Effects of Antibiotic Residues on Fecal Microbiota Composition and Antimicrobial Resistance Gene Profiles in Cattle from Northwestern China
Abstract
1. Introduction
2. Materials and Methods
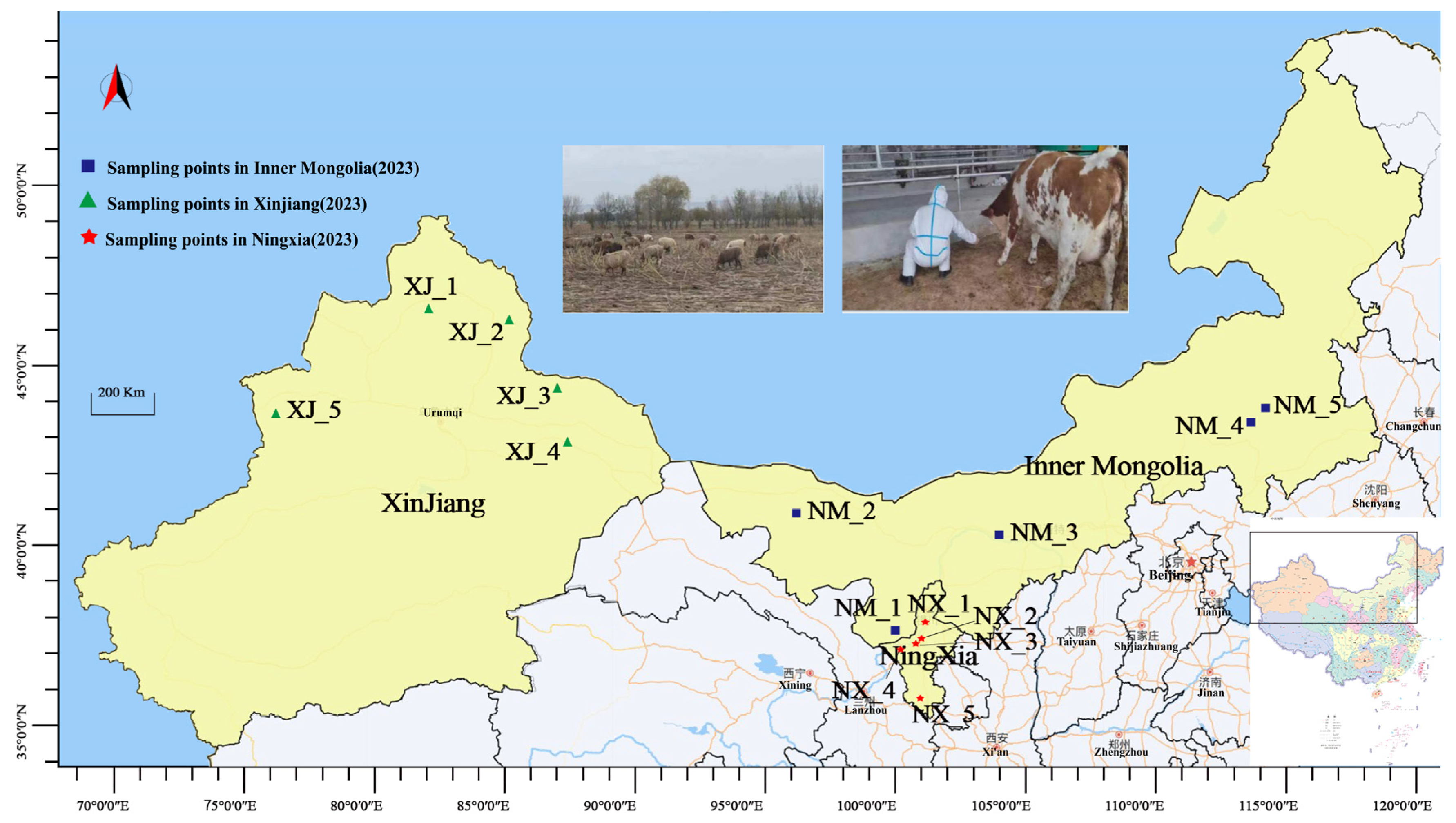
2.1. Sample Collection
2.2. Analysis of Antibiotic Residues
2.3. Metagenomic Sequencing
2.4. Library Construction and Sequencing
2.5. Metagenomic Data Processing
2.6. Statistical Analysis
3. Results
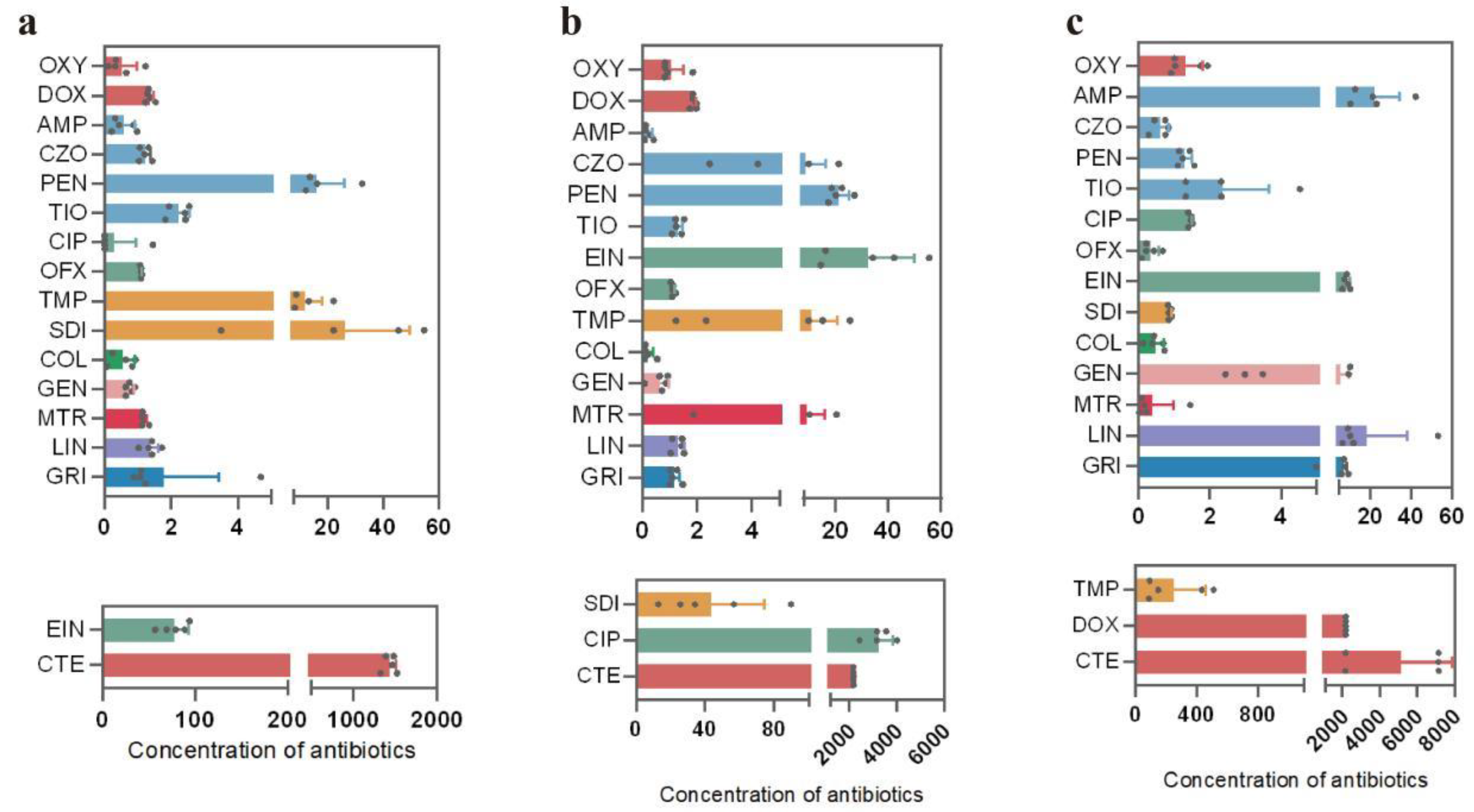
3.1. Antibiotic Residues in Cattle Feces
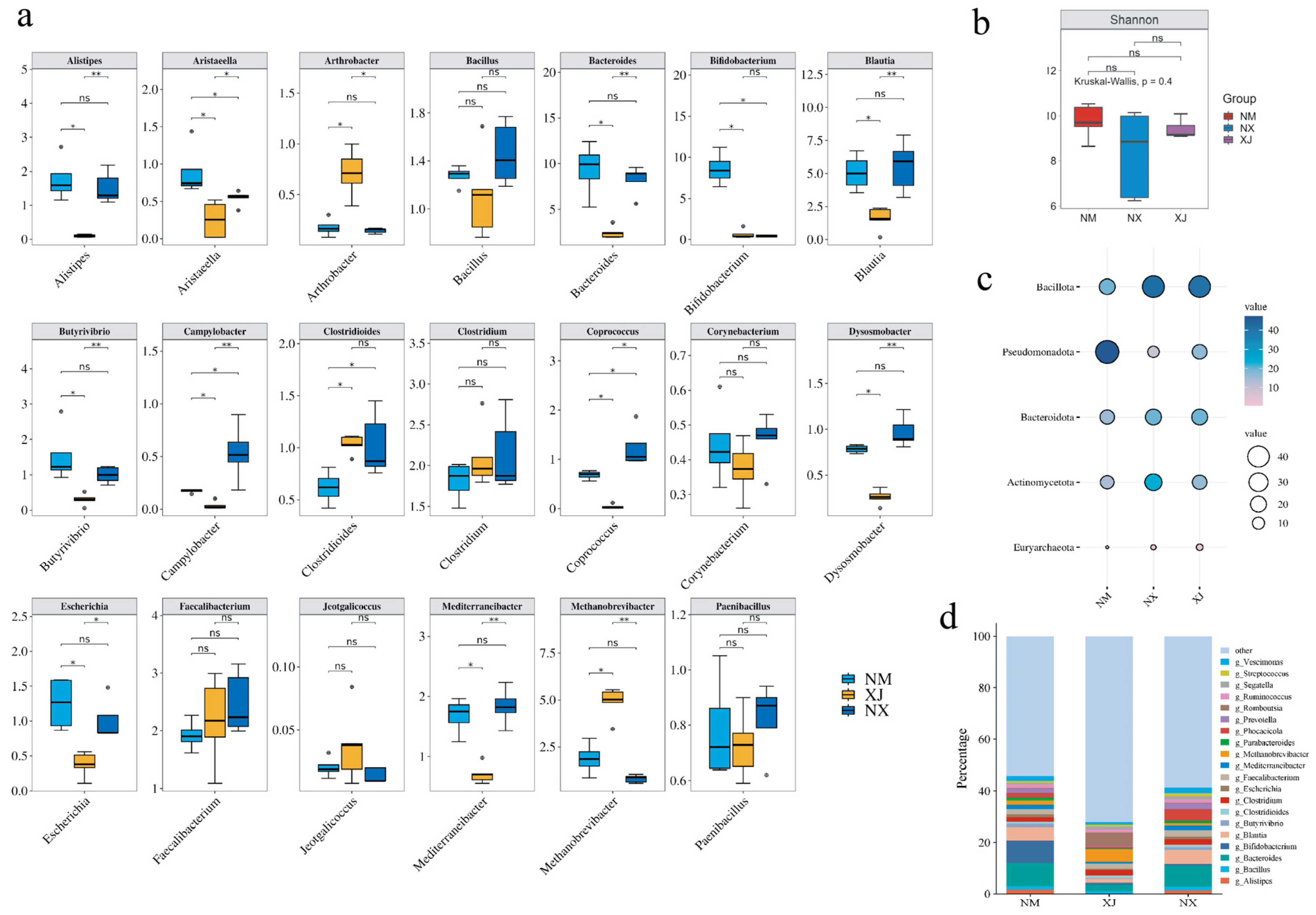
3.2. Characteristics of the Intestinal Microbiota of Grazing Cattle in Northwestern China
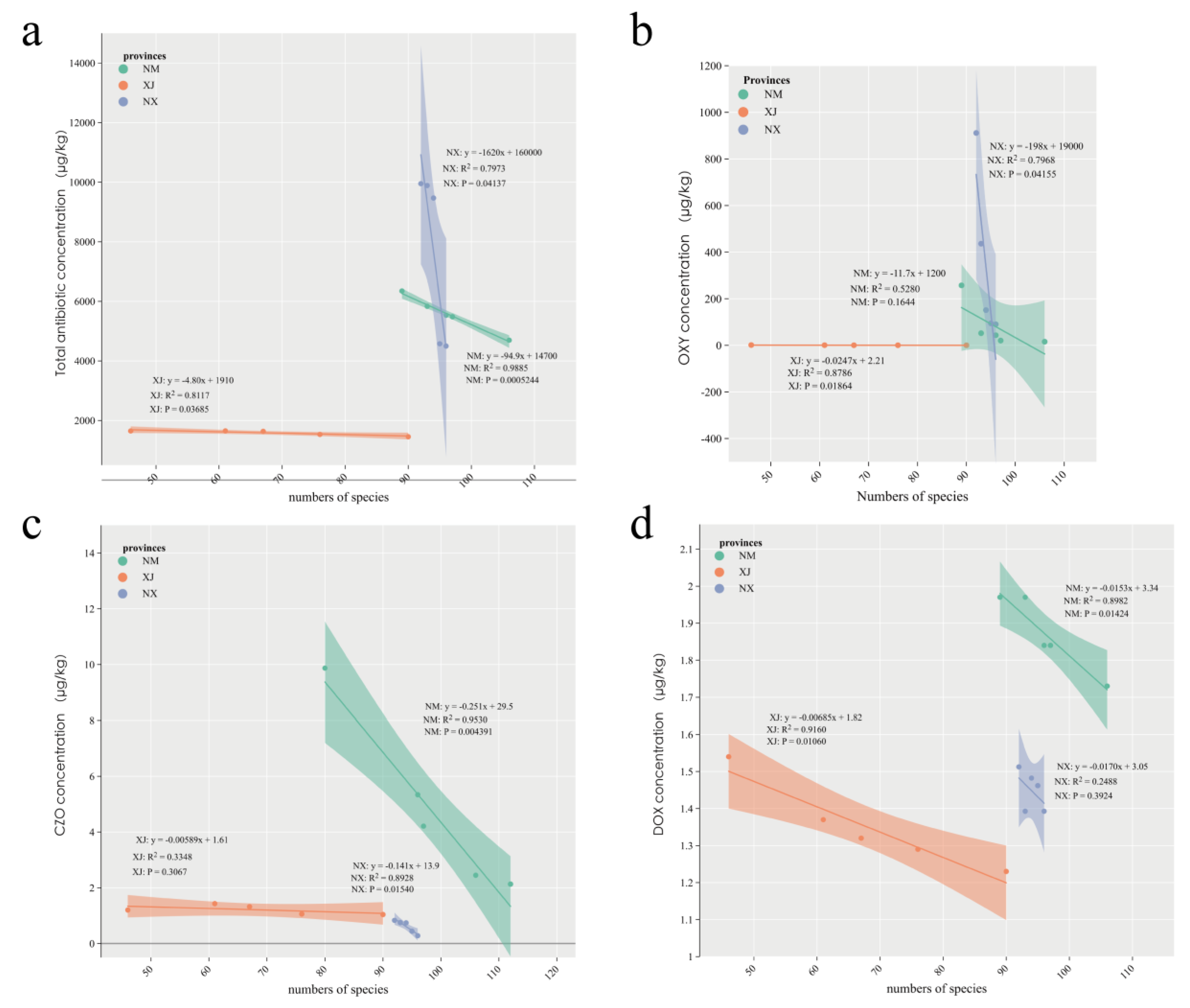
3.3. The Diversity of Intestinal Microbiota Is Strongly Associated with Antibiotic Use
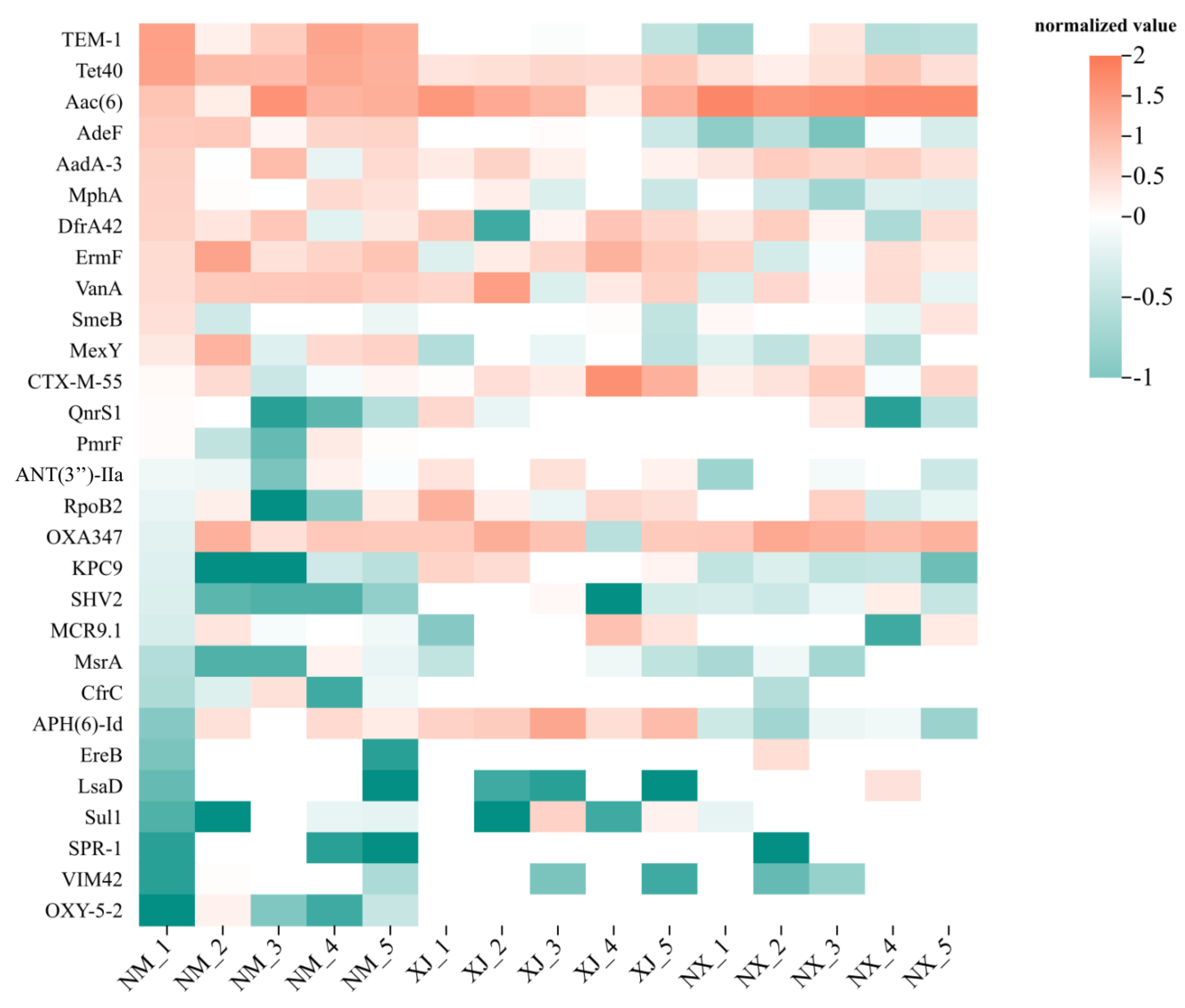
3.4. Relative Abundance of ARGs, MEGs, and HBPs
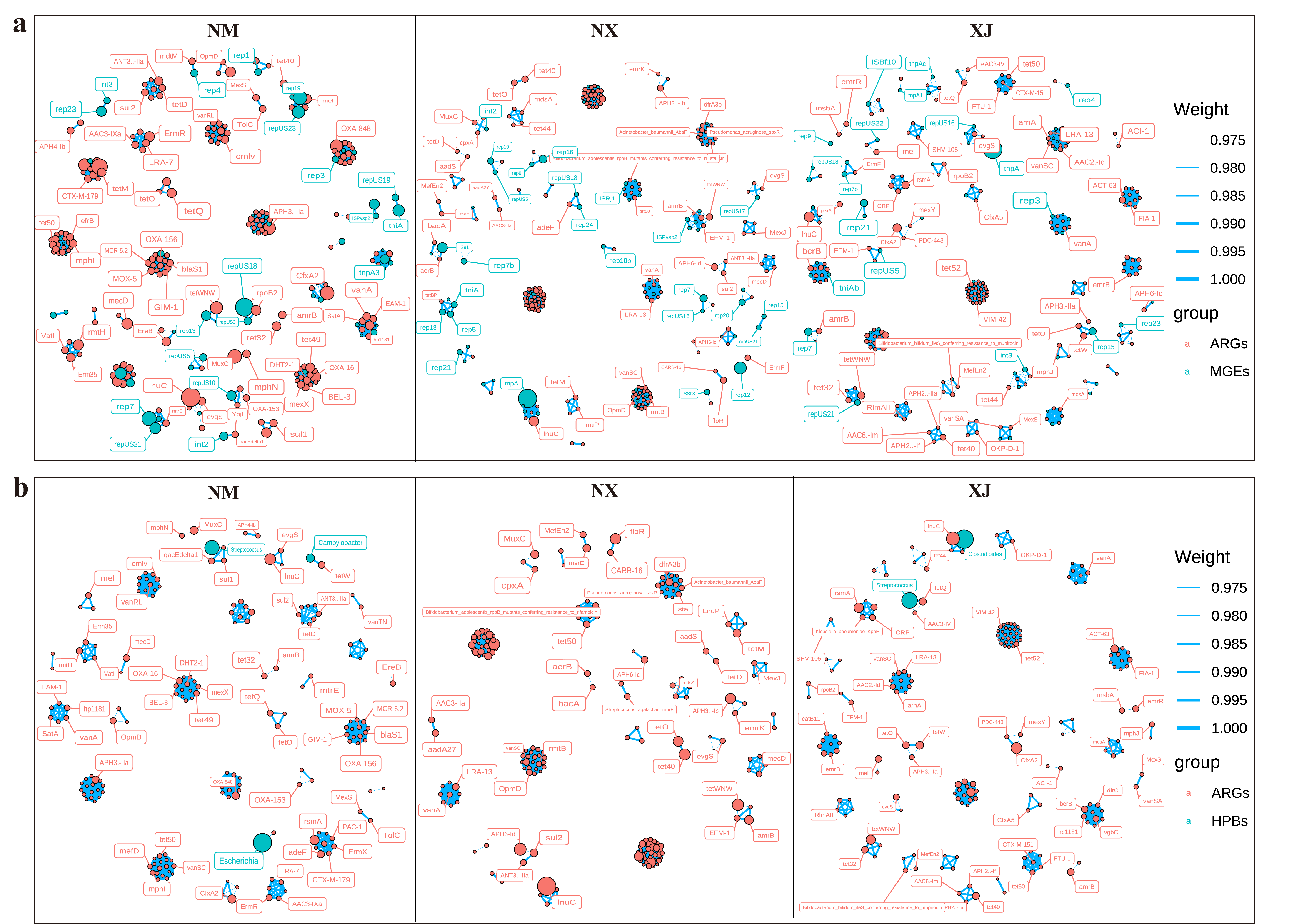
3.5. Analysis of Co-Correlation of ARGs, MGEs, and HBPs
3.6. Association Analysis of Multidrug-Resistant HPBs and ARGs in Feces Samples
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Briske, D.D.; Zhao, M.; Han, G.; Xiu, C.; Kemp, D.R.; Willms, W.; Havstad, K.; Kang, L.; Wang, Z.; Wu, J.; et al. Strategies to alleviate poverty and grassland degradation in Inner Mongolia: Intensification vs production efficiency of livestock systems. J. Environ. Manag. 2015, 152, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yan, W. Evaluation of Herbivorous Animal Husbandry Productivity and Development Potential of Pastoral Areas in Northwest China. Master’s Thesis, Northwest A&F University, Xianyang, China, 2017. [Google Scholar]
- Godde, C.M.; Garnett, T.; Thornton, P.K.; Ash, A.J.; Herrero, M. Grazing systems expansion and intensification: Drivers, dynamics, and trade-offs. Glob. Food Secur. 2018, 16, 93–105. [Google Scholar] [CrossRef]
- Liu, W.-R.; Zeng, D.; She, L.; Su, W.-X.; He, D.-C.; Wu, G.-Y.; Ma, X.-R.; Jiang, S.; Jiang, C.-H.; Ying, G.-G. Comparisons of pollution characteristics, emission situations, and mass loads for heavy metals in the manures of different livestock and poultry in China. Sci. Total Environ. 2020, 734, 139023. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, Q.; Wang, S.; Xu, J.; Fang, Z.; Chen, J.; Zhu, L. Influence of site-specific factors on antibiotic resistance in agricultural soils of Yangtze River Delta: An integrated study of multi-factor modeling. Sci. Total Environ. 2022, 838, 156474. [Google Scholar] [CrossRef]
- Hong, B.; Li, Q.; Li, J.; Zhou, M.; Wang, X.; He, B.; Yu, S. Spectrum of pharmaceutical residues in commercial manure-based organic fertilizers from multi-provinces of China mainland in relation to animal farming and possible environmental risks of fertilization. Sci. Total Environ. 2023, 894, 165029. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Q.; Jin, M.; Huang, L.; Hui, D.; Sardans, J.; Peñuelas, J.; O’cOnnor, P.; Zhu, Y.; Yang, X.; et al. From grasslands to genes: Exploring the major microbial drivers of antibiotic-resistance in microhabitats under persistent overgrazing. Microbiome 2024, 12, 245. [Google Scholar] [CrossRef]
- Van Goethem, M.W.; Pierneef, R.; Bezuidt, O.K.I.; Van De Peer, Y.; Cowan, D.A.; Makhalanyane, T.P. A reservoir of ’historical’ antibiotic resistance genes in remote pristine Antarctic soils. Microbiome 2018, 6, 40. [Google Scholar] [CrossRef]
- Gaballah, M.S.; Guo, J.; Sun, H.; Aboagye, D.; Sobhi, M.; Muhmood, A.; Dong, R. A review targeting veterinary antibiotics removal from livestock manure management systems and future outlook. Bioresour. Technol. 2021, 333, 125069. [Google Scholar] [CrossRef]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as deep modulators of gut microbiota: Between good and evil. Gut 2016, 65, 1906–1915. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, L.; Chen, S.; Wu, H.; Li, R.; Li, J.; Yuan, J.; Wen, T.; Xue, C.; Shen, Q. Risk assessment and dissemination mechanism of antibiotic resistance genes in compost. Environ. Int. 2023, 178, 108126. [Google Scholar] [CrossRef]
- Salam, A.; Al-Amin, Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.-G.; Singer, A.C.; Zhu, Y.-G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Wang, F.; Han, W.; Chen, S.; Dong, W.; Qiao, M.; Hu, C.; Liu, B. Fifteen-Year Application of Manure and Chemical Fertilizers Differently Impacts Soil ARGs and Microbial Community Structure. Front. Microbiol. 2020, 11, 62. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Y.; Yan, X.; Ma, X.; Duan, A.; Hassan, F.-U.; Wang, W.; Deng, T.; Ishaq, S.L. Metagenomic and metabolomic analyses reveal the role of gut microbiome-associated metabolites in diarrhea calves. Msystems 2023, 8, e0058223. [Google Scholar] [CrossRef]
- Gao, F.-Z.; He, L.-Y.; He, L.-X.; Bai, H.; Zhang, M.; Chen, Z.-Y.; Qiao, L.-K.; Liu, Y.-S.; Ying, G.-G. Swine farming shifted the gut antibiotic resistome of local people. J. Hazard. Mater. 2024, 465, 133082. [Google Scholar] [CrossRef]
- Gan, L.; Hu, X. The pollutants from livestock and poultry farming in China-geographic distribution and drivers. Environ. Sci. Pollut. Res. 2016, 23, 8470–8483. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, X.; Yang, T.; Su, J.; Qin, Y.; Wang, S.; Gillings, M.; Wang, C.; Ju, F.; Lan, B.; et al. Air pollution could drive global dissemination of antibiotic resistance genes. ISME J. 2021, 15, 270–281. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, L.; Makhalanyane, T.P.; Xu, C.; Li, K.; Xue, K.; Xu, C.; Qian, R.; Zhang, B.; Du, J.; et al. The composition of antibiotic resistance genes is not affected by grazing but is determined by microorganisms in grassland soils. Sci. Total Environ. 2021, 761, 143205. [Google Scholar] [CrossRef]
- Tyrrell, C.; Do, T.T.; Leigh, R.J.; Burgess, C.M.; Brennan, F.P.; Walsh, F. Differential impact of swine, bovine and poultry manure on the microbiome and resistome of agricultural grassland. Sci. Total Environ. 2023, 886, 163926. [Google Scholar] [CrossRef]
- Butcherine, P.; Kelaher, B.P.; Benkendorff, K. Assessment of acetylcholinesterase, catalase, and glutathione S-transferase as biomarkers for imidacloprid exposure in penaeid shrimp. Aquat. Toxicol. 2022, 242, 106050. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.A.; Park, J.L.; Park, S.J.; Kim, J.H.; Goh, S.H.; Han, J.Y.; Kim, S.Y. Comparison between MGI and Illumina sequencing platforms for whole genome sequencing. Genes Genom. 2021, 43, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Peng, G.-H.; He, X.-S.; Yang, X.-S. Global convergence analysis of the bat algorithm using a Markovian framework and dynamical system theory. Expert Syst. Appl. 2018, 114, 173–182. [Google Scholar] [CrossRef]
- Li, N.; Liu, C.; Zhang, Z.; Li, H.; Song, T.; Liang, T.; Li, B.; Li, L.; Feng, S.; Su, Q.; et al. Research and Technological Advances Regarding the Study of the Spread of Antimicrobial Resistance Genes and Antimicrobial-Resistant Bacteria Related to Animal Husbandry. Int. J. Environ. Res. Public Health 2019, 16, 4896. [Google Scholar] [CrossRef]
- Kang, D.D.; Froula, J.; Egan, R.; Wang, Z. MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ 2015, 3, e11656. [Google Scholar] [CrossRef]
- Shuken, S.R.; McNerney, M.W. Costs and Benefits of Popular P-Value Correction Methods in Three Models of Quantitative Omic Experiments. Anal. Chem. 2023, 95, 2732–2740. [Google Scholar] [CrossRef]
- Chen, Z.; Boehnke, M.; Wen, X.; Mukherjee, B. Revisiting the genome-wide significance threshold for common variant GWAS. G3 2021, 11, jkaa056. [Google Scholar] [CrossRef]
- Chaumeil, P.-A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics 2020, 36, 1925–1927. [Google Scholar] [CrossRef]
- Bowers, R.M.; Kyrpides, N.C.; Stepanauskas, R.; Harmon-Smith, M.; Doud, D.; Reddy, T.B.K.; Schulz, F.; Jarett, J.; Rivers, A.R.; Eloe-Fadrosh, E.A.; et al. Minimum information about a single amplified genome (MISAG) and a metagenome-assembled genome (MIMAG) of bacteria and archaea. Nat. Biotechnol. 2017, 35, 725–731. [Google Scholar] [CrossRef]
- Jang, J.; Kim, M.; Baek, S.; Shin, J.; Shin, J.; Shin, S.G.; Kim, Y.M.; Cho, K.H. Hydrometeorological Influence on Antibiotic-Resistance Genes (ARGs) and Bacterial Community at a Recreational Beach in Korea. J. Hazard. Mater. 2021, 403, 123599. [Google Scholar] [CrossRef]
- Alcock, L.; Galna, B.; Hausdorff, J.M.; Lord, S.; Rochester, L. Enhanced Obstacle Contrast to Promote Visual Scanning in Fallers with Parkinson’s Disease: Role of Executive Function. Neuroscience 2020, 436, 82–92. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Chen, R.-H.; Liu, J.-L.; Shang, T.-H.; Shen, C. Distribution Characteristics of Antibiotics and Antibiotic Resistance Genesin Manure and Surrounding Soil of Cattle Farms in Ningxia. Environ. Sci. 2021, 42, 2981–2991. [Google Scholar]
- Li, S.; Zhu, Y.; Zhong, G.; Huang, Y.; Jones, K.C. Comprehensive Assessment of Environmental Emissions, Fate, and Risks of Veterinary Antibiotics in China: An Environmental Fate Modeling Approach. Environ. Sci. Technol. 2024, 58, 5534–5547. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Rong, H.; Zhao, M.; Luo, H.; Chu, Z.; Wang, R. Interaction between tetracycline and microorganisms during wastewater treatment: A review. Sci. Total Environ. 2021, 757, 143981. [Google Scholar] [CrossRef] [PubMed]
- Pate, L.A.; Milne, C.E.; McMorran, R.; Roberts, D.J.; Macrae, A.I. Factors influencing Scottish dairy farmers’ antibiotic use. Vet. Rec. 2023, 192, e2997. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, H.; Song, D.; Chen, H.; Lin, X.; Wang, Y.; Ji, L. Distribution of antibiotic, heavy metals and antibiotic resistance genes in livestock and poultry feces from different scale of farms in Ningxia, China. J. Hazard. Mater. 2022, 440, 129719. [Google Scholar] [CrossRef]
- Kang, J.; Liu, Y.; Chen, X.; Xu, F.; Wang, H.; Xiong, W.; Li, X. Metagenomic insights into the antibiotic resistomes of typical Chinese dairy farm environments. Front. Microbiol. 2022, 13, 990272. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, G.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, G.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Jung, J.Y.; Ahn, Y.; Khare, S.; Gokulan, K.; Piñeiro, S.A.; Cerniglia, C.E. An in vitro study to assess the impact of tetracycline on the human intestinal microbiome. Anaerobe 2018, 49, 85–94. [Google Scholar] [CrossRef]
- Grudzinski, B.; Fritz, K.; Dodds, W. Dodds, Does Riparian Fencing Protect Stream Water Quality in Cattle-Grazed Lands? Environ. Manag. 2020, 66, 121–135. [Google Scholar] [CrossRef]
- Slizovskiy, I.B.; Bonin, N.; Bravo, J.E.; Ferm, P.M.; Singer, J.; Boucher, C.; Noyes, N.R. Factors impacting target-enriched long-read sequencing of resistomes and mobilomes. Genome Res. 2024, 34, 2048–2060. [Google Scholar] [CrossRef]
- Heuer, H.; Schmitt, H.; Smalla, K. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr. Opin. Microbiol. 2011, 14, 236–243. [Google Scholar] [CrossRef]
- Karkman, A.; Johnson, T.A.; Lyra, C.; Stedtfeld, R.D.; Tamminen, M.; Tiedje, J.M.; Virta, M.; Topp, E. High-throughput quantification of antibiotic resistance genes from an urban wastewater treatment plant. FEMS Microbiol. Ecol. 2016, 92, fiw014. [Google Scholar] [CrossRef]
- Yang, T.; Wang, X.; Hui, X.; Jiang, L.; Bi, X.; Ng, H.Y.; Zheng, X.; Huang, S.; Jiang, B.; Zhou, X. Antibiotic resistome associated with inhalable bioaerosols from wastewater to atmosphere: Mobility, bacterial hosts, source contributions and resistome risk. Water Res. 2023, 243, 120403. [Google Scholar] [CrossRef]
- Liu, C.; Feng, C.; Duan, Y.; Wang, P.; Peng, C.; Li, Z.; Yu, L.; Liu, M.; Wang, F. Ecological risk under the dual threat of heavy metals and antibiotic resistant Escherichia coli in swine-farming wastewater in Shandong Province, China. Environ. Pollut. 2023, 319, 120998. [Google Scholar] [CrossRef]
- Xie, J.; Jin, L.; Wu, D.; Pruden, A.; Li, X. Inhalable Antibiotic Resistome from Wastewater Treatment Plants to Urban Areas: Bacterial Hosts, Dissemination Risks, and Source Contributions. Environ. Sci. Technol. 2022, 56, 7040–7051. [Google Scholar] [CrossRef]
- Yang, T.; Jiang, L.; Bi, X.; Cheng, L.; Zheng, X.; Wang, X.; Zhou, X. Submicron aerosols share potential pathogens and antibiotic resistomes with wastewater or sludge. Sci. Total Environ. 2022, 821, 153521. [Google Scholar] [CrossRef]
- Li, J.; Cao, J.; Zhu, Y.-G.; Chen, Q.-L.; Shen, F.; Wu, Y.; Xu, S.; Fan, H.; Da, G.; Huang, R.-J.; et al. Global Survey of Antibiotic Resistance Genes in Air. Environ. Sci. Technol. 2018, 52, 10975–10984. [Google Scholar] [CrossRef]
- He, P.; Wu, Y.; Huang, W.; Wu, X.; Lv, J.; Liu, P.; Bu, L.; Bai, Z.; Chen, S.; Feng, W.; et al. Characteristics of and variation in airborne ARGs among urban hospitals and adjacent urban and suburban communities: A metagenomic approach. Environ. Int. 2020, 139, 105625. [Google Scholar] [CrossRef]
- Agarwal, V.; Yue, Y.; Zhang, X.; Feng, X.; Tao, Y.; Wang, J. Spatial and temporal distribution of endotoxins, antibiotic resistance genes and mobile genetic elements in the air of a dairy farm in Germany. Environ. Pollut. 2023, 336, 122404. [Google Scholar] [CrossRef]
- Sun, W.; Wang, J.; Wang, G.; Jiang, L.; Feng, W.; Dang, S.; Li, M.; Jiao, S.; Wei, G.; Gu, J.; et al. Exposure and health risks of livestock air resistomes. Proc. Natl. Acad. Sci. USA 2025, 122, e2403866122. [Google Scholar] [CrossRef] [PubMed]
- McClung, R.P.; Roth, D.M.; Vigar, M.; Roberts, V.A.; Kahler, A.M.; Cooley, L.A.; Hilborn, E.D.; Wade, T.J.; Fullerton, K.E.; Yoder, J.S.; et al. Waterborne Disease Outbreaks Associated With Environmental and Undetermined Exposures to Water—United States, 2013–2014. MMWR Morb. Mortal. Wkly Rep. 2017, 66, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, Y.; Zhou, Y.; Pei, Y.; Qu, M.; Lv, P.; Zhang, J.; Xu, X.; Hu, Y.; Wang, Y. Deciphering antibiotic resistance genes and plasmids in pathogenic bacteria from 166 hospital effluents in Shanghai, China. J. Hazard. Mater. 2025, 483, 136641. [Google Scholar] [CrossRef]
- Han, R.R.; Shi, Q.; Wu, S.; Yin, D.; Peng, M.; Dong, D.; Zheng, Y.; Guo, Y.; Zhang, R.; Hu, F.; et al. Dissemination of Carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) Among Carbapenem-Resistant Enterobacteriaceae Isolated From Adult and Children Patients in China. Front. Cell. Infect. Microbiol. 2020, 10, 314. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Wang, X.; Cao, Y.; Liu, C.; Qin, Z.; Zuo, Y.; Li, Y.; Tang, F.; Dai, J.; Wang, S.; et al. Effects of Antibiotic Residues on Fecal Microbiota Composition and Antimicrobial Resistance Gene Profiles in Cattle from Northwestern China. Microorganisms 2025, 13, 1658. https://doi.org/10.3390/microorganisms13071658
He W, Wang X, Cao Y, Liu C, Qin Z, Zuo Y, Li Y, Tang F, Dai J, Wang S, et al. Effects of Antibiotic Residues on Fecal Microbiota Composition and Antimicrobial Resistance Gene Profiles in Cattle from Northwestern China. Microorganisms. 2025; 13(7):1658. https://doi.org/10.3390/microorganisms13071658
Chicago/Turabian StyleHe, Wei, Xiaoming Wang, Yuying Cao, Cong Liu, Zihui Qin, Yang Zuo, Yiming Li, Fang Tang, Jianjun Dai, Shaolin Wang, and et al. 2025. "Effects of Antibiotic Residues on Fecal Microbiota Composition and Antimicrobial Resistance Gene Profiles in Cattle from Northwestern China" Microorganisms 13, no. 7: 1658. https://doi.org/10.3390/microorganisms13071658
APA StyleHe, W., Wang, X., Cao, Y., Liu, C., Qin, Z., Zuo, Y., Li, Y., Tang, F., Dai, J., Wang, S., & Xue, F. (2025). Effects of Antibiotic Residues on Fecal Microbiota Composition and Antimicrobial Resistance Gene Profiles in Cattle from Northwestern China. Microorganisms, 13(7), 1658. https://doi.org/10.3390/microorganisms13071658





