Chronic Leptospirosis in a Breeding Bull: A Case Report
Abstract
1. Introduction
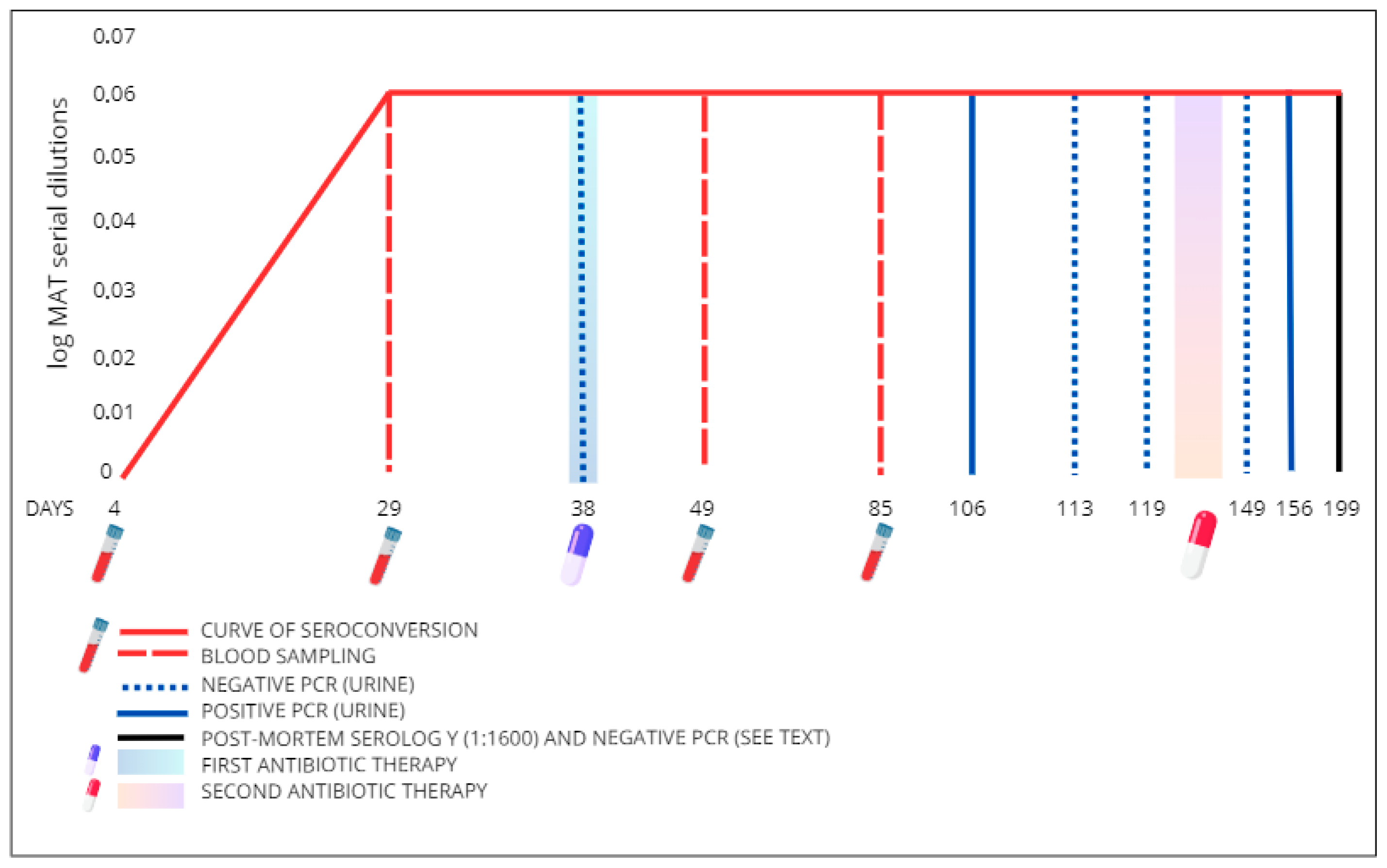
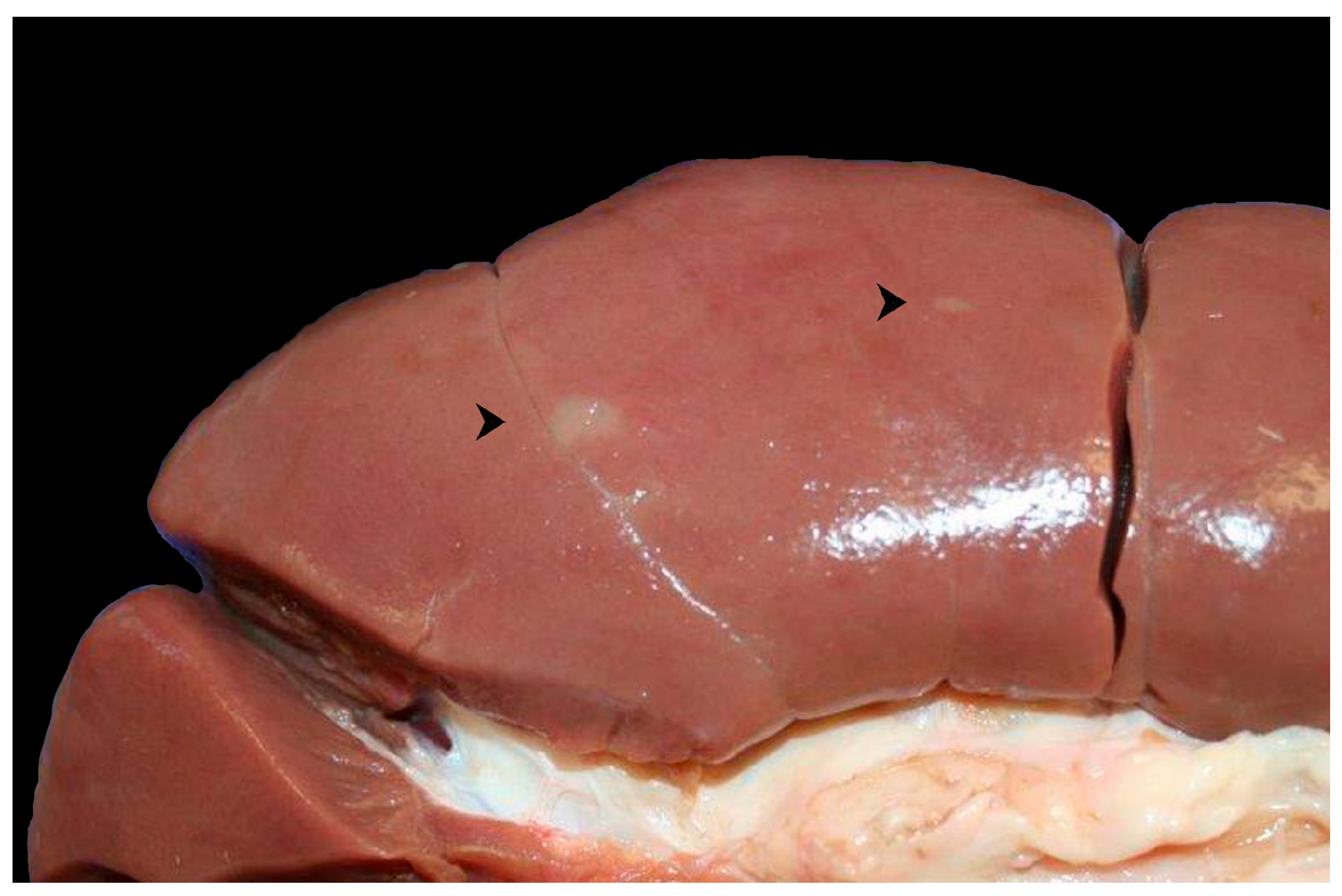
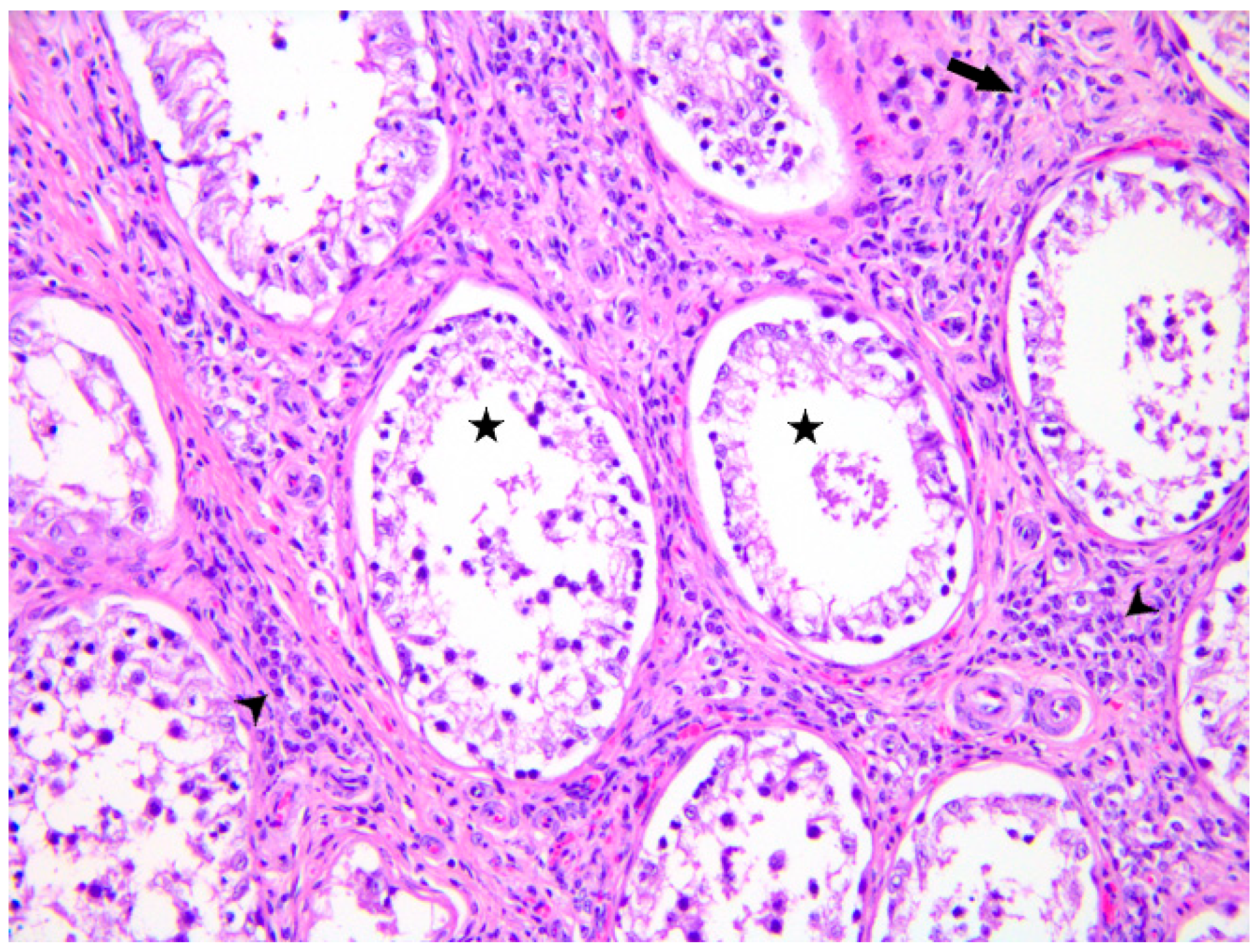
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef] [PubMed]
- Goarant, C.; Picardeau, M.; Morand, S.; McIntyre, K.M. Leptospirosis under the bibliometrics radar: Evidence for a vicious circle of neglect. J. Glob. Health 2019, 9, 010302. [Google Scholar] [CrossRef] [PubMed]
- Di Bari, C.; Venkateswaran, N.; Fastl, C.; Gabriël, S.; Grace, D.; Havelaar, A.H.; Huntington, B.; Patterson, G.T.; Rushton, J.; Speybroeck, N.; et al. The global burden of neglected zoonotic diseases: Current state of evidence. One Health 2023, 17, 100595. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Sean, T.C.; Bhavya, K.S.; Sahithya, C.S.; Chan-Drasekaran, S.; Palanisamy, R.; Robinson, E.R.; Subbiah, S.K.; Mok, P.L. Leptospiral Infection, Pathogenesis and Its Diagnosis—A Review. Pathogens 2021, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Leptospirosis. In ECDC. Annual Epidemiological Report for 2022; ECDC: Stockholm, Sweden, 2024. [Google Scholar]
- Almeida, D.S.; Paz, L.N.; de Oliveira, D.S.; Silva, D.N.; Ristow, P.; Hamond, C.; Costa, F.; Portela, R.W.; Estrela-Lima, A.; Pinna, M.H. Investigation of chronic infection by Leptospira spp. in asymptomatic sheep slaughtered in slaughterhouse. PLoS ONE 2019, 14, e0217391. [Google Scholar] [CrossRef] [PubMed]
- Dufour, B.; Moutou, F.; Hattenberger, A.M.; Rodhain, F. Global change: Impact, management, risk approach and health measures-the case of Europe. Rev. Sci. Tech. 2008, 27, 529–550. [Google Scholar] [CrossRef]
- Semenza, J.C.; Menne, B. Climate change and infectious diseases in Europe. Lancet Infect. Dis. 2009, 9, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Sohm, C.; Steiner, J.; Jöbstl, J.; Wittek, T.; Firth, C.; Steinparzer, R.; Desvars-Larrive, A. A systematic review on leptospirosis in cattle: A European perspective. One Health 2023, 17, 100608. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.P.; Lilenbaum, W. Genital bovine leptospirosis: A new look for an old disease. Theriogenology 2020, 141, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on Transmissible Animal Diseases and Amending and Repealing Certain Acts in the Area of Animal Health (‘Animal Health Law’). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0429 (accessed on 16 July 2025).
- Ellis, W.A. Animal leptospirosis. Curr. Top. Microbiol. Immunol. 2015, 387, 99–137. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, J.; Mendes, J.; Zambrano, J.; Carvalho-Costa, F.A.; Di Azevedo, M.I.N.; Aymée, L.; Lilenbaum, W. How Is Bovine Genital Leptospirosis Diagnosed Under Field Conditions? Animals 2025, 15, 443. [Google Scholar] [CrossRef] [PubMed]
- Sohm, C.; Willixhofer, D.; Fasching, E.; Waldner, K.; Deitzer, N.; Steiner, J.; Jöbstl, J.; Schleicher, C.; Schwarz, M.; Fuchs, R.; et al. First isolation and genotyping of pathogenic Leptospira spp. from Austria. Sci. Rep. 2024, 14, 4467. [Google Scholar] [CrossRef] [PubMed]
- Aymée, L.; Gregg, W.R.R.; Loureiro, A.P.; Di Azevedo, M.I.N.; Pedrosa, J.S.; Melo, J.D.S.L.; Carvalho-Costa, F.A.; de Souza, G.N.; Lilenbaum, W. Bovine Genital Leptospirosis and reproductive disorders of live subfertile cows under field conditions. Vet. Microbiol. 2021, 261, 109213. [Google Scholar] [CrossRef] [PubMed]
- Aymée, L.; Mendes, J.; Lilenbaum, W. Bovine Genital Leptospirosis: An Update of This Important Reproductive Disease. Animals 2024, 14, 322. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.L. Sexually Transmitted Diseases of Bulls. Vet. Clin. N. Am. Food Anim. Pract. 2024, 40, 111–119. [Google Scholar] [CrossRef] [PubMed]
- O’ Doherty, E.; Sayers, R.; O’ Grady, L.; Shalloo, L. Effect of exposure to Neospora caninum, Salmonella, and Leptospira interrogans serovar Hardjo on the economic performance of Irish dairy herds. J. Dairy Sci. 2015, 98, 2789–2800. [Google Scholar] [CrossRef]
- Carvalho, H.G.A.C.; Silva, D.M.; Rodrigues, G.R.D.; Gameiro, A.H.; Dos Santos, R.F.; Raineri, C.; Lima, A.M.C. Estimation of economic losses due to leptospirosis in dairy cattle. Prev. Vet. Med. 2024, 229, 106255. [Google Scholar] [CrossRef]
- Grégoire, F.; Bakinahe, R.; Petitjean, T.; Boarbi, S.; Delooz, L.; Fretin, D.; Saulmont, M.; Mori, M. Laboratory Diagnosis of Bovine Abortions Caused by Non-Maintenance Pathogenic Leptospira spp.: Necropsy, Serology and Molecular Study Out of a Belgian Experience. Pathogens 2020, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, S.R.; Cortez, A.; Langoni, H.; Giuffrida, R.; Dos Santos, J.R.; de Nardi Júnior, G.; Lara, G.H.B.; Motta, R.G.; Chacur, M.G.M.; Monteiro, F.M.; et al. Sperm viability, serological, molecular, and modified seminal plasma agglutination tests in the diagnosis of Leptospira in the semen and serum of bovine bulls. Braz. J. Microbiol. 2021, 52, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health (WOAH). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 12th ed.; OIE Terrestrial Manual; World Organization for Animal Health (WOAH): Paris, France, 2023; Chapter 3.1.12. [Google Scholar]
- Mazzotta, E.; Bellinati, L.; Bertasio, C.; Boniotti, M.B.; Lucchese, L.; Ceglie, L.; Martignago, F.; Leopardi, S.; Natale, A. Synanthropic and Wild Animals as Sentinels of Zoonotic Agents: A Study of Leptospira Genotypes Circulating in Northeastern Italy. Int. J. Environ. Res. Public Health 2023, 20, 3783. [Google Scholar] [CrossRef] [PubMed]
- Smythe, L.D.; Smith, I.L.; Smith, G.A.; Dohnt, M.F.; Symonds, M.L.; Barnett, L.J.; McKay, D.B. A quantitative PCR (TaqMan) assay for pathogenic Leptospira spp. BMC Infect. Dis. 2002, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Bedir, O.; Kilic, A.; Atabek, E.; Kuskucu, A.M.; Turhan, V.; Basustaoglu, A.C. Simultaneous detection and differentiation of pathogenic and nonpathogenic Leptospira spp. by multiplex real-time PCR (TaqMan) assay. Pol. J. Microbiol. 2010, 59, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, R.A.; Gee, J.E.; Wilkins, P.P.; McCaustland, K.; Hoffmaster, A.R. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn. Microbiol. Infect. Dis. 2009, 64, 247–255. [Google Scholar] [CrossRef]
- Boonsilp, S.; Thaipadungpanit, J.; Amornchai, P.; Wuthiekanun, V.; Bailey, M.S.; Holden, M.T.G.; Zhang, C.; Jiang, X.; Koizumi, N.; Taylor, K.; et al. A Single Multilocus Sequence Typing (MLST) Scheme for Seven Pathogenic Leptospira Species. PLoS Negl. Trop. Dis. 2013, 7, e1954. [Google Scholar] [CrossRef] [PubMed]
- Bertasio, C.; Papetti, A.; Scaltriti, E.; Tagliabue, S.; D’Incau, M.; Boniotti, M.B. Serological Survey and Molecular Typing Reveal New Leptospira Serogroup Pomona Strains among Pigs of Northern Italy. Pathogens 2020, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Recordati, C.; Radaelli, E.; Simpson, K.W.; Scanziani, E. A simple method for the production of bacterial controls for immunohistochemistry and fluorescent in situ hybridization. J. Mol. Histol. 2008, 39, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Moinet, M.; Wilkinson, D.A.; Aberdein, D.; Russell, J.C.; Vallée, E.; Collins-Emerson, J.M.; Heuer, C.; Benschop, J. Of Mice, Cattle, and Men: A Review of the Eco-Epidemiology of Leptospira borgpetersenii Serovar Ballum. Trop. Med. Infect. Dis. 2021, 6, 189. [Google Scholar] [CrossRef] [PubMed]
- Delooz, L.; Czaplicki, G.; Gregoire, F.; Dal Pozzo, F.; Pez, F.; Kodjo, A.; Saegerman, C. Serogroups and genotypes of Leptospira spp. strains from bovine aborted foetuses. Transbound. Emerg. Dis. 2018, 65, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, A.; Blanda, V.; Di Marco Lo Presti, V.; Macaluso, G.; Galluzzo, P.; Bertasio, C.; Sciacca, C.; Arcuri, F.; D’Agostino, R.; Ippolito, D.; et al. Leptospira interrogans Serogroup Pomona in a Dairy Cattle Farm in a Multi-Host Zootechnical System. Vet. Sci. 2022, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Yupiana, Y.; Wilson, P.R.; Weston, J.F.; Vallée, E.; Collins-Emerson, J.M.; Benschop, J.; Scotland, T.; Heuer, C. Epidemiological investigation of Leptospira spp. in a dairy farming enterprise after the occurrence of three human leptospirosis cases. Zoonoses Public Health 2019, 66, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.R.; Martins, G.; Lilenbaum, W. An historical view of the experimental leptospiral infection in ruminants. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101532. [Google Scholar] [CrossRef]
- Molinari, P.C.C.; Nally, J.E.; Bromfield, J.J. Bovine endometrial cells do not mount an inflammatory response to Leptospira. Reprod. Fertil. 2021, 2, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Thiermann, A.B. Experimental leptospiral infections in pregnant cattle with organisms of the Hebdomadis serogroup. Am. J. Vet. Res. 1982, 43, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Nally, J.E.; Ahmed, A.A.A.; Putz, E.J.; Palmquist, D.E.; Goris, M.G.A. Comparison of Real-Time PCR, Bacteriologic Culture and Fluorescent Antibody Test for the Detection of Leptospira borgpetersenii in Urine of Naturally Infected Cattle. Vet. Sci. 2020, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, J.; Zuerner, R.L.; Alt, D.; Bolin, C.A. Comparison of polymerase chain reaction assays with bacteriologic culture, immunofluorescence, and nucleic acid hybridization for detection of Leptospira borgpetersenii serovar hardjo in urine of cattle. Am. J. Vet. Res. 2000, 61, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Zarantonelli, L.; Suanes, A.; Meny, P.; Buroni, F.; Nieves, C.; Salaberry, X.; Briano, C.; Ashfield, N.; Da Silva Silveira, C.; Dutra, F.; et al. Isolation of pathogenic Leptospira strains from naturally infected cattle in Uruguay reveals high serovar diversity, and uncovers a relevant risk for human leptospirosis. PLoS Negl. Trop. Dis. 2018, 12, e0006694. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, P.; Díaz, C.A.; Dalmau, E.A.; Quintero, G.M. A comparison between polymerase chain reaction (PCR) and traditional techniques for the diagnosis of leptospirosis in bovines. J. Microbiol. Methods 2011, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hamond, C.; LeCount, K.; Putz, E.J.; Bayles, D.O.; Camp, P.; Goris, M.G.A.; van der Linden, H.; Stone, N.E.; Schlater, L.K.; Sahl, J.W.; et al. Bovine Leptospirosis Due to Persistent Renal Carriage of Leptospira borgpetersenii Serovar Tarassovi. Front. Vet. Sci. 2022, 9, 848664. [Google Scholar] [CrossRef] [PubMed]
- Monti, G.; Montes, V.; Tortosa, P.; Tejeda, C.; Salgado, M. Urine shedding patterns of pathogenic Leptospira spp. in dairy cows. Vet. Res. 2023, 54, 64. [Google Scholar] [CrossRef] [PubMed]
- Jubb, Kennedy & Palmer’s Pathology of Domestic Animals, 6th ed.; Elsevier: Maryland Heights, MO, USA, 2016; Volume 2, pp. 435–437.
- Bonaparte, A.; Page, C.; Beeler, E. Orchitis and balanoposthitis in a dog with Leptospira interrogans serovar Canicola in Southern California. Vet. Rec. Case Rep. 2018, 6, e000463. [Google Scholar] [CrossRef]

| Target | Oligonucleotide Sequence (5′-3′) | Fragment Length | Reference |
|---|---|---|---|
| 16S rRNA gene of pathogenic leptospires | Forward: 5′-CCCGCGTCCGATTAG-3′ Reverse: 5′-TCCATTGTGGCCGRACAC-3′ Probe: 5′-FAM-CTCACCAAGGCGACGATCGGTAGC-TMR-3′ | 87 bp | [23] |
| 16S rRNA gene for Leptospira genus | Forward: 5′-TAGTGAACGGGATTAGATAC-3′ Reverse: 5′-GGTCTACTTAATCCGTTAGG-3′ Probe: 5′-Cy5-AATCCACGCCCTAAACGTTGTCTAC-BHQ1-3′ | 103 bp | [24] |
| lipL32 gene of pathogenic leptospires | Forward: 5′-AAGCATTACCGCTTGTGGTG-3′ Reverse: 5′-GAACTCCCATTTCAGCGATT-3′ Probe: 5′-FAM- AAAGCCAGGACAAGCGCCG –BHQ1-3′ | 242 bp | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Zan, G.; Carminato, A.; Cocchi, M.; Catarin, G.; Pascuci, I.; Lucchese, L.; Bellinati, L.; Ceglie, L.; Mazzotta, E.; D’Incau, M.; et al. Chronic Leptospirosis in a Breeding Bull: A Case Report. Microorganisms 2025, 13, 1695. https://doi.org/10.3390/microorganisms13071695
De Zan G, Carminato A, Cocchi M, Catarin G, Pascuci I, Lucchese L, Bellinati L, Ceglie L, Mazzotta E, D’Incau M, et al. Chronic Leptospirosis in a Breeding Bull: A Case Report. Microorganisms. 2025; 13(7):1695. https://doi.org/10.3390/microorganisms13071695
Chicago/Turabian StyleDe Zan, Gabrita, Antonio Carminato, Monia Cocchi, Giacomo Catarin, Irene Pascuci, Laura Lucchese, Laura Bellinati, Letizia Ceglie, Elisa Mazzotta, Mario D’Incau, and et al. 2025. "Chronic Leptospirosis in a Breeding Bull: A Case Report" Microorganisms 13, no. 7: 1695. https://doi.org/10.3390/microorganisms13071695
APA StyleDe Zan, G., Carminato, A., Cocchi, M., Catarin, G., Pascuci, I., Lucchese, L., Bellinati, L., Ceglie, L., Mazzotta, E., D’Incau, M., Ustulin, M., Grassi, L., & Natale, A. (2025). Chronic Leptospirosis in a Breeding Bull: A Case Report. Microorganisms, 13(7), 1695. https://doi.org/10.3390/microorganisms13071695











