Early Warning Approach to Identify Positive Cases of SARS-CoV-2 in School Settings in Italy
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Procedures in the Experimental Schools
2.3. Procedures by Region
2.4. Data Management
2.5. Statistical Analysis
3. Results
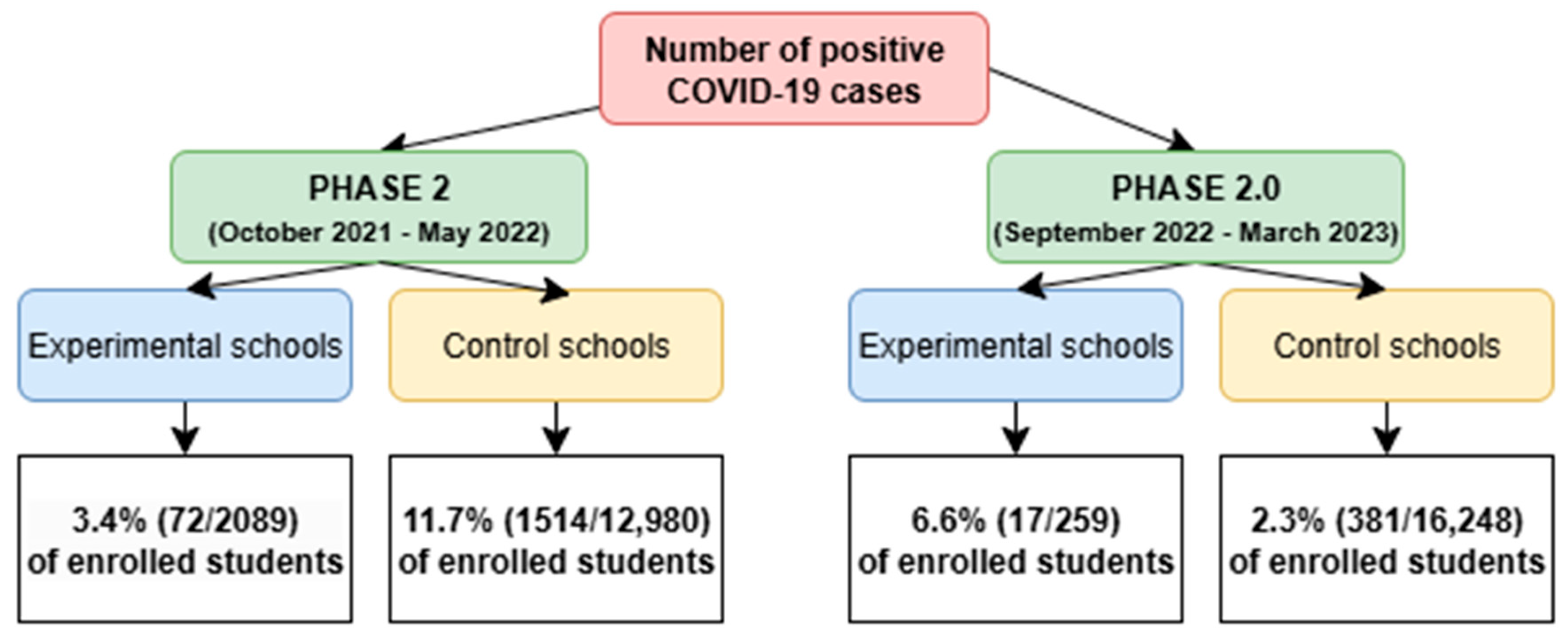
3.1. Study Population
3.2. Adherence to Screening and Incidence of SARS-CoV-2 Cases
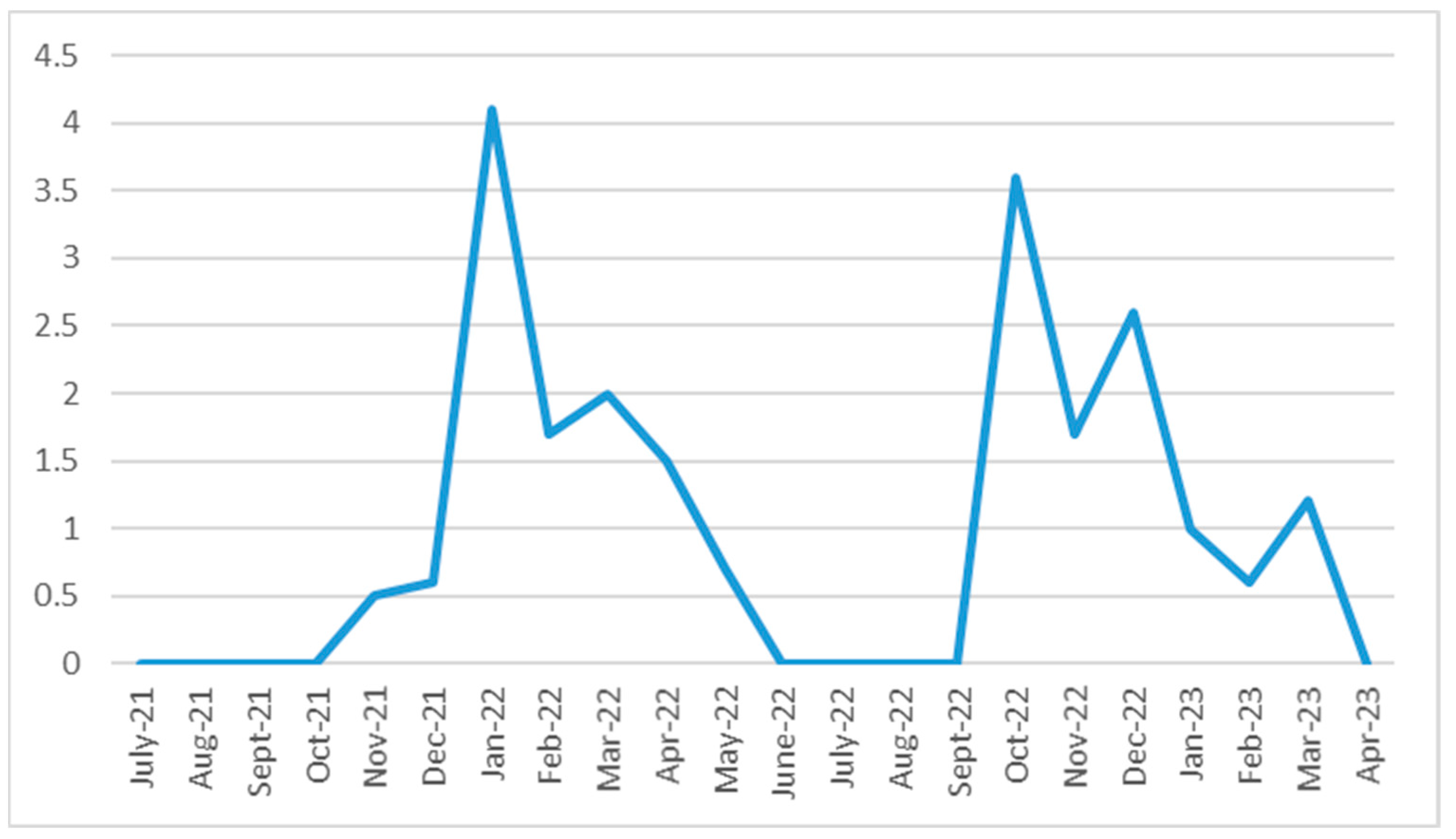
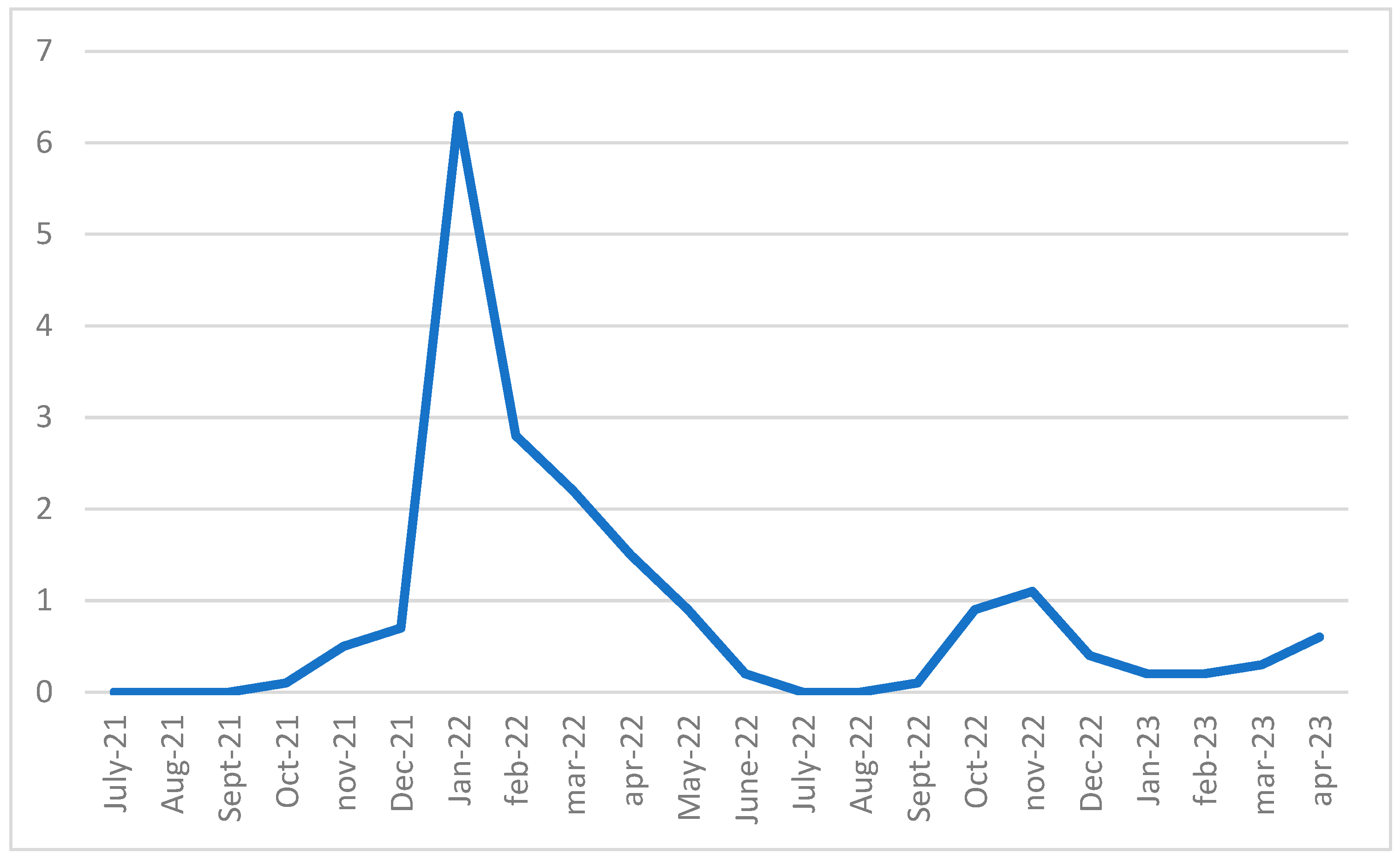
3.3. Temporal Profile of the Epidemic Spread Within the Schools
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Bio. Medica Atenei Parm. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- Sanyaolu, A.; Okorie, C.; Hosein, Z.; Patidar, R.; Desai, P.; Prakash, S.; Jaferi, U.; Mangat, J.; Marinkovic, A. Global Pandemicity of COVID-19: Situation Report as of June 9, 2020. Infect. Dis. 2021, 14, 1178633721991260. [Google Scholar] [CrossRef] [PubMed]
- Misure Profilattiche Contro il Nuovo Coronavirus (2019–nCoV), Ordinanza Min. Salute 30.01.2020: Gazzetta Ufficiale n. 26 del 01.02.2020 Martedì 4 Febbraio 2020. Available online: https://www.regioni.it/news/2020/02/04/misure-profilattiche-contro-il-nuovo-coronavirus-2019-ncov-ordinanza-min-salute-30-01-2020-gazzetta-ufficiale-n-26-del-01-02-2020-604897/ (accessed on 1 February 2020).
- Giovanetti, M.; Benvenuto, D.; Angeletti, S.; Ciccozzi, M. The first two cases of 2019-nCoV in Italy: Where they come from? J. Med. Virol. 2020, 92, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Decreto del Presidente del Consiglio Dei Ministri 4 Marzo 2020. Ulteriori Disposizioni Attuative del Decreto-Legge 23 Febbraio 2020, N. 6, Recante Misure Urgenti in Materia di Contenimento e Gestione Dell’emergenza Epidemiologica Da COVID-19, Applicabili Sull’intero Territorio Nazionale. (20A01475) (GU Serie Generale N.55 Del 04-03-2020). Available online: https://www.gazzettaufficiale.it/eli/id/2020/03/04/20A01475/s (accessed on 4 March 2020).
- Decreto del Presidente del Consiglio Dei Ministri 9 Marzo 2020. Ulteriori Disposizioni Attuative del Decreto-Legge 23 Febbraio 2020, N. 6, Recante Misure Urgenti in Materia Di Contenimento E Gestione Dell’emergenza Epidemiologica Da COVID-19, Applicabili Sull’intero Territorio Nazionale. (20A01558) (GU Serie Generale N. 62 Del 09-03-2020). Available online: https://www.gazzettaufficiale.it/eli/id/2020/03/09/20A01558/s (accessed on 9 March 2020).
- Prezioso, C.; Pietropaolo, V. COVID-19: Update of the Italian situation. J. Neurovirology 2020, 26, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Decreto del Presidente del Consiglio Dei Ministri 11 Giugno 2020. Ulteriori Disposizioni Attuative del Decreto-Legge 25 Marzo 2020, N. 19, Recante Misure Urgenti per Fronteggiare L’emergenza Epidemiologica Da COVID-19, E del Decreto-Legge 16 Maggio 2020, N. 33, Recante Ulteriori Misure Urgenti per Fronteggiare L’emergenza Epidemiologica Da COVID-19. (20A03194) (GU Serie Generale N.147 del 11-06-2020). Available online: https://www.gazzettaufficiale.it/eli/id/2020/06/11/20A03194/s (accessed on 11 June 2020).
- Decreto del Presidente del Consiglio dei Ministri 3 Novembre 2020. Ulteriori Disposizioni Attuative del Decreto-Legge 25 Marzo 2020, N. 19, Convertito, Con Modificazioni, Dalla Legge 25 Maggio 2020, N. 35, Recante «Misure Urgenti Per Fronteggiare L’emergenza Epidemiologica Da COVID-19, E del Decreto-Legge 16 Maggio 2020, N. 33, Convertito, Con Modificazioni, Dalla Legge 14 Luglio 2020, N. 74, Recante «Ulteriori Misure Urgenti Per Fronteggiare L’emergenza Epidemiologica Da COVID-19». (20A06109) (GU Serie Generale N.275 del 04-11-2020—Suppl. Ordinario N. 41). Available online: https://www.gazzettaufficiale.it/eli/id/2020/11/04/20A06109/s (accessed on 4 November 2020).
- Istituto Superiore di Sanità. Epicentro–Epidemiology for Public Health National COVID-19 Vaccination Plan. Available online: https://www.epicentro.iss.it/en/vaccines/covid-19-vaccination-plan#:~:text=%E2%80%9CVaccine%20day%E2%80%9D%2C%20on%2027,started%20on%2031%20December%202021 (accessed on 10 August 2021).
- Ministero Della Salute. Ordinanza 22 Giugno 2021. Ulteriori Misure Urgenti in Materia di Contenimento E Gestione Dell’emergenza Epidemiologica Da COVID-19 in «Zona Bianca». (21A03849) (GU Serie Generale N.148 Del 23-06-2021). Available online: https://www.gazzettaufficiale.it/eli/id/2021/06/23/21A03849/SG (accessed on 23 June 2021).
- Decreto del Presidente del Consiglio Dei Ministri 17 Giugno 2021. Disposizioni Attuative Dell’articolo 9, Comma 10, del Decreto-Legge 22 Aprile 2021, N. 52, Recante «Misure Urgenti Per La Graduale Ripresa Delle Attivita’ Economiche E Sociali Nel Rispetto Delle Esigenze Di Contenimento Della Diffusione Dell’epidemia Da COVID-19». (21A03739) (GU Serie Generale N.143 Del 17-06-2021). Available online: https://www.gazzettaufficiale.it/eli/id/2021/06/17/21A03739/SG (accessed on 17 June 2021).
- DECRETO-LEGGE 5 Gennaio 2021, N. 1 (Raccolta 2021). Ulteriori Disposizioni Urgenti in Materia Di Contenimento E Gestione Dell’emergenza Epidemiologica Da COVID-19. (21G00001) (GU Serie Generale N.3 del 05-01-2021). Available online: https://www.gazzettaufficiale.it/eli/id/2021/01/05/21G00001/sg (accessed on 5 January 2021).
- Parotto, E.; Lamberti-Castronuovo, A.; Censi, V.; Valente, M.; Atzori, A.; Ragazzoni, L. Exploring Italian healthcare facilities response to COVID-19 pandemic: Lessons learned from the Italian Response to COVID-19 initiative. Front. Public Health 2023, 10, 1016649. [Google Scholar] [CrossRef] [PubMed]
- Doz, E.; Cuder, A.; Caputi, M.; Pellizzoni, S.; Passolunghi, M.C. Distance learning environment: Perspective of Italian primary and secondary teachers during COVID-19 pandemic. Learn. Environ. Res. 2023, 26, 555–571. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. COVID-19 in Children and the Role of School Settings in COVID-19 Transmission. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-schools-transmission-August%202020.pdf (accessed on 6 August 2020).
- Silverberg, S.L.; Zhang, B.Y.; Li, S.N.J.; Burgert, C.; Shulha, H.P.; Kitchin, V.; Sauvé, L.; Sadarangani, M. Child transmission of SARS-CoV-2: A systematic review and meta-analysis. BMC Pediatr. 2022, 22, 172. [Google Scholar] [CrossRef] [PubMed]
- Meuris, C.; Kremer, C.; Geerinck, A.; Locquet, M.; Bruyère, O.; Defêche, J.; Meex, C.; Hayette, M.P.; Duchene, L.; Dellot, P.; et al. Transmission of SARS-CoV-2 After COVID-19 Screening and Mitigation Measures for Primary School Children Attending School in Liège, Belgium. JAMA 2021, 4, e2128757. [Google Scholar] [CrossRef] [PubMed]
- Pistellato, I.; Fonzo, M.; Calzavara, A.; Sorrentino, P.; Selle, V.; Sbrogiò, L.G.; Bertoncello, C. The spread of SARS-CoV-2 at school through the different pandemic waves: A population-based study in Italy. Eur. J. Pediatr. 2023, 182, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Aslaner, H.; Benli, A.R.; Şimşek, E.; Korkmaz, Z. Prevalence of COVID-19 infection in asymptomatic school children. Turk. J. Pediatr. 2022, 64, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Djuric, O.; Larosa, E.; Cassinadri, M.; Cilloni, S.; Bisaccia, E.; Pepe, D.; Vicentini, M.; Venturelli, F.; Bonvicini, L.; Giorgi Rossi, P.; et al. Surveillance, contact tracing and characteristics of SARS-CoV-2 transmission in educational settings in Northern Italy, September 2020 to April 2021. PLoS ONE 2022, 17, e0275667. [Google Scholar] [CrossRef] [PubMed]
- Bert, F.; Lo Moro, G.; Barbaro, S.; Barbero, S.; Boietti, E.; Minutiello, E.; Sinigaglia, T.; Fagioli, F.; Siliquini, R. Analysis of a direct access testing system for the detection of SARS-CoV-2 in the paediatric population attending school. Ann. Ig. Med. Prev. Comunita 2023, 35, 617–630. [Google Scholar] [CrossRef]
- Paduano, S.; Facchini, M.C.; Borsari, L.; D’Alterio, A.; Iacuzio, L.; Greco, A.; Fioretti, E.; Creola, G.; Kahfian, Z.; Zona, S.; et al. Health surveillance for SARS-CoV-2: Infection spread and vaccination coverage in the schools of Modena province, Italy. Front. Public Health 2023, 11, 1240315. [Google Scholar] [CrossRef] [PubMed]
- Farina, E.; Eboli, I.; Spadea, T.; Saugo, C.; Richiardi, L.; Maule, M.; Presti, P.; Bena, A. ‘Scuola sicura’: A school screening testing programme to prevent the spread of COVID-19 in students in Piedmont. Epidemiol. Prev. 2021, 45, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, G.; Paoli, S.; Biamonte, M.A.; Moscadelli, A.; Baggiani, L.; Nerattini, M.; Lastrucci, V.; Zanobini, P.; Lorini, C. COVID-19 and schools: What is the risk of contagion? Results of a rapid-antigen-test-based screening campaign in Florence, Italy. Int. J. Infect. Dis. 2021, 112, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Borghi, E.; Massa, V.; Carmagnola, D.; Dellavia, C.; Parodi, C.; Ottaviano, E.; Sangiorgio, A.; Barcellini, L.; Gambacorta, G.; Forlanini, F.; et al. Saliva sampling for chasing SARS-CoV-2: A Game-changing strategy. Pharmacol. Res. 2021, 165, 105380. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Technical Report COVID-19. Children and the Role of School Settings in Transmission—First Update. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-in-children-and-the-role-of-school-settings-in-transmission-first-update_1.pdf (accessed on 23 December 2020).
- Istituto Superiore di Sanità. Rapporto ISS COVID-19–N. 63/2020. Apertura Delle Scuole E Andamento Dei Casi Confermati di SARS-Cov-2: La Situazione in Italia. Available online: https://www.iss.it/documents/20126/0/Rapporto+ISS+COVID-19+n.+63_2020.pdf/7b3d3626-3982-f7a1-86ef-1ede83e170a4?t=1609758939391 (accessed on 30 December 2020).
- Ministero Della Salute. Report Vaccini Anti COVID-19. Available online: https://www.governo.it/it/cscovid19/report-vaccini/ (accessed on 17 July 2025).
- Chen, C.C.; Lee, M.H.; Chen, S.Y.; Lu, S.C.; Bai, C.H.; Ko, Y.L.; Wang, C.Y.; Wang, Y.H. Assessment of the detection accuracy of SARS-CoV-2 rapid antigen test in children and adolescents: An updated meta-analysis. J. Chin. Med. Assoc. 2023, 86, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Ejima, K.; Kim, K.S.; Ludema, C.; Bento, A.I.; Iwanami, S.; Fujita, Y.; Ohashi, H.; Koizumi, Y.; Watashi, K.; Aihara, K.; et al. Estimation of the incubation period of COVID-19 using viral load data. Epidemics 2021, 35, 100454. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, J.J.; Vos, M.B.; Tyburski, E.A.; Nguyen, P.V.; Ingersoll, J.M.; Miller, C.; Sullivan, J.; Griffiths, M.; Stone, C.; Benoit, M.; et al. Concordance of SARS-CoV-2 Results in Self-collected Nasal Swabs vs. Swabs Collected by Health Care Workers in Children and Adolescents. JAMA 2022, 328, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.M.; Mullane, M.J.; Ang, S.; Barrow, T.; Leahy, A.; Whelan, A.; Lombardi, K.; Cooper, M.; Stevenson, P.G.; Lester, L.; et al. Acceptability of OP/Na swabbing for SARS-CoV-2: A prospective observational cohort surveillance study in Western Australian schools. BMJ Open 2022, 12, e055217. [Google Scholar] [CrossRef] [PubMed]
- Agenzia Italiana del Farmaco. Primo Vaccino Anti-COVID-19 Approvato Nell’ue Per Bambini Di Età Compresa Tra 12 E 15 Anni. Available online: https://www.aifa.gov.it/-/primo-vaccino-anti-covid-19-approvato-nell-ue-per-bambini-di-et%C3%A0-compresa-tra-12-e-15-anni (accessed on 28 May 2021).
| Regions | Experimental Schools | Control Schools |
|---|---|---|
| FVG * | 4 secondary schools | 8 secondary schools |
| Veneto | 4 secondary schools | 8 secondary schools |
| Tuscany | 3 secondary schools | 6 secondary schools |
| Apulia | 5 secondary schools | 10 secondary schools |
| Total | 16 secondary schools | 32 secondary schools |
| Region | Total Enrolled Students | Total Enrolled with at Least 1 Valid Swabs | % Positives Out of Total Enrolled | % Positives Out of Total Enrolled with at Least 1 Valid Swab |
|---|---|---|---|---|
| Friuli-Venezia Giulia | 355 | 329 | 3.9 | 4.3 |
| Apulia | 182 | 175 | 10.4 | 10.9 |
| Tuscany | 222 | 153 | 2.7 | 3.9 |
| Veneto | 1474 | 1432 | 2.2 | 2.2 |
| Total | 2233 | 2089 | 3.2 | 3.4 |
| Region | Total Enrolled Students | Total Enrolled with at Least 1 Valid Swab | % Positives Out of Total Enrolled | % Positives Out of Total Enrolled with at Least 1 Valid Swab |
|---|---|---|---|---|
| Friuli-Venezia Giulia | 105 | 101 | 8.6 | 8.9 |
| Apulia | 148 | 120 | 4.7 | 5.8 |
| Tuscany | 2 | 2 | 0.0 | 0.0 |
| Veneto | 39 | 36 | 2.6 | 2.8 |
| Total | 294 | 259 | 5.8 | 6.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milli, C.; Stasi, C.; Profili, F.; Silvestri, C.; Pacifici, M.; Baccini, M.; Rossolini, G.M.; Mealli, F.; Antonelli, A.; Chilleri, C.; et al. Early Warning Approach to Identify Positive Cases of SARS-CoV-2 in School Settings in Italy. Microorganisms 2025, 13, 1775. https://doi.org/10.3390/microorganisms13081775
Milli C, Stasi C, Profili F, Silvestri C, Pacifici M, Baccini M, Rossolini GM, Mealli F, Antonelli A, Chilleri C, et al. Early Warning Approach to Identify Positive Cases of SARS-CoV-2 in School Settings in Italy. Microorganisms. 2025; 13(8):1775. https://doi.org/10.3390/microorganisms13081775
Chicago/Turabian StyleMilli, Caterina, Cristina Stasi, Francesco Profili, Caterina Silvestri, Martina Pacifici, Michela Baccini, Gian Maria Rossolini, Fabrizia Mealli, Alberto Antonelli, Chiara Chilleri, and et al. 2025. "Early Warning Approach to Identify Positive Cases of SARS-CoV-2 in School Settings in Italy" Microorganisms 13, no. 8: 1775. https://doi.org/10.3390/microorganisms13081775
APA StyleMilli, C., Stasi, C., Profili, F., Silvestri, C., Pacifici, M., Baccini, M., Rossolini, G. M., Mealli, F., Antonelli, A., Chilleri, C., Morecchiato, F., Giovacchini, N., Baldo, V., Ruscio, M., Malacarne, F., Martin, F., Occoni, E., Prato, R., Martinelli, D., ... Voller, F. (2025). Early Warning Approach to Identify Positive Cases of SARS-CoV-2 in School Settings in Italy. Microorganisms, 13(8), 1775. https://doi.org/10.3390/microorganisms13081775











