pathogenesis but also identifies PapB and its homologs as potential targets for inhibiting some types of fimbriae as a targeted strategy to prevent bacterial infections dependent on Pap B-family regulators.
2. P Fimbriae in E. coli
2.1. Structural Architecture and Biogenesis
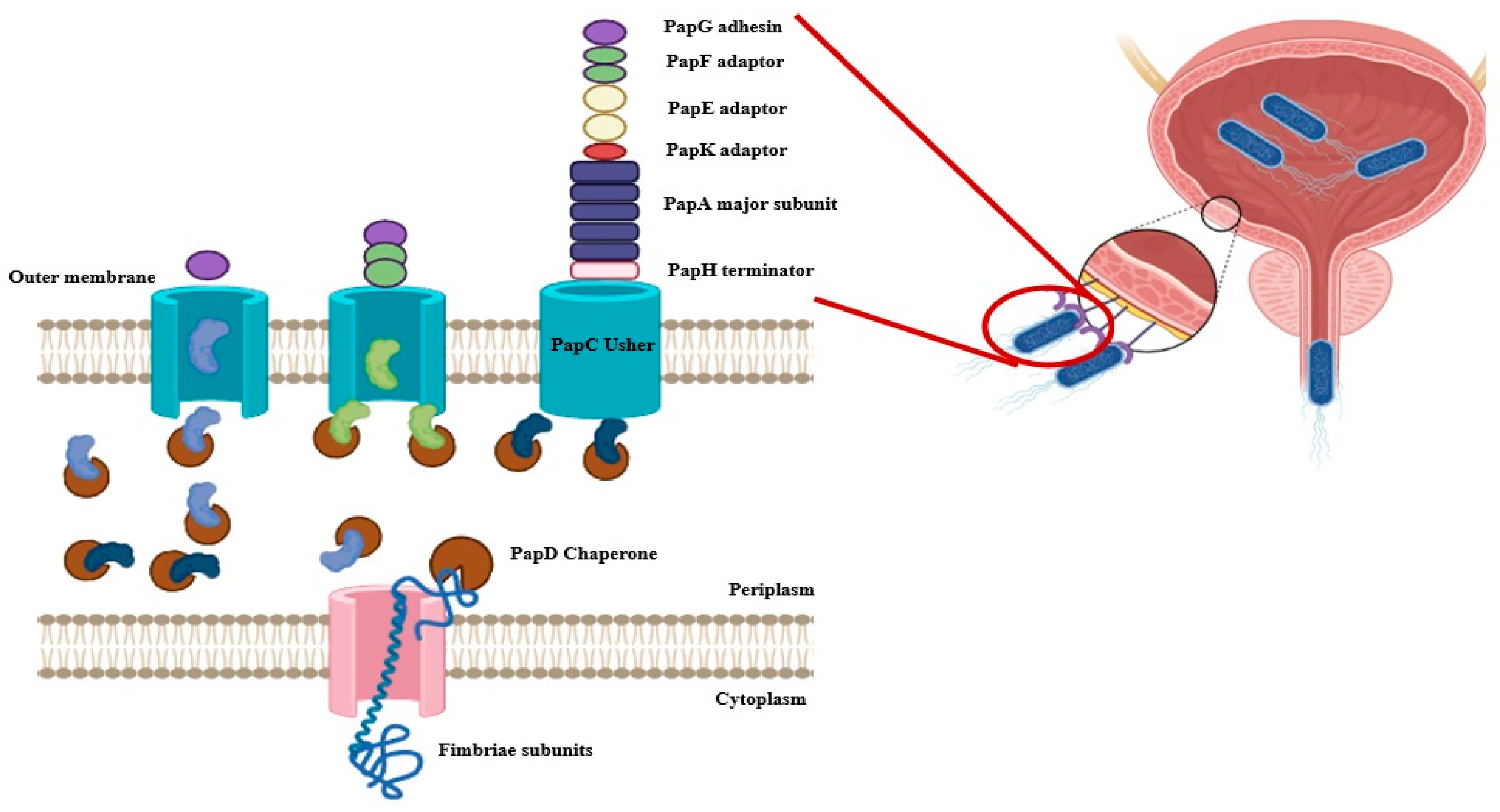
The biosynthesis of P fimbriae in E. coli is governed by the pap operon (papBAHCDJKEFG), which encodes both structural and regulatory components. The fimbrial structure is primarily composed of the major subunit PapA, while the tip adhesin PapG mediates host receptor binding. The minor subunits PapE, PapF, and PapK form the tip fibrillum, contributing to host specificity (Figure 1). Assembly is orchestrated by the periplasmic chaperone PapD and the outer membrane usher PapC (chaperon-usher system), which coordinate the ordered secretion and polymerization of subunits, ensuring functional fimbrial display on the bacterial surface (Figure 2) [9,13].
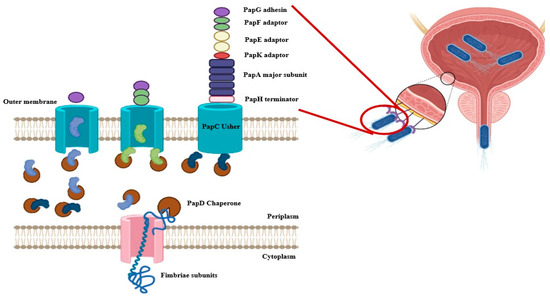
Figure 2.
Biogenesis and Assembly of P Fimbriae in Escherichia coli. The figure illustrates the chaperone-usher pathway responsible for the assembly and surface localization of P fimbriae in E. coli. Fimbrial subunits, including PapG (adhesin tip, purple), PapF (green), PapE (minor fibrillin, yellow), PapK (adaptor, red), PapA (major rod subunit, blue), and PapH (terminator subunit, pink), are translocated into the periplasm through the inner membrane (IM) via the Sec translocon. In the periplasm, PapD acts as a chaperone to stabilize these subunits and prevent premature aggregation. PapD-subunit complexes are then delivered to the outer membrane (OM) usher protein PapC, which facilitates the ordered assembly and secretion of fimbrial subunits onto the bacterial surface. PapC enables the polymerization of PapA subunits into the rod structure, capping the assembly with the tip adhesin PapG and anchoring it with PapH. This highly coordinated process enables the formation of functional P fimbriae that mediate adherence to host epithelial cells, a key step in uropathogenic E. coli colonization (Created by Biorender).
2.2. Epigenetic and Global Regulatory Control
The expression of P fimbriae in E. coli is governed by a finely tuned regulatory system that integrates epigenetic modifications, environmental signals, and global transcriptional control. Central to this system are the divergently transcribed papB and papI genes, which encode key regulators of the pap gene cluster. The regulatory logic of this locus is mediated through phase variation, controlled by the accessibility of promoter regions marked by GATC motifs, which are substrates for DNA adenine methylation by Dam [24,25,27,30]. Leucine-responsive regulatory protein (Lrp) binds to two distinct clusters flanking the promoter, and its interaction is mutually exclusive with Dam-mediated methylation, providing a reversible ON/OFF switch for fimbrial expression [27,30]. In the OFF state, unmethylated GATC sites near the papBA promoter are bound by Lrp, which represses papBA transcription while simultaneously enhancing papI expression. PapI subsequently interacts with Lrp to form a complex that promotes transcriptional activation by displacing Lrp from repressive sites and allowing Dam to methylate the DNA. Methylation of GATC sites diminishes Lrp binding affinity, thereby shifting the operon into the ON state and enabling expression of the fimbrial subunits. This system allows E. coli to respond dynamically to changes in the host environment by controlling fimbrial adhesin expression through reversible epigenetic modifications [31,32,33]. PapB acts downstream of PapI and serves a dual role in regulating PapBA depending on its intracellular concentration. At low to moderate concentrations, PapB enhances transcription of papBA by modulating DNA topology and possibly counteracting Lrp repression. At higher concentrations, however, PapB autoregulates its own expression and represses papBA to avoid excessive fimbrial production [23,34,35,36]. The intermediate inactive state of the pap gene cluster is marked by low papBA transcription and a slight elevation of papI expression. In this state, Lrp binds to the unmethylated GATC site near the papBA promoter, inhibiting papBA transcription while promoting papI expression. The binding of Lrp to either the upstream or downstream clusters prevents DNA adenine methyltransferase (Dam) from methylating the GATC site. Methylation by Dam reduces Lrp’s affinity for the DNA, modulating its regulatory role [25,27,32]. Despite this, the binding of Lrp to the GATC site is not entirely abolished by methylation. PapI, in complex with Lrp, binds to an unmethylated GATC site near the papI promoter, activating papBA transcription and turning the operon ON. Additionally, PapB autoregulates its expression to maintain controlled fimbrial production under varying conditions (Figure 1) [23,27,36].
In addition to the Lrp-Dam-PapI-PapB axis, global regulators such as H-NS contribute to environmental modulation of pap expression. Under non-permissive conditions—such as low temperature, high osmolarity, or nutrient-rich conditions—H-NS binds to AT-rich regions within the pap locus to silence papBA and papI expression by forming repressive nucleoprotein filaments [31]. This repression is reversible and is influenced by environmental cues, reinforcing the phase-variable nature of P fimbrial expression and enhancing the pathogen’s ability to adapt to changing host niches [27].
Recent studies also highlight the role of a small RNA papR in regulating phase variation at the pap locus during UPEC infection in bladder epithelial cells. Transcriptionally activated by Lrp, papR acts as a post-transcriptional repressor of papI. Deletion of papR enhances bacterial adhesion to kidney and bladder cells, even in the absence of type 1 fimbriae, allowing rapid adaptation to changing host environments [28].
3. PapB-like Regulators Controlling Fimbrial Expression and Bacterial Pathogenesis
PapB-like regulators are small transcription factors crucial for controlling the expression of fimbrial adhesins in various bacterial species. These regulators often regulate fimbriae encoding systems through response to environmental and host-specific cues. PapB, the prototype of this family, regulates P fimbriae in Escherichia coli, with homologs such as SfaB and FocB controlling S and F1C fimbriae, respectively. Similar regulators, like MrpB in Proteus mirabilis, govern mannose-resistant fimbriae production in this species [20,21,22].
Some other E. coli fimbrial systems also feature PapB-like regulators, including Pix. The pix protein, found in uropathogenic E. coli (UPEC), aids in bladder colonization, though its role in pathogenesis remains to be fully elucidated [20]. PlfB is another PapB family protein, although at present it is unknown what specific role it may play in the regulation of PL fimbriae [18].
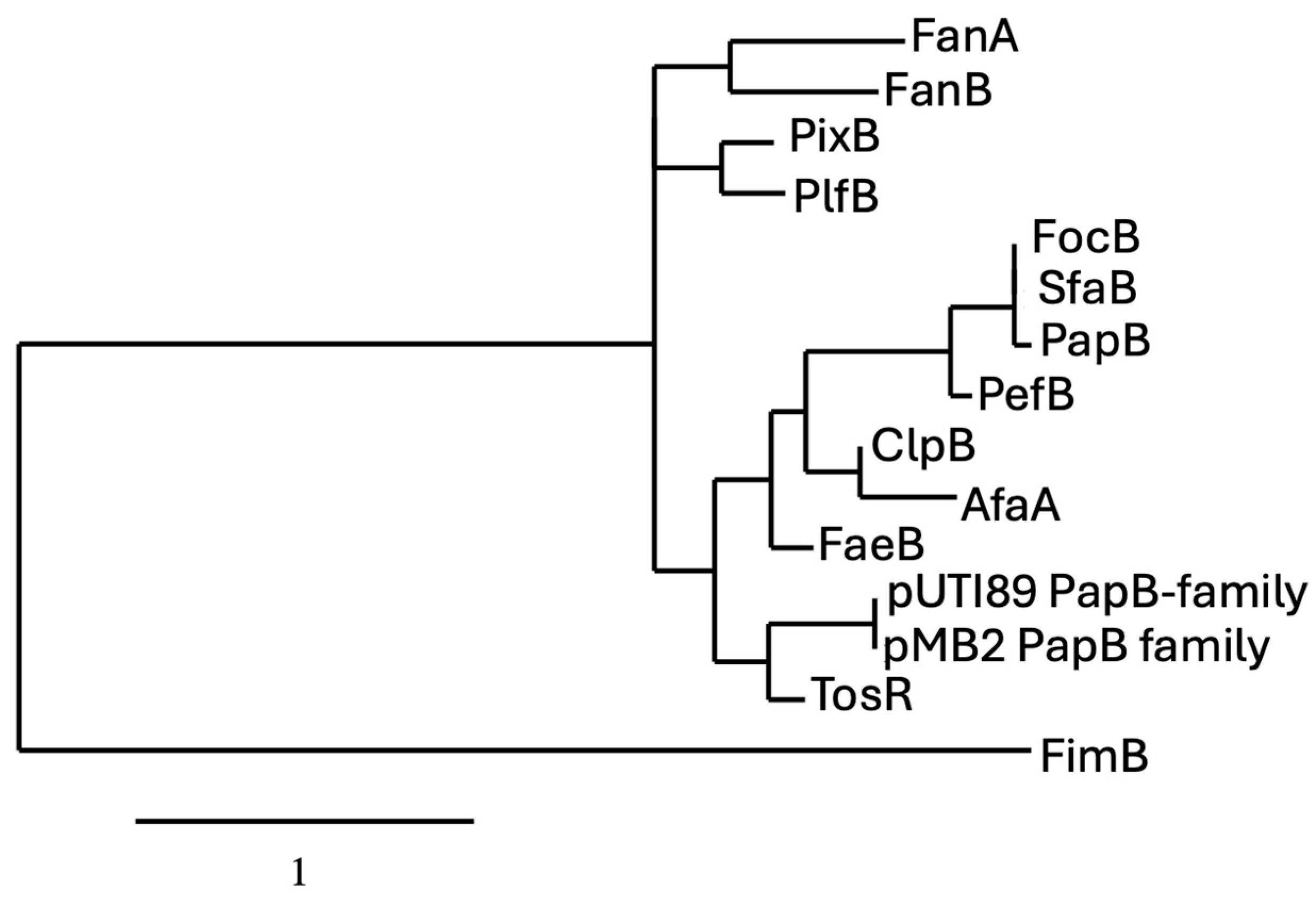
The phylogenetic analysis of PapB-family regulators reveals evolutionary clustering that reflects both functional conservation and divergence among fimbrial systems in Escherichia coli and related enterobacteria. The proteins PapB, SfaB, and PefB form a tightly grouped clade, sharing high sequence identity and short branch lengths, indicating strong evolutionary conservation likely linked to their roles as transcriptional regulators of fimbrial operons. This suggests that even closely related regulators can acquire unique regulatory roles depending on the genetic and ecological context. These patterns support the idea that while a conserved regulatory backbone exists among certain fimbrial systems, others have evolved distinct mechanisms, possibly through horizontal gene transfer or niche-specific adaptation (Figure 3). The presence of PapB-like regulators in these gene clusters suggests a shared regulatory strategy that enables bacteria to fine-tune adhesion, balancing colonization and dispersal in response to dynamic environments.
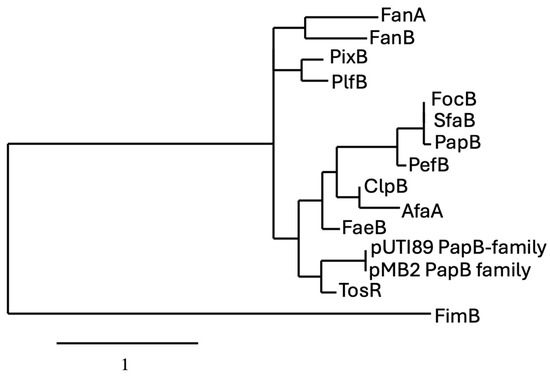
Figure 3.
Phylogram of proteins belonging to the PapB regulator family. For comparison of the protein sequences, entries were obtained from either the NCBI (https://www.ncbi.nlm.nih.gov) or the Universal Protein Resource (UniProt) (www.uniprot.org) website. Phylogenetic analyses of protein sequences were conducted with the platform at Phylogeny (http://www.phylogeny.fr) using the default (“one click”) parameters [37]. Analyses consisted of multiple-sequence alignment with MUSCLE [38], alignment curation with GBlocks [39], maximum likelihood phylogeny analysis using PhyML 3.0 [40], and TreeDyn for generation and editing of trees (https://www.phylogeny.fr/one_task.cgi?task_type=treedyn). Specific parameters are described at the Phylogeny.fr website platform. Sequences included in the tree are from Uniprot.org. unless indicated otherwise and include from top to bottom: FanA (P07104); FanB (P07105); PixB (Q83XC7); PlfB (NCBI: AKG46875.1); FocB (Q93K76); SfaB (Q93K76); PapB (P0474); PefB (H9L498); ClpB (Q47101); AfaA (P53515); FaeB (Q47205); pUTI89 PapB-family (Q1R1Z3); pBM2 PapB-family (NCBI: WP_001322642); TosR (A0AAD2S3G5); FimB (P0ADH5), which was used as an outlier for the tree, (P0ADH5).
3.1. F1C and SFA Fimbriae and the FocB/SfaB Regulators
F1C fimbriae, encoded by the foc operon, facilitate the adherence of uropathogenic Escherichia coli (UPEC) to urinary tract epithelial cells. More frequently detected in UPEC strains from cystitis cases (14–38%) than in fecal isolates (7%), the foc gene cluster closely resembles the pap operon in gene organization, differing only in minor rearrangements [41]. It comprises seven structural genes and two regulatory genes near the promoter region. The transcriptional regulator FocB, a member of the PapB family, shares 81% identity with PapB and complete identity with SfaB, which regulates S fimbriae in neonatal meningitis-associated E. coli. FocB exhibits partial similarity to PefB, a fimbrial regulator in Salmonella enterica, as well as to PapB and SfaB in E. coli. [42,43]. F1C fimbriae mediate binding to glycosphingolipids, such as galactosyl ceramides and globotriosyl ceramides, prevalent in the bladder, ureters, and kidneys, supporting UPEC colonization and persistence [41]. Structurally, the F1C fimbriae are composed of FocA as the major subunit and a tip adhesin complex including FocF, FocG, and FocH. Its biogenesis follows the chaperone-usher pathway, involving the periplasmic chaperone FocC and outer membrane usher FocD. The expression of the Foc operon is regulated by SfaC and FocB, homologs of PapI and PapB, respectively [2,42].
FocB specifically functions as a transcriptional regulator that binds to upstream regulatory regions of the foc and pap operons, modulating their expression through interaction with global regulators such as H-NS and Lrp. As demonstrated by Hultdin et al. [42], FocB has a winged helix-turn-helix (wHTH) domain structurally analogous to PapB and can displace the repressor H-NS from operator DNA, relieving repression and enabling Lrp-mediated activation [42]. This cross-talk enables FocB to not only activate the Foc operon but also influence expression of pap genes under certain conditions, effectively integrating the regulation of multiple fimbrial systems. Co-regulation by FocB or SfaC provides tight control and environmental responsiveness, ensuring that energetically costly fimbrial systems are expressed only when beneficial to the pathogen [44]. Emerging evidence also suggests a role for F1C fimbriae in biofilm formation, contributing to UPEC adaptability in host environments [41].
S fimbriae are important adhesins associated with extra-intestinal pathogenic E. coli that facilitate attachment to sialylated glycoproteins in brain endothelial and renal tissues, contributing to neonatal meningitis and sepsis. Encoded by the sfa gene cluster, this ~6.5 kb locus includes at least seven genes, among them the 16.5 kDa fimbrial subunit protein [44]. Sfa expression is governed by three promoters—pA, pB, and pC—with pB serving as the primary driver of transcription. While the sfa operon’s primary promoter, pA, remains largely inactive under non-inducing conditions, it can produce a 700-base transcript spanning sfaA, along with additional 500- and 1400-base transcripts corresponding to sfaC and sfaBA, respectively [45]. In contrast to the pap gene cluster, where PapB and CRP-cAMP facilitate activation, sfa expression is positively regulated by SfaB and SfaC. A proposed repressor, SfaR, may inhibit transcription in the absence of these activators [21,46], and hns mutations have also been shown to relieve repression and activate sfaA expression [46,47].
Previously thought to be governed solely by direct transcriptional control, emerging evidence has shown that sfa expression is also modulated by phase variation mechanisms involving the leucine-responsive regulatory protein (Lrp) and DNA adenine methylase (Dam). Lrp binding influences local DNA methylation at GATC sites within the sfa promoter region, creating mutually exclusive methylation states that determine whether the operon is in the transcriptionally active (ON) or inactive (OFF) phase. This mechanism parallels the regulation of the pap operon and highlights that S fimbriae, like P fimbriae, are subject to epigenetically controlled, reversible phase variation mediated by Lrp and Dam [48]. This regulatory divergence likely reflects an evolutionary adaptation favoring rapid and transient adhesion during systemic infections [25,45].
3.2. Pef Fimbriae and the PefB Regulator
Pef fimbriae are fimbrial adhesins that promote S. enterica Typhimurium colonization of intestinal epithelium, facilitating resistance to peristalsis and initiating gastrointestinal infection. This adhesion triggers virulence mechanisms leading to inflammation, fluid secretion, and diarrhea. The pef operon, comprising genes pefA, B, C, D, and I, is finely regulated to function under the acidic and fluctuating pH of the intestine [22].
Regulation of pef transcription mirrors that of the pap system, relying on Dam-mediated methylation of GATC motifs within the promoter region. Lrp acts as a positive regulator, enhancing transcription, while H-NS and RpoS suppress expression under non-inducing conditions. Notably, pefI, although homologous to papI, functions as a repressor rather than an activator. PefB, a paralog of PapB, plays an important but less-characterized role in the regulatory control of the pef operon. Although its function in Salmonella remains underexplored, comparative studies suggest that PefB likely contributes to transcriptional regulation of fimbrial genes by interacting with promoter regions or modulating nucleoid-associated silencing proteins [49,50]. In Escherichia coli, PefB has been shown to repress fimB-mediated recombination, suggesting that it may also influence phase variation or expression of other fimbriae indirectly [44,46].
Its sequence similarity to PapB (~46% identity) and conservation of a helix-turn-helix DNA-binding motif support the likelihood that it participates in fine-tuning the expression of pef genes under specific environmental conditions [22]. Moreover, the pef operon contains two promoters, PpefB and PpefA, and is subject to repression by H-NS, Hha, and StpA, especially in the absence of host-inducing cues [51]. While the precise mechanisms by which PefB integrates into this network are not fully delineated, it is likely that PefB acts in coordination with Lrp and Dam methylation to modulate expression in a context-dependent manner. This distinct regulatory arrangement ensures optimal expression of fimbriae for intestinal colonization and pathogenesis [51,52].
3.3. Pix Fimbriae and PixB Regulator
Pix fimbriae, part of the π-fimbrial family, are adhesins that contribute to the virulence of uropathogenic Escherichia coli (UPEC) by promoting attachment to uroepithelial cells during urinary tract infections. These surface structures enhance bacterial colonization and persistence in the bladder and kidneys, exacerbating infection severity [20].
Encoded by the pix operon, which mirrors the genetic organization of the pap locus, Pix fimbriae are assembled via the chaperone-usher pathway. Core components include PixA (major pilin), PixG (tip adhesin), PixD (periplasmic chaperone), and PixC (outer membrane usher), with PixB functioning as a regulatory factor. There is, however, no PapI-type protein present in the pix regulatory region. Expression of the pix operon is controlled by phase variation, enabling reversible ON/OFF switching in response to environmental cues and immune pressures, thus facilitating bacterial adaptability and possible immune evasion during infection [35].
3.4. Orf G from Plasmid pMB2: A Non-Fimbrial Member of the PapB Regulatory Family
Orf G, encoded by the conjugative plasmid pMB2, is a plasmid-borne transcriptional regulator identified in Escherichia coli transconjugants. Unlike chromosomally encoded PapB-family members typically associated with fimbrial operons, orf G is not linked to any fimbrial structural gene cluster, yet it modulates phenotypes related to surface behavior, particularly auto-aggregation [49]. The gene was initially described by Monárrez and Okeke [49] who demonstrated that its expression significantly altered cell aggregation patterns, suggesting a role in host cell interaction or plasmid dissemination. Structurally, Orf G belongs to the PapB family of transcriptional regulators and shares sequence homology with PapB, SfaB, and FocB, although it shares the highest level of identity with TosR [53]. While Orf G lacks an associated chaperone-usher fimbrial operon, it likely retains a conserved helix-turn-helix (HTH) domain, enabling it to influence gene expression through DNA binding. Its plasmid location and functional divergence suggest that PapB-like regulators have been horizontally transferred and adapted to new regulatory contexts beyond fimbrial control. As such, Orf G represents a unique expansion of the PapB regulatory family, emphasizing the plasticity and regulatory versatility of these small transcription factors in both chromosomal and extrachromosomal environments [44]. Although the downstream targets of Orf G remain to be fully characterized, its influence on bacterial aggregation hints at broader roles in plasmid biology, host colonization, or interbacterial interactions [49].
3.5. Additional Regulators Belonging to the PapB Family
Other regulators belonging to the PapB family include FaeA F4 (K88) fimbriae, DaaA of F1845 fimbriae, ClpB of CS31 fimbriae, Fan A and Fan B of K99 fimbriae, AfaA regulating the Afa adhesins, DaaA of F1845 fimbriae and TosR. These other systems will be described in the following sections.
4. FaeA: Regulation of F4 (K88) Fimbriae in Enterotoxigenic E. coli
F4 (K88) fimbriae, a key virulence factor of enterotoxigenic Escherichia coli (ETEC), play a crucial role in the colonization of the porcine small intestine, leading to diarrhea in neonatal and postweaning pigs. The ability of ETEC to adhere to the intestinal epithelium via F4 fimbriae facilitates bacterial persistence and subsequent secretion of enterotoxins, which drive disease pathology [16]. The fae operon consists of nine genes involved in fimbrial biosynthesis, structure, and regulation. The operon includes genes encoding structural subunits (faeG, faeC, faeF, faeH, faeI, and faeJ), as well as the periplasmic chaperone FaeE and the outer membrane usher FaeD. The operon also contains regulatory proteins, including FaeA and FaeB, which influence transcriptional control. Unlike many fimbrial systems where adhesins are confined to the tip structure, the binding properties of F4 fimbriae are distributed along the entire shaft of the fiber, allowing for enhanced interaction with host receptors. Transcription of the fae operon is tightly regulated by the operon-specific regulator FaeA and the global regulator Lrp, ensuring controlled expression under appropriate environmental conditions. Although FaeB is encoded within the operon, its function remains unclear, unlike its counterpart PapB, which serves as a positive regulator in the pap gene cluster [15].
5. Functional and Regulatory Comparison of DaaA and PapB
The daa operon of diffusely adherent Escherichia coli (DAEC) encodes the F1845 fimbriae, a virulence factor critical for colonization of the intestinal epithelium. Among the operon’s gene products, DaaA is a small regulatory protein that shares structural similarity with the PapB family of transcriptional regulators, including PapB, SfaB, and FocB [31]. These proteins are known for their role in controlling the expression of fimbrial adhesins through direct interaction with upstream regulatory regions and global nucleoid-associated factors. DaaA, however, while structurally related to PapB, exhibits important differences in regulatory activity and functional scope [1,46].
Sequence analyses reveal that DaaA contains a conserved helix-turn-helix (HTH) DNA-binding motif similar to that of PapB, yet it lacks critical residues in the carboxy-terminal domain required for PapB’s broader regulatory function. Functional assays show that while PapB represses type 1 fimbriae expression by inhibiting fimB-mediated recombination at the fim switch (fimS), DaaA does not influence the orientation of fimS, nor does it affect fimA transcription. Substitution of PapB’s C-terminal residues with those from DaaA significantly diminishes its ability to repress type 1 fimbriae, suggesting that the unique sequence composition of DaaA confines its regulatory influence to its native operon rather than allowing cross-regulation between fimbrial systems [10,31,54].
Regulatory mechanisms within the daa operon also differ markedly from those of the pap operon. While PapB integrates environmental signals via Lrp, Dam methylation, and H-NS to coordinate phase variation in P fimbriae, DaaA’s regulation operates primarily at the transcriptional and post-transcriptional levels. Transcription of daaE, the major fimbrial subunit gene, is repressed under non-inducing conditions, such as low temperature or high osmolarity by the nucleoid structuring protein H-NS [31]. This repression is relieved in hns mutants, indicating an environmental responsiveness analogous to other fimbrial operons. Interestingly, expression of daaE is also regulated post-transcriptionally by the upstream open reading frame daaP. Translation of daaP is required for proper mRNA processing of the downstream daaE, a regulatory feature not observed in the pap system [55].
In contrast to PapB, which is deeply embedded in a network of cross-operon regulatory influences—such as repressing type 1 fimbriae and responding to the CRP-cAMP pathway—DaaA appears to function as a more isolated, operon-specific regulator. This divergence may reflect evolutionary adaptation to niche-specific regulatory demands of DAEC in the intestinal tract. Taken together, while DaaA and PapB belong to the same structural family of fimbrial regulators, their differing roles in cross-talk, operon regulation, and environmental responsiveness underscore the functional diversification of fimbrial control mechanisms in E. coli pathotypes [56].
6. ClpB of the CS31A Fimbriae
The CS31A fimbriae, encoded by the clp operon in certain pathogenic E. coli strains, play a key role in adherence to host tissues during colonization of the intestinal tract, particularly in calves. Central to the regulation of this system is ClpB, a small transcriptional regulator structurally related to PapB, the well-characterized regulator of P fimbriae in uropathogenic E. coli. While both ClpB and PapB belong to the same helix-turn-helix (HTH) protein family, they differ markedly in their regulatory roles and scope of influence [57]. ClpB and PapB share conserved structural elements, especially in their DNA-binding domains, suggesting a common evolutionary origin. However, functional divergence is apparent. PapB not only activates the expression of its own pap operon but also exerts trans-repressive effects on the type 1 fimbrial operon by interfering with fimB-dependent recombination and promoting fimE-mediated switching to the “OFF” orientation of the fimS phase switch. In contrast, ClpB lacks the ability to influence type 1 fimbriae expression, indicating the absence of regulatory cross-talk. Experimental evidence confirms that ClpB does not repress fimB or modulate fimbrial phase variation, underscoring its specificity to the CS31A system [57].
The regulation of the clp operon is driven by both local and global transcriptional factors, primarily the leucine-responsive regulatory protein (Lrp). Lrp binds to upstream regions of the clp promoter and represses transcription under nutrient-rich conditions. This repression is alleviated by branched-chain amino acids, especially leucine and alanine, indicating a fine-tuned regulatory mechanism responsive to host nutrient availability [57]. Notably, the clp operon is not under the control of Dam methylation or known phase variation systems. Furthermore, current evidence suggests that ClpB does not engage in cross-regulation of other fimbrial systems such as type 1 or P fimbriae, distinguishing it from regulators like PapB, which orchestrate broader regulatory effects influencing the adhesin expression hierarchy. The absence of cross-talk implies that ClpB acts exclusively within its native regulatory circuit, maintaining tight control over CS31A expression without interfering with the transcriptional programs of other adhesin operons [54,57].
The transcriptional regulation of the clp operon also diverges mechanistically from that of pap. PapB integrates multiple regulatory inputs, including Lrp, Dam methylation, and environmental signals, to coordinate P fimbriae expression. In contrast, ClpB operates within a more streamlined network, primarily responding to nutrient cues through Lrp. These features highlight a more specialized and environmentally responsive regulation of CS31A fimbriae, in contrast to the complex, multilayered control seen in P fimbriae [57].
7. Regulatory Functions of FanA and FanB of the K99 Fimbrial Operon
The K99 fimbriae (also known as F5 fimbriae) are important virulence factors expressed by enterotoxigenic E. coli (ETEC), especially in neonatal and young animals, where they mediate adherence to the small intestinal epithelium. The biogenesis and expression of K99 fimbriae are controlled by the fan operon, which includes several genes (fanA through fanH) encoding structural subunits, chaperone-usher assembly machinery, and two regulatory proteins, FanA and FanB. While PapB—best studied in the context of the pap operon encoding P fimbriae in uropathogenic E. coli (UPEC)—has been shown to participate in broader fimbrial cross-regulation and phase variation, FanA and FanB appear to represent a more localized and specialized form of transcriptional control [58,59].
FanA and FanB are small basic proteins encoded at the 5′ end of the fan operon and expressed from a common promoter upstream of fanA. Structurally, they share similarities with PapB, particularly in predicted DNA-binding domains, suggesting a conserved mechanism of interaction with target promoters. However, their regulatory functions differ significantly. While PapB is capable of repressing the expression of type 1 fimbriae, there is no evidence that FanA or FanB participates in such cross-talk with heterologous fimbrial systems. Their role appears to be confined to the activation of downstream genes within the fan operon, facilitating the assembly and surface expression of K99 fimbriae [59].
The transcriptional regulation of the fan operon is responsive to global regulatory factors such as the Lrp and CRP. Lrp acts as a transcriptional activator under nutrient-limited conditions, enhancing the expression of fanA and fanB, while CRP mediates catabolite repression, integrating environmental nutrient signals. This regulatory logic allows ETEC to express K99 fimbriae in the nutrient-limited conditions typical of the small intestine, where colonization occurs. Unlike the pap operon, the fan operon is not phase-variable, nor is it subject to Dam methylation-dependent silencing, suggesting a simpler and more targeted expression strategy tailored to a defined ecological niche [58].
8. Comparative Analysis of AfaA and PapB
The Afa/Dr family of adhesins represents a class of afimbrial adhesins expressed by diffusely adherent E. coli (DAEC) and certain uropathogenic E. coli (UPEC) strains. These adhesins are implicated in urinary tract infections and chronic diarrhea, particularly in children and immunocompromised patients [60,61]. Central to the expression of these adhesins is the afa operon, which encodes several proteins involved in assembly, transport, and regulation of the adhesin system. Among these, AfaA serves as a key regulatory protein and shares structural and functional similarities with PapB, the prototypical transcriptional regulator of the pap operon encoding P fimbriae. A comparison of AfaA and PapB reveals both evolutionary homology and significant functional divergence in their mechanisms of regulation, operon organization, and roles in pathogenesis [62].
The afa operon typically comprises five genes: afaA, afaB, afaC, afaD, and afaE. AfaA is located at the 5′ end of the operon and encodes a small cytoplasmic protein with predicted helix-turn-helix motifs similar to those found in PapB. Like PapB, AfaA is believed to function as a DNA-binding protein that modulates the transcription of downstream genes required for adhesin production. However, while PapB not only activates its operon but also participates in trans-regulation, notably repressing type 1 fimbriae by influencing the fim switch, AfaA appears to act exclusively in a cis-regulatory manner within the afa operon. To date, there is no evidence that AfaA influences the expression of other fimbrial or afimbrial systems, nor does it appear to play a role in phase variation mechanisms that typify PapB function [1,63,64].
The regulation of the afa operon is also shaped by interactions with global regulators and environmental cues. For instance, host-derived factors such as temperature and osmolarity can affect the transcriptional activity of the afa promoter. Although the precise molecular interactions remain less well-characterized than those of PapB, it is clear that AfaA operates within a streamlined, operon-specific regulatory architecture, optimized for the persistent, low-level expression of Afa/Dr adhesins in host mucosal environments. By contrast, PapB functions within a broader regulatory network, coordinating fimbrial expression hierarchies in response to environmental inputs through interactions with Dam methylation, Lrp, H-NS, and recombinase systems. This allows PapB to exert control over both phase-variable and non-phase-variable operons, tailoring fimbrial expression profiles across stages of infection and tissue niches [1,60,65].
9. PapB in Relation to Other Fimbrial Regulators
FimB and FimE, the key regulators of type 1 fimbrial phase variation in Escherichia coli, have a distinct mechanism of action when compared to PapB, the master regulator of the pap (P fimbriae) fimbrial gene cluster. FimB and FimE are site-specific recombinases that control expression via an invertible DNA element, with FimB mediating bidirectional switching, while FimE favors the OFF orientation [66]. Notably, the fim and pap gene clusters are genetically distinct but evolutionarily and mechanistically analogous. PapB itself is a small DNA-binding protein that acts as both a positive and negative regulator by modulating promoter accessibility within the pap gene cluster [35,67,68,69,70]. Sequence homology and regulatory behavior suggest that fimbrial regulators, such as TosR, homologous to PapB, may have co-evolved to govern fimbrial and non-fimbrial adhesin expression across diverse pathogenic E. coli strains. Studies on the Tos operon in uropathogenic E. coli further support the evolutionary plasticity of PapB-like regulators. TosR, for example, represses its own operon while simultaneously interacting with promoters of other adhesins like Pap, suggesting a broad-reaching regulatory network among fimbrial and non-fimbrial loci. These findings support the concept that non-PapB regulators, such as FimB, while lacking identical sequences, fulfill overlapping roles in coordinating adhesin expression in response to environmental or host-derived cues [70].
10. Cross-Talk Between Fim Regulation and the PapB Family Regulators
Cross-regulatory mechanisms exist between different fimbrial systems in E. coli, enabling precise temporal control of surface structures to adapt to host environments. In uropathogenic strains, expression of type 1 fimbriae inversely correlates with the expression of P fimbriae, a phenomenon termed fimbrial cross-talk [71]. This mutual exclusivity is mediated by regulators such as PapB, Lrp, and H-NS, which directly or indirectly influence the orientation of the fim switch (fimS). The interplay ensures energy-efficient and host-context-appropriate expression of adhesins, allowing bacteria to modulate adherence profiles during different stages of infection. Notably, PapB itself can repress type 1 fimbrial expression by interfering with the transcriptional activity at the fim switch region. Engström and Mobley demonstrated that PapB homologs like TosR bind AT-rich promoter regions and downregulate P fimbriae expression, which in turn may permit type 1 fimbrial activation depending on the chromatin context [70]. Such hierarchical regulation suggests a broader adhesin regulatory axis, wherein the expression of one adhesin system dynamically suppresses another.
This regulation is reinforced by environmental sensing systems and nucleoid-associated proteins like H-NS and Lrp, which participate in fine-tuning fimbrial gene accessibility [27].
Comparative studies on regulatory cistrons across fimbrial systems have shown that PapB and its homologs—SfaB, FocB, PefB, and TosR—share a conserved helix-turn-helix DNA-binding motif and can functionally substitute for one another to a certain extent [72]. These regulators typically bind to upstream AT-rich regions and modulate transcription either by antagonizing global repressors like H-NS or by recruiting activators such as Lrp. For example, SfaB and FocB—although sometimes encoded in different contexts—have both been shown to influence the expression of unrelated fimbrial operons and even affect the fim switch orientation indirectly. This modularity indicates a shared evolutionary strategy among ExPEC strains, allowing for regulatory flexibility during infection [72,73].
Furthermore, PapB-family proteins do not limit their influence to adhesin expression alone. TosR, for instance, has been implicated in regulating motility by modulating the flhDC flagellar master operon, thereby linking fimbrial control to flagellar function. This coupling enables E. coli to shift between sessile (adherent) and motile states depending on environmental signals, infection phase, or anatomical niche. Such integrated regulation enhances bacterial adaptability by coordinating surface structure deployment with metabolic and motility programs, ensuring successful colonization and immune evasion. These insights underscore that fimbrial regulation in E. coli is not isolated, but rather interconnected with broader transcriptional networks and pathogenesis strategies [74].
11. Discussion and Conclusions
The comparative analysis of fimbrial gene clusters such as pap, pix, sfa, foc, and pef highlights a conserved modular architecture, with regulatory genes like papB playing pivotal roles in modulating downstream fimbrial expression. The striking similarities in operon structure and regulatory sequences, especially the conserved positioning of genes such as papB and the involvement of transcriptional regulators like H-NS, Lrp, and CRP, underscore the functional significance of precise regulatory control in pathogenic contexts. These systems are tightly regulated to ensure optimal expression during host colonization, preventing unnecessary energy expenditure and immune detection. The detailed regulatory circuit of the Foc operon, which mirrors that of pap, demonstrates how bacterial pathogens integrate environmental cues to fine-tune fimbrial gene expression.
Importantly, a broader inclusion of PapB family homologs—including SfaB, FocB, PefB, PixB, and TosR—reveals that this family of regulators extends well beyond the canonical Pap system and serves as a central regulatory network in multiple fimbrial operons. These proteins often share helix-turn-helix motifs and bind to AT-rich regions near promoter elements, though their regulatory capacity and interaction networks may differ significantly. For example, while PapB and SfaB show similar repression of type 1 fimbriae by modulating the fim switch, FocB may act in a more confined regulatory context, and PefB (though homologous) has been shown to repress rather than activate transcription. Furthermore, TosR, a PapB-like transcriptional regulator encoded on pathogenicity islands in UPEC, has been implicated in both repression of P fimbriae and activation of other loci such as iron uptake systems and motility genes, indicating a broader role in virulence regulation. These functional differences support the idea that PapB homologs have evolved diverse regulatory strategies despite their conserved structural domains.
From a therapeutic standpoint, targeting fimbrial regulation remains a promising anti-virulence strategy. Inhibitors that block PapB-family regulators or mimic competitive global regulators like Lrp could disrupt transcriptional activation of fimbrial genes, effectively silencing bacterial adhesion without applying selective pressure typically associated with antibiotics. This could be particularly valuable in managing recurrent urinary tract infections (UTIs), where type 1 and P fimbriae play critical roles in bladder colonization and biofilm formation. Further, identifying cues or regulatory signals that can increase expression of PapB family regulators, could also lead to effective strategies to inhibit expression of other fimbriae such as type 1 fimbriae, that play a critical role in pathogenesis of urinary tract infections caused by UPEC.
Moreover, small molecules that interfere with protein-DNA interactions at conserved regulatory sites, such as Site 1 and Site 2 found in pap and foc promoters, could have broad-spectrum utility against multiple fimbrial operons that rely on structurally similar regulatory architectures.
From an evolutionary perspective, the recurrence of PapB-like regulatory modules across diverse fimbrial systems supports the idea of horizontal gene transfer and strong selective pressures to maintain such regulators in uropathogenic E. coli lineages. Phylogenetic comparisons show that PapB, SfaB, and FocB cluster closely together, suggesting recent divergence from a common ancestor, whereas more distantly related homologs like PefB branch separately, reflecting possible specialization or regulatory rewiring. The inclusion of TosR in this comparison further supports the expansion of the PapB regulatory family into horizontally acquired and chromosomally encoded loci that control a wider array of virulence functions. The preservation of gene order and the use of similar promoter elements across operons such as pap, sfa, foc, and pef imply convergent evolution toward a successful regulatory template for adhesion control. Understanding these evolutionary and mechanistic relationships enhances our grasp of fimbrial plasticity and provides a strong foundation for developing molecular inhibitors targeting PapB-family regulators to prevent or limit infections dependent on adhesin expression controlled by PapB-family protein-mediated regulatory mechanisms.
Author Contributions
F.A.: supervision, conceptualization, investigation, supervision, validation, visualization, writing—original draft, writing—review and editing. H.J.: visualization, writing—review and editing. M.K.: writing—review and editing. C.M.D.: supervision, phylogenetic analysis, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Sciences and Engineering Research Council (NSERC) Canada: Grant number RGPIN-2025-07045.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
During the preparation of this work, the authors used ChatGPT4 to correct potential grammatical errors. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Le Bouguénec, C.; Servin, A.L. Diffusely adherent Escherichia coli strains expressing Afa/Dr adhesins (Afa/Dr DAEC): Hitherto unrecognized pathogens. FEMS Microbiol. Lett. 2006, 256, 185–194. [Google Scholar] [CrossRef]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The population genetics of pathogenic Escherichia coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, P.; Zhao, Y.; Ma, X. Enterotoxigenic Escherichia coli: Intestinal pathogenesis mechanisms and colonization resistance by gut microbiota. Gut Microbes 2022, 14, 2055943. [Google Scholar] [CrossRef]
- Poolman, J.T.; Wacker, M. Extraintestinal pathogenic Escherichia coli, a common human pathogen: Challenges for vaccine development and progress in the field. J. Infect. Dis. 2016, 213, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Zacchè, M.M.; Giarenis, I. Therapies in early development for the treatment of urinary tract inflammation. Expert Opin. Investig. Drugs 2016, 25, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Bonten, M.; Johnson, J.R.; van den Biggelaar, A.H.; Georgalis, L.; Geurtsen, J.; de Palacios, P.I.; Gravenstein, S.; Verstraeten, T.; Hermans, P.; Poolman, J.T. Epidemiology of Escherichia coli bacteremia: A systematic literature review. Clin. Infect. Dis. 2021, 72, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Allard, M.W.; Strain, E.; Melka, D.; Bunning, K.; Musser, S.M.; Brown, E.W.; Timme, R. Practical value of food pathogen traceability through building a whole-genome sequencing network and database. J. Clin. Microbiol. 2016, 54, 1975–1983. [Google Scholar] [CrossRef]
- Gahlot, D.K.; Taheri, N.; MacIntyre, S. Diversity in genetic regulation of bacterial fimbriae assembled by the chaperone usher pathway. Int. J. Mol. Sci. 2022, 24, 161. [Google Scholar] [CrossRef]
- Low, D.; Robinson, E.; McGee, Z.; Falkow, S. The frequency of expression of pyelonephritis-associated pili is under regulatory control. Mol. Microbiol. 1987, 1, 335–346. [Google Scholar] [CrossRef]
- Holden, N.J.; Totsika, M.; Mahler, E.; Roe, A.J.; Catherwood, K.; Lindner, K.; Dobrindt, U.; Gally, D.L. Demonstration of regulatory cross-talk between P fimbriae and type 1 fimbriae in uropathogenic Escherichia coli. Microbiology 2006, 152, 1143–1153. [Google Scholar] [CrossRef]
- Buckles, E.L.; Luterbach, C.L.; Wang, X.; Lockatell, C.V.; Johnson, D.E.; Mobley, H.L.; Donnenberg, M.S. Signature-tagged mutagenesis and co-infection studies demonstrate the importance of P fimbriae in a murine model of urinary tract infection. Pathog. Dis. 2015, 73, ftv014. [Google Scholar] [CrossRef]
- Luterbach, C.L.; Mobley, H.L. Cross talk between MarR-like transcription factors coordinates the regulation of motility in uropathogenic Escherichia coli. Infect. Immun. 2018, 86, e00338-18. [Google Scholar] [CrossRef]
- Wurpel, D.J.; Beatson, S.A.; Totsika, M.; Petty, N.K.; Schembri, M.A. Chaperone-usher fimbriae of Escherichia coli. PLoS ONE 2013, 8, e52835. [Google Scholar] [CrossRef]
- Bayliss, C.D.; Clark, J.L.; van der Woude, M.W. 100+ years of phase variation: The premier bacterial bet-hedging phenomenon. Microbiology 2025, 171, 001537. [Google Scholar] [CrossRef]
- Huisman, T.T.; De Graaf, F.K. Negative control of fae (K88) expression by the ‘global’regulator Lrp is modulated by the ‘local’regulator FaeA and affected by DNA methylation. Mol. Microbiol. 1995, 16, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Mol, O.; Oudhuis, W.C.; Oud, R.P.; Sijbrandi, R.; Luirink, J.; Harms, N.; Oudega, B. Biosynthesis of K88 fimbriae in Escherichia coli: Interaction of tip-subunit FaeC with the periplasmic chaperone FaeE and the outer membrane usher FaeD. J. Mol. Microbiol. Biotechnol. 2001, 3, 135–142. [Google Scholar]
- Payne, D.; O’Reilly, M.; Williamson, D. The K88 fimbrial adhesin of enterotoxigenic Escherichia coli binds to beta 1-linked galactosyl residues in glycosphingolipids. Infect. Immun. 1993, 61, 3673–3677. [Google Scholar] [CrossRef]
- Habouria, H.; Bessaiah, H.; Pokharel, P.; Dhakal, S.; Maris, S.; Buron, J.; Houle, S.; Dozois, C.M. A Newly Identified Group of P-like (PL) Fimbria Genes from Extraintestinal Pathogenic Escherichia coli (ExPEC) Encode Distinct Adhesin Subunits and Mediate Adherence to Host Cells. Appl. Environ. Microbiol. 2022, 88, e0142121. [Google Scholar] [CrossRef]
- Hernday, A.D.; Braaten, B.A.; Low, D.A. The mechanism by which DNA adenine methylase and PapI activate the pap epigenetic switch. Mol. Cell 2003, 12, 947–957. [Google Scholar] [CrossRef]
- Lugering, A.; Benz, I.; Knochenhauer, S.; Ruffing, M.; Schmidt, M.A. The Pix pilus adhesin of the uropathogenic Escherichia coli strain X2194 (O2: K−:H6) is related to Pap pili but exhibits a truncated regulatory region. Microbiology 2003, 149, 1387–1397. [Google Scholar] [CrossRef]
- Ott, M.; Hoschützky, H.; Jann, K.; Van Die, I.; Hacker, J. Gene clusters for S fimbrial adhesin (sfa) and F1C fimbriae (foc) of Escherichia coli: Comparative aspects of structure and function. J. Bacteriol. 1988, 170, 3983–3990. [Google Scholar] [CrossRef]
- Bäumler, A.J.; Tsolis, R.M.; Bowe, F.A.; Kusters, J.G.; Hoffmann, S.; Heffron, F. The pef fimbrial operon of Salmonella typhimurium mediates adhesion to murine small intestine and is necessary for fluid accumulation in the infant mouse. Infect. Immun. 1996, 64, 61–68. [Google Scholar] [CrossRef]
- Totsika, M.; Beatson, S.A.; Holden, N.; Gally, D.L. Regulatory interplay between pap operons in uropathogenic Escherichia coli. Mol. Microbiol. 2008, 67, 996–1011. [Google Scholar] [CrossRef]
- Båga, M.; Göransson, M.; Normark, S.; Uhlin, B. Transcriptional activation of a pap pilus virulence operon from uropathogenic Escherichia coli. EMBO J. 1985, 4, 3887–3893. [Google Scholar] [CrossRef] [PubMed]
- Blyn, L.B.; Braaten, B.A.; White-Ziegler, C.A.; Rolfson, D.H.; Low, D.A. Phase-variation of pyelonephritis-associated pili in Escherichia coli: Evidence for transcriptional regulation. EMBO J. 1989, 8, 613–620. [Google Scholar] [CrossRef]
- White-Ziegler, C.A.; Black, A.M.; Eliades, S.H.; Young, S.; Porter, K. The N-acetyltransferase RimJ responds to environmental stimuli to repress pap fimbrial transcription in Escherichia coli. J. Bacteriol. 2002, 184, 4334–4342. [Google Scholar] [CrossRef]
- Graveline, R.; Mourez, M.; Hancock, M.A.; Martin, C.; Boisclair, S.; Harel, J. Lrp–DNA complex stability determines the level of ON cells in type P fimbriae phase variation. Mol. Microbiol. 2011, 81, 1286–1299. [Google Scholar] [CrossRef]
- Khandige, S.; Kronborg, T.; Uhlin, B.E.; Møller-Jensen, J. sRNA-mediated regulation of P-fimbriae phase variation in uropathogenic Escherichia coli. PLoS Pathog. 2015, 11, e1005109. [Google Scholar] [CrossRef]
- Zamora, M.; Ziegler, C.A.; Freddolino, P.L.; Wolfe, A.J. A thermosensitive, phase-variable epigenetic switch: Pap revisited. Microbiol. Mol. Biol. Rev. 2020, 84, e00030-17. [Google Scholar] [CrossRef] [PubMed]
- Hernday, A.D.; Braaten, B.A.; Broitman-Maduro, G.; Engelberts, P.; Low, D.A. Regulation of the pap epigenetic switch by CpxAR: Phosphorylated CpxR inhibits transition to the phase ON state by competition with Lrp. Mol. Cell 2004, 16, 537–547. [Google Scholar] [CrossRef]
- White-Ziegler, C.A.; Villapakkam, A.; Ronaszeki, K.; Young, S. H-NS controls pap and daa fimbrial transcription in Escherichia coli in response to multiple environmental cues. J. Bacteriol. 2000, 182, 6391–6400. [Google Scholar] [CrossRef]
- Braaten, B.A.; Nou, X.; Kaltenbach, L.S.; Low, D.A. Methylation patterns in pap regulatory DNA control pyelonephritis-associated pili phase variation in E. coli. Cell 1994, 76, 577–588. [Google Scholar] [CrossRef]
- Xia, Y.; Forsman, K.; Jass, J.; Uhlin, B.E. Oligomeric interaction of the PapB transcriptional regulator with the upstream activating region of pili adhesin gene promoters in Escherichia coli. Mol. Microbiol. 1998, 30, 513–523. [Google Scholar] [CrossRef]
- Xia, Y.; Uhlin, B.E. Mutational Analysis of the PapB Transcriptional Regulator in Escherichia coli: Regions important for DNA binding and oligomerization. J. Biol. Chem. 1999, 274, 19723–19730. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrowicz, A.; Khan, M.M.; Sidorczuk, K.; Noszka, M.; Kolenda, R. Whatever makes them stick–Adhesins of avian pathogenic Escherichia coli. Vet. Microbiol. 2021, 257, 109095. [Google Scholar] [CrossRef]
- Bakker, A.; Smith, D. Methylation of GATC sites is required for precise timing between rounds of DNA replication in Escherichia coli. J. Bacteriol. 1989, 171, 5738–5742. [Google Scholar] [CrossRef] [PubMed]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.-F.; Guindon, S.; Lefort, V.; Lescot, M. Phylogeny. fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Khan, A.S.; Kniep, B.; Oelschlaeger, T.A.; Van Die, I.; Korhonen, T.; Hacker, J.r. Receptor structure for F1C fimbriae of uropathogenic Escherichia coli. Infect. Immun. 2000, 68, 3541–3547. [Google Scholar] [CrossRef]
- Hultdin, U.W.; Lindberg, S.; Grundström, C.; Huang, S.; Uhlin, B.E.; Sauer-Eriksson, A.E. Structure of FocB–a member of a family of transcription factors regulating fimbrial adhesin expression in uropathogenic Escherichia coli. FEBS J. 2010, 277, 3368–3381. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, T.K.; Valtonen, M.V.; Parkkinen, J.; Väisänen-Rhen, V.; Finne, J.; Orskov, F.; Orskov, I.; Svenson, S.B.; Mäkelä, P.H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Med. Microbiol. Immunol. 1985, 48, 486–491. [Google Scholar] [CrossRef]
- Ott, M.; Hacker, J.; Schmoll, T.; Jarchau, T.; Korhonen, T.; Goebel, W. Analysis of the genetic determinants coding for the S-fimbrial adhesin (sfa) in different Escherichia coli strains causing meningitis or urinary tract infections. Infect. Immun. 1986, 54, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Balsalobre, C.; Morschhäuser, J.; Jass, J.; Hacker, J.; Uhlin, B.E. Transcriptional analysis of the sfa determinant revealing multiple mRNA processing events in the biogenesis of S fimbriae in pathogenic Escherichia coli. J. Bacteriol. 2003, 185, 620–629. [Google Scholar] [CrossRef]
- Morschhäuser, J.; Uhlin, B.-E.; Hacker, J. Transcriptional analysis and regulation of the sfa determinant coding for S fimbriae of pathogenic Escherichia coli strains. Mol. Gen. Genet. MGG 1993, 238, 97–105. [Google Scholar] [CrossRef]
- Korhonen, T.; Uhlin, B.E.; Hacker, J. Regulation and Binding Properdes of S Fimbriae Cloned from E. coli Strains Causing Urinary Tract Infection and Meningitis. Zentralbl. Bakteriol. 1993, 278, 165–176. [Google Scholar] [CrossRef]
- Van der Woude, M.; Low, D. Leucine-responsive regulatory protein and deoxyadenosine methylase control the phase variation and expression of the sfa and daa pili operons in Escherichia coli. Mol. Microbiol. 1994, 11, 605–618. [Google Scholar] [CrossRef]
- Monárrez, R.; Okeke, I.N. A plasmid-encoded papB paralogue modulates autoaggregation of Escherichia coli transconjugants. BMC Res. Notes 2020, 13, 565. [Google Scholar] [CrossRef]
- Woodward, M.J.; Allen-Vercoe, E.; Redstone, J. Distribution, gene sequence and expression in vivo of the plasmid encoded fimbrial antigen of Salmonella serotype Enteritidis. Epidemiol. Infect. 1996, 117, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Escobar, G.A.; Grépinet, O.; Raymond, P.; Abed, N.; Velge, P.; Virlogeux-Payant, I. H-NS is the major repressor of Salmonella Typhimurium Pef fimbriae expression. Virulence 2019, 10, 849–867. [Google Scholar] [CrossRef]
- Nicholson, B.; Low, D. DNA methylation-dependent regulation of pef expression in Salmonella typhimurium. Mol. Microbiol. 2000, 35, 728–742. [Google Scholar] [CrossRef]
- Luterbach, C.L.; Forsyth, V.S.; Engstrom, M.D.; Mobley, H.L. TosR-mediated regulation of adhesins and biofilm formation in uropathogenic Escherichia coli. Msphere 2018, 3, e00222-18. [Google Scholar] [CrossRef]
- Holden, N.J.; Uhlin, B.E.; Gally, D.L. PapB paralogues and their effect on the phase variation of type 1 fimbriae in Escherichia coli. Mol. Microbiol. 2001, 42, 319–330. [Google Scholar] [CrossRef]
- Loomis, W.P.; Koo, J.T.; Cheung, T.P.; Moseley, S.L. A tripeptide sequence within the nascent DaaP protein is required for mRNA processing of a fimbrial operon in Escherichia coli. Mol. Microbiol. 2001, 39, 693–707. [Google Scholar] [CrossRef]
- Koo, J.T. Identification of Factors Involved in Processing of mRNA in a Fimbrial Operon of Escherichia coli. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 2004. [Google Scholar]
- Martin, C. The clp (CS31A) operon is negatively controlled by Lrp, ClpB, and L-alanine at the transcriptional level. Mol. Microbiol. 1996, 21, 281–292. [Google Scholar] [CrossRef]
- Braaten, B.A.; Platko, J.V.; van der Woude, M.W.; Simons, B.H.; de Graaf, F.K.; Calvo, J.M.; Low, D.A. Leucine-responsive regulatory protein controls the expression of both the pap and fan pili operons in Escherichia coli. Proc. Natl. Acad. Sci. USA 1992, 89, 4250–4254. [Google Scholar] [CrossRef]
- Roosendaal, E.; Boots, M.; de Graaf, F.K. Two novel genes, fanA and fanB, involved in the biogenesis of K99 fimbriae. Nucleic Acids Res. 1987, 15, 5973–5984. [Google Scholar] [CrossRef]
- Alvarez-Fraga, L.; Phan, M.-D.; Goh, K.G.; Nhu, N.T.K.; Hancock, S.J.; Allsopp, L.P.; Peters, K.M.; Forde, B.M.; Roberts, L.W.; Sullivan, M.J. Differential Afa/Dr fimbriae expression in the multidrug-resistant Escherichia coli ST131 clone. mBio 2022, 13, e03519-21. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, B.; Selvarangan, R.; Nowicki, S. Family of Escherichia coli Dr adhesins: Decay-accelerating factor receptor recognition and invasiveness. J. Infect. Dis. 2001, 183 (Suppl. 1), S24–S27. [Google Scholar] [CrossRef]
- Le Bouguenec, C.; Archambaud, M.; Labigne, A. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1189–1193. [Google Scholar] [CrossRef]
- Lalioui, L.; Le Bouguénec, C. afa-8 Gene cluster is carried by a pathogenicity island inserted into the tRNA(Phe) of human and bovine pathogenic Escherichia coli isolates. Infect. Immun. 2001, 69, 937–948. [Google Scholar] [CrossRef]
- Xia, Y.; Gally, D.; Forsman-Semb, K.; Uhlin, B.E. Regulatory cross-talk between adhesin operons in Escherichia coli: Inhibition of type 1 fimbriae expression by the PapB protein. EMBO J. 2000, 19, 1450–1457. [Google Scholar] [CrossRef]
- Boisen, N.; Struve, C.; Scheutz, F.; Krogfelt, K.A.; Nataro, J.P. New adhesin of enteroaggregative Escherichia coli related to the Afa/Dr/AAF family. Infect. Immun. 2008, 76, 3281–3292. [Google Scholar] [CrossRef]
- Klemm, P. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 1986, 5, 1389–1393. [Google Scholar] [CrossRef]
- Eisenstein, B.I. Type 1 fimbriae of Escherichia coli: Genetic regulation, morphogenesis, and role in pathogenesis. Rev. Infect. Dis. 1988, 10 (Suppl. 2), S341–S344. [Google Scholar] [CrossRef]
- Behzadi, P. Classical chaperone-usher (CU) adhesive fimbriome: Uropathogenic Escherichia coli (UPEC) and urinary tract infections (UTIs). Folia Microbiol. 2020, 65, 45–65. [Google Scholar] [CrossRef]
- Elpers, L.; Hensel, M. Expression and functional characterization of various chaperon-usher fimbriae, curli fimbriae, and type 4 pili of enterohemorrhagic Escherichia coli O157: H7 Sakai. Front. Microbiol. 2020, 11, 378. [Google Scholar] [CrossRef]
- Engstrom, M.D.; Mobley, H.L. Regulation of expression of uropathogenic Escherichia coli nonfimbrial adhesin TosA by PapB homolog TosR in conjunction with H-NS and Lrp. Infect. Immun. 2016, 84, 811–821. [Google Scholar] [CrossRef]
- Bessaiah, H.; Anamalé, C.; Sung, J.; Dozois, C.M. What flips the switch? Signals and stress regulating extraintestinal pathogenic Escherichia coli type 1 fimbriae (pili). Microorganisms 2021, 10, 5. [Google Scholar] [CrossRef]
- Göransson, M.; Forsman, K.; Uhlin, B.E. Functional and structural homology among regulatory cistrons of pili-adhesin determinants in Escherichia coli. Mol. Gen. Genet. MGG 1988, 212, 412–417. [Google Scholar] [CrossRef]
- Isidro-Coxca, M.I.; Ortiz-Jiménez, S.; Puente, J.L. Type 1 fimbria and P pili: Regulatory mechanisms of the prototypical members of the chaperone-usher fimbrial family. Arch. Microbiol. 2024, 206, 373. [Google Scholar] [CrossRef]
- Hirakawa, H.; Shimokawa, M.; Noguchi, K.; Tago, M.; Matsuda, H.; Takita, A.; Suzue, K.; Tajima, H.; Kawagishi, I.; Tomita, H. The PapB/FocB family protein TosR acts as a positive regulator of flagellar expression and is required for optimal virulence of uropathogenic Escherichia coli. Front. Microbiol. 2023, 14, 1185804. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).