Dynamics of Water Quality and Microbial Communities in the Middle Route of the South-to-North Water Diversion Project: Characterization and Driving Mechanisms
Abstract
1. Introduction
2. Materials and Methods
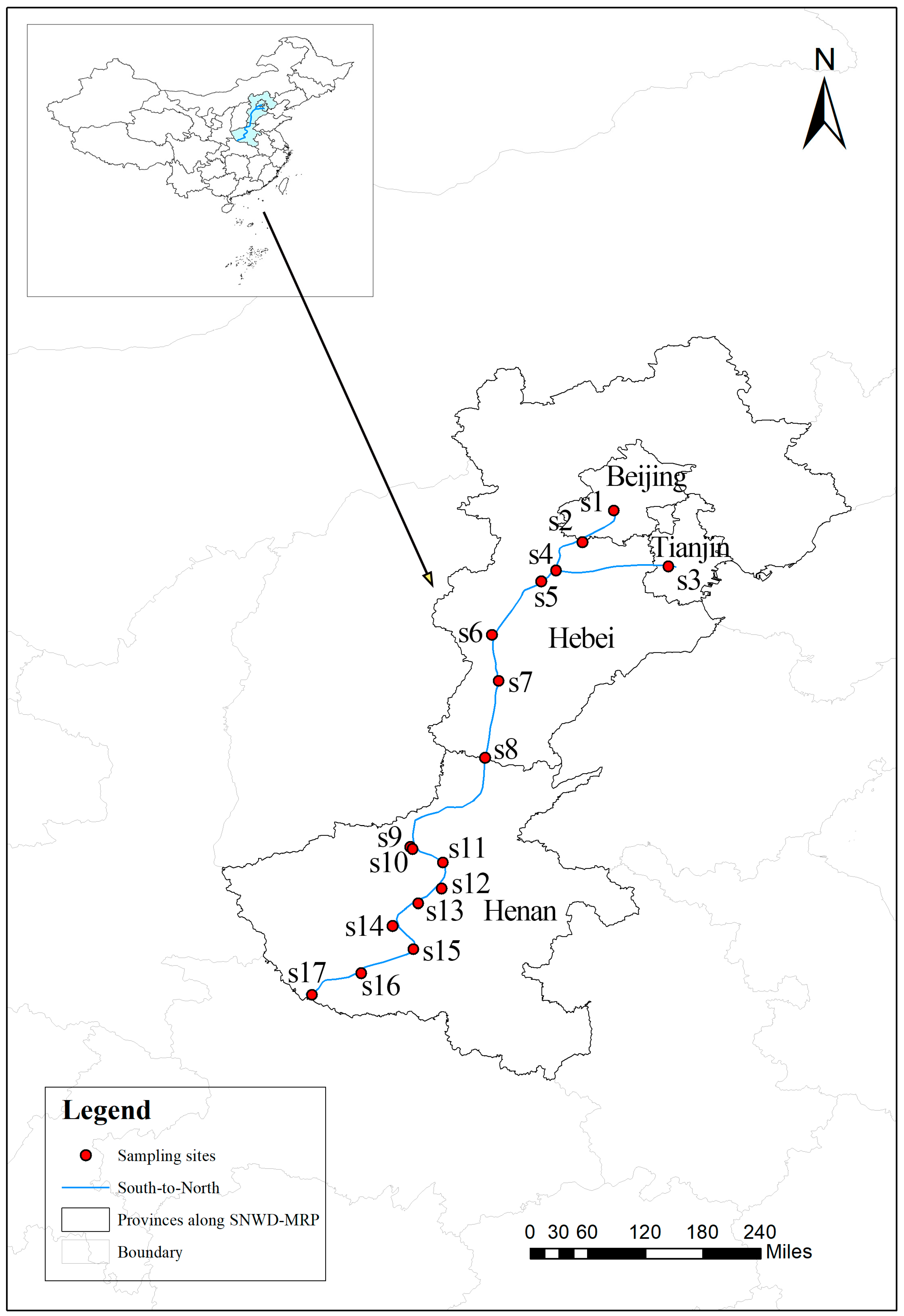
2.1. Study Area and Sampling
2.2. DNA Extraction, Sequencing, and Processing
2.3. Bioinformatics Processing
2.4. Statistical Analysis
3. Results
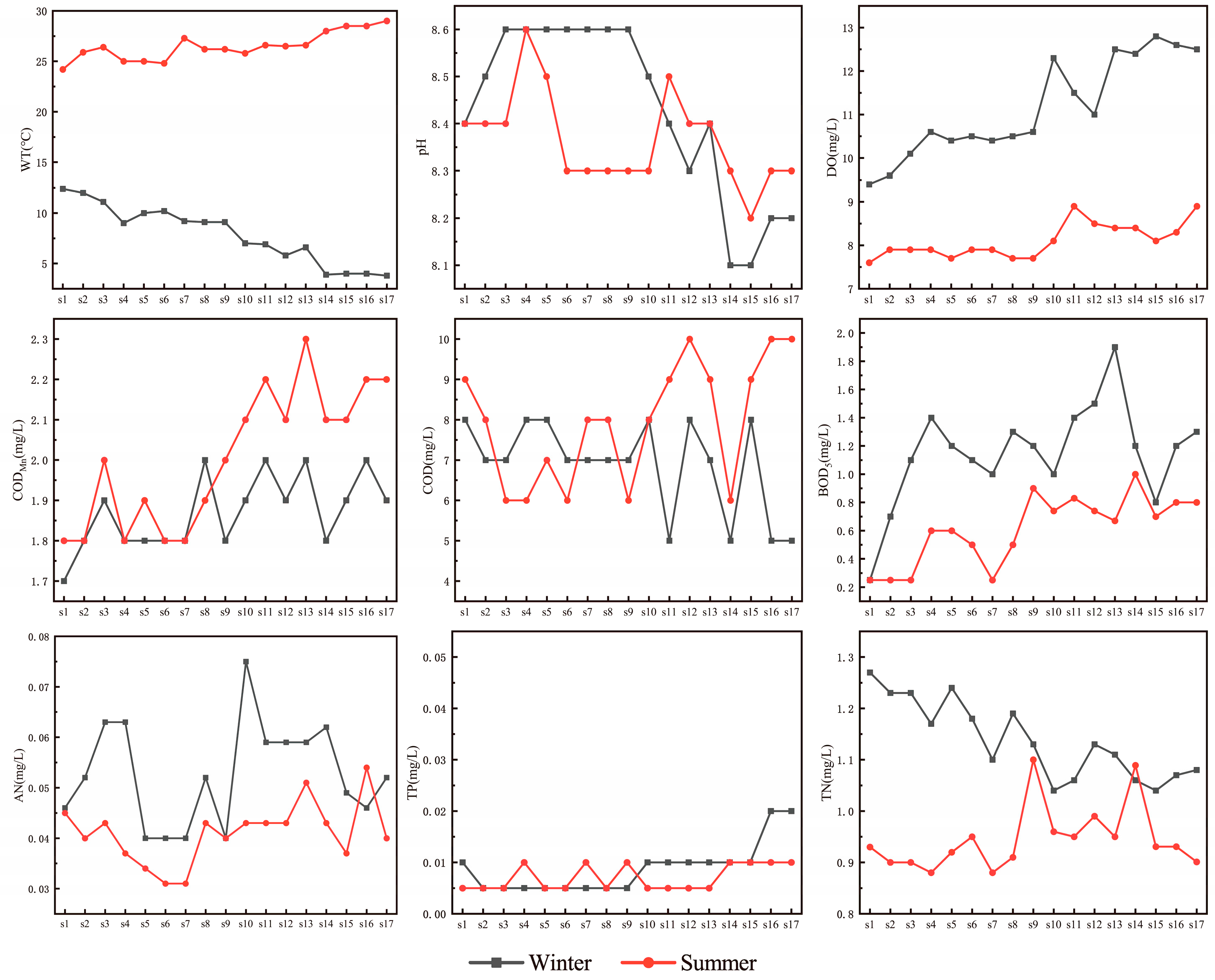
3.1. Spatiotemporal Variations in Water Quality Parameters Along the SNWD-MRP
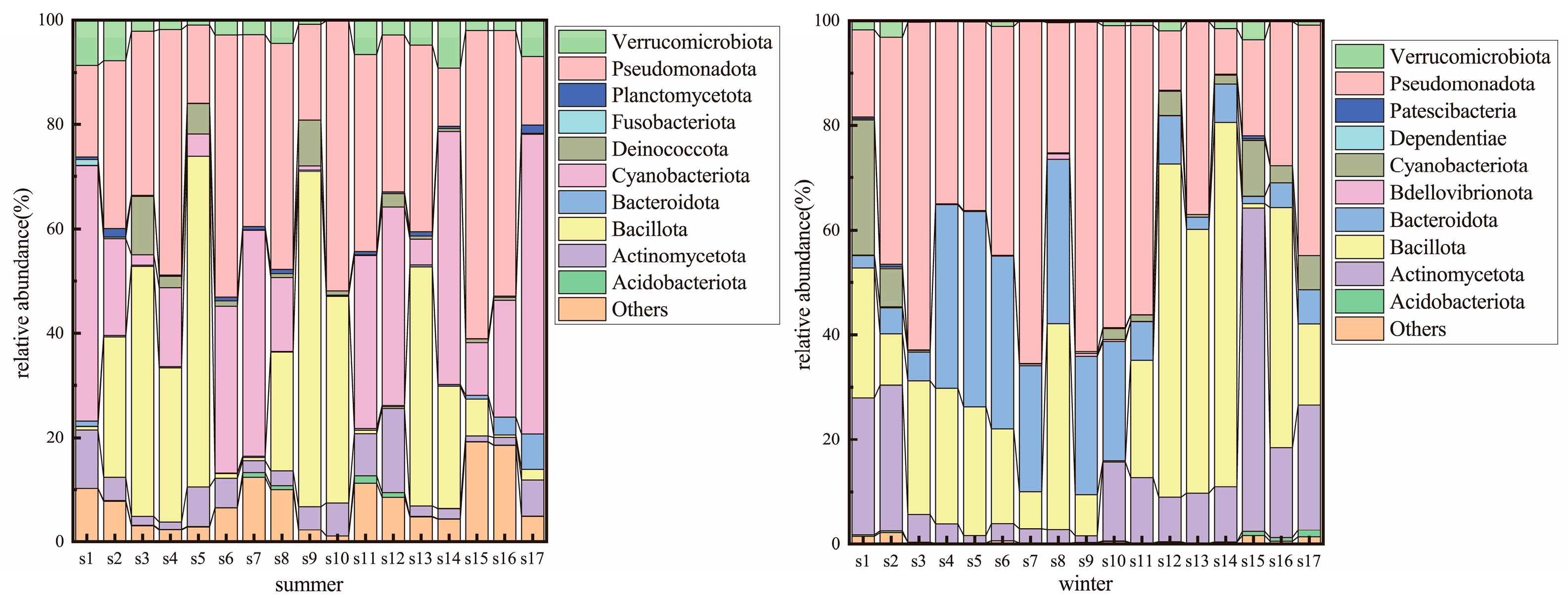
3.2. Microbial Community Composition Along the SNWD-MRP
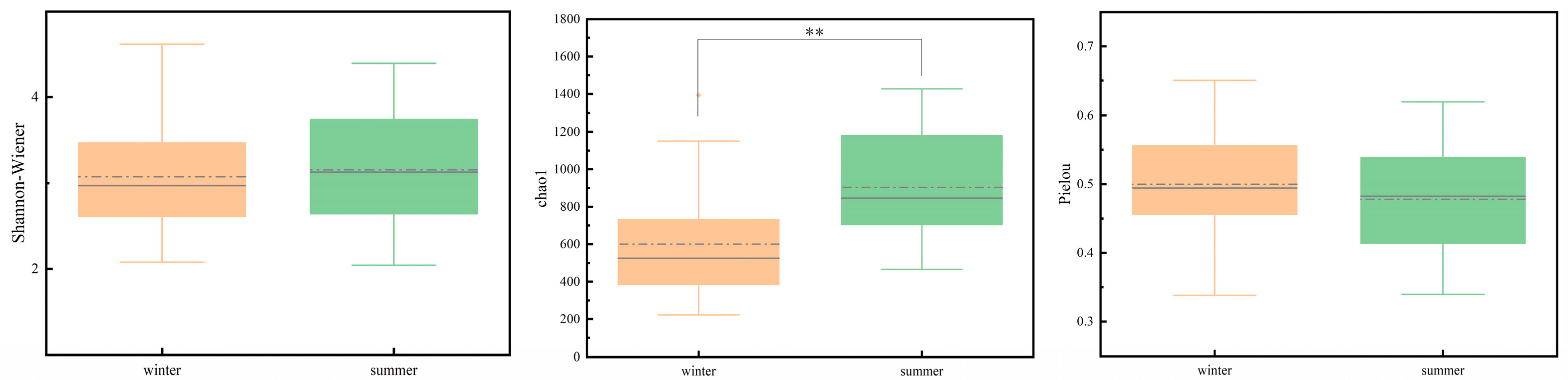
3.3. Microbial Diversity Patterns
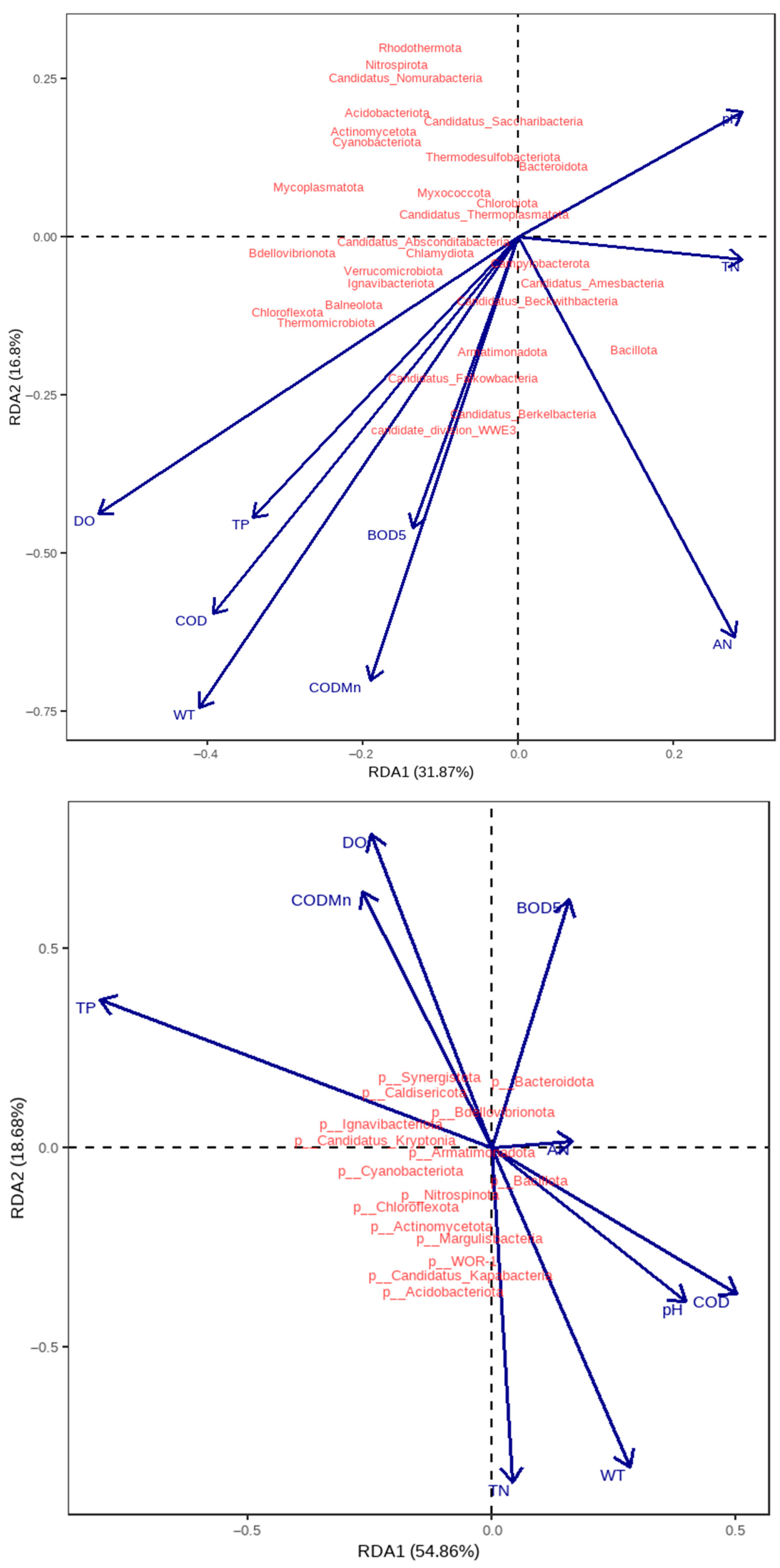
3.4. Impacts of Water Quality Factors on Microbial Communities
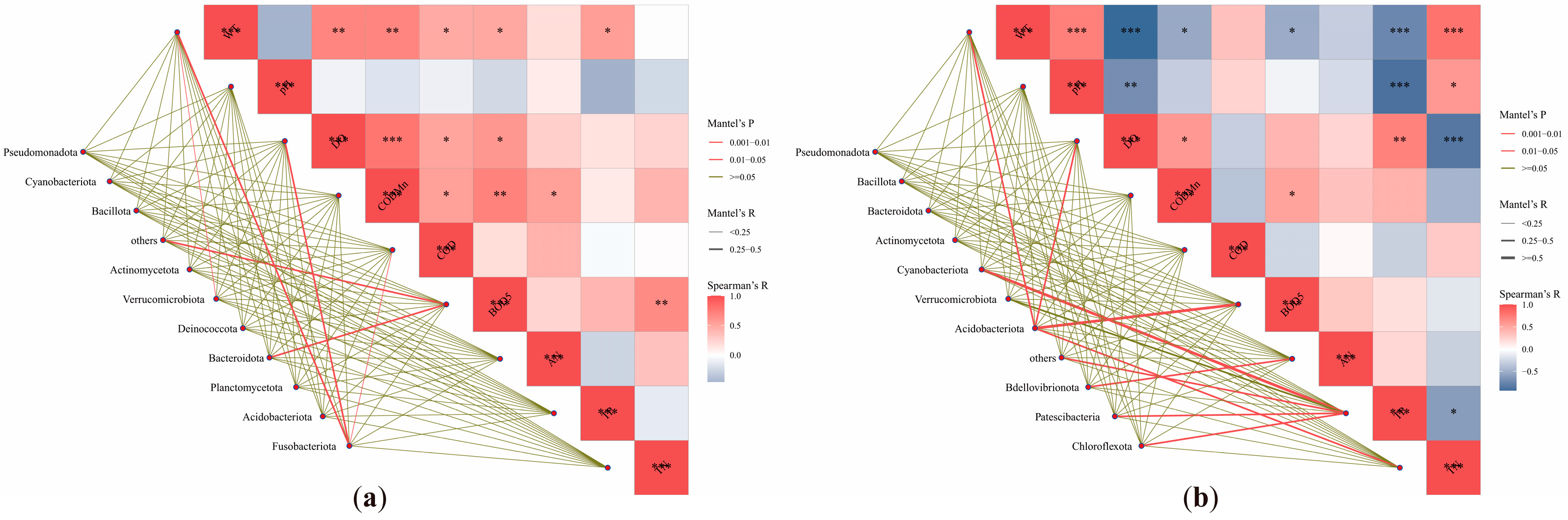
3.5. Microbial Community-Environment Interactions
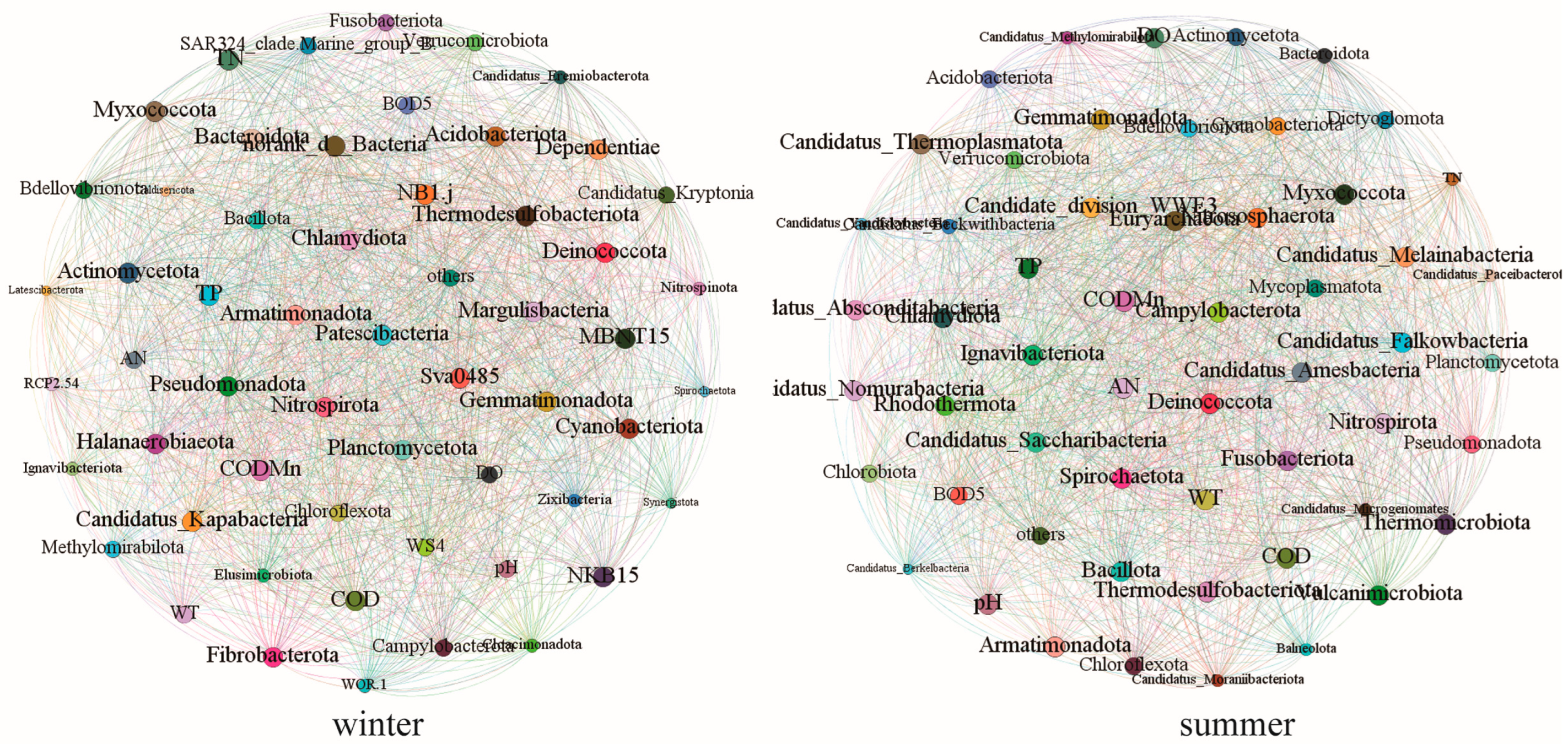
3.6. Seasonal Characteristics of Ecological Networks Between Water Quality and Microbial Communities
4. Discussion
4.1. Temporal and Spatial Differences in Microbial Communities
4.2. Water Quality-Microbial Community Interactions
4.3. Seasonal Drivers of Water Quality–Microbial Networks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madsen, E.L. Microorganisms and Their Roles in Fundamental Biogeochemical Cycles. Curr. Opin. Biotechnol. 2011, 22, 456–464. [Google Scholar] [CrossRef]
- Zhi, E.; Song, Y.; Duan, L.; Yu, H.; Peng, J. Spatial Distribution and Diversity of Microbial Community in Large-Scale Constructed Wetland of the Liao River Conservation Area. Environ. Earth Sci. 2015, 73, 5085–5094. [Google Scholar] [CrossRef]
- Hu, Y.-X.; Zhang, J.; Huang, J.; Duan, C.-J.; Li, T.-C.; Liu, W.; Wang, Y.-C.; Hu, S. Characteristics of Bacterioplankton Community between River and Lake/Reservoir in the Yangtze River Basin. Environ. Sci. 2022, 43, 1414–1423. [Google Scholar]
- Findlay, S. Stream Microbial Ecology. J. N. Am. Benthol. Soc. 2010, 29, 170–181. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Yu, X.; Chen, G.; Yu, Z. Patterns in the Composition of Microbial Communities from a Subtropical River: Effects of Environmental, Spatial and Temporal Factors. PLoS ONE 2013, 8, e81232. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, L.; Zhang, X.; He, M.; Wang, D.; Ren, Y.; Yao, H.; Pan, H. Others Effects of Different Types of Anthropogenic Disturbances and Natural Wetlands on Water Quality and Microbial Communities in a Typical Black-Odor River. Ecol. Indic. 2022, 136, 108613. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; Liu, L.; Zhang, W.; Guo, P. Algae Community and Trophic State of Subtropical Reservoirs in Southeast Fujian, China. Environ. Sci. Pollut. Res. 2012, 19, 1432–1442. [Google Scholar] [CrossRef]
- Hu, Y.; Peng, Y.; Li, R.; Huang, J.; Zhou, Z.; Hu, S.; Wang, Y.; Qiu, G. Plankton Diversity and Community Characteristics in Danjiangkou Reservoir Based on Environmental DNA Metabarcoding. J. Lake Sci. 2021, 33, 1650–1659. [Google Scholar] [CrossRef]
- Buysschaert, B.; Favere, J.; Vermijs, L.; Baetens, V.; Naka, A.; Boon, N.; De Gusseme, B. Flow Cytometric Fingerprinting to Assess the Microbial Community Response to Changing Water Quality and Additives. Environ. Sci. Water Res. Technol. 2019, 5, 1672–1682. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Wang, Y.; Hu, S.; Zhang, J.; Huang, X.; Li, R.; Hu, Y.; Yao, H.; Wang, Z. Microbiome Analysis in Asia’s Largest Watershed Reveals Inconsistent Biogeographic Pattern and Microbial Assembly Mechanisms in River and Lake Systems. iScience 2024, 27, 110053. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, X.; Xing, W.; Hu, Z.; Liu, G. Marker Gene Analysis Reveals the Spatial and Seasonal Variations in the Eukaryotic Phytoplankton Community Composition in the Yangtze River, Three Gorges Reservoir, China. J. Plankton Res. 2019, 41, 835–848. [Google Scholar] [CrossRef]
- Staley, C.; Gould, T.J.; Wang, P.; Phillips, J.; Cotner, J.B.; Sadowsky, M.J. Species Sorting and Seasonal Dynamics Primarily Shape Bacterial Communities in the Upper Mississippi River. Sci. Total Environ. 2015, 505, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-X.; Cao, L.; Qu, R.-C.; Huang, J.; Hu, S.; Zhou, Z.; Wang, Y.-C.; Zhang, J. Community Composition and Assessment of the Aquatic Ecosystem of Periphytic Algae in the Yangtze River Basin. Environ. Sci. 2022, 43, 3998–4007. [Google Scholar]
- Wan, W.; Gadd, G.M.; Gu, J.-D.; He, D.; Liu, W.; Yuan, W.; Ye, L.; Yang, Y. Dredging Alleviates Cyanobacterial Blooms by Weakening Diversity Maintenance of Bacterioplankton Community. Water Res. 2021, 202, 117449. [Google Scholar] [CrossRef]
- Kosten, S.; Huszar, V.L.M.; Bécares, E.; Costa, L.S.; Van Donk, E.; Hansson, L.; Jeppesen, E.; Kruk, C.; Lacerot, G.; Mazzeo, N.; et al. Warmer Climates Boost Cyanobacterial Dominance in Shallow Lakes. Glob. Change Biol. 2012, 18, 118–126. [Google Scholar] [CrossRef]
- Han, B.; Meng, N.; Zhang, J.; Cai, W.; Wu, T.; Kong, L.; Ouyang, Z. Assessment and Management of Pressure on Water Quality Protection along the Middle Route of the South-to-North Water Diversion Project. Sustainability 2019, 11, 3087. [Google Scholar] [CrossRef]
- Ren, H. Others Spatial Distribution and Variation of Suspended Solids in the Main Channel of the Middle Route of the South-to North Water Diversion Project and Relationships with Phytoplankton Community. Resour. Environ. Yangtze Basin 2022, 31, 2473–2480. [Google Scholar]
- Qian, J.; Pu, N.; Qian, L.; Xue, X.; Bi, Y.; Norra, S. Identification of Driving Factors of Algal Growth in the South-to-North Water Diversion Project by Transformer-Based Deep Learning. Water Biol. Secur. 2023, 2, 100184. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, B.; Zhu, X.; Zhao, X.; Sun, H.; He, H.; Jiang, W. Patterns of Microbial Communities and Their Relationships with Water Quality in a Large-Scale Water Transfer System. J. Environ. Manag. 2022, 319, 115678. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, W.; Wang, C.; Zhang, A.; Zhang, H.; Zhang, T.; Ju, F. Untangling Microbiota Diversity and Assembly Patterns in the World’s Largest Water Diversion Canal. Water Res. 2021, 204, 117617. [Google Scholar] [CrossRef]
- Huang, S.; Wang, H.; Tang, Y.; Wang, Z.; Li, G.; Li, D. New Insights into the Assembly Processes of Biofilm Microbiota Communities: Taking the World’s Largest Water Diversion Canal as a Case Study. Sci. Total Environ. 2025, 968, 178827. [Google Scholar] [CrossRef]
- Chambers, P. Standard Methods for the Examination of Water and Wastewater; Scientific e-Resources: Washington, DC, USA, 2019. [Google Scholar]
- GB 3838-2002; The Environmental Quality Standards for Surface Water in China. National Standard of the People’s Republic of China: Beijing, China, 2002.
- Hu, Y.; Zhang, J.; Wang, Y.; Hu, S. Distinct Mechanisms Shape Prokaryotic Community Assembly across Different Land-Use Intensification. Water Res. 2023, 245, 120601. [Google Scholar] [CrossRef]
- Hu, Y.; Su, H.; Song, H.; Xiong, Q.; Zhou, Z.; Huang, B.; Wang, X.; Xiao, N.; Yu, M. Synergistic Impacts of Anthropogenic and Climatic Drivers on Total Phosphorus Dynamics in a Mega River Basin. Environ. Technol. Innov. 2025, 39, 104259. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Huang, J.; Zhou, M.; Hu, S. The Biogeography of Colonial Volvocine Algae in the Yangtze River Basin. Front. Microbiol. 2023, 14, 1078081. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, J.; Pan, C.; Xu, S.; Wu, Y.; Lv, W.; Hong, M.; Hu, Y.; Wang, Y. Assessing Fish Diversity in the Chishui River Using Environmental DNA (eDNA) Metabarcoding. Fishes 2025, 10, 279. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Huang, J.; Hu, S. Environmental Drivers and Aquatic Ecosystem Assessment of Periphytic Algae at Inflow Rivers in Six Lakes over the Yangtze River Basin. Water 2022, 14, 2184. [Google Scholar] [CrossRef]
- Kelly, L.A.; Hassall, C. The Spatial Ecology of Phytoplankton Blooms in UK Canals. Inland Waters 2018, 8, 422–433. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, F.; Wang, Y.; Zhu, Y.; Song, G.; Mi, W.; Bi, Y. Assembly Processes of Eukaryotic Plankton Communities in the World’s Largest Drinking Water Diversion Project. Sci. Total Environ. 2023, 884, 163665. [Google Scholar] [CrossRef]
- Zhu, X.; Chang, W.; Kong, Y.; Cai, Y.; Huang, Z.; Wu, T.; Zhang, M.; Nie, H.; Wang, Y. Effects of Low Temperature on the Microbial Community of MBBR Filler Biofilm. Water Sci. Technol. 2024, 90, 3166–3179. [Google Scholar] [CrossRef]
- Cai, W.; Huang, Q.; Li, H.; Cheng, H.; Li, Y.; Hu, J. Longitudinal Patterns of Microbial Communities in the Water Diversion Rivers of South-to-North Water Diversion Project. CLEAN–Soil Air Water 2022, 50, 2100303. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, S.; Ma, B.; Huang, T.; Kosolapov, D.B.; Ma, M.; Liu, X.; Liu, H.; Liu, X. Multivariate Statistical and Bioinformatic Analyses for the Seasonal Variations of Actinobacterial Community Structures in a Drinking Water Reservoir. J. Environ. Sci. 2024, 137, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Ding, L.; Hu, H.; Ma, H.; Xu, K.; Huang, H.; Geng, J.; Ren, H. Cell Membrane Characteristics and Microbial Population Distribution of MBBR and IFAS with Different Dissolved Oxygen Concentration. Bioresour. Technol. 2018, 265, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, B.; Yang, M.; Li, W.; Shi, X.; Liu, C.-Q. The Different Responses of Planktonic Bacteria and Archaea to Water Temperature Maintain the Stability of Their Community Diversity in Dammed Rivers. Ecol. Process. 2023, 12, 25. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, J.; Tu, Q.; Deng, Y.; Qiu, Q.; Xiong, J. Bacterioplankton Assembly and Interspecies Interaction Indicating Increasing Coastal Eutrophication. Chemosphere 2017, 177, 317–325. [Google Scholar] [CrossRef]
- Niu, A.; Song, L.-Y.; Xiong, Y.-H.; Lu, C.-J.; Junaid, M.; Pei, D.-S. Impact of Water Quality on the Microbial Diversity in the Surface Water along the Three Gorge Reservoir (TGR), China. Ecotoxicol. Environ. Saf. 2019, 181, 412–418. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Z.; Yang, G.; Wu, G.; Yang, Q.; Chen, Q. Microbial Communities and Sediment Nitrogen Cycle in a Coastal Eutrophic Lake with Salinity and Nutrients Shifted by Seawater Intrusion. Environ. Res. 2023, 225, 115590. [Google Scholar] [CrossRef]
- Jia, P.; Tian, M.; Zhang, B.; Wu, X.; He, X.; Zhang, W. Habitat Changes Due to Glacial Freezing and Melting Reshape Microbial Networks. Environ. Int. 2024, 189, 108788. [Google Scholar] [CrossRef]
- Gibson, G.; Carlson, R.; Simpson, J.; Smeltzer, E.; Gerritson, J.; Chapra, S.; Heiskary, S.; Jones, J.; Kennedy, R. Nutrient Criteria Technical Guidance Manual Lake and Reservoirs; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2000.
- Zhang, J.; Hu, Y.; Hu, S.; Huang, J. Environmental Driving Factors and Ecological Assessment of Phytoplankton Communities in the Yangtze River Basin. Environ. Sci. 2023, 44, 2072–2082. [Google Scholar]
| Parameter | Summer | Winter |
|---|---|---|
| nodes number | 55 | 56 |
| edges number | 1464 | 1523 |
| average degree | 53.24 | 54.39 |
| nodes connectivity | 50 | 52 |
| edges connectivity | 50 | 52 |
| average path length | 0.011 | 0.005 |
| graph diameter | 0.044 | 0.048 |
| graph density | 0.986 | 0.989 |
| clustering coefficient | 0.986 | 0.989 |
| betweenness centralization | 2.26 × 10−5 | 1.23 × 10−5 |
| degree_centralization | 0.014 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Chang, Z.; Liu, L.; Li, J.; Gao, J.; Wang, Y.; Su, Y.; Hu, Y.; Peng, Y. Dynamics of Water Quality and Microbial Communities in the Middle Route of the South-to-North Water Diversion Project: Characterization and Driving Mechanisms. Microorganisms 2025, 13, 1895. https://doi.org/10.3390/microorganisms13081895
Liu X, Chang Z, Liu L, Li J, Gao J, Wang Y, Su Y, Hu Y, Peng Y. Dynamics of Water Quality and Microbial Communities in the Middle Route of the South-to-North Water Diversion Project: Characterization and Driving Mechanisms. Microorganisms. 2025; 13(8):1895. https://doi.org/10.3390/microorganisms13081895
Chicago/Turabian StyleLiu, Xinyong, Zhibing Chang, Li Liu, Juechun Li, Jing Gao, Yingcai Wang, Yuming Su, Yuxin Hu, and Yu Peng. 2025. "Dynamics of Water Quality and Microbial Communities in the Middle Route of the South-to-North Water Diversion Project: Characterization and Driving Mechanisms" Microorganisms 13, no. 8: 1895. https://doi.org/10.3390/microorganisms13081895
APA StyleLiu, X., Chang, Z., Liu, L., Li, J., Gao, J., Wang, Y., Su, Y., Hu, Y., & Peng, Y. (2025). Dynamics of Water Quality and Microbial Communities in the Middle Route of the South-to-North Water Diversion Project: Characterization and Driving Mechanisms. Microorganisms, 13(8), 1895. https://doi.org/10.3390/microorganisms13081895





