IL-24 Is a Promising Molecular Adjuvant for Enhancing Protective Immunity Induced by DNA Vaccination Against Toxoplasma gondii
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Parasites, Cells, and Antigens
2.3. Construction of the Eukaryotic Expression Plasmid
2.4. Expression of pVAX-IL-24 Plasmid In Vitro
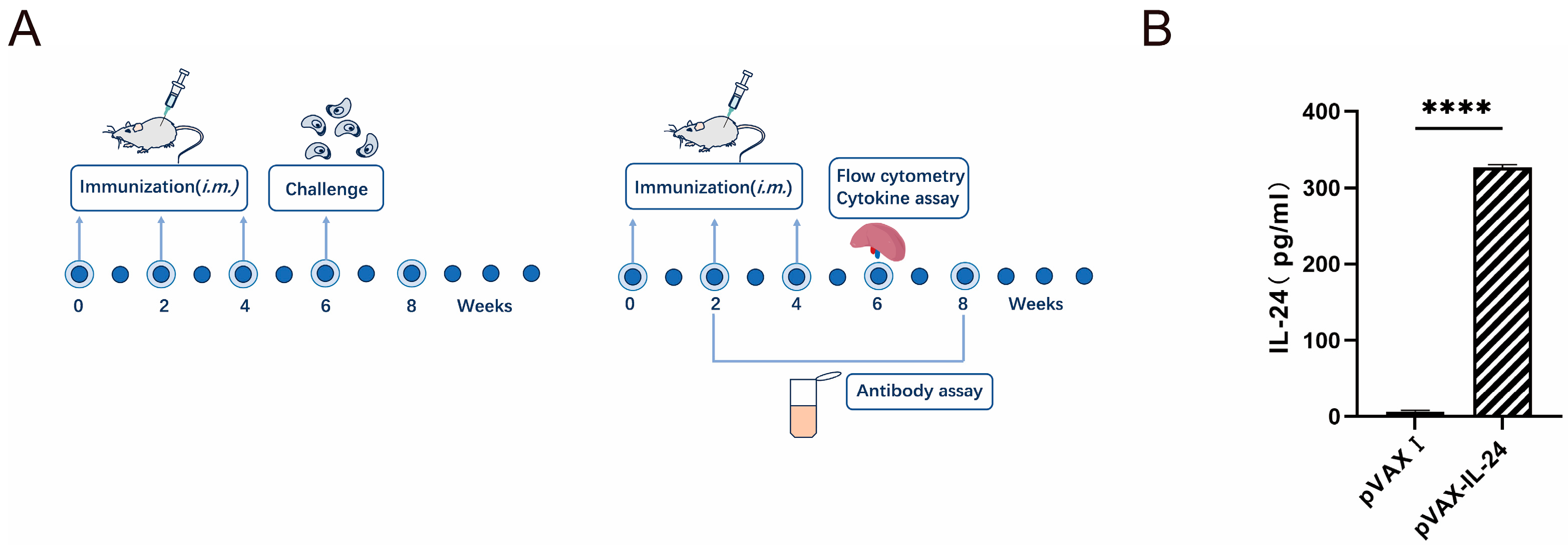
2.5. DNA Immunization and Challenge Infection
2.6. Measurement of Humoral Immune Responses
2.7. Lymphocyte Proliferation Assays
2.8. Cytokine Assays
2.9. Flow Cytometry Analysis
2.10. Statistical Analysis
3. Results
3.1. Identification of Plasmids
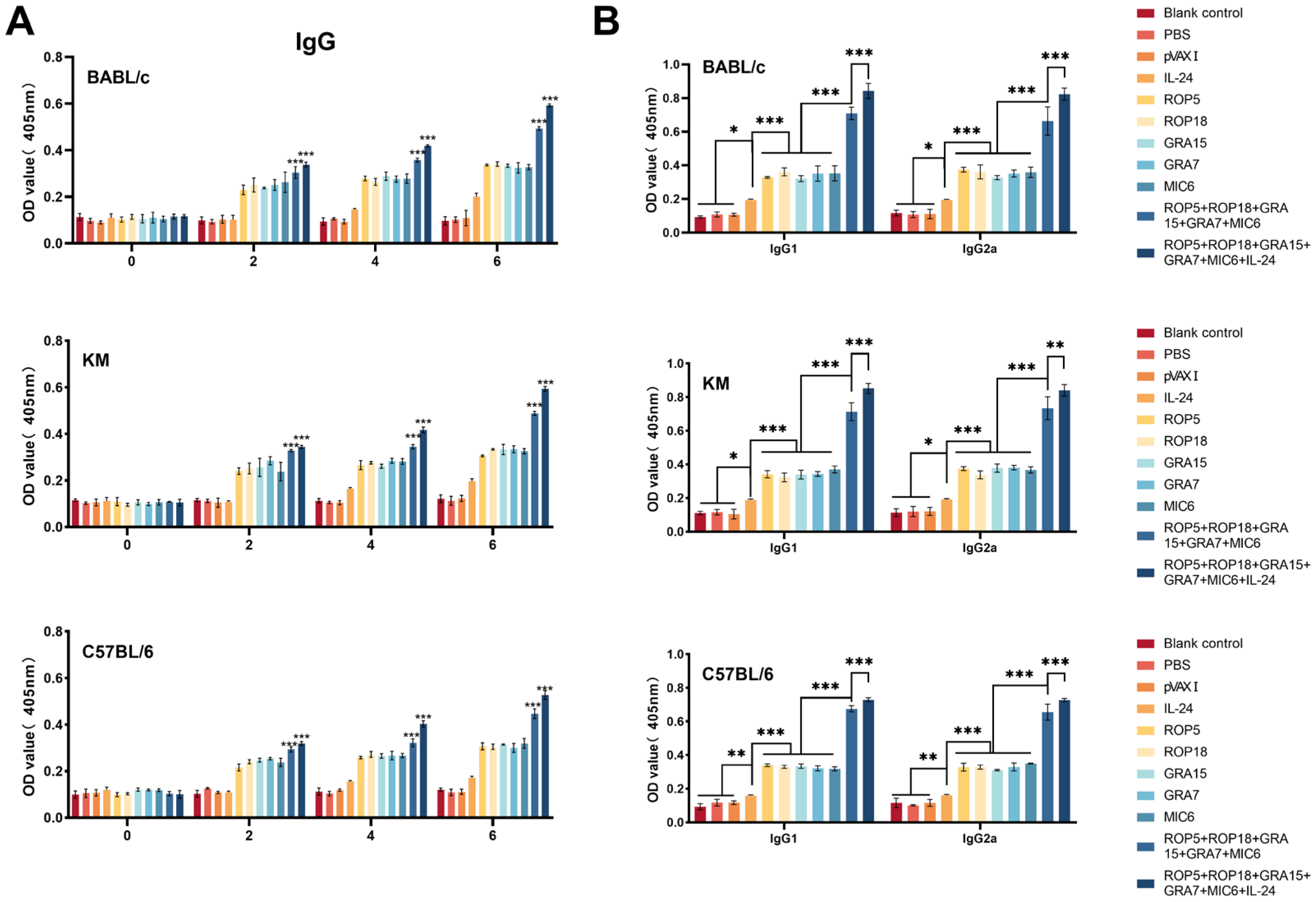
3.2. Humoral Immune Responses
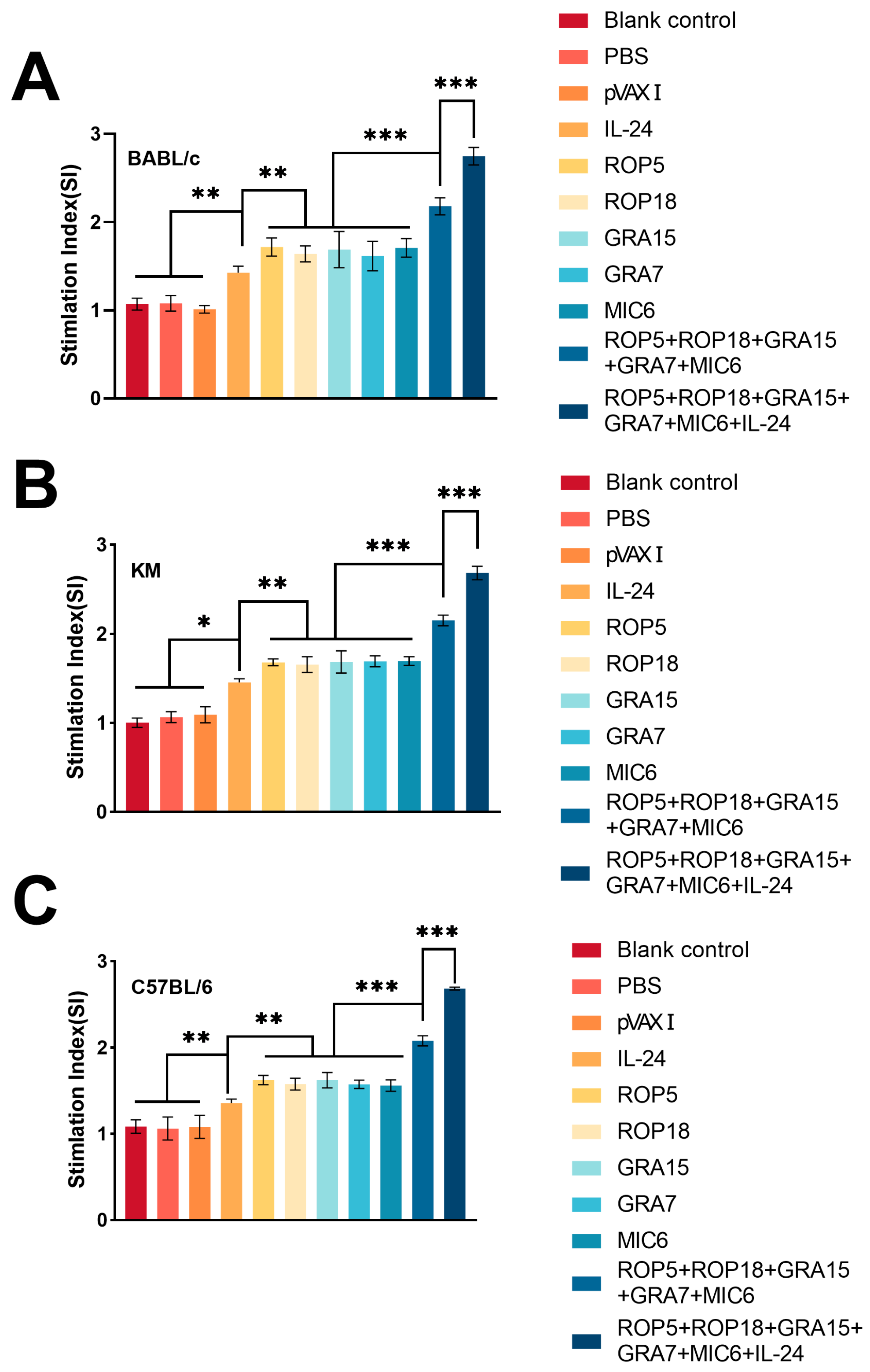
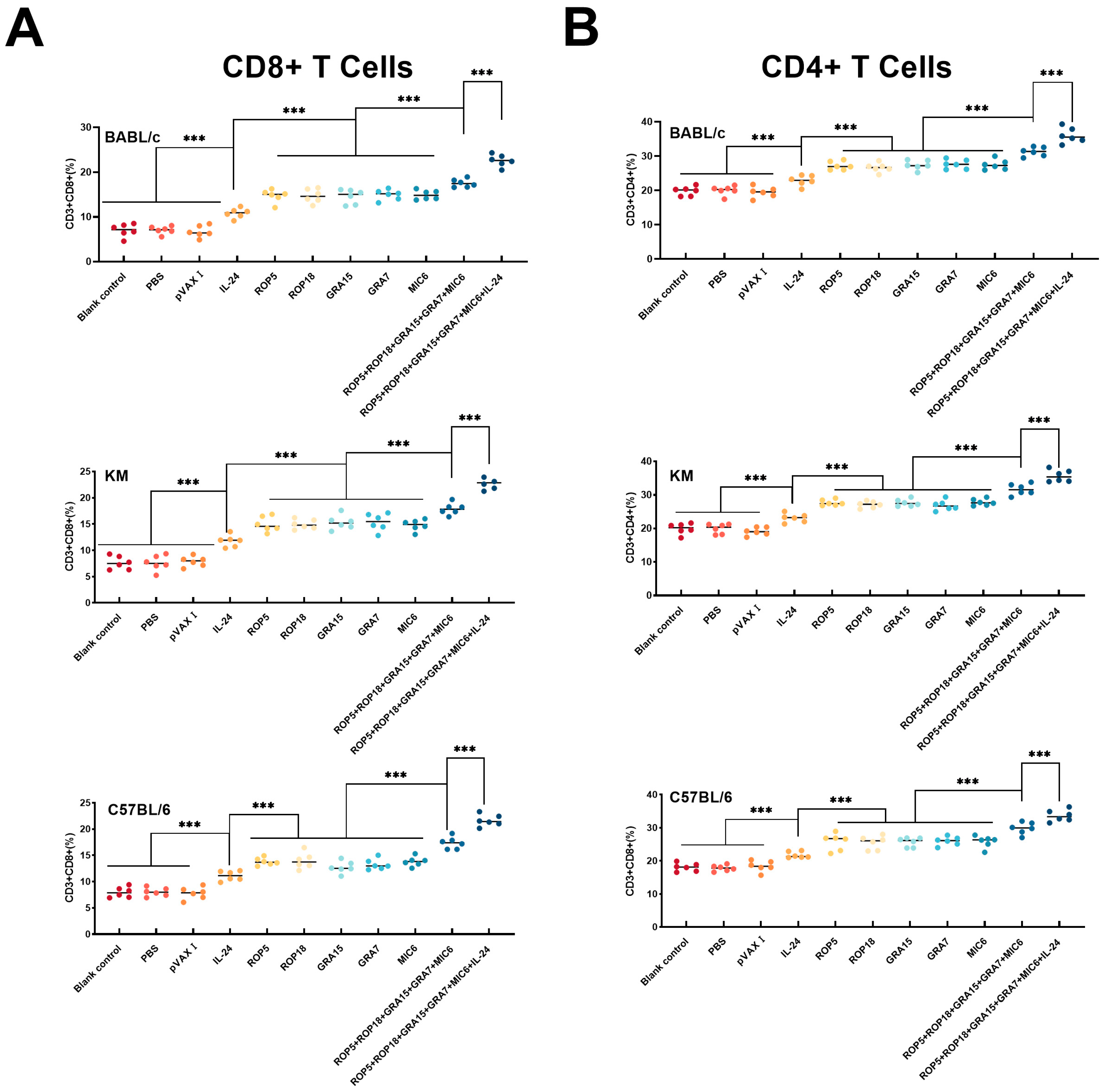
3.3. Cellular Immune Responses
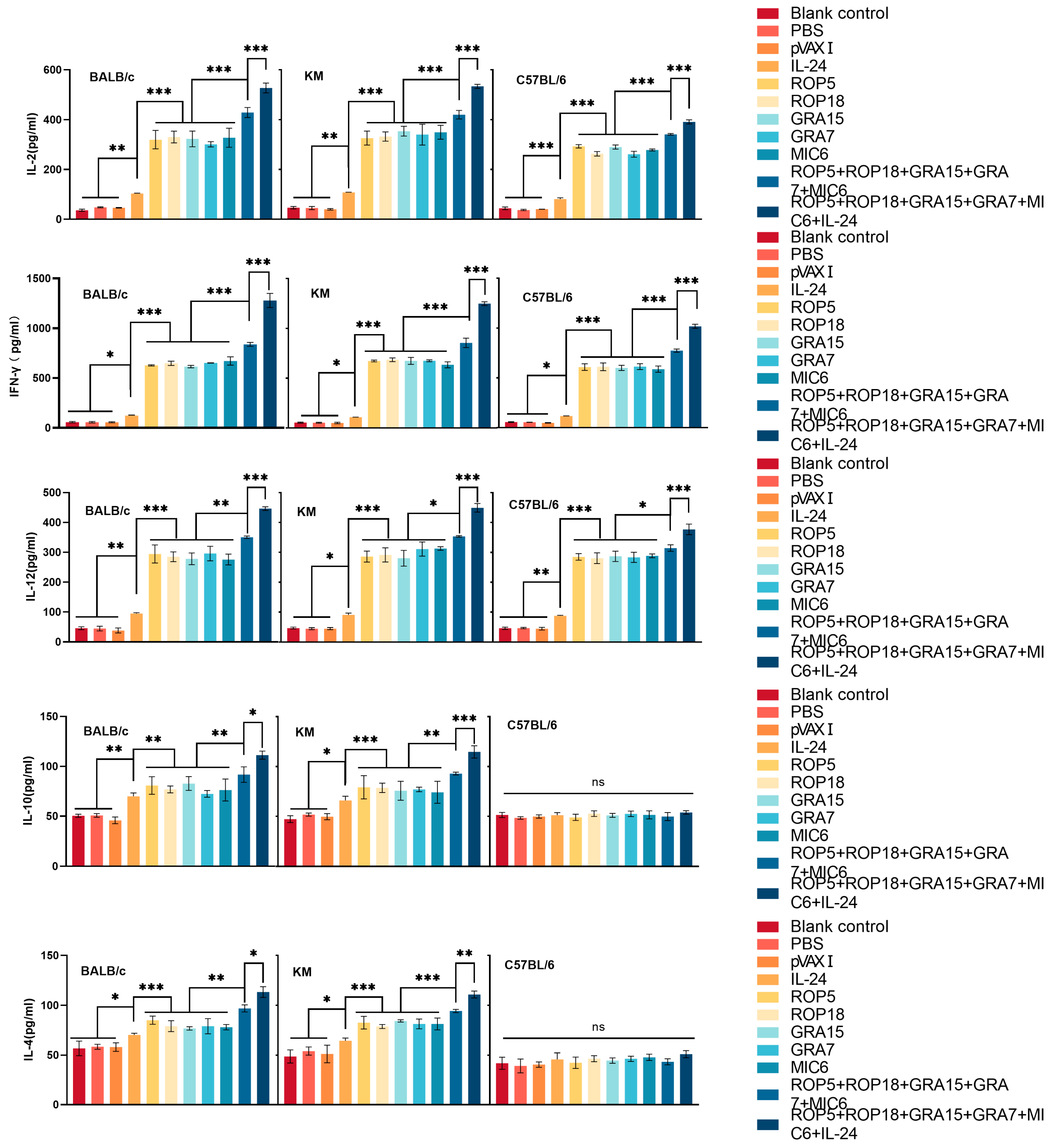
3.4. Cytokine Production
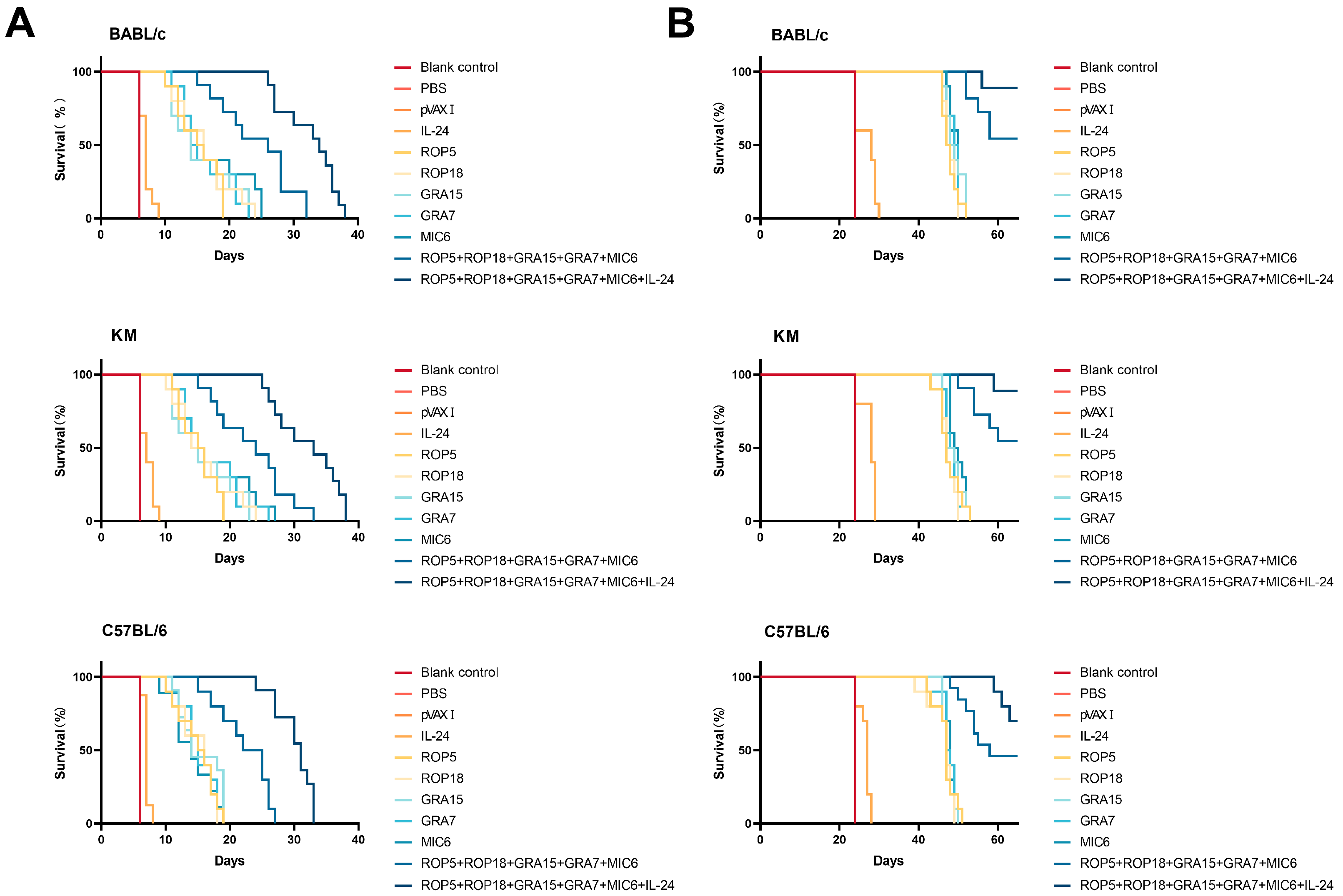
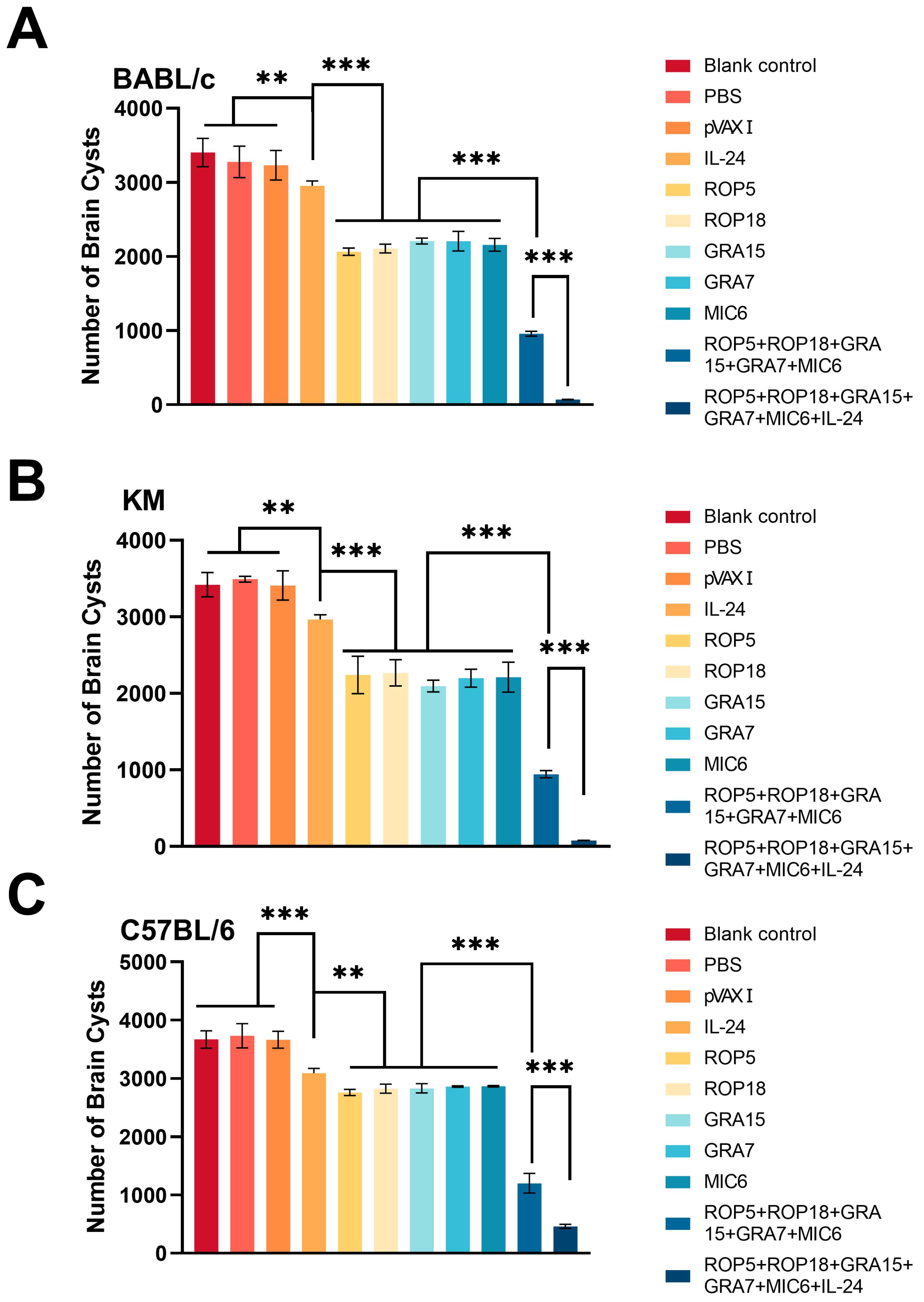
3.5. Assessment of Protective Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bisetegn, H.; Debash, H.; Ebrahim, H.; Mahmood, N.; Gedefie, A.; Tilahun, M.; Alemayehu, E.; Mohammed, O.; Feleke, D.G. Global seroprevalence of Toxoplasma gondii infection among patients with mental and neurological disorders: A systematic review and meta-analysis. Health Sci. Rep. 2023, 6, e1319. [Google Scholar] [CrossRef] [PubMed]
- Elsheikha, H.M.; Marra, C.M.; Zhu, X.Q. Epidemiology, Pathophysiology, Diagnosis, and Management of Cerebral Toxoplasmosis. Clin. Microbiol. Rev. 2021, 34, 10–1128. [Google Scholar] [CrossRef]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef] [PubMed]
- Burrells, A.; Benavides, J.; Cantón, G.; Garcia, J.L.; Bartley, P.M.; Nath, M.; Thomson, J.; Chianini, F.; Innes, E.A.; Katzer, F. Vaccination of pigs with the S48 strain of Toxoplasma gondii–safer meat for human consumption. Vet. Res. 2015, 46, 47. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; He, J.; Meng, J.; Zhang, D.; Li, D.; Wang, H.; Fan, A.; Xu, G.; Ma, S.; Zuo, Z.; et al. Vaccination with a DNA vaccine cocktail encoding TgROP2, TgROP5, TgROP9, TgROP16, TgROP17, and TgROP18 confers limited protection against Toxoplasma gondii in BALB/c mice. Parasitol. Res. 2024, 123, 420. [Google Scholar] [CrossRef]
- Warner, R.C.; Chapman, R.C.; Davis, B.N.; Davis, P.H. Review of DNA vaccine approaches against the parasite Toxoplasma gondii. J. Parasitol. 2021, 107, 882–903. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, H.; Mahmmod, Y.S.; Yang, Z.; Zhao, M.; Song, Y.; Luo, S.; Zhang, X.X.; Yuan, Z.G. Insight into the current Toxoplasma gondii DNA vaccine: A review article. Expert Rev. Vaccines 2023, 22, 66–89. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.Y.; Petersen, E.; Liu, W.G.; Zhu, X.Q. Co-administration of interleukins 7 and 15 with DNA vaccine improves protective immunity against Toxoplasma gondii. Exp. Parasitol. 2016, 162, 18–23. [Google Scholar] [CrossRef]
- Zhu, Y.C.; He, Y.; Liu, J.F.; Chen, J. Adjuvantic cytokine IL-33 improves the protective immunity of cocktailed DNA vaccine of ROP5 and ROP18 against Toxoplasma gondii infection in mice. Parasite 2020, 27, 26. [Google Scholar] [CrossRef]
- Feng, K.; Cen, J.; Zou, X.; Zhang, T. Novel insight into MDA-7/IL-24: A potent therapeutic target for autoimmune and inflammatory diseases. Clin. Immunol. 2024, 266, 110322. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Y.; Hong, L.; Zhou, C.; Chen, J. Immunization with a DNA Vaccine Encoding the Toxoplasma gondii’ s GRA39 Prolongs Survival and Reduce Brain Cyst Formation in a Murine Model. Front. Microbiol. 2021, 12, 630682. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Ma, L.J.; Zhang, J.L.; Liu, J.F.; He, Y.; Feng, J.Y.; Chen, J. Protective Immunity Induced by TgMIC5 and TgMIC16 DNA Vaccines Against Toxoplasmosis. Front. Cell. Infect. Microbiol. 2021, 11, 686004. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Yang, W.; Chen, J. Protective immunity induced by DNA vaccine containing TgGRA35, TgGRA42, and TgGRA43 against Toxoplasma gondii infection in Kunming mice. Front. Cell. Infect. Microbiol. 2023, 13, 1236130. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.Y.; Petersen, E.; Huang, S.Y.; Zhou, D.H.; Zhu, X.Q. DNA vaccination with genes encoding Toxoplasma gondii antigens ROP5 and GRA15 induces protective immunity against toxoplasmosis in Kunming mice. Expert Rev. Vaccines 2015, 14, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.Z.; Gao, Q.; Wang, M.; Elsheikha, H.M.; Wang, B.; Wang, J.L.; Zhang, F.K.; Hu, L.Y.; Zhu, X.Q. Immunization with a DNA Vaccine Cocktail Encoding TgPF, TgROP16, TgROP18, TgMIC6, and TgCDPK3 Genes Protects Mice Against Chronic Toxoplasmosis. Front. Immunol. 2018, 9, 1505. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H. Moving towards improved vaccines for Toxoplasma gondii. Expert Opin. Biol. Ther. 2018, 18, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Zhang, N.Z.; Li, T.T.; He, J.J.; Elsheikha, H.M.; Zhu, X.Q. Advances in the Development of Anti-Toxoplasma gondii Vaccines: Challenges, Opportunities, and Perspectives. Trends Parasitol. 2019, 35, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Mu, J.; Chen, J. IL-36 Gamma: A Novel Adjuvant Cytokine Enhancing Protective Immunity Induced by DNA Immunization with TGIST and TGNSM Against Toxoplasma gondii Infection in Mice. Microorganisms 2024, 12, 2258. [Google Scholar] [CrossRef]
- Zhang, N.Z.; Wang, M.; Xu, Y.; Petersen, E.; Zhu, X.Q. Recent advances in developing vaccines against Toxoplasma gondii: An update. Expert Rev. Vaccines 2015, 14, 1609–1621. [Google Scholar] [CrossRef]
- Vercammen, M.; Scorza, T.; Huygen, K.; De Braekeleer, J.; Diet, R.; Jacobs, D.; Saman, E.; Verschueren, H. DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2 induces partially protective immunity against lethal challenge in mice. Infect. Immun. 2000, 68, 38–45. [Google Scholar] [CrossRef]
- Pifer, R.; Yarovinsky, F. Innate responses to Toxoplasma gondii in mice and humans. Trends Parasitol. 2011, 27, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Sayles, P.C.; Gibson, G.W.; Johnson, L.L. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect. Immun. 2000, 68, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Yarovinsky, F. Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 2014, 14, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Frickel, E.M.; Hunter, C.A. Lessons from Toxoplasma: Host responses that mediate parasite control and the microbial effectors that subvert them. J. Exp. Med. 2021, 218, e20201314. [Google Scholar] [CrossRef]
- Lüder, C.G.K. IFNs in host defence and parasite immune evasion during Toxoplasma gondii infections. Front. Immunol. 2024, 15, 1356216. [Google Scholar] [CrossRef]
- LaRosa, D.F.; Stumhofer, J.S.; Gelman, A.E.; Rahman, A.H.; Taylor, D.K.; Hunter, C.A.; Turka, L.A. T cell expression of MyD88 is required for resistance to Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 2008, 105, 3855–3860. [Google Scholar] [CrossRef]
- Matowicka-Karna, J.; Dymicka-Piekarska, V.; Kemona, H. Does Toxoplasma gondii infection affect the levels of IgE and cytokines (IL-5, IL-6, IL-10, IL-12, and TNF-alpha)? J. Immunol. Res. 2009, 2009, 374696. [Google Scholar] [CrossRef]
- Sonaimuthu, P.; Ching, X.T.; Fong, M.Y.; Kalyanasundaram, R.; Lau, Y.L. Induction of Protective Immunity against Toxoplasmosis in BALB/c Mice Vaccinated with Toxoplasma gondii Rhoptry-1. Front. Microbiol. 2016, 7, 808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.Z.; Xu, Y.; Wang, M.; Petersen, E.; Chen, J.; Huang, S.Y.; Zhu, X.Q. Protective efficacy of two novel DNA vaccines expressing Toxoplasma gondii rhomboid 4 and rhomboid 5 proteins against acute and chronic toxoplasmosis in mice. Expert Rev. Vaccines 2015, 14, 1289–1297. [Google Scholar] [CrossRef]
- Guo, X.D.; Zhou, C.X.; Cui, L.L.; Qiu, H.J.; Wang, Y.L.; Fu, M.; Liu, D.A.; Han, B.; Zhou, H.Y.; Zhou, D.H. Evaluation of protective immunity induced by a DNA vaccine encoding SAG2 and SRS2 against Toxoplasma gondii infection in mice. Acta Trop. 2024, 257, 107302. [Google Scholar] [CrossRef]
- Bao, Y.; Cao, X. The immune potential and immunopathology of cytokine-producing B cell subsets: A comprehensive review. J. Autoimmun. 2014, 55, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.L.; Sun, L.X.; Elsheikha, H.M.; Cao, X.Z.; Nie, L.B.; Li, T.T.; Li, T.S.; Zhu, X.Q.; Wang, J.L. RHΔgra17Δnpt1 Strain of Toxoplasma gondii Elicits Protective Immunity Against Acute, Chronic and Congenital Toxoplasmosis in Mice. Microorganisms 2020, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Pittman, K.J.; Knoll, L.J. Long-Term Relationships: The Complicated Interplay between the Host and the Developmental Stages of Toxoplasma gondii during Acute and Chronic Infections. Microbiol. Mol. Biol. Rev. 2015, 79, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Chyb, M.; Dziadek, B.; Dzitko, K.; Ferra, B.T.; Kawka, M.; Holec-Gąsior, L.; Gatkowska, J. Evaluation of long-term immunity and protection against T. gondii after immunization with multivalent recombinant chimeric T. gondii proteins. Sci. Rep. 2023, 13, 12976. [Google Scholar] [CrossRef]
- Li, J.; Kang, Y.; Wu, Z.X.; Yang, S.F.; Tian, Y.Y.; Zhu, X.Q.; Zheng, X.N. Live-attenuated PruΔgra72 strain of Toxoplasma gondii induces strong protective immunity against acute and chronic toxoplasmosis in mice. Parasites Vectors 2024, 17, 377. [Google Scholar] [CrossRef]
- Menezes, M.E.; Bhoopathi, P.; Pradhan, A.K.; Emdad, L.; Das, S.K.; Guo, C.; Wang, X.Y.; Sarkar, D.; Fisher, P.B. Role of MDA-7/IL-24 a Multifunction Protein in Human Diseases. Adv. Cancer Res. 2018, 138, 143–182. [Google Scholar] [CrossRef]
- Miri, S.M.; Pourhossein, B.; Hosseini, S.Y.; Keshavarz, M.; Shahmahmoodi, S.; Zolfaghari, M.R.; Mohebbi, S.R.; Gorji, A.; Ghaemi, A. Enhanced synergistic antitumor effect of a DNA vaccine with anticancer cytokine, MDA-7/IL-24, and immune checkpoint blockade. Virol. J. 2022, 19, 106. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, W.; Li, F.; Wen, C.; Zhou, L.; Zhang, L.; Lian, J.; Liu, S.; Wang, S.; Zhang, Y. IL-24 improves efficacy of CAR-T cell therapy by targeting stemness of tumor cells. Br. J. Cancer 2024, 130, 1337–1347. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Xu, Y. Interleukin-24 Regulates T Cell Activity in Patients with Colorectal Adenocarcinoma. Front. Oncol. 2019, 9, 1401. [Google Scholar] [CrossRef]
- Cao, X.; Fu, Y.X.; Peng, H. Promising Cytokine Adjuvants for Enhancing Tuberculosis Vaccine Immunity. Vaccines 2024, 12, 477. [Google Scholar] [CrossRef]
- Rahman, N.A.A.; Balasubramaniam, V.; Yap, W.B. Potential of Interleukin (IL)-12 Group as Antivirals: Severe Viral Disease Prevention and Management. Int. J. Mol. Sci. 2023, 24, 7350. [Google Scholar] [CrossRef]
- Rahman, T.; Das, A.; Abir, M.H.; Nafiz, I.H.; Mahmud, A.R.; Sarker, M.R.; Emran, T.B.; Hassan, M.M. Cytokines and their role as immunotherapeutics and vaccine Adjuvants: The emerging concepts. Cytokine 2023, 169, 156268. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.; Nishikawa, Y. Advances in vaccine development and the immune response against toxoplasmosis in sheep and goats. Front. Vet. Sci. 2022, 9, 951584. [Google Scholar] [CrossRef]
- Jafari, M.M.; Tabrizi, Z.A.; Dayer, M.S.; Kazemi-Sefat, N.A.; Mohtashamifard, M.; Mohseni, R.; Bagheri, A.; Bahadory, S.; Karimipour-Saryazdi, A.; Ghaffarifar, F. Immune system roles in pathogenesis, prognosis, control, and treatment of Toxoplasma gondii infection. Int. Immunopharmacol. 2023, 124, 110872. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Chen, J.; Petersen, E.; Zhou, D.H.; Huang, S.Y.; Song, H.Q.; Zhu, X.Q. Synergy of mIL-21 and mIL-15 in enhancing DNA vaccine efficacy against acute and chronic Toxoplasma gondii infection in mice. Vaccine 2014, 32, 3058–3065. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Chakraborty, A.R.; DePamphilis, M.L. PIKFYVE inhibitors trigger interleukin-24-dependent cell death of autophagy-dependent melanoma. Mol. Oncol. 2024, 18, 988–1011. [Google Scholar] [CrossRef]
- Li, Y.; Luo, H.; Lin, X.; Hua, L.; Wang, J.; Xie, J.; Zhang, Z.; Shi, Z.; Li, M.; Peng, Q.; et al. Triterpenes of Prunella vulgaris Inhibit Triple-Negative Breast Cancer by Regulating PTP1B/PI3K/AKT/mTOR and IL-24/CXCL12/CXCR4 Pathways. Int. J. Mol. Sci. 2025, 26, 1959. [Google Scholar] [CrossRef]
| Group | Content | Volume | Administration |
|---|---|---|---|
| pVAX I plasmids expressing ROP5 + ROP18 + GRA7 + GRA15 + MIC6 + IL-24 | 17 μg/plasmid | 100 μL | Intramuscular |
| pVAX I plasmids expressing ROP5 + ROP18 + GRA7 + GRA15 + MIC6 | 20 μg/plasmid | 100 μL | Intramuscular |
| pVAX I | 100 μg/plasmid | 100 μL | Intramuscular |
| pVAX-ROP5 | 100 μg/plasmid | 100 μL | Intramuscular |
| pVAX-ROP18 | 100 μg/plasmid | 100 μL | Intramuscular |
| pVAX-GRA7 | 100 μg/plasmid | 100 μL | Intramuscular |
| pVAX-GRA15 | 100 μg/plasmid | 100 μL | Intramuscular |
| pVAX-MIC6 | 100 μg/plasmid | 100 μL | Intramuscular |
| pVAX-IL-24 | 100 μg/plasmid | 100 μL | Intramuscular |
| Phosphate-buffered saline | - | 100 μL | Intramuscular |
| Blank control | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Zhang, X.; Wang, Y.; Chen, J. IL-24 Is a Promising Molecular Adjuvant for Enhancing Protective Immunity Induced by DNA Vaccination Against Toxoplasma gondii. Microorganisms 2025, 13, 1661. https://doi.org/10.3390/microorganisms13071661
Xu B, Zhang X, Wang Y, Chen J. IL-24 Is a Promising Molecular Adjuvant for Enhancing Protective Immunity Induced by DNA Vaccination Against Toxoplasma gondii. Microorganisms. 2025; 13(7):1661. https://doi.org/10.3390/microorganisms13071661
Chicago/Turabian StyleXu, Bohuai, Xiuqiang Zhang, Yaowen Wang, and Jia Chen. 2025. "IL-24 Is a Promising Molecular Adjuvant for Enhancing Protective Immunity Induced by DNA Vaccination Against Toxoplasma gondii" Microorganisms 13, no. 7: 1661. https://doi.org/10.3390/microorganisms13071661
APA StyleXu, B., Zhang, X., Wang, Y., & Chen, J. (2025). IL-24 Is a Promising Molecular Adjuvant for Enhancing Protective Immunity Induced by DNA Vaccination Against Toxoplasma gondii. Microorganisms, 13(7), 1661. https://doi.org/10.3390/microorganisms13071661







