Investigating the Alleviating Effects of Dihydromyricetin on Subclinical Mastitis in Dairy Cows: Insights from Gut Microbiota and Metabolomic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Design
2.2. Milk Yield and Composition Analysis
2.3. Blood and Feces Specimen Collection
2.4. Serum Biochemical and Antioxidant Indices and Inflammatory Cytokines Measurements
2.5. Gut Microbiota Profiling with 16S rDNA Amplicon Sequencing
2.6. Untargeted Metabolomics Profiling
2.7. Statistical Analysis
3. Results
3.1. Effects of Dietary DMY Supplementation on Milk Composition
3.2. Effects of Dietary DMY Supplementation on Blood Parameters
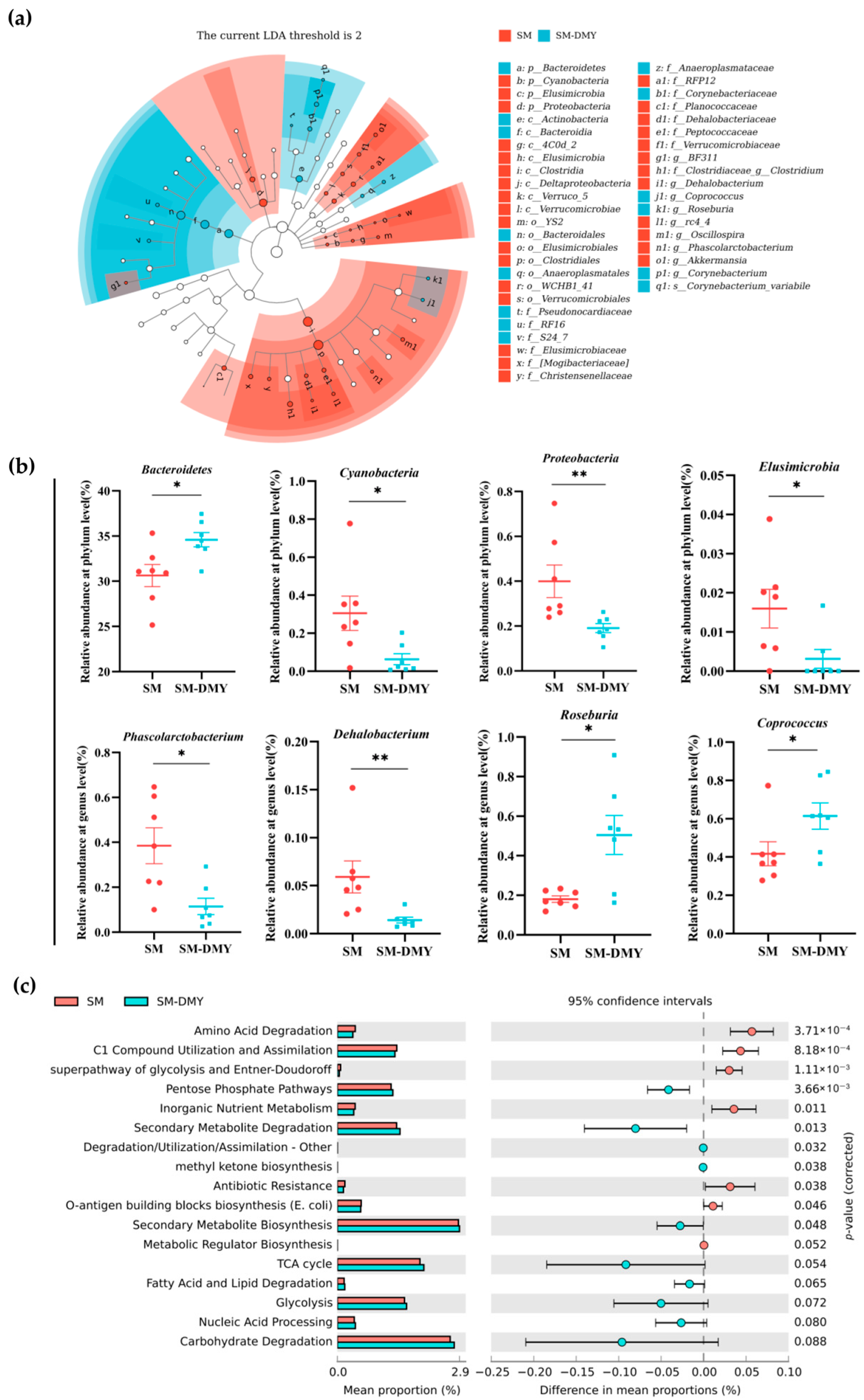
3.3. Effects of Dietary DMY Supplementation on the Diversity and Taxonomic Composition of Gut Microbiota
3.4. Differences in Gut Microbial Composition and Function Between Groups
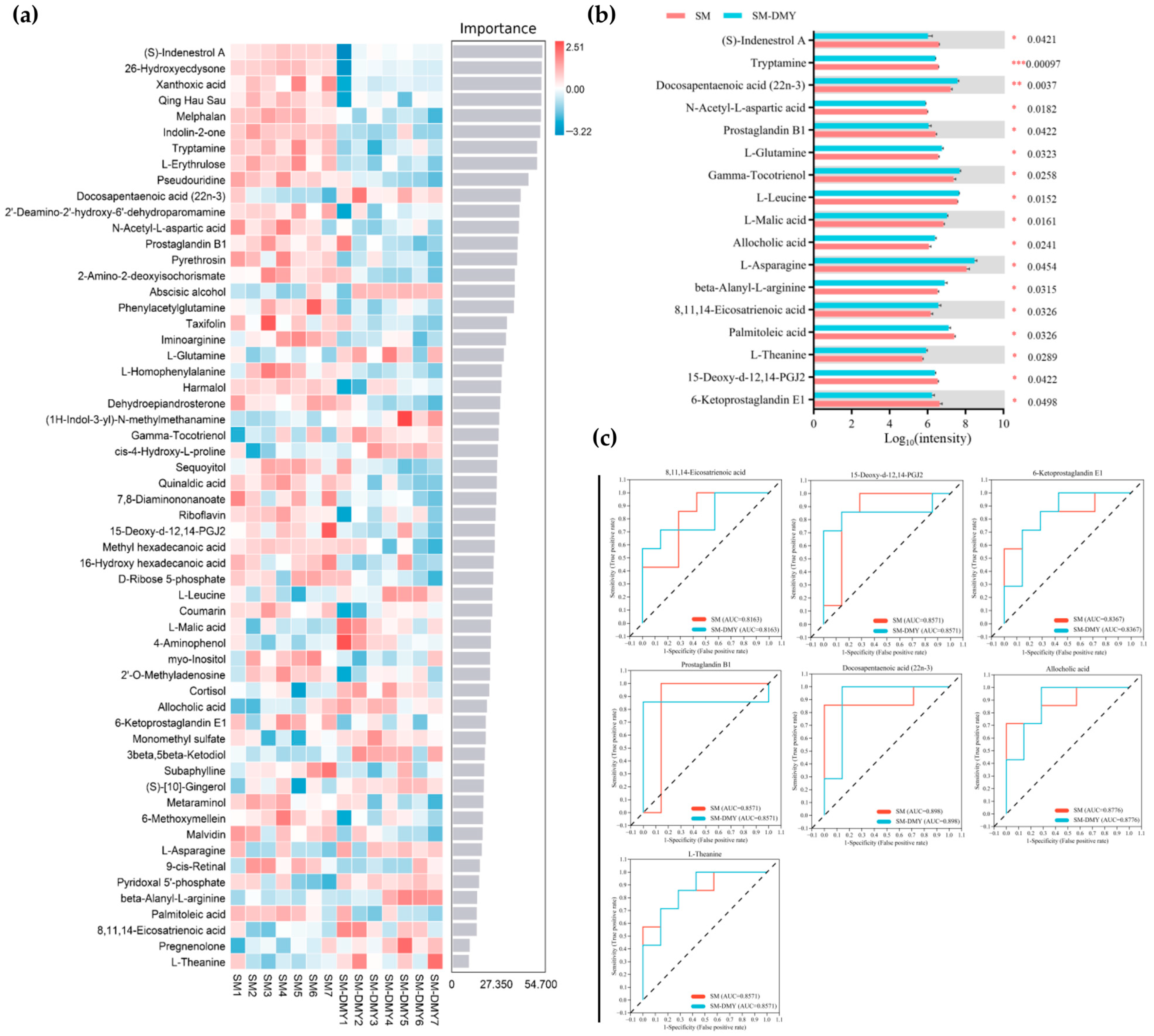
3.5. Effects of Dietary DMY Supplementation on Fecal Metabolomic Profiles
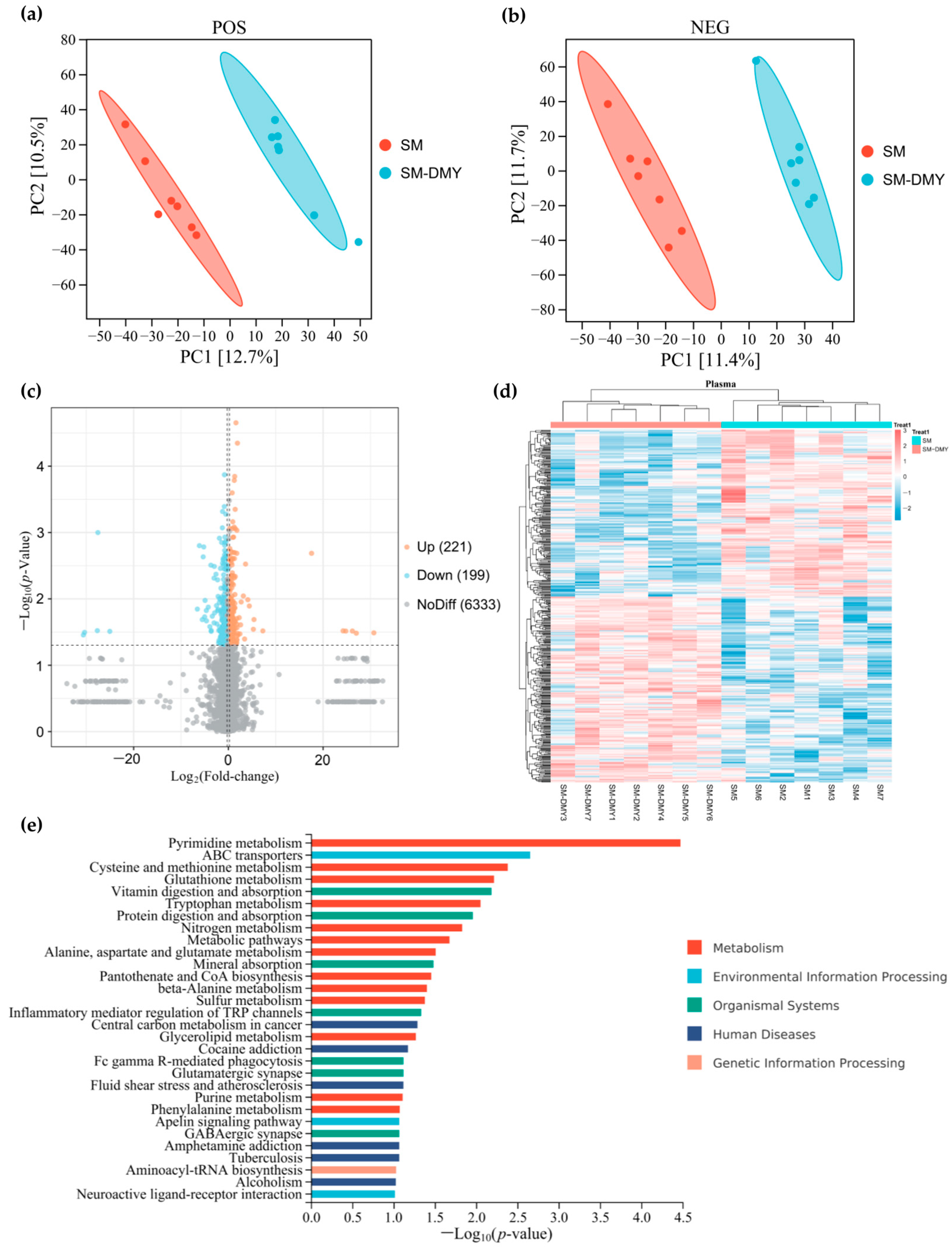
3.6. Effects of Dietary DMY Supplementation on Plasma Metabolomic Profiles
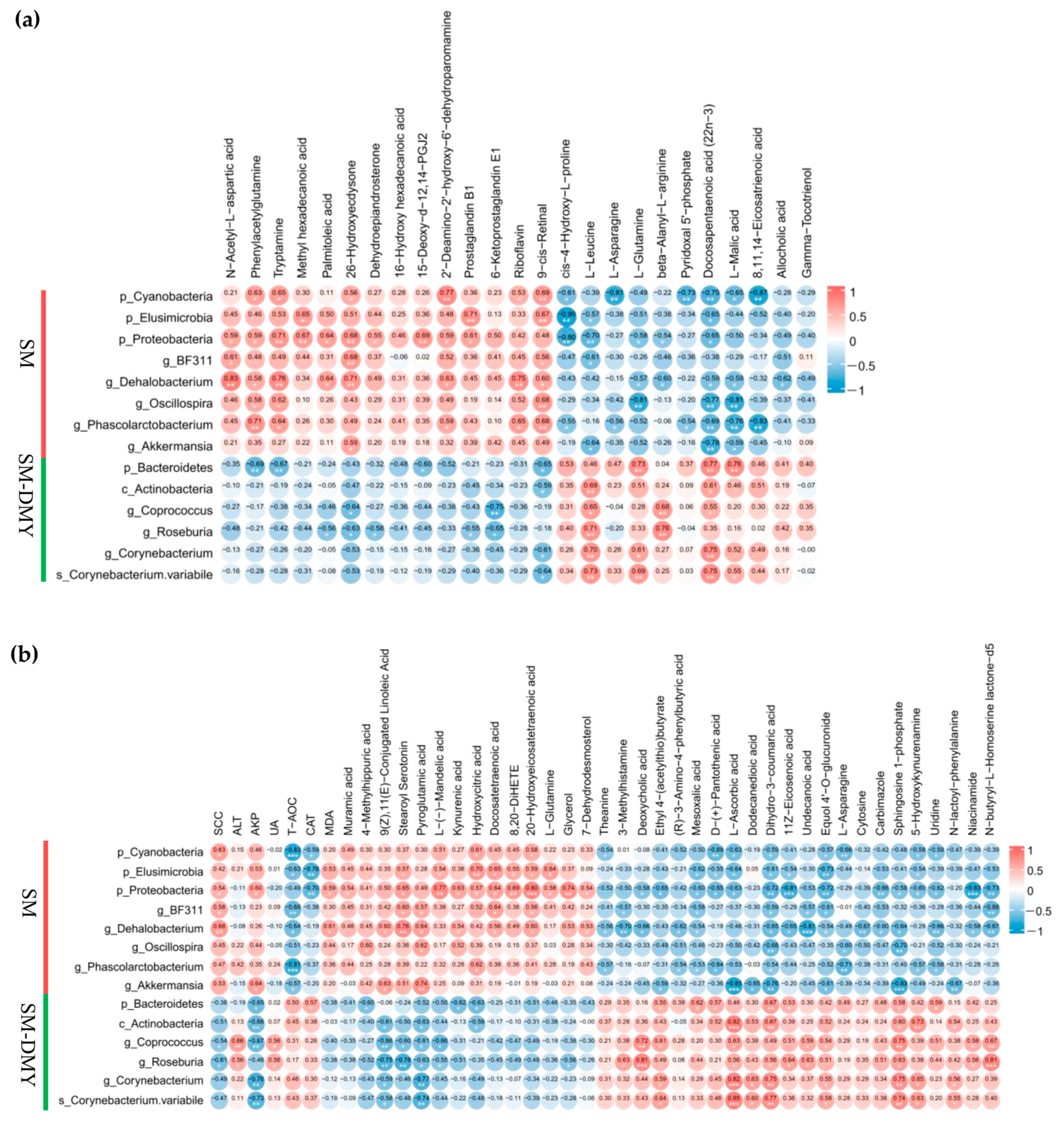
3.7. Associations Between Significantly Different Fecal Microbiome and Metabolites in Feces
3.8. Correlation Analysis Among Significantly Different Fecal Microbiome, Plasma Metabolites, SCC, and Blood Parameters
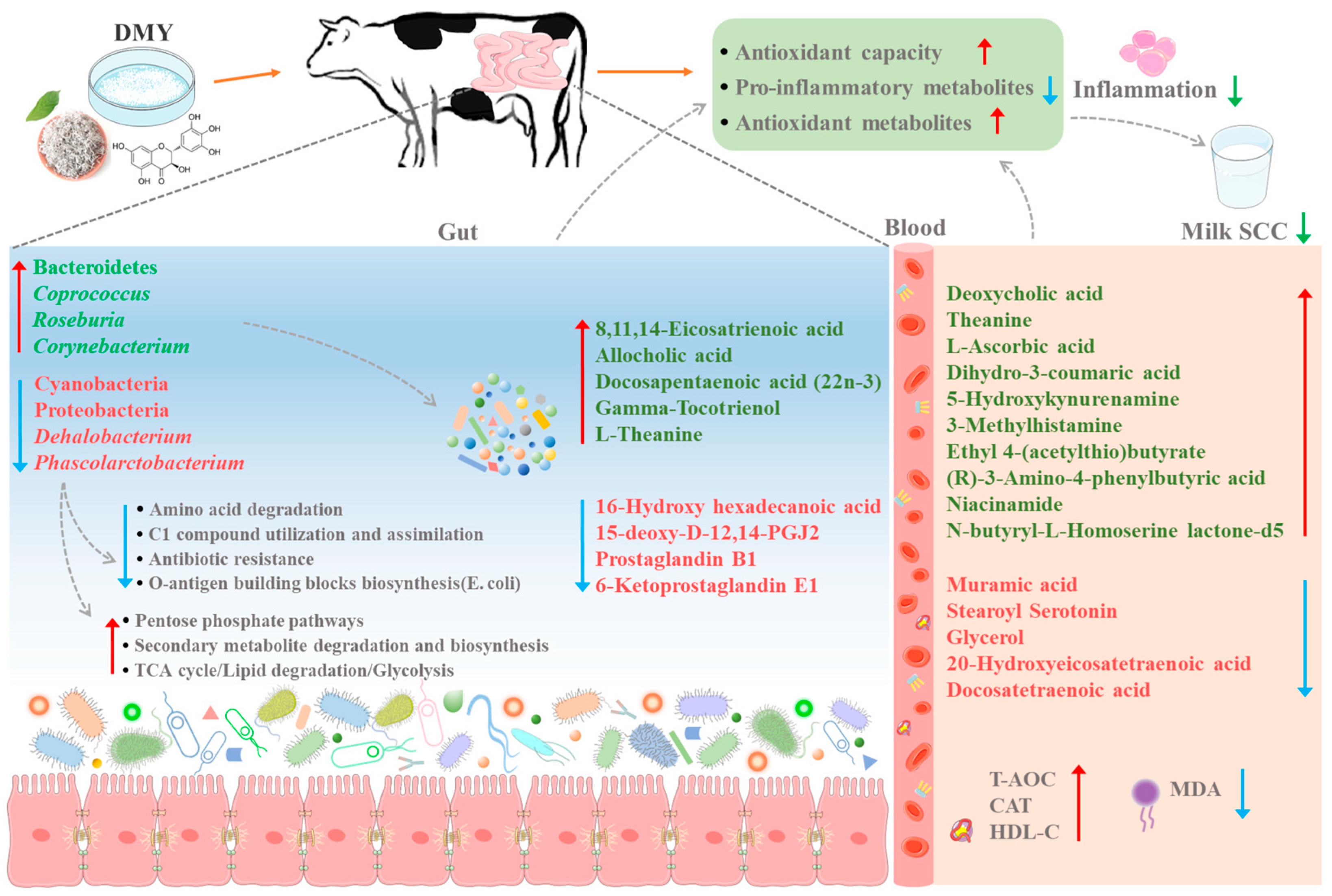
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKP | Alkaline phosphatase |
| ALB | Albumin |
| ASVs | Amplicon sequence variants |
| AUC | Area under the curve |
| CAT | Catalase |
| CM | Clinical mastitis |
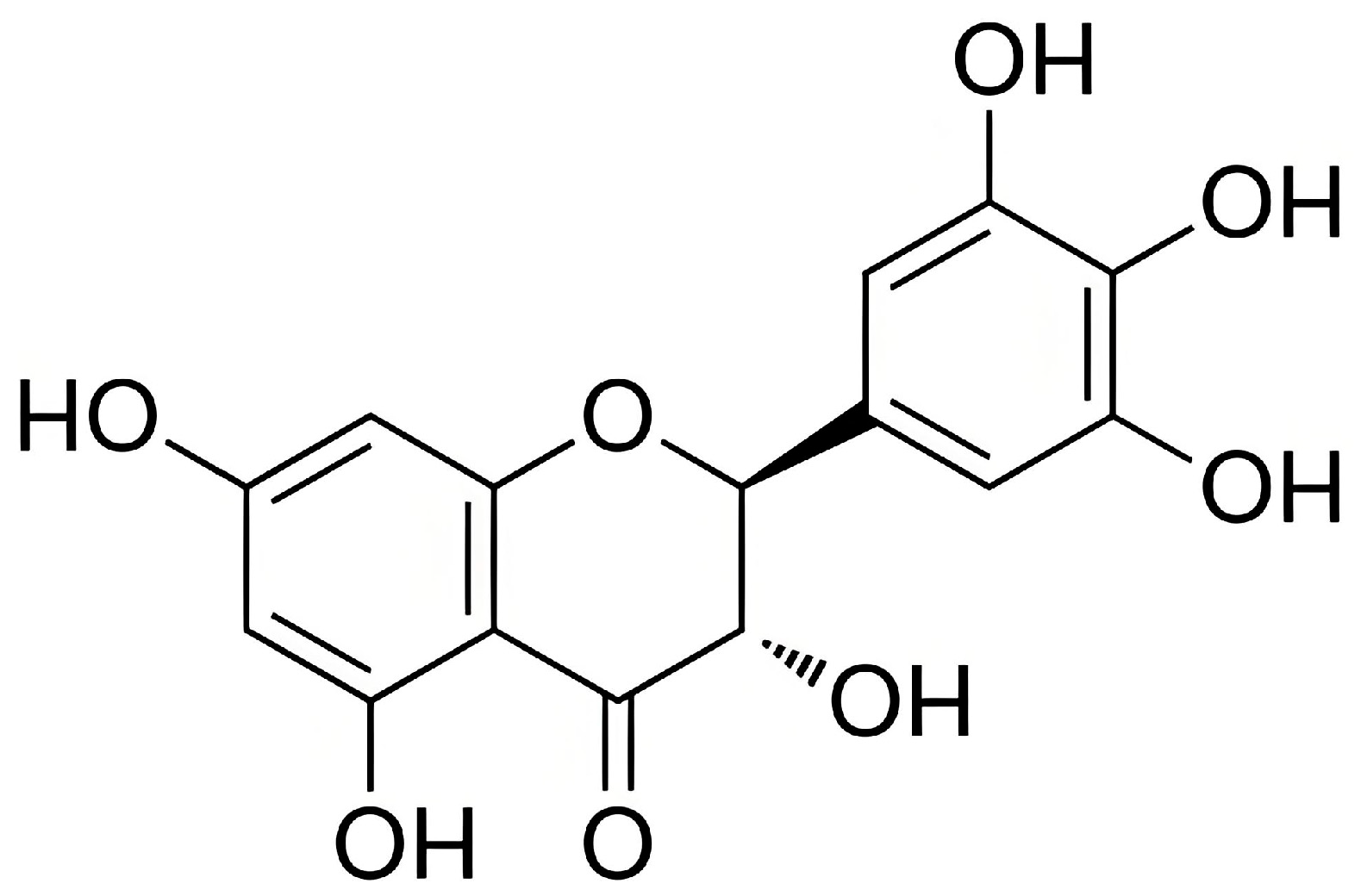
| DMY | Dihydromyricetin |
| DSS | Dextran sulfate sodium |
| GLB | Globulin |
| GSH-PX | Glutathione peroxidase |
| HDL-C | High-density lipoprotein cholesterol |
| LDH | Lactate dehydrogenase |
| LDL-C | Low-density lipoprotein cholesterol |
| LEfSe | Linear discriminant analysis effect size |
| MDA | Malondialdehyde |
| NEFA | Non-esterified fatty acid |
| PCoA | Principal coordinate analysis |
| PICRUSt | Phylogenetic investigation of communities by reconstruction of unobserved states |
| PUFA | Polyunsaturated fatty acid |
| ROC | Receiver operating characteristic |
| SBAs | Secondary bile acids |
| SCC | Somatic cell count |
| SCFAs | Short-chain fatty acids |
| SM | Subclinical mastitis |
| SOD | Superoxide dismutase |
| T-AOC | Total antioxidant capacity |
| TC | Total cholesterol |
| TG | Triglyceride |
| TMR | Total mixed ration |
| TP | Total protein |
| UA | Uric acid |
| VIP | Variable importance in projection |
References
- Angelopoulou, A.; Field, D.; Ryan, C.A.; Stanton, C.; Hill, C.; Ross, R.P. The microbiology and treatment of human mastitis. Med. Microbiol. Immunol. 2018, 207, 83–94. [Google Scholar] [CrossRef]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.P.; Murinda, S.E.; Jayarao, B.M. Impact of antibiotic use in adult dairy cows on antimicrobial resistance of veterinary and human pathogens: A comprehensive review. Foodborne Pathog. Dis. 2011, 8, 337–355. [Google Scholar] [CrossRef] [PubMed]
- De Vliegher, S.; Fox, L.K.; Piepers, S.; McDougall, S.; Barkema, H.W. Invited review: Mastitis in dairy heifers: Nature of the disease, potential impact, prevention, and control. J. Dairy Sci. 2012, 95, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, N.; Laterrière, M.; Gagné, D.; Omonijo, F.; Ibeagha-Awemu, E.M. Multi-omics integration identifies regulatory factors underlying bovine subclinical mastitis. J. Anim. Sci. Biotechnol. 2024, 15, 46. [Google Scholar] [CrossRef]
- Deluyker, H.A.; Chester, S.T.; Van Oye, S.N. A multilocation clinical trial in lactating dairy cows affected with clinical mastitis to compare the efficacy of treatment with intramammary infusions of a lincomycin/neomycin combination with an ampicillin/cloxacillin combination. J. Vet. Pharmacol. Ther. 1999, 22, 274–282. [Google Scholar] [CrossRef]
- Viveros, M.; Lopez Ordaz, R.; Gutiérrez, L.; Miranda Calderón, J.E.; Sumano, H. Efficacy assessment of an intramammary treatment with a new recrystallized enrofloxacin vs ceftiofur and parenteral enrofloxacin in dairy cows with nonsevere clinical mastitis. J. Vet. Pharmacol. Ther. 2018, 41, e1–e9. [Google Scholar] [CrossRef]
- Maalaoui, A.; Trimeche, A.; Marnet, P.G. Alternative approaches to antibiotics in the control of mastitis in dairy cows: A review. Vet. Res. Commun. 2025, 49, 150. [Google Scholar] [CrossRef]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- Lee, J.; Park, J. Host-microbial interactions in metabolic diseases: From diet to immunity. J. Microbiol. 2022, 60, 561–575. [Google Scholar] [CrossRef]
- Ma, C.; Sun, Z.; Zeng, B.; Huang, S.; Zhao, J.; Zhang, Y.; Su, X.; Xu, J.; Wei, H.; Zhang, H. Cow-to-mouse fecal transplantations suggest intestinal microbiome as one cause of mastitis. Microbiome 2018, 6, 200. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Álvarez-Mercado, A.I.; Ruiz-Marín, C.M.; Reina-Pérez, I.; Pérez-Alonso, A.J.; Sánchez-Andujar, M.B.; Torné, P.; Gallart-Aragón, T.; Sánchez-Barrón, M.T.; Reyes Lartategui, S.; et al. Association of breast and gut microbiota dysbiosis and the risk of breast cancer: A case-control clinical study. BMC Cancer 2019, 19, 495. [Google Scholar] [CrossRef] [PubMed]
- Soto-Pantoja, D.R.; Gaber, M.; Arnone, A.A.; Bronson, S.M.; Cruz-Diaz, N.; Wilson, A.S.; Clear, K.Y.J.; Ramirez, M.U.; Kucera, G.L.; Levine, E.A.; et al. Diet alters entero-mammary signaling to regulate the breast microbiome and tumorigenesis. Cancer Res. 2021, 81, 3890–3904. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, H.; Zhang, F.; Hua, D.; Liu, J.; Yang, L.; Yao, J.; et al. Discrepancies among healthy, subclinical mastitic, and clinical mastitic cows in fecal microbiome and metabolome and serum metabolome. J. Dairy Sci. 2022, 105, 7668–7688. [Google Scholar] [CrossRef]
- Du, Q.; Cai, W.; Xia, M.; Ito, Y. Purification of (+)-dihydromyricetin from leaves extract of ampelopsis grossedentata using high-speed countercurrent chromatograph with scale-up triple columns. J. Chromatogr. A 2002, 973, 217–220. [Google Scholar] [CrossRef]
- Liu, D.; Mao, Y.; Ding, L.; Zeng, X.A. Dihydromyricetin: A review on identification and quantification methods, biological activities, chemical stability, metabolism and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2019, 91, 586–597. [Google Scholar] [CrossRef]
- Liang, X.; Wu, Y.P.; Qiu, J.H.; Zhong, K.; Gao, H. A potent antibrowning agent from pine needles of cedrus deodara: 2r,3r-dihydromyricetin. J. Food Sci. 2014, 79, C1643–C1648. [Google Scholar] [CrossRef]
- Mohammed, F.S.; Kına, E.; Uysal, I.; Sevindik, M. Total phenolic, flavonoid contents, antioxidant and antimicrobial activities of hesperis pendula. Prospect. Pharm. Sci. 2023, 21, 57–61. [Google Scholar] [CrossRef]
- Çömlekçioğlu, N.; Aygan, A.; Sevindik, M.; Çömlekçioğlu, U. Comparative analysis of phytochemical properties of extracts obtained from flowers and leaves of astragalus angustifolius collected from different locations. Prospect. Pharm. Sci. 2024, 22, 62–68. [Google Scholar] [CrossRef]
- Xie, K.; He, X.; Chen, K.; Chen, J.; Sakao, K.; Hou, D. Antioxidant properties of a traditional vine tea, ampelopsis grossedentata. Antioxidants 2019, 8, 295. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, S.; Yang, S.; Chen, C.; Yang, Y.; Lin, M.; Liu, C.; Wang, W.; Zhou, X.; Ai, Q.; et al. Mechanism of dihydromyricetin on inflammatory diseases. Front. Pharmacol. 2021, 12, 794563. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, Z.; Yue, Z.; Yang, Z. Research progress of dihydromyricetin in the treatment of diabetes mellitus. Front. Endocrinol. 2023, 14, 1216907. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, J.; Hu, O.; Huang, J.; Ran, L.; Chen, M.; Zhang, Y.; Zhou, X.; Zhu, J.; Zhang, Q.; et al. Dihydromyricetin ameliorates nonalcoholic fatty liver disease by improving mitochondrial respiratory capacity and redox homeostasis through modulation of SIRT3 signaling. Antioxid. Redox Signal. 2019, 30, 163–183. [Google Scholar] [CrossRef]
- Dong, S.; Zhu, M.; Wang, K.; Zhao, X.; Hu, L.; Jing, W.; Lu, H.; Wang, S. Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol. Res. 2021, 171, 105767. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; Yu, J.; Yan, H.; Zheng, P.; Luo, Y. Dietary dihydromyricetin supplementation enhances antioxidant capacity and improves lipid metabolism in finishing pigs. Food Funct. 2021, 12, 6925–6935. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Qi, J.; Deng, L.; Yu, B.; Luo, Y.; Huang, Z.; Mao, X.; Yu, J.; Zheng, P.; Yan, H.; et al. Protective effect of dihydromyricetin on intestinal epithelium in weaned pigs upon enterotoxigenic escherichia coli challenge. Int. Immunopharmacol. 2024, 140, 112806. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Tong, Q.; Dong, W.; Yang, G.; Hou, X.; Xiong, W.; Shi, C.; Fang, J.; Wang, W. Tissue distribution, excretion, and metabolic profile of dihydromyricetin, a flavonoid from vine tea (ampelopsis grossedentata) after oral administration in rats. J. Agric. Food. Chem. 2017, 65, 4597–4604. [Google Scholar] [CrossRef]
- Fang, Y.; Liang, F.; Liu, K.; Qaiser, S.; Pan, S.; Xu, X. Structure characteristics for intestinal uptake of flavonoids in caco-2 cells. Food Res. Int. 2018, 105, 353–360. [Google Scholar] [CrossRef]
- Hou, G.; Xu, C.; Cheng, K.; Mei, S.; Kang, Y.; Zhang, C.; Shang, L.; Chen, S. Metabolic mechanisms of dihydromyricetin and strategies for enhancing its bioavailability: A recent review. Food Chem. 2025, 485, 144470. [Google Scholar] [CrossRef]
- Sharma, C.P.; Kaushal, G.P.; Sareen, V.K.; Singh, S.; Bhatia, I.S. The in vitro metabolism of flavonoids by whole rumen contents and its fractions. Zentralblatt Für Veterinärmedizin Reihe A 1981, 28, 27–34. [Google Scholar] [CrossRef]
- Aldian, D.; Harisa, L.D.; Tomita, H.; Tian, K.; Takashima, S.; Iwasawa, A.; Yayota, M. Insights into rutin and quercetin biotransformations in ruminants revealed by molecular networking. J. Anim. Sci. 2025, 103, skaf154. [Google Scholar] [CrossRef]
- Martins, L.; Barcelos, M.M.; Cue, R.I.; Anderson, K.L.; Santos, M.V.; Gonçalves, J.L. Chronic subclinical mastitis reduces milk and components yield at the cow level. J. Dairy Res. 2020, 87, 298–305. [Google Scholar] [CrossRef]
- Petzer, I.M.; Karzis, J.; Donkin, E.F.; Webb, E.C.; Etter, E.M. Somatic cell count thresholds in composite and quarter milk samples as indicator of bovine intramammary infection status. Onderstepoort J. Vet. Res. 2017, 84, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Turk, R.; Piras, C.; Kovacic, M.; Samardzija, M.; Ahmed, H.; De Canio, M.; Urbani, A.; Mestric, Z.F.; Soggiu, A.; Bonizzi, L.; et al. Proteomics of inflammatory and oxidative stress response in cows with subclinical and clinical mastitis. J. Proteom. 2012, 75, 4412–4428. [Google Scholar] [CrossRef] [PubMed]
- McDougall, E.I. Studies on ruminant saliva. 1. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Sumon, S.M.M.R.; Parvin, M.S.; Ehsan, M.A.; Islam, M.T. Relationship between somatic cell counts and subclinical mastitis in lactating dairy cows. Vet. World 2020, 13, 1709–1713. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Liu, L.; Ma, G.; Zhang, Y.; Ren, J. Effect of moringa leaf flavonoids on the production performance, immune system, and rumen fermentation of dairy cows. Vet. Med. Sci. 2023, 9, 917–923. [Google Scholar] [CrossRef]
- Jiang, B.; Le, L.; Pan, H.; Hu, K.; Xu, L.; Xiao, P. Dihydromyricetin ameliorates the oxidative stress response induced by methylglyoxal via the AMPK/GLUT4 signaling pathway in PC12 cells. Brain Res. Bull. 2014, 109, 117–126. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef]
- Da Silva, I.V.; Mlinaric, M.; Lourenco, A.R.; Perez-Garcia, O.; Cipak Gasparovic, A.; Soveral, G. Peroxiporins and oxidative stress: Promising targets to tackle inflammation and cancer. Int. J. Mol. Sci. 2024, 25, 8381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, M.; Wang, H.; Zhang, F.; Xue, F.; Hua, D.; Liu, J.; et al. Rumen microbiome structure and metabolites activity in dairy cows with clinical and subclinical mastitis. J. Anim. Sci. Biotechnol. 2021, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Mu, Y.; Zhang, D.; Lin, X.; Wang, Z.; Hou, Q.; Wang, Y.; Hu, Z. Determination of microbiological characteristics in the digestive tract of different ruminant species. Microbiologyopen 2019, 8, e00769. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.; Sundralingam, U.; Palanisamy, U.D. Polyphenols as prebiotics in the management of high-fat diet-induced obesity: A systematic review of animal studies. Foods 2021, 10, 299. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-chain fatty-acid-producing bacteria: Key components of the human gut microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Zhao, C.; Bao, L.; Qiu, M.; Wu, K.; Zhao, Y.; Feng, L.; Xiang, K.; Zhang, N.; Hu, X.; Fu, Y. Commensal cow roseburia reduces gut-dysbiosis-induced mastitis through inhibiting bacterial translocation by producing butyrate in mice. Cell Rep. 2022, 41, 111681. [Google Scholar] [CrossRef]
- Mustonen, A.; Nieminen, P. Dihomo-γ-linolenic acid (20:3n-6)—metabolism, derivatives, and potential significance in chronic inflammation. Int. J. Mol. Sci. 2023, 24, 2116. [Google Scholar] [CrossRef]
- Han, X.; Lin, C.; Liu, H.; Li, S.; Hu, B.; Zhang, L. Allocholic acid protects against α-naphthylisothiocyanate-induced cholestasis in mice by ameliorating disordered bile acid homeostasis. J. Appl. Toxicol. 2024, 44, 582–594. [Google Scholar] [CrossRef]
- Mendoza, M.E.; Monte, M.J.; Serrano, M.A.; Pastor-Anglada, M.; Stieger, B.; Meier, P.J.; Medarde, M.; Marin, J.J.G. Physiological characteristics of allo-cholic acid. J. Lipid Res. 2003, 44, 84–92. [Google Scholar] [CrossRef]
- Kaur, G.; Cameron-Smith, D.; Garg, M.; Sinclair, A.J. Docosapentaenoic acid (22:5n-3): A review of its biological effects. Prog. Lipid Res. 2011, 50, 28–34. [Google Scholar] [CrossRef]
- Reiter, E.; Jiang, Q.; Christen, S. Anti-inflammatory properties of α- and γ-tocopherol. Mol. Asp. Med. 2007, 28, 668–691. [Google Scholar] [CrossRef]
- Shrum, S.A.; Nukala, U.; Shrimali, S.; Pineda, E.N.; Krager, K.J.; Thakkar, S.; Jones, D.E.; Pathak, R.; Breen, P.J.; Aykin-Burns, N.; et al. Tocotrienols provide radioprotection to multiple organ systems through complementary mechanisms of antioxidant and signaling effects. Antioxidants 2023, 12, 1987. [Google Scholar] [CrossRef]
- Chen, S.; Kang, J.; Zhu, H.; Wang, K.; Han, Z.; Wang, L.; Liu, J.; Wu, Y.; He, P.; Tu, Y.; et al. L-theanine and immunity: A review. Molecules 2023, 28, 3846. [Google Scholar] [CrossRef]
- Liu, S.; Wang, B.; Lin, L.; Xu, W.; Gong, Z.H.; Xiao, W.J. L-theanine alleviates heat stress through modulation of gut microbiota and immunity. J. Sci. Food Agric. 2024, 104, 2059–2072. [Google Scholar] [CrossRef]
- Aoki, T.; Narumiya, S. Prostaglandins and chronic inflammation. Trends Pharmacol. Sci. 2012, 33, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, Z.; Moaiedi, M.Z.; Gorji, A.V.; Mansouri, E. P-coumaric acid mitigates doxorubicin-induced nephrotoxicity through suppression of oxidative stress, inflammation and apoptosis. Arch. Med. Res. 2020, 51, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Malaczewska, J.; Siwicki, A.K.; Wojcik, R.M.; Kaczorek, E.; Turski, W.A. Effect of oral administration of kynurenic acid on the activity of the peripheral blood leukocytes in mice. Central Eur. J. Immunol. 2014, 39, 6–13. [Google Scholar] [CrossRef]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic acid as a ligand for orphan g protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef]
- Zhao, M.; Li, W.; Liu, K.; Li, H.; Lan, X. C4-HSL aptamers for blocking qurom sensing and inhibiting biofilm formation in pseudomonas aeruginosa and its structure prediction and analysis. PLoS ONE 2019, 14, e0212041. [Google Scholar] [CrossRef]
- Garcia, V.; Gilani, A.; Shkolnik, B.; Pandey, V.; Zhang, F.F.; Dakarapu, R.; Gandham, S.K.; Reddy, N.R.; Graves, J.P.; Gruzdev, A.; et al. 20-HETE signals through g-protein–coupled receptor GPR75 (gq) to affect vascular function and trigger hypertension. Circ. Res. 2017, 120, 1776–1788. [Google Scholar] [CrossRef] [PubMed]
- Pascale, J.V.; Park, E.J.; Adebesin, A.M.; Falck, J.R.; Schwartzman, M.L.; Garcia, V. Uncovering the signalling, structure and function of the 20-HETE-GPR75 pairing: Identifying the chemokine CCL5 as a negative regulator of GPR75. Br. J. Pharmacol. 2021, 178, 3813–3828. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Ishikawa, H.; Beppu, F.; Gotoh, N. Incorporation of dietary arachidonic and docosatetraenoic acid into mouse brain. J. Agric. Food Chem. 2021, 69, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, D.; Lu, D.; Zhang, R.; Zhao, Y.; Zhong, Q.; Ji, S.; Wang, L.; Tang, D. Lipid metabolism disorders and lipid mediator changes of mice in response to long-term exposure to high-fat and high sucrose diets and ameliorative effects of mulberry leaves. Food Funct. 2022, 13, 4576–4591. [Google Scholar] [CrossRef]
- Nunzi, E.; Pariano, M.; Costantini, C.; Garaci, E.; Puccetti, P.; Romani, L. Host–microbe serotonin metabolism. Trends Endocrinol. Metab. 2025, 36, 83–95. [Google Scholar] [CrossRef]
- Lappalainen, M.H.; Hyvarinen, A.; Hirvonen, M.R.; Rintala, H.; Roivainen, J.; Renz, H.; Pfefferle, P.I.; Nevalainen, A.; Roponen, M.; Pekkanen, J. High indoor microbial levels are associated with reduced th1 cytokine secretion capacity in infancy. Int. Arch. Allergy Immunol. 2012, 159, 194–203. [Google Scholar] [CrossRef]
- Yu, J.; Liu, C.; Wang, D.; Wan, P.; Cheng, L.; Yan, X. Integrated microbiome and metabolome analysis reveals altered gut microbial communities and metabolite profiles in dairy cows with subclinical mastitis. BMC Microbiol. 2025, 25, 115. [Google Scholar] [CrossRef]



| Items | Time | SM | SM-DMY | p-Value |
|---|---|---|---|---|
| Milk yield (kg/d) | 0 d | 19.48 ± 1.98 | 19.53 ± 1.99 | 0.9860 |
| 60 d | 13.73 ± 2.83 | 15.87 ± 1.17 | 0.4283 | |
| Milk fat (%) | 0 d | 4.28 ± 0.38 | 3.89 ± 0.29 | 0.4225 |
| 60 d | 3.62 ± 0.55 | 3.81 ± 0.21 | 0.7090 | |
| Milk protein (%) | 0 d | 3.30 ± 0.10 | 3.28 ± 0.078 | 0.8978 |
| 60 d | 3.44 ± 0.074 | 3.34 ± 0.055 | 0.2979 | |
| F/P | 0 d | 1.30 ± 0.11 | 1.20 ± 0.11 | 0.5075 |
| 60 d | 1.05 ± 0.14 | 1.14 ± 0.057 | 0.4895 | |
| SCC (×104 cells/mL) | 0 d | 64 ± 9.0 | 64 ± 9.7 | 0.9873 |
| 60 d | 183 ± 113.0 a | 28 ± 13.4 b | 0.0064 | |
| MUN (mg/dL) | 0 d | 16.27 ± 0.31 | 16.32 ± 0.37 | 0.9187 |
| 60 d | 19.00 ± 0.80 | 19.60 ± 0.73 | 0.6112 |
| Items | SM | SM-DMY | p-Value |
|---|---|---|---|
| TP (g/L) | 71.34 ± 1.50 | 74.61 ± 1.39 | 0.1357 |
| ALB (g/L) | 32.79 ± 0.85 | 33.50 ± 0.56 | 0.4976 |
| GLB (g/L) | 38.56 ± 1.94 | 41.11 ± 1.63 | 0.3336 |
| AST (U/L) | 73.96 ± 8.54 | 69.07 ± 3.23 | 0.6024 |
| ALT (U/L) | 26.24 ± 1.86 | 28.49 ± 1.83 | 0.4064 |
| AKP (U/L) | 355.66 ± 38.94 | 273.15 ± 20.74 | 0.0860 |
| BUN (mmol/L) | 6.32 ± 0.38 | 6.11 ± 0.32 | 0.6857 |
| UA (µmol/L) | 30.60 ± 3.55 | 35.91 ± 2.45 | 0.2411 |
| TG (mmol/L) | 0.18 ± 0.035 | 0.12 ± 0.0088 | 0.1405 |
| TC (mmol/L) | 2.49 ± 0.23 | 3.03 ± 0.29 | 0.1744 |
| HDL-C (mmol/L) | 1.66 ± 0.15 | 2.10 ± 0.16 | 0.0656 |
| LDL-C (mmol/L) | 0.59 ± 0.063 | 0.72 ± 0.12 | 0.3504 |
| NEFA (mmol/L) | 0.12 ± 0.023 | 0.095 ± 0.014 | 0.4348 |
| LDH (U/L) | 1440.92 ± 66.44 | 1444.62 ± 39.72 | 0.9626 |
| Items | SM | SM-DMY | p-Value |
|---|---|---|---|
| IL-1β (ng/L) | 423.91 ± 36.75 | 451.92 ± 12.44 | 0.4842 |
| IL-2 (ng/L) | 265.40 ± 24.31 | 283.16 ± 11.58 | 0.5221 |
| TNF-α (ng/L) | 165.74 ± 11.60 | 165.72 ± 4.81 | 0.9988 |
| IL-8 (ng/L) | 161.48 ± 9.79 | 175.02 ± 4.77 | 0.2489 |
| IL-10 (ng/L) | 122.13 ± 8.51 | 129.82 ± 8.98 | 0.5458 |
| Items | SM | SM-DMY | p-Value |
|---|---|---|---|
| T-AOC (mM) | 0.68 ± 0.026 a | 0.85 ± 0.039 b | 0.0035 |
| SOD (U/mL) | 13.46 ± 0.79 | 14.41 ± 0.48 | 0.3206 |
| GSH-PX (U/mL) | 239.99 ± 32.82 | 261.06 ± 12.66 | 0.5604 |
| CAT (U/mL) | 1.99 ± 0.41 a | 3.69 ± 0.65 b | 0.0462 |
| MDA (nmol/mL) | 3.11 ± 0.82 a | 1.45 ± 0.28 b | 0.0482 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Ao, Y.; Chen, H.; Deng, T.; Liu, C.; Wang, D.; Wan, P.; Xiang, M.; Cheng, L. Investigating the Alleviating Effects of Dihydromyricetin on Subclinical Mastitis in Dairy Cows: Insights from Gut Microbiota and Metabolomic Analysis. Microorganisms 2025, 13, 1890. https://doi.org/10.3390/microorganisms13081890
Yu J, Ao Y, Chen H, Deng T, Liu C, Wang D, Wan P, Xiang M, Cheng L. Investigating the Alleviating Effects of Dihydromyricetin on Subclinical Mastitis in Dairy Cows: Insights from Gut Microbiota and Metabolomic Analysis. Microorganisms. 2025; 13(8):1890. https://doi.org/10.3390/microorganisms13081890
Chicago/Turabian StyleYu, Jie, Yingnan Ao, Hongbo Chen, Tinxian Deng, Chenhui Liu, Dingfa Wang, Pingmin Wan, Min Xiang, and Lei Cheng. 2025. "Investigating the Alleviating Effects of Dihydromyricetin on Subclinical Mastitis in Dairy Cows: Insights from Gut Microbiota and Metabolomic Analysis" Microorganisms 13, no. 8: 1890. https://doi.org/10.3390/microorganisms13081890
APA StyleYu, J., Ao, Y., Chen, H., Deng, T., Liu, C., Wang, D., Wan, P., Xiang, M., & Cheng, L. (2025). Investigating the Alleviating Effects of Dihydromyricetin on Subclinical Mastitis in Dairy Cows: Insights from Gut Microbiota and Metabolomic Analysis. Microorganisms, 13(8), 1890. https://doi.org/10.3390/microorganisms13081890






