The Role of Plant Growth-Promoting Bacteria in Soil Restoration: A Strategy to Promote Agricultural Sustainability
Abstract
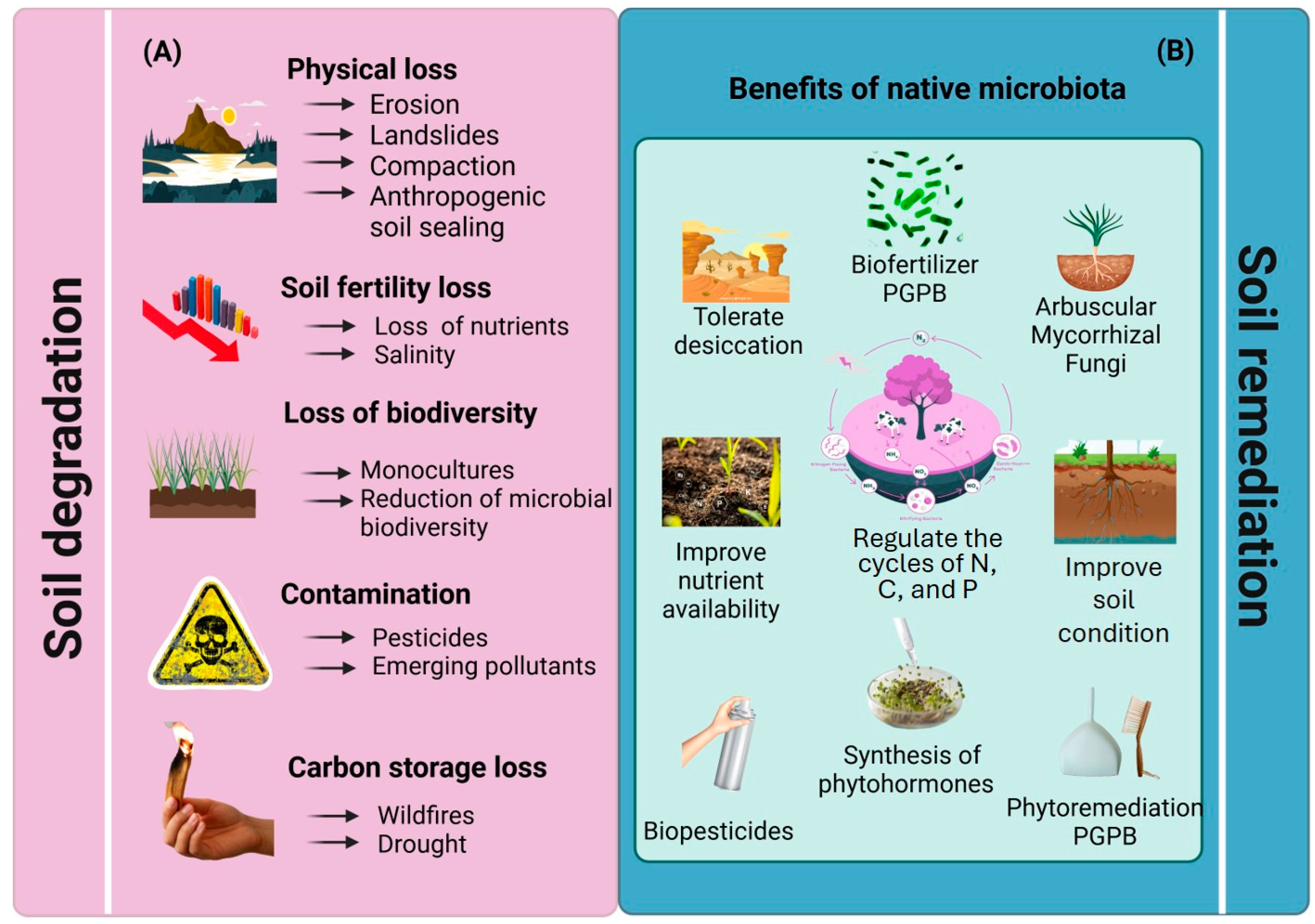
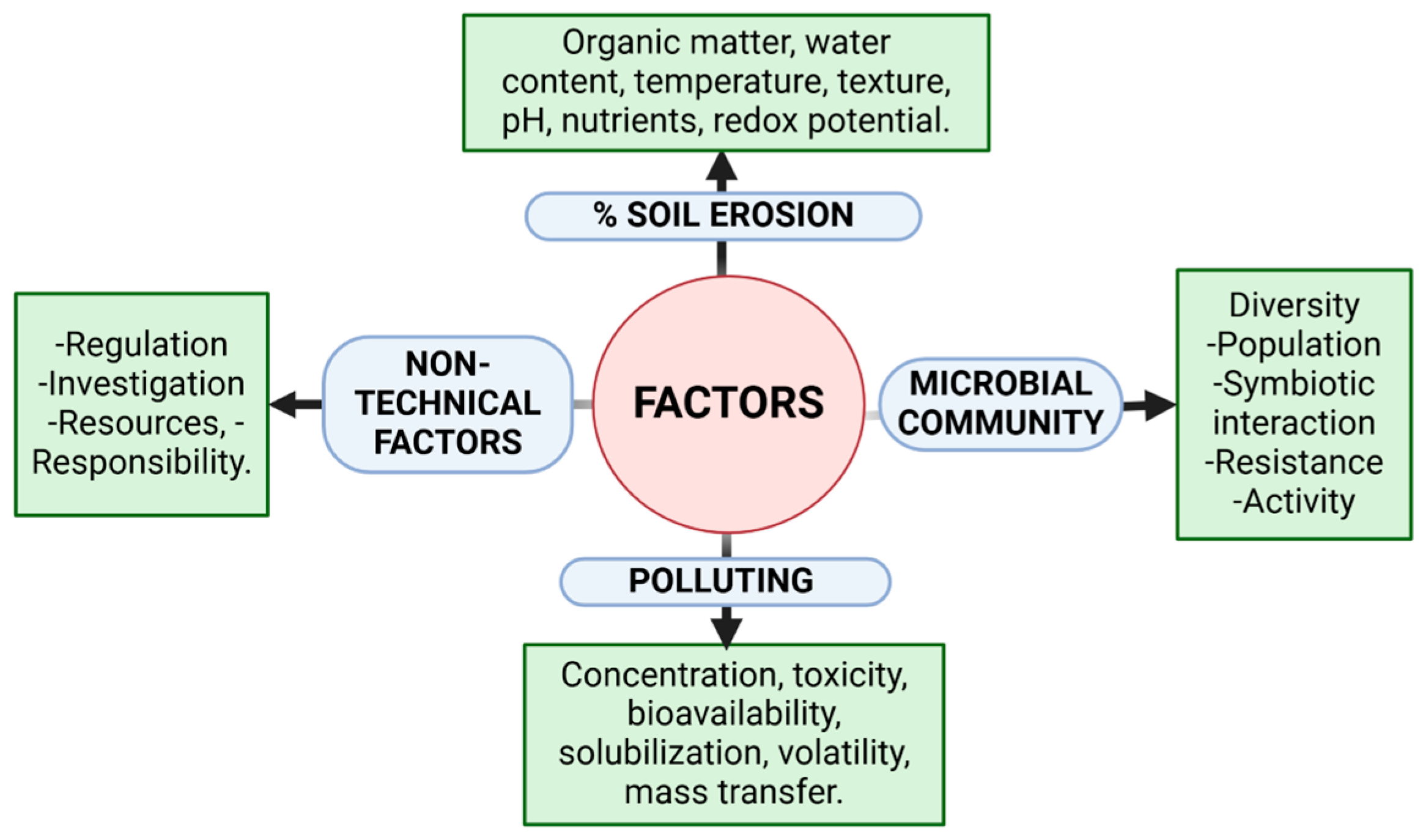
1. Soil Degradation Challenges Stemming from Excessive Agricultural Cultivation
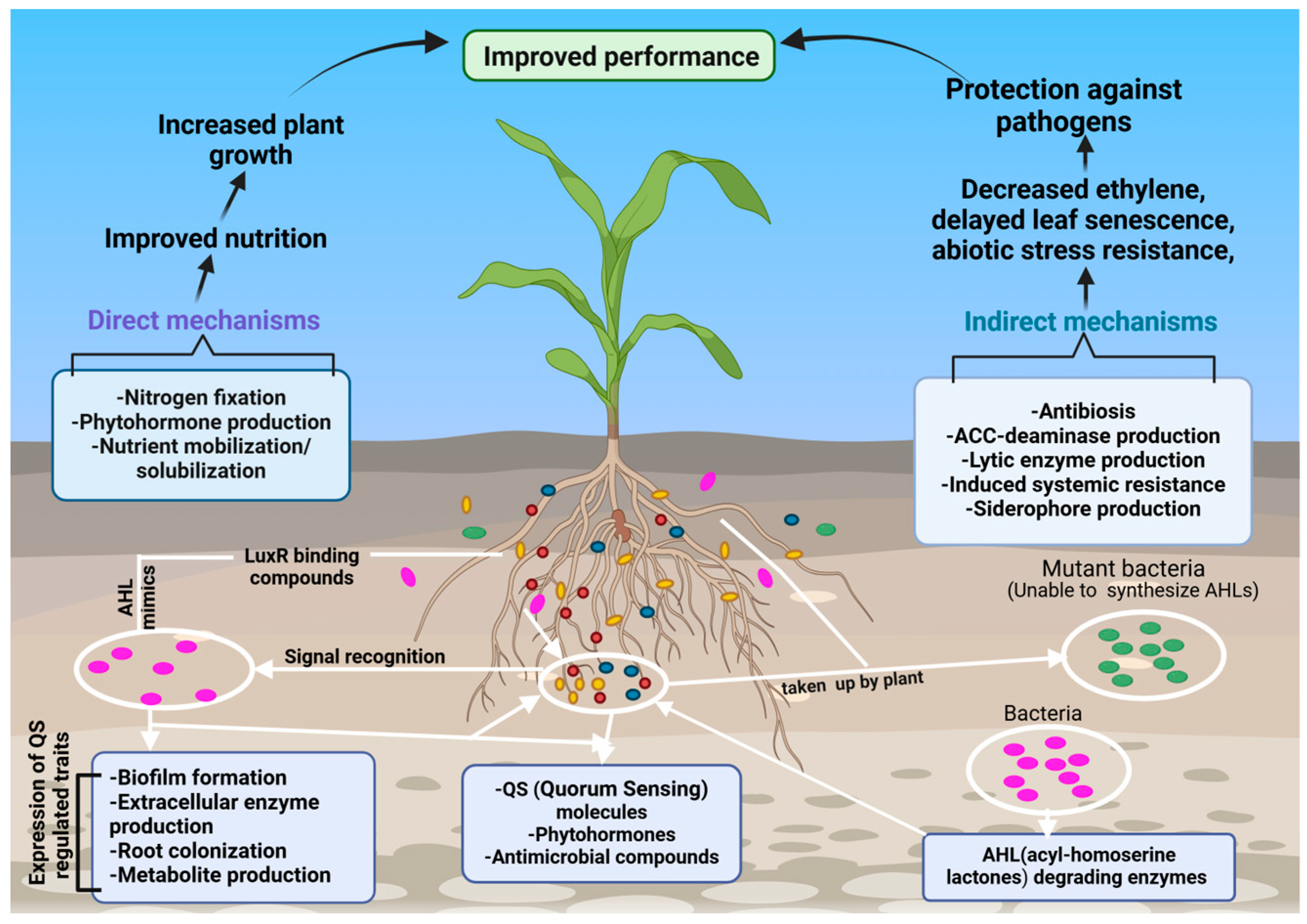
2. Direct and Indirect Mechanisms of Plant Growth-Promoting Bacteria
2.1. Indirect Mechanisms
2.1.1. Siderophores Production
2.1.2. Enzymatic Mechanisms in Indirect Plant Defense
2.2. Direct Mechanisms
2.2.1. Plant Growth Regulators and Their Role in Plant Growth and Signaling
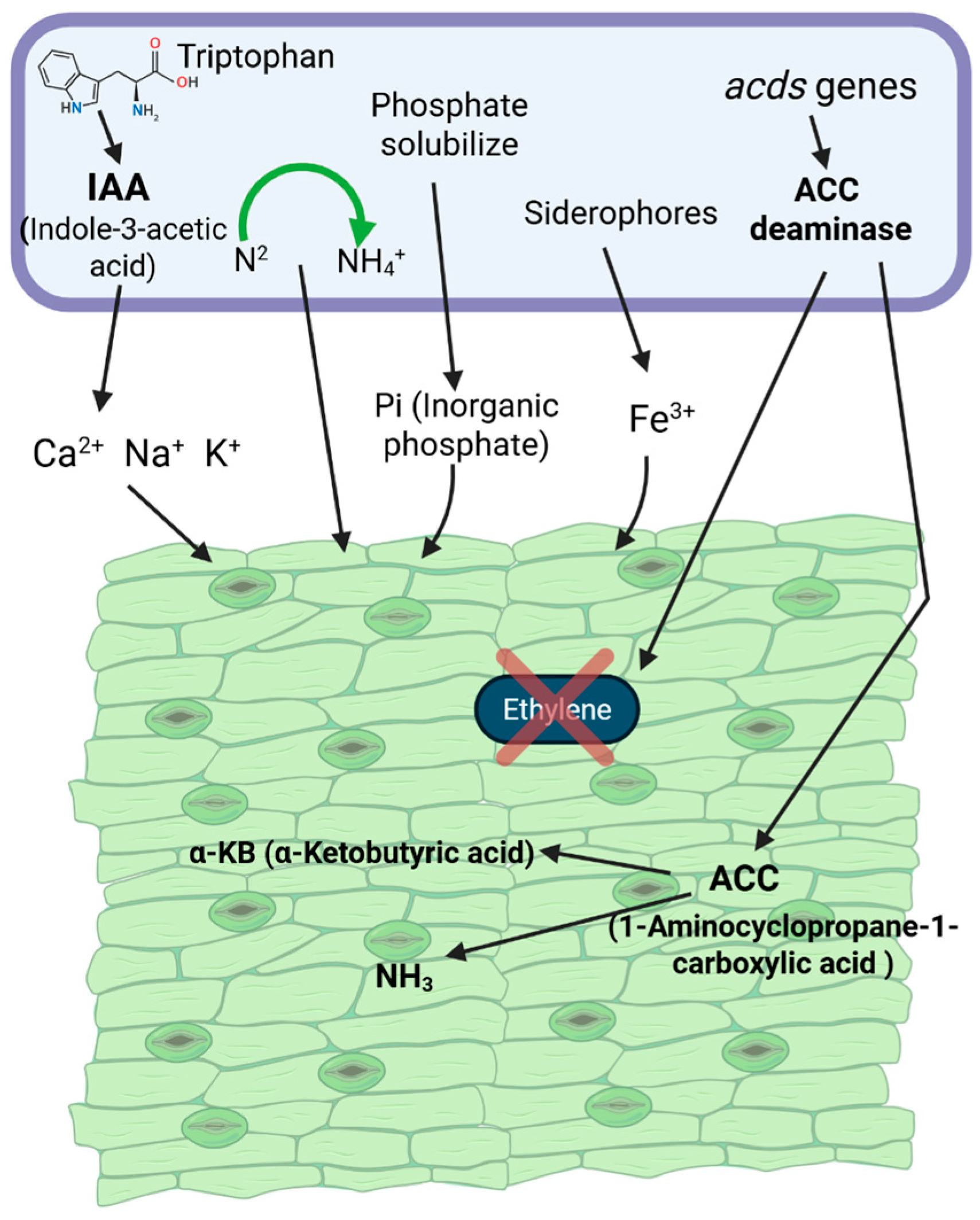
2.2.2. Contribution of Microbial Activity to Nutrient Solubilization and Plant Growth
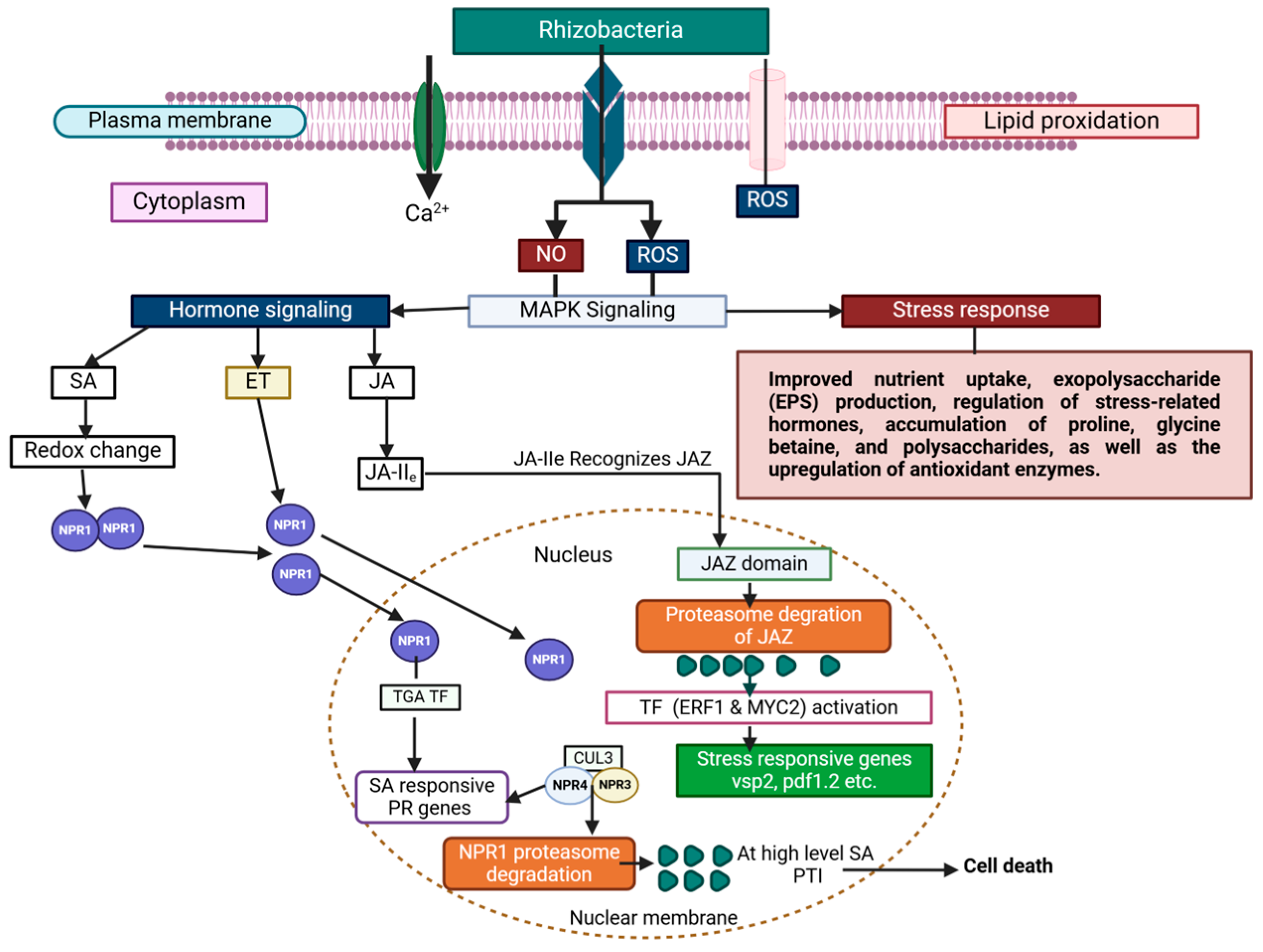
3. Physiological Mechanisms of Plant-Microorganism Interaction
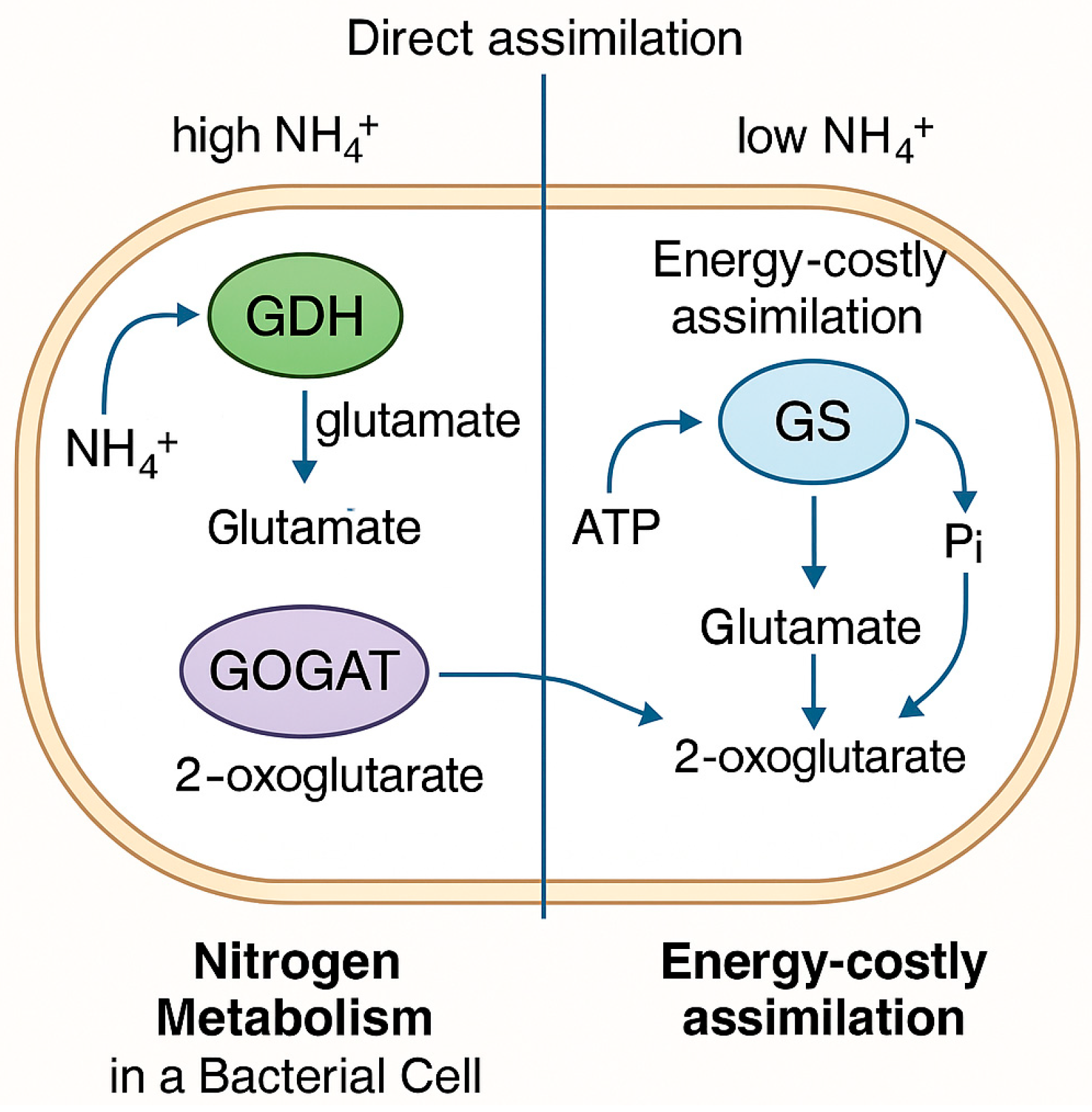
3.1. Bacterial Contribution to Plant Nutrient Acquisition
3.1.1. Nitrogen
3.1.2. Phosphorus
3.2. Bacterial Modulation of Plant Growth Regulator Pathways and Signaling Mechanisms
3.2.1. Plant Growth Regulators: How Do PGPB Influence Growth Regulator Signaling Networks to Enhance Growth and Stress Tolerance?
- Auxins (IAA): How does microbial IAA shape root system architecture and stress responses?
- Cytokinins: How do PGPB-derived cytokinins sustain meristem activity and shoot growth?
- Gibberellins: How Do Microbial Gibberellins Support Stem Elongation and Seed Germination?
- Abscisic Acid (ABA): How Does ABA Signaling Mediate Stress Responses and Water Balance?
- Ethylene: How Do PGPB Reduce Ethylene-Induced Growth Inhibition Under Stress?
3.2.2. Volatile Organic Compounds (VOCs): How Do Bacterial VOCs Activate Defense Pathways and Improve Stress Resilience?
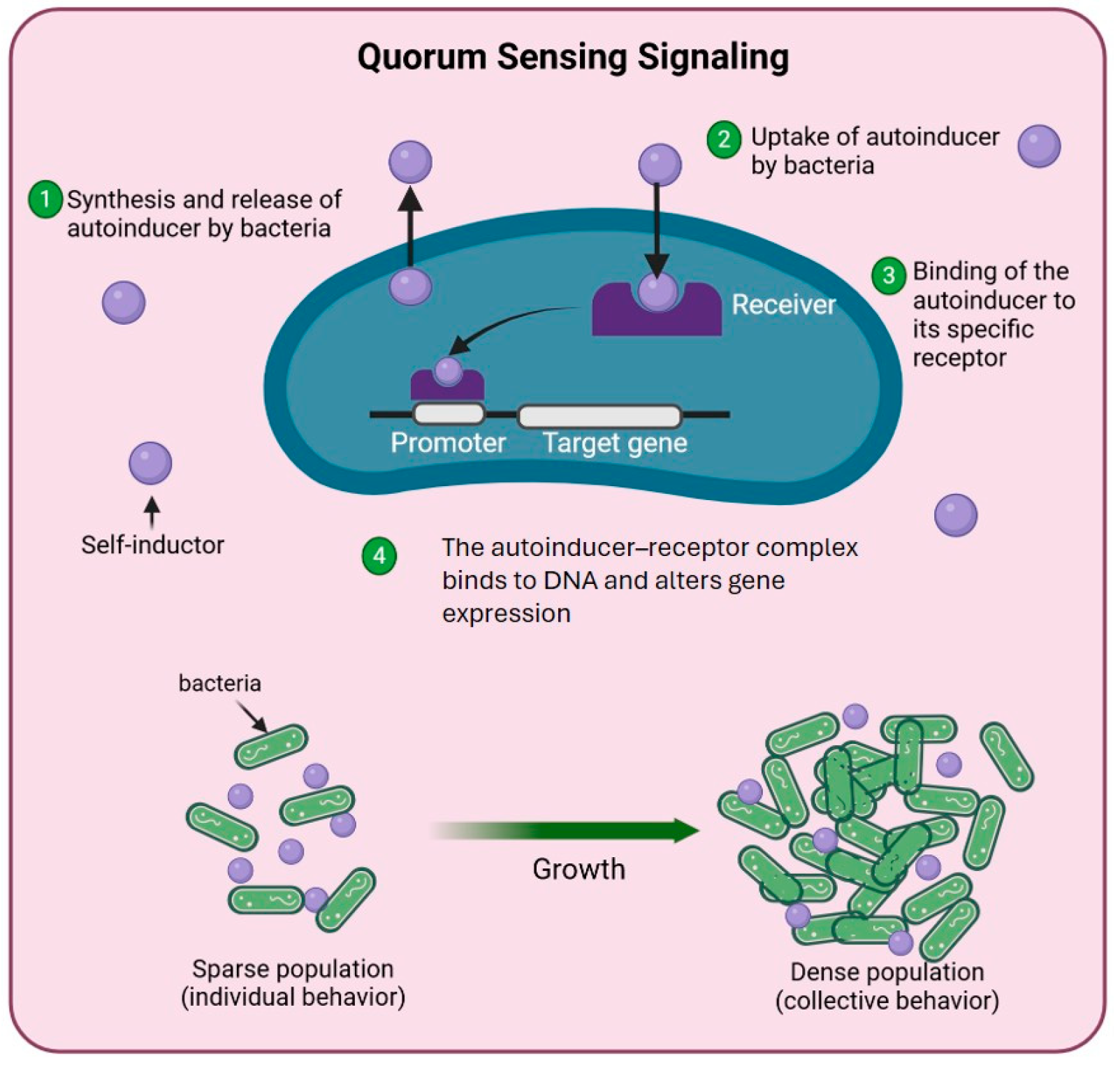
3.2.3. Quorum Sensing Detection
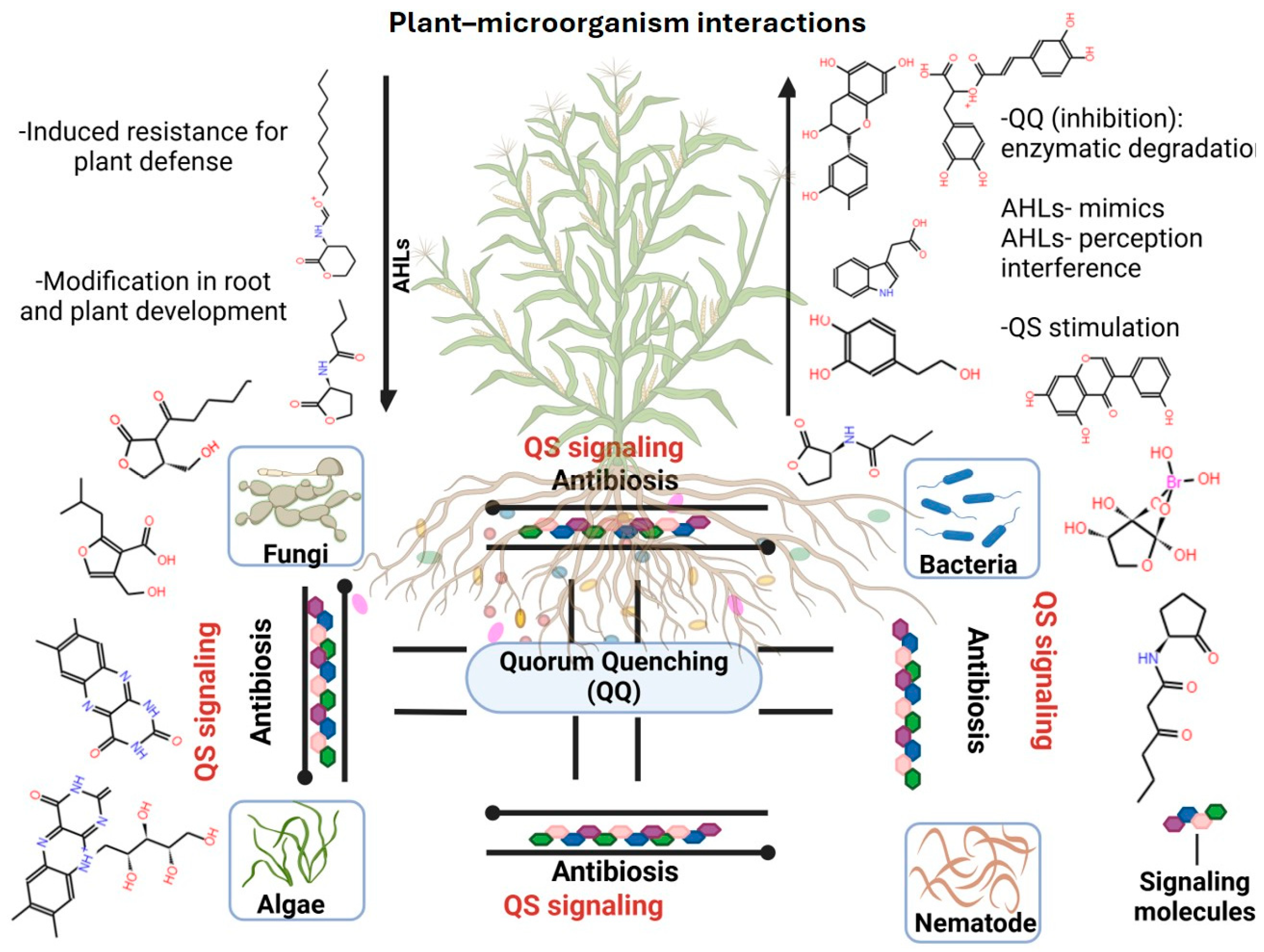
3.3. Plant Molecular Signaling and Microbial Communication in the Rhizosphere
4. PGPB-Mediated Soil Restoration of Soils Degraded by Excessive Cultivation
4.1. Nutrient Dynamics in the Restoration of Degraded Soils
4.2. Biological Strategies for Mitigating Salt-Induced Stress in Degraded Soils
4.3. Bioremediation of Heavy Metal-Contaminated Soils Using PGPB
5. Perspectives on Innovative Strategies for Soil Restoration Using PGPB in Overcultivated Lands
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PGPB | Plant Growth-Promoting Bacteria |
| PGRs | Plant Growth regulators |
| PSB | Phosphate Solubilizing Bacteria |
| QS | Quorum Sensing |
| HM | Heavy Metals |
References
- Subramaniam, Y.; Masron, T.A. Food security and environmental degradation: Evidence from developing countries. GeoJournal 2021, 86, 1141–1153. [Google Scholar] [CrossRef]
- Sartori, M.; Ferrari, E.; M’Barek, R.; Philippidis, G.; Boysen-Urban, K.; Borrelli, P.; Montanarella, L.; Panagos, P. Remaining Loyal to Our Soil: A Prospective Integrated Assessment of Soil Erosion on Global Food Security. Ecol. Econ. 2024, 219, 108103. [Google Scholar] [CrossRef]
- Pozza, L.E.; Field, D.J. The science of Soil Security and Food Security. Soil. Secur. 2020, 1, 100002. [Google Scholar] [CrossRef]
- Bravo-Espinosa, M.; Mendoza, M.E.; Medina-Orozco, L.; Prat, C.; García-Oliva, F.; López-Granados, E. Runoff, soil loss, and nutrient depletion under traditional and alternative cropping systems in the Transmexican Volcanic Belt, Central Mexico. Land Degrad. Dev. 2009, 20, 640–653. [Google Scholar] [CrossRef]
- Luo, P.; Yang, J.; Zhang, X.; Wang, Z. Uncovering the drivers; impacts, and urgent solutions to soil erosion: A global synthesis. Front. Enviorn. Sci. 2024, 12, 1521611. [Google Scholar] [CrossRef]
- Kumari, V.V.; Balloli, S.S.; Kumar, M.; Ramana, D.B.V.; Prabhakar, M.; Osman, M.; Indoria, A.K.; Manjunath, M.; Maruthi, V.; Chary, G.R.; et al. Diversified cropping systems for reducing soil erosion and nutrient loss and for increasing crop productivity and profitability in rainfed environments. Agric. Syst. 2024, 217, 103919. [Google Scholar] [CrossRef]
- Yang, T.; Siddique, K.H.M.; Liu, K. Cropping systems in agriculture and their impact on soil health-A review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Hunt, N.D.; Hill, J.D.; Liebman, M. Cropping System Diversity Effects on Nutrient Discharge, Soil Erosion, and Agronomic Performance. Enviorn. Sci. Technol. 2019, 53, 1344–1352. [Google Scholar] [CrossRef]
- Goel, R.; Chaturvedi, P.; Kumar, S.; Soni, R.; Suyal, D.C. Editorial: Hazardous pollutants in agricultural soil and environment. Front. Microbiol. 2024, 15, 1411735. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.; Zheng, H.; Olesen, J.E.; Rees, R.M.; Zou, J.; Zhang, L.; Hu, S.; Qiao, B.; Wang, X.; et al. Excessive N applications reduces yield and biological N fixation of summer-peanut in the North China Plain. Field Crops Res. 2023, 302, 109021. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, L.; Jiang, S.; Su, B.; Li, Z.; Shuai, Y.; Wang, J. Improved Salt Tolerance in Brassica napus L. Overexpressing a Synthetic Deinocuccus Stress-Resistant Module DICW. Int. J. Mol. Sci. 2025, 26, 2500. [Google Scholar] [CrossRef]
- Thomas, E.; Thomas Kurien, V.; Prabha, V.; Thomas, S. Monoculture vs mixed-species plantation impact on the soil quality of an ecologically sensitive area. J. Agric. Environ. Int. Dev. 2020, 112, 41–62. [Google Scholar] [CrossRef]
- Qian, L.U.; Zhang, J.; Lisha, C.H. Impact of monoculture of poplar on rhizosphere microbial communities over time. Pedosphere 2020, 30, 487–495. [Google Scholar] [CrossRef]
- Gui, H.; Fan, L.; Wang, D.; Yan, P.; Li, X.; Pang, Y.; Zhang, L.; Zamanian, K.; Shi, L.; Xu, J.; et al. Variations in Soil Nutrient Dynamics and Bacterial Communities After the Conversion of Forests to Long-Term Tea Monoculture Systems. Front. Microbiol. 2022, 13, 896530. [Google Scholar] [CrossRef] [PubMed]
- Ilampooranan, I.; Van Meter, K.J.; Basu, N.B. Intensive agriculture, nitrogen legacies, and water quality: Intersections and implications. Environ. Res. Lett. 2022, 17, 035006. [Google Scholar] [CrossRef]
- Mera, J.L.A.; Vinces, L.M.G.; Murillo, D.M.S.; Chilán, G.R.M. The monoculture of corn (Zea mayz) and its impact on fertility soil. Int. J. Chem. Mater. Sci. 2021, 4, 7–12. [Google Scholar]
- Purwanto, B.H.; Alam, S. Impact of intensive agricultural management on carbon and nitrogen dynamics in the humid tropics. Soil. Sci. Plant Nutr. 2020, 66, 50–59. [Google Scholar] [CrossRef]
- Rodríguez, B.C.; Durán-Zuazo, V.H.; Rodríguez, M.S.; García-Tejero, I.F.; Ruiz, B.G.; Cuadros Tavira, S. Conservation Agriculture as a Sustainable System for Soil Health: A Review. Soil. Syst. 2022, 6, 87. [Google Scholar] [CrossRef]
- Hamada, K.; Inoue, H.; Mochizuki, H.; Miyamoto, T.; Asakura, M.; Shimizu, Y. Effect of hardpan on the vertical distribution of water stress in a converted paddy field. Soil. Tillage Res. 2021, 214, 105161. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, S.; Gul, S.; He, L.; Chen, H.; Liu, D. Effect of Plow Pan on the Redistribution Dynamics of Water and Nutrient Transport in Soils. Sustainability 2024, 16, 8859. [Google Scholar] [CrossRef]
- Issaka, S.; Ashraf, M.A. Impact of soil erosion and degradation on water quality: A review. Geol. Ecol. Landsc. 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Shaheb, M.R.; Venkatesh, R.; Shearer, S.A. A Review on the Effect of Soil Compaction and its Management for Sustainable Crop Production. J. Biosyst. Eng. 2021, 46, 417–439. [Google Scholar] [CrossRef]
- Fernández-Ojeda, P.R.; Acevedo, D.C.; Villanueva-Morales, A.; Uribe-Gómez, M. State of the essential chemical elements in the soils of natural, agroforestry and monoculture systems. Rev. Mex. Cienc. For. 2016, 7, 65–77. [Google Scholar]
- Haruna, S.I.; Anderson, S.H.; Udawatta, R.P.; Gantzer, C.J.; Phillips, N.C.; Cui, S.; Gao, Y. Improving soil physical properties through the use of cover crops: A review. Agrosyst. Geosci. Environ. 2020, 3, e20105. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; Shahzad, B.; Yang, G.; Fahad, S.; Ali, S.; Bukhari, M.A.; Tung, S.A.; Hafeez, A.; Souliyanonh, B. Soil compaction effects on soil health and cropproductivity: An overview. Environ. Sci. Pollut. Res. 2017, 24, 10056–10067. [Google Scholar] [CrossRef]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems. Soil. Tillage Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- An, Y.; Wang, L.; Zhang, M.; Tong, S.; Li, Y.; Wu, H.; Jiang, M.; Wang, X.; Guo, Y.; Jiang, L. Synergies and trade-offs between aboveground and belowground traits explain the dynamics of soil organic carbon and nitrogen in wetlands undergoing agricultural management changes in semi-arid regions. Agric. Ecosyst. Environ. 2025, 381, 109432. [Google Scholar] [CrossRef]
- Piccoli, I.; Seehusen, T.; Bussell, J.; Vizitu, O.; Calciu, I.; Berti, A.; Börjesson, G.; Kirchmann, H.; Kätterer, T.; Sartori, F.; et al. Opportunities for Mitigating Soil Compaction in Europe—Case Studies from the Soil Care Project Using Soil-Improving Cropping Systems. Land 2022, 11, 223. [Google Scholar] [CrossRef]
- Swain, C.K. Environmental pollution indices: A review on concentration of heavy metals in air, water, and soil near industrialization and urbanisation. Discov. Environ. 2024, 2, 5. [Google Scholar] [CrossRef]
- Bedolla-Rivera, H.I.; Negrete-Rodríguez, M.D.L.L.X.; Gámez-Vázquez, F.P.; Álvarez-Bernal, D.; Conde-Barajas, E. Analyzing the Impact of Intensive Agriculture on Soil Quality: A Systematic Review and Global Meta-Analysis of Quality Indexes. Agronomy 2023, 13, 2166. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive agriculture reduces soil biodiversity across Europe. Glob. Change Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef]
- Tripathi, S.; Srivastava, P.; Devi, R.S.; Bhadouria, R. Influence of synthetic fertilizers and pesticides on soil health and soil microbiology. In Agrochemicals Detection, Treatment and Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–54. [Google Scholar] [CrossRef]
- Baweja, P.; Kumar, S.; Kumar, G. Fertilizers and Pesticides: Their Impact on Soil Health and Environment. Soil Health 2020, 59 27, 265–285. [Google Scholar] [CrossRef]
- Bourke, P.M.; Evers, J.B.; Bijma, P.; van Apeldoorn, D.F.; Smulders, M.J.M.; Kuyper, T.W.; Mommer, L.; Bonnema, G. Breeding Beyond Monoculture: Putting the ‘Intercrop’ Into Crops. Front. Plant Sci. 2021, 12, 734167. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, H.; Ma, C.; Li, S.; Wu, H.; Yuan, K.; Meng, X.; Song, Z.; Fang, X.; Zhao, Z. Unraveling the Impact of Long-Term Rice Monoculture Practice on Soil Fertility in a Rice-Planting Meadow Soil: A Perspective from Microbial Biomass and Carbon Metabolic Rate. Microorganisms 2022, 10, 2153. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, L.; Lv, M.; Wang, R.; Wang, L.; Yu, S.; Gao, Z.; Li, X. PGPR: Key to enhancing crop productivity and achieving sustainable agriculture. Curr. Microbiol. 2024, 81, 377. [Google Scholar] [CrossRef]
- Reang, L.; Bhatt, S.; Tomar, R.S.; Joshi, K.; Padhiyar, S.; Vyas, U.M.; Kheni, J.K. Plant growth promoting characteristics of halophilic and halotolerant bacteria isolated from coastal regions of Saurashtra Gujarat. Sci. Rep. 2022, 12, 4699. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.-A.; Song, J.; Choe, S.; Jang, G.; Kim, Y. Plant growth-promoting rhizobacterium Bacillus megaterium modulates the expression of antioxidant-related and drought-responsive genes to protect rice (Oryza sativa L.) from drought. Front. Microbiol. 2024, 15, 1430546. [Google Scholar] [CrossRef]
- Khan, A.; Wang, T.; Hussain, T.; Amna Ali, F.; Shi, F.; Latef, A.; Ali, O.; Hayat, K.; Mehmood, S.; Zainab, N.; et al. Halotolerant-Koccuria rhizophila (14asp)-induced amendment of salt stress in pea plants by limiting Na⁺ uptake and elevating production of antioxidants. Agronomy 2021, 11, 1907. [Google Scholar] [CrossRef]
- Molina-Romero, D.; Baez, A.; Quintero-Hernández, V.; Castañeda-Lucio, M.; Fuentes-Ramírez, L.E.; Bustillos-Cristales, M.; del Rocío Bustillos-Cristales, M.; Rodríguez-Andrade, O.; Morales-García, Y.E.; Munive, A.; et al. Compatible bacterial mixture, tolerant to desiccation, improves maize plant growth. PLoS ONE 2017, 12, e0187913. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, J.; Shao, X.; Yan, X.; Liu, K. Effective microorganisms input efficiently improves the vegetation and microbial community of degraded alpine grassland. Front. Microbiol. 2024, 14, 1330149. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.F.; Olivares, F.L. Plant microbiome structure and benefits for sustainable agriculture. Curr. Plant Biol. 2021, 26, 100198. [Google Scholar] [CrossRef]
- Fitzpatrick, C.R.; Salas-González, I.; Conway, J.M.; Finkel, O.M.; Gilbert, S.; Russ, D.; Teixeira, P.J.P.L.; Dangl, J.L. The Plant Microbiome: From Ecology to Reductionism and Beyond. Annu. Rev. Microbiol. 2020, 74, 81–100. [Google Scholar] [CrossRef]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant–microbiome interactions under a changing world: Responses, consequences and perspectives. New Phytol. 2022, 234, 1951–1959. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Tack, A.J.M.; Lobato, C.; Wassermann, B.; Berg, G. From seed to seed: The role of microbial inheritance in the assembly of the plant microbiome. Trends Microbiol. 2023, 31, 346–355. [Google Scholar] [CrossRef]
- Liu, X.; Mei, S.; Salles, J.F. Inoculated microbial consortia perform better than single strains in living soil: A meta-analysis. Appl. Soil. Ecol. 2023, 190, 105011. [Google Scholar] [CrossRef]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving crop yield and nutrient use efficiency via biofertilization—A global meta-analysis. Front. Plant Sci. 2018, 8, 2204. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Jia, Z.; Zhai, L.; Zhang, B.; Grüters, U.; Ma, S.; Qian, J.; Liu, X.; Zhang, J.; et al. Meta-analysis reveals the effects of microbial inoculants on the biomass and diversity of soil microbial communities. Nat. Ecol. Evol. 2024, 8, 1270–1284. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.; Chen, L.; Liu, H.; Wu, Y.; Huang, M.; Fang, L. Biological roles of soil microbial consortium on promoting safe crop production in heavy metal(loid) contaminated soil: A systematic review. Sci. Total Environ. 2024, 912, 168994. [Google Scholar] [CrossRef]
- Molina-Romero, D.; Juárez-Sánchez, S.; Venegas, B.; Ortíz-González, C.S.; Baez, A.; Morales-García, Y.E.; Muñoz-Rojas, J. A Bacterial Consortium Interacts With Different Varieties of Maize, Promotes the Plant Growth, and Reduces the Application of Chemical Fertilizer Under Field Conditions. Front. Sustain. Food Syst. 2021, 4, 616757. [Google Scholar] [CrossRef]
- Heredia-Acuña, C.; Almaraz-Suarez, J.J.; Arteaga-Garibay, R.; Ferrera-Cerrato, R.; Pineda-Mendoza, D.Y. Isolation, characterization and effect of plant-growth-promoting rhizobacteria on pine seedlings (Pinus pseudostrobus Lindl). J. For. Res. 2019, 30, 1727–1734. [Google Scholar] [CrossRef]
- Domínguez-Castillo, C.; Alatorre-Cruz, J.M.; Castañeda-Antonio, D.; Munive, J.A.; Guo, X.; López-Olguín, J.F.; Fuentes-Ramírez, L.E.; Carreño-López, R. Potential seed germination-enhancing plant growth-promoting rhizobacteria for restoration of Pinus chiapensis ecosystems. J. For. Res. 2021, 32, 2143–2153. [Google Scholar] [CrossRef]
- Patel, J.S.; Yadav, S.K.; Bajpai, R.; Teli, B.; Rashid, M. PGPR secondary metabolites: An active syrup for improvement of plant health. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–208. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Bacteria, S.E.S.P.G.-P.S.; Solubilization, P.; Production, S. Plant Growth-Promoting Soil Bacteria: Nitrogen Fixation, Phosphate Solubilization, Siderophore Production, and Other Biological Activities. Plants 2023, 12, 4074. [Google Scholar] [CrossRef]
- Saha, R.; Saha, N.; Donofrio, R.S.; Bestervelt, L.L. Microbial siderophores: A mini review. J. Basic. Microbiol. 2013, 53, 303–317. [Google Scholar] [CrossRef]
- Sayyed, R.Z.; Chincholkar, S.B.; Reddy, M.S.; Gangurde, N.S.; Patel, P.R. Siderophore Producing PGPR for Crop Nutrition and Phytopathogen Suppression. In Bacteria in Agrobiology: Disease Management; Springer: Berlin/Heidelberg, Germany, 2013; pp. 449–471. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Rossano, S.; Trcera, N.; Verney-Carron, A.; Rommevaux, C.; Fourdrin, C.; Agnello, A.C.; Huguenot, D.; Guyot, F. Direct and indirect impact of the bacterial strain Pseudomonas aeruginosa on the dissolution of synthetic Fe(III)-and Fe(II)-bearing basaltic glasses. Chem. Geol. 2019, 523, 9–18. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Huang, Y.; Guo, M.; Song, J.; Zhang, T.; Long, Y.; Wang, B.; Liu, H. A Potential Biofertilizer—Siderophilic Bacteria Isolated From the Rhizosphere of Paris polyphylla var. yunnanensis. Front. Microbiol. 2022, 13, 870413. [Google Scholar] [CrossRef] [PubMed]
- Bilitewski, U.; Blodgett, J.A.V.; Duhme-Klair, A.; Dallavalle, S.; Laschat, S.; Routledge, A.; Schobert, R. Chemical and Biological Aspects of Nutritional Immunity—Perspectives for New Anti-Infectives that Target Iron Uptake Systems. Angew. Chem. Int. Ed. 2017, 56, 14360–14382. [Google Scholar] [CrossRef] [PubMed]
- Grobelak, A.; Napora, A.; Kacprzak, M. Using plant growth-promoting rhizobacteria (PGPR) to improve plant growth. Ecol. Eng. 2015, 84, 22–28. [Google Scholar] [CrossRef]
- Glick, B.R. Beneficial Plant-Bacterial Interactions; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalolaand, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- ALKahtani, M.D.F.; Fouda, A.; Attia, K.A.; Al-Otaibi, F.; Eid, A.M.; Ewais, E.E.-D.; Hijri, M.; St-Arnaud, M.; Hassan, S.E.-D.; Khan, N.; et al. Isolation and Characterization of Plant Growth Promoting Endophytic Bacteria from Desert Plants and Their Application as Bioinoculants for Sustainable Agriculture. Agronomy 2020, 10, 1325. [Google Scholar] [CrossRef]
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial Proteases Applications. Front. Bioeng. Biotechnol. 2019, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Bumunang, E.W.; Babalola, O.O. Characterization of Rhizobacteria from field grown Genetically Modified (GM) and non-GM maizes. Braz. Arch. Biol. Technol. 2014, 57, 1–8. [Google Scholar] [CrossRef]
- Atouei, M.T.; Pourbabaee, A.A.; Shorafa, M. Alleviation of Salinity Stress on Some Growth Parameters of Wheat by Exopolysaccharide-Producing Bacteria. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 2725–2733. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sindhu, S.S.; Glick, B.R. Hydrogen cyanide production by soil bacteria: Biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere 2022, 32, 15–38. [Google Scholar] [CrossRef]
- Ismail, M.A.; Amin, M.A.; Eid, A.M.; Hassan, S.E.-D.; Mahgoub, H.A.M.; Lashin, I.; Abdelwahab, A.T.; Azab, E.; Gobouri, A.A.; Elkelish, A.; et al. Comparative Study between Exogenously Applied Plant Growth Hormones versus Metabolites of Microbial Endophytes as Plant Growth-Promoting for Phaseolus vulgaris L. Cells 2021, 10, 1059. [Google Scholar] [CrossRef] [PubMed]
- Far, B.E.; Ahmadi, Y.; Khosroshahi, A.Y.; Dilmaghani, A. Microbial alpha-amylase production: Progress, challenges and perspectives. Adv. Pharm. Bull. 2020, 10, 350–358. [Google Scholar] [CrossRef]
- Witte, C.-P. Urea metabolism in plants. Plant Sci. 2011, 180, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.-J.; Teng, A.; Chu, J.; Cao, B.A. quantitative, high-throughput urease activity assay for comparison and rapid screening of ureolytic bacteria. Environ. Res. 2022, 208, 112738. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Mekureyaw, M.F.; Pandey, C.; Hennessy, R.C.; Nicolaisen, M.H.; Liu, F.; Nybroe, O.; Roitsch, T. The cytokinin-producing plant beneficial bacterium Pseudomonas fluorescens G20-18 primes tomato (Solanum lycopersicum) for enhanced drought stress responses. J. Plant Physiol. 2022, 270, 153629. [Google Scholar] [CrossRef]
- Davies, P.J. The Plant Hormones: Their Nature, Occurrence, and Functions. In Plant Hormones; Springer: Dordrecht, The Netherlands, 1995; pp. 1–12. [Google Scholar] [CrossRef]
- Smith, S.M.; Li, C.; Li, J. Hormone function in plants. In Hormone Metabolism and Signaling in Plants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–38. [Google Scholar] [CrossRef]
- Arc, E.; Sechet, J.; Corbineau, F.; Rajjou, L.; Marion-Poll, A. ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front. Plant Sci. 2013, 4, 63. [Google Scholar] [CrossRef]
- Gutiérrez-Mañero, F.J.; Ramos-Solano, B.; Probanza, A.; Mehouachi, J.; Tadeo, F.R.; Talon, M. The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol. Plant 2001, 111, 206–211. [Google Scholar] [CrossRef]
- Rehman, F.; Kalsoom, M.; Adnan, M.; Toor, M.D.; Zulfiqar, A. Plant Growth Promoting Rhizobacteria and their Mechanisms Involved in Agricultural Crop Production: A Review. SunText Rev. Biotechnol. 2020, 1, 110. [Google Scholar] [CrossRef]
- Chandra, S.; Askari, K.; Kumari, M. Optimization of indole acetic acid production by isolated bacteria from Stevia rebaudiana rhizosphere and its effects on plant growth. J. Genet. Eng. Biotechnol. 2018, 16, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, S.; Friml, J. Auxin: A Trigger for Change in Plant Development. Cell 2009, 136, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Nikitha, M.S.T.; Sadhana, E.U.B.R.B.; Vani, S.S. Phosphorous and Phosphate Solubilising Bacteria and their Role in Plant Nutrition. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2133–2144. [Google Scholar] [CrossRef]
- Verma, J.P.; Jaiswal, D.K.; Singh, S.; Kumar, A.; Prakash, S.; Curá, J.A. Consequence of phosphate solubilising microbes in sustainable agriculture as efficient microbial consortium: A review. Clim. Change Environ. Sustain. 2017, 5, 1–19. [Google Scholar] [CrossRef]
- Suleimanova, A.; Bulmakova, D.; Sokolnikova, L.; Egorova, E.; Itkina, D.; Kuzminova, O.; Gizatullina, A.; Sharipova, M. Phosphate Solubilization and Plant Growth Promotion by Pantoea brenneri Soil Isolates. Microorganisms 2023, 11, 1136. [Google Scholar] [CrossRef]
- Saha, K.K.; Mandal, S.; Barman, A.; Mondal, S.; Chatterjee, S.; Mandal, N.C. Genomic insight of phosphate solubilization and plant growth promotion of two taxonomically distinct winter crops by Enterobacter sp. DRP3. J. Appl. Microbiol. 2024, 135, lxae146. [Google Scholar] [CrossRef]
- Bharwad, K.; Rajkumar, S. Modulation of PQQ-dependent glucose dehydrogenase (mGDH and sGDH) activity by succinate in phosphate solubilizing plant growth promoting Acinetobacter sp. SK2. 3 Biotech 2020, 10, 5. [Google Scholar] [CrossRef]
- An, Y.; Wang, Y.; Liu, S.; Wu, W.; Wang, W.; Liu, M.; Xiao, H.; Dong, J.; Ren, H.; Xu, H.; et al. Impact of Split Nitrogen Topdressing on Rhizobacteria Community of Winter Wheat. Agriculture 2025, 15, 794. [Google Scholar] [CrossRef]
- Gullì, M.; Cangioli, L.; Frusciante, S.; Graziano, S.; Caldara, M.; Fiore, A.; Klonowski, A.M.; Maestri, E.; Brunori, A.; Mengoni, A.; et al. The relevance of biochar and co-applied SynComs on maize quality and sustainability: Evidence from field experiments. Sci. Total Environ. 2025, 968, 178872. [Google Scholar] [CrossRef]
- Kerbab, S.; Silini, A.; Chenari Bouket, A.; Cherif-Silini, H.; Eshelli, M.; el Houda Rabhi, N.; Belbahri, L. Mitigation of NaCl Stress in Wheat by Rhizosphere Engineering Using Salt Habitat Adapted PGPR Halotolerant Bacteria. Appl. Sci. 2021, 11, 1034. [Google Scholar] [CrossRef]
- Ayub, A.; Shabaan, M.; Malik, M.; Asghar, H.N.; Zulfiqar, U.; Ejaz, M.; Alarjani, K.M.; al Farraj, D.A. Synergistic application of Pseudomonas strains and compost mitigates lead (Pb) stress in sunflower (Helianthus annuus L.) via improved nutrient uptake, antioxidant defense and physiology. Ecotoxicol. Environ. Saf. 2024, 274, 116194. [Google Scholar] [CrossRef]
- Eshaghi, E.; Nosrati, R.; Owlia, P.; Malboobi, M.A.; Ghaseminejad, P.; Ganjali, M.R. Zinc solubilization characteristics of efficient siderophore-producing soil bacteria. Iran. J. Microbiol. 2019, 11, 419–430. Available online: https://ijm.tums.ac.ir/index.php/ijm/article/view/1766 (accessed on 17 August 2024). [CrossRef]
- Fasim, F.; Ahmed, N.; Parsons, R.; Gadd, G.M. Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiol. Lett. 2002, 213, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.A.; Upadhyay, S.K.; Srivastava, A.K.; Bhojiya, A.A. Biofertilizers: A Nexus between soil fertility and crop productivity under abiotic stress. Curr. Res. Environ. Sustain. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Olaniyan, F.T.; Alori, E.T.; Adekiya, A.O.; Ayorinde, B.B.; Daramola, F.Y.; Osemwegie, O.O.; Babalola, O.O. The use of soil microbial potassium solubilizers in potassium nutrient availability in soil and its dynamics. Ann. Microbiol. 2022, 72, 45. [Google Scholar] [CrossRef]
- Rawat, J.; Pandey, N.; Saxena, J. Role of Potassium in Plant Photosynthesis, Transport, Growth and Yield. In Role of Potassium in Abiotic Stress; Springer Nature: Singapore, 2022; pp. 1–14. [Google Scholar] [CrossRef]
- Nawaz, A.; Qamar, Z.U.; Marghoob, M.U.; Imtiaz, M.; Imran, A.; Mubeen, F. Contribution of potassium solubilizing bacteria in improved potassium assimilation and cytosolic K+/Na+ ratio in rice (Oryza sativa L.) under saline-sodic conditions. Front. Microbiol. 2023, 14, 1196024. [Google Scholar] [CrossRef]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A review of plants strategies to resist biotic and abiotic environmental stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef] [PubMed]
- Bukhat, S.; Imran, A.; Javaid, S.; Shahid, M.; Majeed, A.; Naqqash, T. Communication of plants with microbial world: Exploring the regulatory networks for PGPR mediated defense signaling. Microbiol. Res. 2020, 238, 126486. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, Y.; Wang, N.R.; Haney, C.H. Mechanisms in plant–microbiome interactions: Lessons from model systems. Curr. Opin. Plant Biol. 2021, 62, 102003. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, S.M.; Mahmud, A.A.; AIbdullahi, M.; Haruna, A. Recent advances in the chemistry of nitrogen, phosphorus and potassium as fertilizers in soil: A review. Pedosphere 2023, 33, 385–406. [Google Scholar] [CrossRef]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Rhizosphere Colonization Determinants by Plant Growth-Promoting Rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef]
- Sharma, P.; Kumawat, K.C.; Kaur, S. Plant Growth Promoting Rhizobacteria in Nutrient Enrichment: Current Perspectives. In Biofortification of Food Crops; Springer: New Delhi, India, 2016; pp. 263–289. [Google Scholar] [CrossRef]
- Geddes, B.A.; Oresnik, I.J. The Mechanism of Symbiotic Nitrogen Fixation. In The Mechanistic Benefits of Microbial Symbionts. Advances in Environmental Microbiology; Springer: Cham, Switzerland, 2016; pp. 69–97. [Google Scholar] [CrossRef]
- Merrick, M. Post-translational modification of PII signal transduction proteins. Front. Microbiol. 2015, 5, 763. [Google Scholar] [CrossRef]
- Bueno Batista, M.; Dixon, R. Manipulating nitrogen regulation in diazotrophic bacteria for agronomic benefit. Biochem. Soc. Trans. 2019, 47, 603–614. [Google Scholar] [CrossRef]
- Gerhardt, E.C.M.; Parize, E.; Gravina, F.; Pontes, F.L.D.; Santos, A.R.S.; Araújo, G.A.T.; Goedert, A.C.; Urbanski, A.H.; Steffens, M.B.R.; Chubatsu, L.S.; et al. The Protein-Protein Interaction Network Reveals a Novel Role of the Signal Transduction Protein PII in the Control of c-di-GMP Homeostasis in Azospirillum brasilense. mSystems 2020, 5, e00817-20. [Google Scholar] [CrossRef]
- Zeng, Y.; Guo, L.; Gao, Y.; Cui, L.; Wang, M.; Huang, L.; Jiang, M.; Liu, Y.; Zhu, Y.; Xiang, H.; et al. Formation of NifA-PII complex represses ammonium-sensitive nitrogen fixation in diazotrophic proteobacteria lacking NifL. Cell Rep. 2024, 43, 114476. [Google Scholar] [CrossRef]
- Plunkett, M.H.; Knutson, C.M.; Barney, B.M. Key factors affecting ammonium production by an Azotobacter vinelandii strain deregulated for biological nitrogen fixation. Microb. Cell Fact. 2020, 19, 107. [Google Scholar] [CrossRef]
- Cherkasov, N.; Ibhadon, A.O.; Fitzpatrick, P. A review of the existing and alternative methods for greener nitrogen fixation. Chem. Eng. Process. Process Intensif. 2015, 90, 24–33. [Google Scholar] [CrossRef]
- Rutledge, H.L.; Tezcan, F.A. Electron Transfer in Nitrogenase. Chem. Rev. 2020, 120, 5158–5193. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Huang, K.; Wang, F.; Mei, Z. Molecular Mechanism and Agricultural Application of the NifA–NifL System for Nitrogen Fixation. Int. J. Mol. Sci. 2023, 24, 907. [Google Scholar] [CrossRef]
- Inomura, K.; Bragg, J.; Follows, M.J. A quantitative analysis of the direct and indirect costs of nitrogen fixation: A model based on Azotobacter vinelandii. ISME J. 2017, 11, 166–175. [Google Scholar] [CrossRef]
- Addo, M.A.; Dos Santos, P.C. Distribution of Nitrogen-Fixation Genes in Prokaryotes Containing Alternative Nitrogenases. ChemBioChem 2020, 21, 1749–1759. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Z.; Liu, X. Engineering nitrogen-fixing symbiosis: Progress and perspectives for non-legume crops. Trends Plant Sci. 2024, 29, 217–229. [Google Scholar] [CrossRef]
- Einsle, O.; Rees, D.C. Structural Enzymology of Nitrogenase Enzymes. Chem. Rev. 2020, 120, 4969–5004. [Google Scholar] [CrossRef]
- Chanderban, M.; Hill, C.A.; Dhamad, A.E.; Lessner, D.J. Expression of V-nitrogenase and Fe-nitrogenase in Methanosarcina acetivorans is controlled by molybdenum, fixed nitrogen, and the expression of Mo-nitrogenase. Appl. Enviorn. Microbiol. 2023, 89, e01033-23. [Google Scholar] [CrossRef]
- Lery, L.M.; Bitar, M.; Costa, M.G.; Rössle, S.C.; Bisch, P.M. Unraveling the molecular mechanisms of nitrogenase conformational protection against oxygen in diazotrophic bacteria. BMC Genom. 2010, 11, S7. [Google Scholar] [CrossRef]
- Grzechowiak, M.; Sliwiak, J.; Link, A.; Ruszkowski, M. Legume-type glutamate dehydrogenase: Structure, activity, and inhibition studies. Int. J. Biol. Macromol. 2024, 278, 134648. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Gates, A.J.; Moreno-Vivián, C.; Ferguson, S.J.; Richardson, D.J.; Roldán, M.D. Bacterial nitrate assimilation: Gene distribution and regulation. Biochem. Soc. Trans. 2011, 39, 1838–1843. [Google Scholar] [CrossRef]
- Beato, V.M.; Rexach, J.; Navarro-Gochicoa, M.T.; Camacho-Cristóbal, J.J.; Herrera-Rodríguez, M.B.; González-Fontes, A. Boron deficiency increases expressions of asparagine synthetase, glutamate dehydrogenase and glutamine synthetase genes in tobacco roots irrespective of the nitrogen source. Soil. Sci. Plant Nutr. 2014, 60, 314–324. [Google Scholar] [CrossRef]
- Mokhele, B.; Zhan, X.; Yang, G.; Zhang, X. Review: Nitrogen assimilation in crop plants and its affecting factors. Can. J. Plant Sci. 2012, 92, 399–405. [Google Scholar] [CrossRef]
- Qiu, X.-M.; Sun, Y.-Y.; Ye, X.-Y.; Li, Z.-G. Signaling Role of Glutamate in Plants. Front. Plant. Sci. 2020, 10, 1743. [Google Scholar] [CrossRef]
- Liu, X.; Hu, B.; Chu, C. Nitrogen assimilation in plants: Current status and future prospects. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 171–190. [Google Scholar] [CrossRef]
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Khan, M.S.; Ahmad, E.; Zaidi, A.; Oves, M. Functional Aspect of Phosphate-Solubilizing Bacteria: Importance in Crop Production. In Bacteria in Agrobiology: Crop Productivity; Springer: Berlin/Heidelberg, Germany, 2013; pp. 237–263. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Bargaz, A.; Elhaissoufi, W.; Khourchi, S.; Benmrid, B.; Borden, K.A.; Rchiad, Z. Benefits of phosphate solubilizing bacteria on belowground crop performance for improved crop acquisition of phosphorus. Microbiol. Res. 2021, 252, 126842. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil. Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Adnan, M.; Shah, Z.; Fahad, S.; Arif, M.; Alam, M.; Khan, I.A.; Mian, I.A.; Basir, A.; Ullah, H.; Arshad, M.; et al. Phosphate-Solubilizing Bacteria Nullify the Antagonistic Effect of Soil Calcification on Bioavailability of Phosphorus in Alkaline Soils. Sci. Rep. 2017, 7, 16131. [Google Scholar] [CrossRef] [PubMed]
- Pande, A.; Pandey, P.; Mehra, S.; Singh, M.; Kaushik, S. Phenotypic and genotypic characterization of phosphate solubilizing bacteria and their efficiency on the growth of maize. J. Genet. Eng. Biotechnol. 2017, 15, 379–391. [Google Scholar] [CrossRef]
- Prabhu, N.; Borkar, S.; Garg, S. Phosphate solubilization by microorganisms. In Advances in Biological Science Research; Elsevier: Amsterdam, The Netherlands, 2019; pp. 161–176. [Google Scholar] [CrossRef]
- Vyas, P.; Gulati, A. Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol. 2009, 9, 174. [Google Scholar] [CrossRef]
- Suleman, M.; Yasmin, S.; Rasul, M.; Yahya, M.; Atta, B.M.; Mirza, M.S. Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and beneficial effects on wheat. PLoS ONE 2018, 13, e0204408. [Google Scholar] [CrossRef]
- Stephen, J.; Shabanamol, S.; Rishad, K.S.; Jisha, M.S. Growth enhancement of rice (Oryza sativa) by phosphate solubilizing Gluconacetobacter sp. (MTCC 8368) and Burkholderia sp. (MTCC 8369) under greenhouse conditions. 3 Biotech 2015, 5, 831–837. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Huang, S.; Peñuelas, J.; Sardans, J.; Wang, L.; Zheng, B. Genome-based identification of phosphate-solubilizing capacities of soil bacterial isolates. AMB Express 2024, 14, 85. [Google Scholar] [CrossRef]
- Li, X.L.; Zhao, X.Q.; Dong, X.Y.; Ma, J.F.; Fang Shen, R. Secretion of Gluconic Acid From Nguyenibacter sp. L1 Is Responsible for Solubilization of Aluminum Phosphate. Front. Microbiol. 2021, 12, 784025. [Google Scholar] [CrossRef]
- Do Amaral Leite, A.; de Souza Cardoso, A.A.; de Almeida Leite, R.; Barrera, A.M.V.; Queiroz, D.D.L.; Viana, T.C.; de Oliveira-Longatti, S.M.; Silva, C.A.; de Souza Moreira, F.M.; Lehmann, J.; et al. Phosphate-solubilizing bacteria increase maize phosphorus uptake from magnesium-enriched poultry manure biochar. Biol. Fertil. Soils 2024, 60, 421–436. [Google Scholar] [CrossRef]
- Mani, G.; Senthilkumar, R.; Venkatesan, K.; Mary Leema, J.T.; Rangamaran, V.R.; Balachandran, K.R.S.; Gopal, D. Halophilic Phosphate-Solubilizing Microbes (Priestia megaterium and Bacillus velezensis) Isolated from Arabian Sea Seamount Sediments for Plant Growth Promotion. Curr. Microbiol. 2024, 81, 405. [Google Scholar] [CrossRef]
- Wan, W.; Qin, Y.; Wu, H.; Zuo, W.; He, H.; Tan, J.; Wang, Y.; He, D. Isolation and Characterization of Phosphorus Solubilizing Bacteria With Multiple Phosphorus Sources Utilizing Capability and Their Potential for Lead Immobilization in Soil. Front. Microbiol. 2020, 11, 752. [Google Scholar] [CrossRef]
- Pan, L.; Cai, B. Phosphate-Solubilizing Bacteria: Advances in Their Physiology, Molecular Mechanisms and Microbial Community Effects. Microorganisms 2023, 11, 2904. [Google Scholar] [CrossRef]
- Cheng, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Phosphate-solubilizing bacteria: Their agroecological function and optimistic application for enhancing agro-productivity. Sci. Total Environ. 2023, 901, 166468. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi—Current perspective. Arch. Agron. Soil. Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Lim, B.L.; Yeung, P.; Cheng, C.; Hill, J.E. Distribution and diversity of phytate-mineralizing bacteria. ISME J. 2007, 1, 321–330. [Google Scholar] [CrossRef]
- Choi, Y.M.; Suh, H.J.; Kim, J.M. Purification and Properties of Extracellular Phytase from Bacillus sp. KHU-10. J. Protein Chem. 2001, 20, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.C.; Singdevsachan, S.K.; Mishra, R.R.; Dutta, S.K.; Thatoi, H.N. Diversity, mechanism and biotechnology of phosphate solubilising microorganism in mangrove—A review. Biocatal. Agric. Biotechnol. 2014, 3, 97–110. [Google Scholar] [CrossRef]
- Krämer, S. Acid and alkaline phosphatase dynamics and their relationship to soil microclimate in a semiarid woodland. Soil Biol. Biochem. 2000, 32, 179–188. [Google Scholar] [CrossRef]
- Panhwar, Q.A.; Naher, U.A.; Jusop, S.; Othman, R.; Latif, M.A.; Ismail, M.R. Biochemical and Molecular Characterization of Potential Phosphate-Solubilizing Bacteria in Acid Sulfate Soils and Their Beneficial Effects on Rice Growth. PLoS ONE 2014, 9, e97241. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Y.; Huang, H.; Li, Z.; Fan, H.; Pan, X.; Wang, Y.; Cao, Y.; Wang, K.; Yang, L. The Effect of Selected Phosphate-Solubilizing Bacteria on the Growth of Cotton Plants in Salinized Farmlands. Microorganisms 2025, 13, 1075. [Google Scholar] [CrossRef]
- Salsabila, N.; Fitriatin, B.N.; Hindersah, R. The Role of Phosphate-Solubilizing Microorganisms in Soil Health and Phosphorus Cycle: A Review. Int. J. Life Sci. Agric. Res. 2023, 2, 281–287. [Google Scholar] [CrossRef]
- Drogue, B.; Combes-Meynet, E.; Moënne-Loccoz, Y.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Control of the Cooperation Between Plant Growth-Promoting Rhizobacteria and Crops by Rhizosphere Signals. In Molecular Microbial Ecology of the Rhizosphere; Wiley: Hoboken, NJ, USA, 2013; pp. 279–293. [Google Scholar] [CrossRef]
- Venturi, V.; Keel, C. Signaling in the Rhizosphere. Trends Plant Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef]
- Agudelo-Morales, C.E.; Lerma, T.A.; Martínez, J.M.; Palencia, M.; Combatt, E.M. Phytohormones and Plant Growth Regulators—A Review. J. Sci. Technol. Appl. 2021, 10, 27–65. [Google Scholar] [CrossRef]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef]
- Emenecker, R.J.; Strader, L.C. Auxin-Abscisic Acid Interactions in Plant Growth and Development. Biomolecules 2020, 10, 281. [Google Scholar] [CrossRef]
- Pandey, V.; Bhatt, I.D.; Nandi, S.K. Role and Regulation of Auxin Signaling in Abiotic Stress Tolerance. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 319–331. [Google Scholar] [CrossRef]
- Jing, H.; Wilkinson, E.G.; Sageman-Furnas, K.; Strader, L.C. Auxin and abiotic stress responses. J. Exp. Bot. 2023, 74, 7000–7014. [Google Scholar] [CrossRef]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic acid in plant–microbe interactions. Antonie Van. Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.; Wu, C.; et al. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef]
- Morel, M.A.; Castro-Sowinski, S. The Complex Molecular Signaling Network in Microbe–Plant Interaction. In Plant Microbe Symbiosis: Fundamentals and Advances; Springer: New Delhi, India, 2013; pp. 169–199. [Google Scholar] [CrossRef]
- Di, D.-W.; Zhang, C.; Luo, P.; An, C.-W.; Guo, G.-Q. The biosynthesis of auxin: How many paths truly lead to IAA? Plant Growth Regul. 2016, 78, 275–285. [Google Scholar] [CrossRef]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- Chandler, J.W. The Hormonal Regulation of Flower Development. J. Plant Growth Regul. 2011, 30, 242–254. [Google Scholar] [CrossRef]
- Roosjen, M.; Paque, S.; Weijers, D. Auxin Response Factors: Output control in auxin biology. J. Exp. Bot. 2018, 69, 179–188. [Google Scholar] [CrossRef]
- Gomes, G.L.B.; Scortecci, K.C. Auxin and its role in plant development: Structure, signalling, regulation and response mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Environ, J.W. Auxin response factors. Plant Cell Enviorn. 2016, 39, 1014–1028. [Google Scholar] [CrossRef]
- Hartmann, A.; Klink, S.; Rothballer, M. Plant Growth Promotion and Induction of Systemic Tolerance to Drought and Salt Stress of Plants by Quorum Sensing Auto-Inducers of the N-acyl-homoserine Lactone Type: Recent Developments. Front Plant Sci. 2021, 12, 683546. [Google Scholar] [CrossRef]
- Verma, S.; Negi, N.P.; Pareek, S.; Mudgal, G.; Kumar, D. Auxin response factors in plant adaptation to drought and salinity stress. Physiol. Plant 2022, 174, e13714. [Google Scholar] [CrossRef]
- Bao, D.; Chang, S.; Li, X.; Qi, Y. Advances in the study of auxin early response genes: Aux/IAA, GH3, and SAUR. Crop J. 2024, 12, 964–978. [Google Scholar] [CrossRef]
- Fanai, A.; Bohia, B.; Lalremruati, F.; Lalhriatpuii, N.; Lalrokimi Lalmuanpuii, R.; Singh, P.K.; Zothanpuia. Plant growth promoting bacteria (PGPB)-induced plant adaptations to stresses: An updated review. PeerJ 2024, 12, e17882. [Google Scholar] [CrossRef]
- Sevim, G.; Ozdemir-Kocak, F.; Unal, D. Hormonal signaling molecules triggered by plant growth-promoting bacteria. In Phytohormones and Stress Responsive Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2023; pp. 187–196. [Google Scholar] [CrossRef]
- Kang, S.-M.; Khan, A.L.; You, Y.-H.; Kim, J.-G.; Kamran, M.; Lee, I.-J. Gibberellin Production by Newly Isolated Strain Leifsonia soli SE134 and Its Potential to Promote Plant Growth. J. Microbiol. Biotechnol. 2014, 24, 106–112. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Santoyo, G.; Glick, B.R. Recent Advances in the Bacterial Phytohormone Modulation of Plant Growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef]
- Castro-Camba, R.; Sánchez, C.; Vidal, N.; Vielba, J.M. Plant Development and Crop Yield: The Role of Gibberellins. Plants 2022, 11, 2650. [Google Scholar] [CrossRef]
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Dumova, V.A.; Shaposhnikov, A.I.; Ladatko, A.G.; Davies, W.J. Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth. Plant Physiol. Biochem. 2014, 74, 84–91. [Google Scholar] [CrossRef]
- Cohen, A.C.; Bottini, R.; Piccoli, P. Role of Abscisic Acid Producing PGPR in Sustainable Agriculture. In Bacterial Metabolites in Sustainable Agroecosystem. Sustainable Development and Biodiversity; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef]
- Bakshi, A.; Shemansky, J.M.; Chang, C.; Binder, B.M. History of Research on the Plant Hormone Ethylene. J. Plant Growth Regul. 2015, 34, 809–827. [Google Scholar] [CrossRef]
- Ju, C.; van de Poel, B.; Cooper, E.D.; Thierer, J.H.; Gibbons, T.R.; Delwiche, C.F.; Chang, C. Conservation of ethylene as a plant hormone over 450 million years of evolution. Nat. Plants 2015, 1, 14004. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Rossi, M.J.; Glick, B.R. Ethylene and 1-Aminocyclopropane-1-carboxylate (ACC) in Plant–Bacterial Interactions. Front. Plant Sci. 2018, 9, 114. [Google Scholar] [CrossRef]
- Shekhawat, K.; Fröhlich, K.; García-Ramírez, G.X.; Trapp, M.A.; Hirt, H. Ethylene: A Master Regulator of Plant–Microbe Interactions under Abiotic Stresses. Cells 2022, 12, 31. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.A.C.J.; Kowalchuk, G.A.; Jousset, A. Microbial modulation of plant ethylene signaling: Ecological and evolutionary consequences. Microbiome 2018, 6, 52. [Google Scholar] [CrossRef]
- Shahid, M.; Singh, U.B.; Khan, M.S.; Singh, P.; Kumar, R.; Singh, R.N.; Kumar, A.; Singh, H.V. Bacterial ACC deaminase: Insights into enzymology, biochemistry, genetics, and potential role in amelioration of environmental stress in crop plants. Front. Microbiol. 2023, 14, 1132770. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Ma, Y.; Shadan, A. Perspective of ACC-deaminase producing bacteria in stress agriculture. J. Biotechnol. 2022, 352, 36–46. [Google Scholar] [CrossRef]
- Effmert, U.; Kalderás, J.; Warnke, R.; Piechulla, B. Volatile Mediated Interactions Between Bacteria and Fungi in the Soil. J. Chem. Ecol. 2012, 38, 665–703. [Google Scholar] [CrossRef]
- Bennett, J.W.; Hung, R.; Lee, S.; Padhi, S. 18 Fungal and Bacterial Volatile Organic Compounds: An Overview and Their Role as Ecological Signaling Agents. In Fungal Associations; Springer: Berlin/Heidelberg, Germany, 2012; pp. 373–393. [Google Scholar] [CrossRef]
- Minerdi, D.; Maggini, V.; Fani, R. Volatile organic compounds: From figurants to leading actors in fungal symbiosis. FEMS Microbiol. Ecol. 2021, 97, fiab067. [Google Scholar] [CrossRef]
- Sánchez Carrillo, R.; Guerra Ramírez, P. Pseudomonas spp. benéficas en la agricultura. Rev. Mex. Cienc. Agric. 2022, 13, 715–725. [Google Scholar] [CrossRef]
- Moreno-Valencia, F.D.; Plascencia-Espinosa, M.Á.; Morales-García, Y.E.; Muñoz-Rojas, J. Selection and Effect of Plant Growth-Promoting Bacteria on Pine Seedlings (Pinus montezumae and Pinus patula). Life 2024, 14, 1320. [Google Scholar] [CrossRef]
- Sabu, R.; Aswani, R.; Jishma, P.; Jasim, B.; Mathew, J.; Radhakrishnan, E.K. Plant Growth Promoting Endophytic Serratia sp. ZoB14 Protecting Ginger from Fungal Pathogens. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 213–220. [Google Scholar] [CrossRef]
- Kankariya, R.A.; Chaudhari, A.B.; Gavit, P.M.; Dandi, N.D. 4-Diacetylphloroglucinol: A Novel Biotech Bioactive Compound for Agriculture. In Microbial Interventions in Agriculture and Environment; Springer: Singapore, 2019; pp. 419–452. [Google Scholar] [CrossRef]
- Jani, J.; Parvez, N.; Mehta, D. Metabolites of Pseudomonads: A New Avenue of Plant Health Management. In New Horizons in Insect Science: Towards Sustainable Pest Management; Springer: New Delhi, India, 2015; pp. 61–69. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Sayyed, R.Z.; Gafur, A.; Ansari, M.J.; Ray, R.R. PGPR in Biofilm Formation and Antibiotic Production. In Antifungal Metabolites of Rhizobacteria for Sustainable Agriculture. Fungal Biology; Springer: Cham, Switzerland, 2022; pp. 65–82. [Google Scholar] [CrossRef]
- Kour, D.; Negi, R.; Khan, S.S.; Kumar, S.; Kaur, S.; Kaur, T.; Sharma, B.; Dasila, H.; Kour, H.; Ramniwas, S.; et al. Microbes mediated induced systemic response in plants: A review. Plant Stress 2024, 11, 100334. [Google Scholar] [CrossRef]
- Singh, A.A.; Singh, A.K. Role of bacterial quorum sensing in plant growth promotion. World J. Microbiol. Biotechnol. 2025, 41, 18. [Google Scholar] [CrossRef]
- Tyagi, S.; Mulla, S.I.; Lee, K.-J.; Chae, J.-C.; Shukla, P. VOCs-mediated hormonal signaling and crosstalk with plant growth promoting microbes. Crit. Rev. Biotechnol. 2018, 38, 1277–1296. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-M.; Zhang, H. The effects of bacterial volatile emissions on plant abiotic stress tolerance. Front. Plant Sci. 2015, 6, 774. [Google Scholar] [CrossRef]
- Laller, R.; Khosla, P.K.; Negi, N.; Avinash, H.; Kusum Thakur, N.; Kashyap, S.; Shukla, S.K.; Hussain, I. Bacterial volatiles as PGPRs: Inducing plant defense mechanisms during stress periods. S. Afr. J. Bot. 2023, 159, 131–139. [Google Scholar] [CrossRef]
- Plyuta, V.; Lipasova, V.; Popova, A.; Koksharova, O.; Kuznetsov, A.; Szegedi, E.; Chernin, L.; Khmel, I. Influence of volatile organic compounds emitted by Pseudomonas and Serratia strains on Agrobacterium tumefaciens biofilms. APMIS 2016, 124, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Raza, W.; Jiang, G.; Yi, Z.; Fields, B.; Greenrod, S.; Friman, V.-P.; Jousset, A.; Shen, Q.; Wei, Z. Bacterial volatile organic compounds attenuate pathogen virulence via evolutionary trade-offs. ISME J. 2023, 17, 443–452. [Google Scholar] [CrossRef]
- Mhlongo, M.I.; Piater, L.A.; Dubery, I.A. Profiling of Volatile Organic Compounds from Four Plant Growth-Promoting Rhizobacteria by SPME–GC–MS: A Metabolomics Study. Metabolites 2022, 12, 763. [Google Scholar] [CrossRef]
- Shrestha, A.; Schikora, A. AHL-priming for enhanced resistance as a tool in sustainable agriculture. FEMS Microbiol. Ecol. 2020, 96, fiaa226. [Google Scholar] [CrossRef]
- Chamkhi, I.; El Omari, N.; Benali, T.; Bouyahya, A. Quorum Sensing and Plant-Bacteria Interaction: Role of Quorum Sensing in the Rhizobacterial Community Colonization in the Rhizosphere. In Quorum Sensing: Microbial Rules of Life; American Chemical Society (ACS): Washington, DC, USA, 2020; pp. 139–153. [Google Scholar] [CrossRef]
- Cortez, M.; Handy, D.; Headlee, A.; Montanez, C.; Pryor, S.; Cutshaw, K.; Vanselow, K.; Perez, A.; Weissman, J.; Ziegler, E.; et al. Quorum Sensing in the Rhizosphere. In Microbial Cross-Talk in the Rhizosphere; Springer: Singapore, 2022; pp. 99–134. [Google Scholar] [CrossRef]
- Hartmann, A. Quorum sensing N-acyl-homoserine lactone signal molecules of plant beneficial Gram-negative rhizobacteria support plant growth and resistance to pathogens. Rhizosphere 2020, 16, 100258. [Google Scholar] [CrossRef]
- Nawaz, M.S.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. Growth-Stimulatory Effect of Quorum Sensing Signal Molecule N-Acyl-Homoserine Lactone-Producing Multi-Trait Aeromonas spp. on Wheat Genotypes Under Salt Stress. Front. Microbiol. 2020, 11, 553621. [Google Scholar] [CrossRef]
- Viswanath, G.; Sekar, J.; Ramalingam, P.V. Detection of Diverse N-Acyl Homoserine Lactone Signalling Molecules Among Bacteria Associated with Rice Rhizosphere. Curr. Microbiol. 2020, 77, 3480–3491. [Google Scholar] [CrossRef]
- Ramírez-Mata, A.; Fernández-Domínguez, I.J.; Nuñez-Reza, K.J.; Xiqui-Vázquez, M.L.; Baca, B.E. Redes de señalización en la producción de biopelículas en bacterias: Quorum sensing, di-GMPc y óxido nítrico. Rev. Argent. Microbiol. 2014, 46, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Bhargava, P.; Goel, R. Quorum Sensing Molecules of Rhizobacteria: A Trigger for Developing Systemic Resistance in Plants. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management: Volume 12: Microorganisms for Sustainability; Springer: Singapore, 2019; pp. 117–138. [Google Scholar] [CrossRef]
- Kenawy, A.; Dailin, D.J.; Abo-Zaid, G.A.; Malek, R.A.; Ambehabati, K.K.; Zakaria, K.H.N.; Sayyed, R.Z.; El Enshasy, H.A. Biosynthesis of Antibiotics by PGPR and Their Roles in Biocontrol of Plant Diseases. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management: Volume 13: Microorganisms for Sustainability; Springer: Singapore, 2019; pp. 1–35. [Google Scholar] [CrossRef]
- Mehmood, A.; Liu, G.; Wang, X.; Meng, G.; Wang, C.; Liu, Y. Fungal Quorum-Sensing Molecules and Inhibitors with Potential Antifungal Activity: A Review. Molecules 2019, 24, 1950. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.; Casadevall, A. Quorum sensing in fungi—A review. Med. Mycol. 2012, 50, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, H.; Xu, S.; Liu, K.; Qi, H.; Wang, M.; Chen, X.; Berg, G.; Ma, Z.; Cernava, T.; et al. Bacterial-fungal interactions under agricultural settings: From physical to chemical interactions. Stress. Biol. 2022, 2, 22. [Google Scholar] [CrossRef]
- Khalid, S.; Keller, N.P. Chemical signals driving bacterial–fungal interactions. Environ. Microbiol. 2021, 23, 1334–1347. [Google Scholar] [CrossRef]
- Yuan, W.A.N.G.; Wenqing, L.I.; Binghai, D.U.; Hanhao, L.I. Effect of biochar applied with plant growth-promoting rhizobacteria (PGPR) on soil microbial community composition and nitrogen utilization in tomato. Pedosphere 2021, 31, 872–881. [Google Scholar] [CrossRef]
- Rahman, M.K.U.; Zhou, X.; Wu, F. The role of root exudates, CMNs, and VOCs in plant–plant interaction. J. Plant Interact. 2019, 14, 630–636. [Google Scholar] [CrossRef]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-Activated Protein Kinase Cascades in Plant Hormone Signaling. Front. Plant Sci. 2018, 9, 1387. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Sinha, A.K. ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Front. Plant Sci. 2015, 6, 769. [Google Scholar] [CrossRef]
- Krysan, P.J.; Colcombet, J. Cellular Complexity in MAPK Signaling in Plants: Questions and Emerging Tools to Answer Them. Front. Plant Sci. 2018, 9, 1674. [Google Scholar] [CrossRef]
- Hamel, L.-P.; Nicole, M.-C.; Sritubtim, S.; Morency, M.-J.; Ellis, M.; Ehlting, J.; Beaudoin, N.; Barbazuk, B.; Klessig, D.; Lee, J.; et al. Ancient signals: Comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci. 2006, 11, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, S. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef]
- Danquah, A.; de Zelicourt, A.; Colcombet, J.; Hirt, H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Bigeard, J.; Hirt, H. Nuclear Signaling of Plant MAPKs. Front. Plant Sci. 2018, 9, 469. [Google Scholar] [CrossRef]
- Solomon, W.; Janda, T.; Molnár, Z. Unveiling the significance of rhizosphere: Implications for plant growth, stress response, and sustainable agriculture. Plant Physiol. Biochem. 2024, 206, 108290. [Google Scholar] [CrossRef] [PubMed]
- Jamil, F.; Mukhtar, H.; Fouillaud, M.; Dufossé, L. Rhizosphere Signaling: Insights into Plant–Rhizomicrobiome Interactions for Sustainable Agronomy. Microorganisms 2022, 10, 899. [Google Scholar] [CrossRef]
- Buitrago, R.R.B.; de Bashan, L.E.G.; Pedraza, R.O.; Bonilla, G.A.E.; Díaz, S.P. Bacterias Promotoras De Crecimiento Vegetal En Sistemas De Agricultura Sostenible; Corporación Colombiana de Investigación Agropecuaria: Mosquera, Cundinamarca, Colombia, 2021. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O. Plant health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef]
- Shah, W.U.H.; Lu, Y.; Liu, J.; Rehman, A.; Yasmeen, R. The impact of climate change and production technology heterogeneity on China’s agricultural total factor productivity and production efficiency. Sci. Total Environ. 2024, 907, 168027. [Google Scholar] [CrossRef]
- Bradáčová, K.; Florea, A.S.; Bar-Tal, A.; Minz, D.; Yermiyahu, U.; Shawahna, R.; Kraut-Cohen, J.; Zolti, A.; Erel, R.; Dietel, K.; et al. Microbial Consortia versus Single-Strain Inoculants: An Advantage in PGPM-Assisted Tomato Production? Agronomy 2019, 9, 105. [Google Scholar] [CrossRef]
- Pervaiz, Z.H.; Iqbal, J.; Zhang, Q.; Chen, D.; Wei, H.; Saleem, M. Continuous Cropping Alters Multiple Biotic and Abiotic Indicators of Soil Health. Soil Syst. 2020, 4, 59. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. Plant responses to multifactorial stress combination. New Phytol. 2022, 234, 1161–1167. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Satish, L.; Pandian, S.K.; Chen, J.-T.; Ahmar, S.; Wang, X.; Mora-Poblete, F.; Ramesh, M. An Overview of Abiotic Stress in Cereal Crops: Negative Impacts, Regulation, Biotechnology and Integrated Omics. Plants 2021, 10, 1472. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.G.; Cellini, F.; Fotopoulos, V.; Balestrini, R.; Arbona, V. New approaches to improve crop tolerance to biotic and abiotic stresses. Physiol. Plant 2022, 174, e13547. [Google Scholar] [CrossRef] [PubMed]
- Teshome, D.T.; Zharare, G.E.; Naidoo, S. The Threat of the Combined Effect of Biotic and Abiotic Stress Factors in Forestry Under a Changing Climate. Front. Plant Sci. 2020, 11, 601009. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Xue, D.; Bao, C.; Zhang, K.; Chen, L.; Li, Q.; Guo, R. Enhanced Plant Growth Through Composite Inoculation of Phosphate-Solubilizing Bacteria: Insights from Plate and Soil Experiments. Agronomy 2024, 14, 2461. [Google Scholar] [CrossRef]
- Liang, J.-L.; Liu, J.; Jia, P.; Yang, T.; Zeng, Q.; Zhang, S.; Liao, B.; Shu, W.; Li, J. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef]
- Bashir, Z.; Hamid, B.; Yatoo, A.M.; Nisa, M.; Sultan, Z.; Popescu, S.M. Phosphorus Solubilizing Microorganisms: An Eco-Friendly Approach for Sustainable Plant Health and Bioremediation. J. Soil. Sci. Plant Nutr. 2024, 24, 6838–6854. [Google Scholar] [CrossRef]
- Liu-Xu, L.; González-Hernández, A.I.; Camañes, G.; Vicedo, B.; Scalschi, L.; Llorens, E. Harnessing Green Helpers: Nitrogen-Fixing Bacteria and Other Beneficial Microorganisms in Plant–Microbe Interactions for Sustainable Agriculture. Horticulturae 2024, 10, 621. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Vanderleyden, J.; Okon, Y. Plant Growth-Promoting Effects of Diazotrophs in the Rhizosphere. CRC Crit. Rev. Plant Sci. 2003, 22, 107–149. [Google Scholar] [CrossRef]
- Ghosh, A.; Sah, D.; Chakraborty, M.; Rai, J.P.N. Mechanism and application of bacterial exopolysaccharides: An advanced approach for sustainable heavy metal abolition from soil. Carbohydr. Res. 2024, 544, 109247. [Google Scholar] [CrossRef]
- Thiebaut, F.; Urquiaga, M.C.d.O.; Rosman, A.C.; da Silva, M.L.; Hemerly, A.S. The Impact of Non-Nodulating Diazotrophic Bacteria in Agriculture: Understanding the Molecular Mechanisms That Benefit Crops. Int. J. Mol. Sci. 2022, 23, 11301. [Google Scholar] [CrossRef]
- Cuevas, J.; Daliakopoulos, I.N.; del Moral, F.; Hueso, J.J.; Tsanis, I.K. A Review of Soil-Improving Cropping Systems for Soil Salinization. Agronomy 2019, 9, 295. [Google Scholar] [CrossRef]
- Mirza, B.S.; McGlinn, D.J.; Bohannan, B.J.M.; Nüsslein, K.; Tiedje, J.M.; Rodrigues, J.L.M. Diazotrophs Show Signs of Restoration in Amazon Rain Forest Soils with Ecosystem Rehabilitation. Appl. Environ. Microbiol. 2020, 86, e00195-20. [Google Scholar] [CrossRef] [PubMed]
- Munir, N.; Hanif, M.; Abideen, Z.; Sohail, M.; El-Keblawy, A.; Radicetti, E.; Mancinelli, R.; Haider, G. Mechanisms and Strategies of Plant Microbiome Interactions to Mitigate Abiotic Stresses. Agronomy 2022, 12, 2069. [Google Scholar] [CrossRef]
- Acosta-Motos, J.; Ortuño, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.; Hernandez, J. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Stavi, I.; Thevs, N.; Priori, S. Soil Salinity and Sodicity in Drylands: A Review of Causes, Effects, Monitoring, and Restoration Measures. Front. Environ. Sci. 2021, 9, 712831. [Google Scholar] [CrossRef]
- Wu, X.; Fan, Y.; Wang, R.; Zhao, Q.; Ali, Q.; Wu, H.; Gu, Q.; Borriss, R.; Xie, Y.; Gao, X. Bacillus halotolerans KKD1 induces physiological, metabolic and molecular reprogramming in wheat under saline condition. Front. Plant. Sci. 2022, 13, 978066. [Google Scholar] [CrossRef]
- Lami, M.J.; Adler, C.; Caram-Di Santo, M.C.; Zenoff, A.M.; Cristóbal, R.E.; Espinosa-Urgel, M.; Vincent, P.A. Pseudomonas stutzeri MJL19, a rhizosphere-colonizing bacterium that promotes plant growth under saline stress. J. Appl. Microbiol. 2020, 129, 1321–1336. [Google Scholar] [CrossRef]
- Degon, Z.; Dixon, S.; Rahmatallah, Y.; Galloway, M.; Gulutzo, S.; Price, H.; Cook, J.; Glazko, G.; Mukherjee, A. Azospirillum brasilense improves rice growth under salt stress by regulating the expression of key genes involved in salt stress response, abscisic acid signaling, and nutrient transport, among others. Front. Agron. 2023, 5, 1216503. [Google Scholar] [CrossRef] [PubMed]
- Okur, B.; Örçen, N. Soil salinization and climate change. In Climate Change and Soil Interactions; Elsevier: Amsterdam, The Netherlands, 2020; pp. 331–350. [Google Scholar] [CrossRef]
- Madline, A.; Benidire, L.; Boularbah, A. Alleviation of salinity and metal stress using plant growth-promoting rhizobacteria isolated from semiarid Moroccan copper-mine soils. Environ. Sci. Pollut. Res. 2021, 28, 67185–67202. [Google Scholar] [CrossRef]
- Etesami, H.; Noori, F. Soil Salinity as a Challenge for Sustainable Agriculture and Bacterial-Mediated Alleviation of Salinity Stress in Crop Plants. In Saline Soil-Based Agriculture by Halotolerant Microorganisms; Springer: Singapore, 2019; pp. 1–22. [Google Scholar] [CrossRef]
- Shultana, R.; Zuan, A.T.K.; Naher, U.A.; Islam, A.K.M.M.; Rana Md, M.; Rashid Md, H.; Irin, I.J.; Islam, S.S.; Rim, A.A.; Hasan, A.K. The PGPR Mechanisms of Salt Stress Adaptation and Plant Growth Promotion. Agronomy 2022, 12, 2266. [Google Scholar] [CrossRef]
- Hnini, M.; Rabeh, K.; Oubohssaine, M. Interactions between beneficial soil microorganisms (PGPR and AMF) and host plants for environmental restoration: A systematic review. Plant Stress. 2024, 11, 100391. [Google Scholar] [CrossRef]
- Sharma, I.; Sharma, S.; Sharma, V.; Singh, A.K.; Sharma, A.; Kumar, A.; Singh, J.; Sharma, A. PGPR-Enabled bioremediation of pesticide and heavy metal-contaminated soil: A review of recent advances and emerging challenges. Chemosphere 2024, 362, 142678. [Google Scholar] [CrossRef]
- Gupta, R.; Khan, F.; Alqahtani, F.M.; Hashem, M.; Ahmad, F. Plant Growth–Promoting Rhizobacteria (PGPR) Assisted Bioremediation of Heavy Metal Toxicity. Appl. Biochem. Biotechnol. 2024, 196, 2928–2956. [Google Scholar] [CrossRef] [PubMed]
- Sobariu, D.L.; Fertu, D.I.T.; Diaconu, M.; Pavel, L.V.; Hlihor, R.-M.; Drăgoi, E.N.; Curteanu, S.; Lenz, M.; Corvini, P.F.-X.; Gavrilescu, M. Rhizobacteria and plant symbiosis in heavy metal uptake and its implications for soil bioremediation. New Biotechnol. 2017, 39, 125–134. [Google Scholar] [CrossRef]
- Hawkes, S.J. What Is a ‘Heavy Metal’? J. Chem. Educ. 1997, 74, 1374. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in Crop Production. Adv. Agron. 2002, 77, 185–268. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Ajwa, H.A. Trace elements in soils and plants: An overview. J. Environ. Sci. Health Part A 1999, 34, 951–974. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, Y.; Lan, X.; Yang, Y.; Wu, X.; Du, L. Comprehensive assessment of harmful heavy metals in contaminated soil in order to score pollution level. Sci. Rep. 2022, 12, 3552. [Google Scholar] [CrossRef]
- Mondal, S.; Mukherjee, S.K.; Hossain, S.T. Exploration of Plant Growth Promoting Rhizobacteria (PGPRs) for Heavy Metal Bioremediation and Environmental Sustainability: Recent Advances and Future Prospects. In Modern Approaches in Waste Bioremediation; Springer International Publishing: Cham, Switzerland, 2023; pp. 29–55. [Google Scholar] [CrossRef]
- Ortiz, J.; Dias, N.; Alvarado, R.; Soto, J.; Sanhueza, T.; Rabert, C.; Jorquera, M.; Arriagada, C. N-acyl homoserine lactones (AHLs) type signal molecules produced by rhizobacteria associated with plants that growing in a metal(oids) contaminated soil: A catalyst for plant growth. Microbiol. Res. 2024, 281, 127606. [Google Scholar] [CrossRef]
- Alves, A.R.A.; Yin, Q.; Oliveira, R.S.; Silva, E.F.; Novo, L.A.B. Plant growth-promoting bacteria in phytoremediation of metal-polluted soils: Current knowledge and future directions. Sci. Total Environ. 2022, 838, 156435. [Google Scholar] [CrossRef]
- Pandey, S.; Ghosh, P.K.; Ghosh, S.; De, T.K.; Maiti, T.K. Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J. Microbiol. 2013, 51, 11–17. [Google Scholar] [CrossRef]
- Hou, J.; Liu, W.; Wang, B.; Wang, Q.; Luo, Y.; Franks, A.E. PGPR enhanced phytoremediation of petroleum contaminated soil and rhizosphere microbial community response. Chemosphere 2015, 138, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Riseh, R.S.; Vazvani, M.G.; Hajabdollahi, N.; Thakur, V.K. Bioremediation of Heavy Metals by Rhizobacteria. Appl. Biochem. Biotechnol. 2023, 195, 4689–4711. [Google Scholar] [CrossRef]
- Chitara, M.K.; Chauhan, S.; Singh, R.P. Bioremediation of Polluted Soil by Using Plant Growth–Promoting Rhizobacteria. In Microbial Rejuvenation of Polluted Environment; Springer: Singapore, 2021; pp. 203–226. [Google Scholar] [CrossRef]
- FAO. El Estado De Los Recursos De Tierras Y Aguas Del Mundo Para La Alimentación Y La Agricultura—Sistemas Al Límite; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Degradation, T.G.S. Land Scarcity and Food Security: Reviewing a Complex Challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef]
- United Nations. United Nations Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/es/2023/05/un-2023-sdg-summit/ (accessed on 28 May 2025).
- Sharma, I.P.; Chandra, S.; Kumar, N.; Chandra, D. PGPR: Heart of Soil and Their Role in Soil Fertility. In Agriculturally Important Microbes for Sustainable Agriculture; Springer: Singapore, 2017; pp. 51–67. [Google Scholar] [CrossRef]
- Hasan, A.; Tabassum, B.; Hashim, M.; Khan, N. Role of Plant Growth Promoting Rhizobacteria (PGPR) as a Plant Growth Enhancer for Sustainable Agriculture: A Review. Bacteria 2024, 3, 59–75. [Google Scholar] [CrossRef]
- Mitra, D.; Pellegrini, M.; Guerra-Sierra, B.E. Interaction between Plants and Growth-Promoting Rhizobacteria (PGPR) for Sustainable Development. Bacteria 2024, 3, 136–140. [Google Scholar] [CrossRef]
- Patel, P.; Sayyed, R.Z.; Patel, H. PGPR: A Sustainable Agricultural Mitigator for Stressed Agro-Environments. In Plant Growth Promoting Microorganisms of Arid Region; Springer: Singapore, 2023; pp. 303–318. [Google Scholar] [CrossRef]
- Morales-García, Y.E.; Juárez-Hernández, D.; Hernández-Tenorio, A.L. Inoculante de segunda generación para incrementar el crecimiento y salud de plantas de jardín. AyTBUAP 2020, 5, 136–154. [Google Scholar]
- Mengual, C.; Schoebitz, M.; Azcón, R.; Roldán, A. Microbial inoculants and organic amendment improves plant establishment and soil rehabilitation under semiarid conditions. J. Environ. Manag. 2014, 134, 1–7. [Google Scholar] [CrossRef]
- Saeed, M.; Ilyas, N.; Jayachandran, K.; Shabir, S.; Akhtar, N.; Shahzad, A.; Sayyed, R.Z.; Bano, A. Advances in Biochar and PGPR engineering system for hydrocarbon degradation: A promising strategy for environmental remediation. Environ. Pollut. 2022, 305, 119282. [Google Scholar] [CrossRef]
- Anbuganesan, V.; Vishnupradeep, R.; Bruno, L.B.; Sharmila, K.; Freitas, H.; Rajkumar, M. Combined Application of Biochar and Plant Growth-Promoting Rhizobacteria Improves Heavy Metal and Drought Stress Tolerance in Zea mays. Plants 2024, 13, 1143. [Google Scholar] [CrossRef] [PubMed]
- Abdelkrim, S.; Jebara, S.H.; Saadani, O.; Abid, G.; Taamalli, W.; Zemni, H.; Mannai, K.; Louati, F.; Jebara, M. In situ effects of Lathyrus sativus- PGPR to remediate and restore quality and fertility of Pb and Cd polluted soils. Ecotoxicol. Environ. Saf. 2020, 192, 110260. [Google Scholar] [CrossRef]
- Hussain, F.; Hussain, I.; Khan, A.H.A.; Muhammad, Y.S.; Iqbal, M.; Soja, G.; Reichenauer, T.G.; Zeshan; Yousaf, S. Combined application of biochar, compost, and bacterial consortia with Italian ryegrass enhanced phytoremediation of petroleum hydrocarbon contaminated soil. Environ. Exp. Bot. 2018, 153, 80–88. [Google Scholar] [CrossRef]
- Ali, M.H.; Khan, M.I.; Bashir, S.; Azam, M.; Naveed, M.; Qadri, R.; Bashir, S.; Mehmood, F.; Shoukat, M.A.; Li, Y.; et al. Biochar and Bacillus sp. MN54 Assisted Phytoremediation of Diesel and Plant Growth Promotion of Maize in Hydrocarbons Contaminated Soil. Agronomy 2021, 11, 1795. [Google Scholar] [CrossRef]
- Minnikova, T.; Kolesnikov, S.; Minin, N.; Gorovtsov, A.; Vasilchenko, N.; Chistyakov, V. The Influence of Remediation with Bacillus and Paenibacillus Strains and Biochar on the Biological Activity of Petroleum-Hydrocarbon-Contaminated Haplic Chernozem. Agriculture 2023, 13, 719. [Google Scholar] [CrossRef]
- Hafez, E.M.; Alsohim, A.S.; Farig, M.; Omara, A.E.-D.; Rashwan, E.; Kamara, M.M. Synergistic Effect of Biochar and Plant Growth Promoting Rhizobacteria on Alleviation of Water Deficit in Rice Plants under Salt-Affected Soil. Agronomy 2019, 9, 847. [Google Scholar] [CrossRef]
- Nehela, Y.; Mazrou, Y.S.A.; Alshaal, T.; Rady, A.M.S.; El-Sherif, A.M.A.; Omara, A.E.-D.; Abd El-Monem, A.M.; Hafez, E.M. The Integrated Amendment of Sodic-Saline Soils Using Biochar and Plant Growth-Promoting Rhizobacteria Enhances Maize (Zea mays L.) Resilience to Water Salinity. Plants 2021, 10, 1960. [Google Scholar] [CrossRef]
- Malik, L.; Sanaullah, M.; Mahmood, F.; Hussain, S.; Shahzad, T. Co-application of biochar and salt tolerant PGPR to improve soil quality and wheat production in a naturally saline soil. Rhizosphere 2024, 29, 100849. [Google Scholar] [CrossRef]
- Wade, M.R.; Gurr, G.M.; Wratten, S.D. Ecological restoration of farmland: Progress and prospects. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 831–847. [Google Scholar] [CrossRef]
- Gairola, S.U.; Bahuguna, R.; Bhatt, S.S. Native Plant Species: A Tool for Restoration of Mined Lands. J. Soil. Sci. Plant Nutr. 2023, 23, 1438–1448. [Google Scholar] [CrossRef]
- Calle, C.; Bonifacio, A.; Villca, M. Arbustos Y Pastos Para Restablecer La Cobertura Vegetal En Zonas Áridas Del Sur De Bolivia; CIMMYT: Texcoco, Mexico, 2022; pp. 1–12. Available online: https://hdl.handle.net/10883/22242 (accessed on 13 June 2024).
- de-Bashan, L.E.; Hernandez, J.-P.; Bashan, Y. The potential contribution of plant growth-promoting bacteria to reduce environmental degradation—A comprehensive evaluation. Appl. Soil. Ecol. 2012, 61, 171–189. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Yao, T.; Ma, Y.; Zhang, H.; Yang, X. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil. Tillage Res. 2020, 199, 104577. [Google Scholar] [CrossRef]
- Khoshru, B.; Mitra, D.; Khoshmanzar, E.; Myo, E.M.; Uniyal, N.; Mahakur, B.; Mohapatra PKdas Panneerselvam, P.; Boutaj, H.; Alizadeh, M.; Cely, M.V.T.; et al. Current scenario and future prospects of plant growth-promoting rhizobacteria: An economic valuable resource for the agriculture revival under stressful conditions. J. Plant Nutr. 2020, 43, 3062–3092. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciel-Rodríguez, M.; Moreno-Valencia, F.D.; Plascencia-Espinosa, M. The Role of Plant Growth-Promoting Bacteria in Soil Restoration: A Strategy to Promote Agricultural Sustainability. Microorganisms 2025, 13, 1799. https://doi.org/10.3390/microorganisms13081799
Maciel-Rodríguez M, Moreno-Valencia FD, Plascencia-Espinosa M. The Role of Plant Growth-Promoting Bacteria in Soil Restoration: A Strategy to Promote Agricultural Sustainability. Microorganisms. 2025; 13(8):1799. https://doi.org/10.3390/microorganisms13081799
Chicago/Turabian StyleMaciel-Rodríguez, Mario, Francisco David Moreno-Valencia, and Miguel Plascencia-Espinosa. 2025. "The Role of Plant Growth-Promoting Bacteria in Soil Restoration: A Strategy to Promote Agricultural Sustainability" Microorganisms 13, no. 8: 1799. https://doi.org/10.3390/microorganisms13081799
APA StyleMaciel-Rodríguez, M., Moreno-Valencia, F. D., & Plascencia-Espinosa, M. (2025). The Role of Plant Growth-Promoting Bacteria in Soil Restoration: A Strategy to Promote Agricultural Sustainability. Microorganisms, 13(8), 1799. https://doi.org/10.3390/microorganisms13081799





