Fusobacterium nucleatum Is Associated with Tumor Characteristics, Immune Microenvironment, and Survival in Appendiceal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort Selection
2.2. Tissue Handling and Acquisition
2.3. Compilation of Clinical Records and Clinicopathologic Features
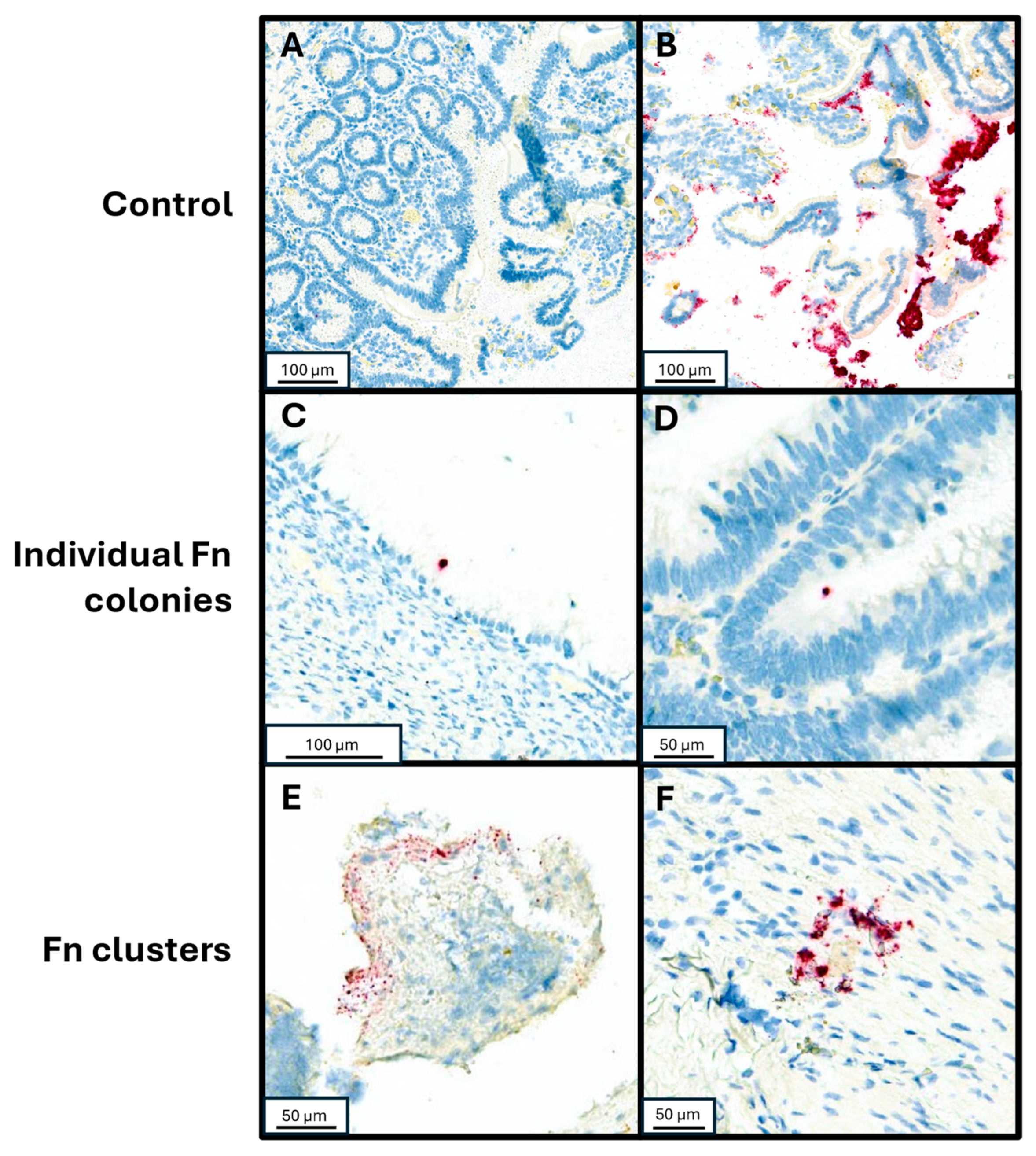
2.4. Detection of Fn Through RNA In Situ Hybridization (RNA-ISH)
2.5. Detection of Fn in Clinical Appendiceal Tumor Specimens and Validation of Density Assay
2.6. Immune Cell Detection Through Immunohistochemistry (IHC), Digital Imagining and Analysis
2.7. Statistical Analysis
3. Results
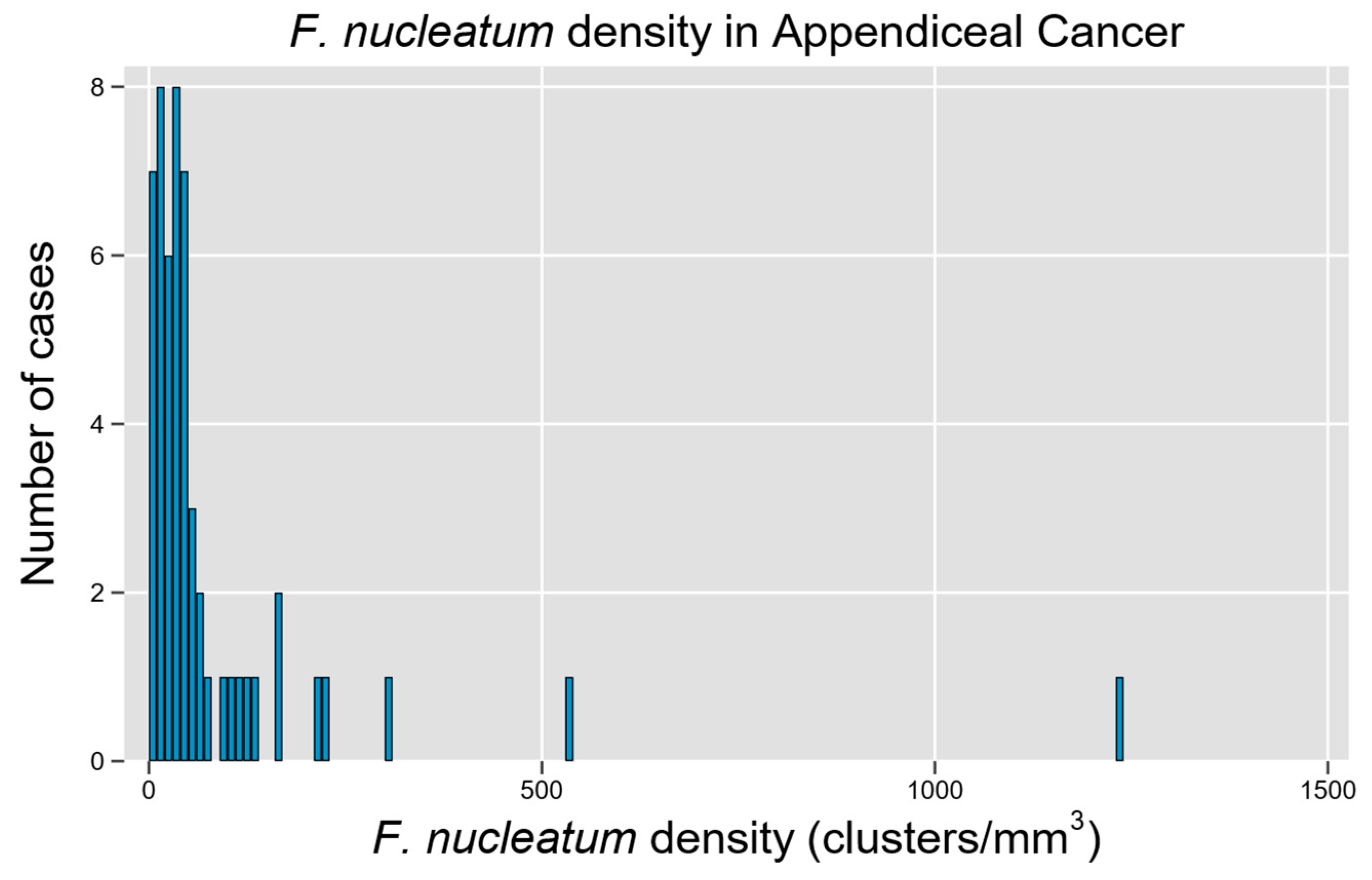
3.1. Cohort Description and Distribution of Fn in Tumor Samples
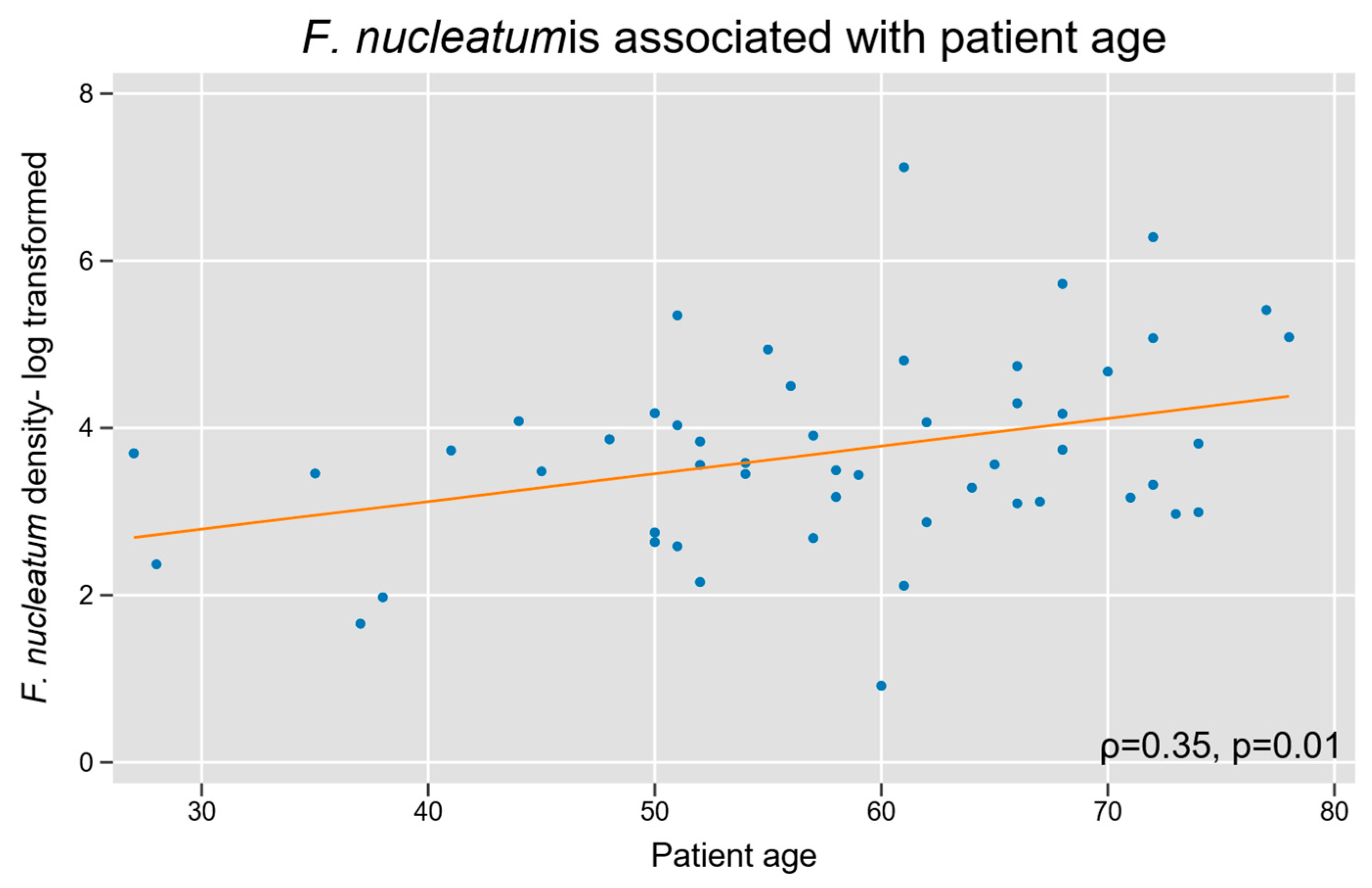
3.2. F. nucleatum with Baseline Patient and Tumor Characteristics
3.3. F. nucleatum and Tumor Immune Microenvironment Within AC
3.4. F. nucleatum and Oncologic Outcome in AC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Appendiceal cancer |
| LAMN | Low-grade appendiceal mucinous neoplasm |
| mAC | Mucinous adenocarcinoma |
| gcAC | Goblet cell adenocarcinoma |
| srcAC | Signet ring cell adenocarcinoma |
| CRC | Colorectal cancer |
| Fn | Fusobacterium nucleatum |
| NF-κB | Nuclear Factor-kappa B |
| PMP | Pseudomyxoma peritonei |
| AHN | Allegheny Health Network |
| FFPE | Formalin-fixed, paraffin-embedded |
| CEA | Carcinoembryonic antigen |
| AJCC | American Joint Committee on Cancer |
| RNA-ISH | Ribonucleic acid in situ hybridization |
| PBS | Phosphate buffered saline |
| ROI | Region of interest |
| IHC | Immunohistochemical |
| PCI | Peritoneal cancer index |
| PFS | Progression free survival |
| OS | Overall survival |
| AC NOS | Adenocarcinoma not otherwise specified |
| NED | No evidence of disease |
| ANOVA | Analysis of Variance |
| HR | Hazard ratio |
| CI | Confidence interval |
| rRNA | Ribosomal ribonucleic acid |
References
- Van de Moortele, M.; De Hertogh, G.; Sagaert, X.; Van Cutsem, E. Appendiceal cancer: A review of the literature. Acta Gastroenterol. Belg. 2020, 83, 441–448. [Google Scholar]
- Collins, D.C. 71,000 Human Appendix Specimens. A Final Report, Summarizing Forty Years’ Study. Am. J. Proctol. 1963, 14, 265–281. [Google Scholar] [PubMed]
- Chua, T.C.; Al-Zahrani, A.; Saxena, A.; Liauw, W.; Zhao, J.; Morris, D.L. Secondary cytoreduction and perioperative intraperitoneal chemotherapy after initial debulking of pseudomyxoma peritonei: A study of timing and the impact of malignant dedifferentiation. J. Am. Coll. Surg. 2010, 211, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.M.; Gupta, P.K.; Carreau, J.H.; Grotz, T.E.; Blas, J.V.; Gatalica, Z.; Nath, S.; Loggie, B.W. Right hemicolectomy is not routinely indicated in pseudomyxoma peritonei. Am. Surg. 2012, 78, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Votanopoulos, K.I.; Russell, G.; Randle, R.W.; Shen, P.; Stewart, J.H.; Levine, E.A. Peritoneal surface disease (PSD) from appendiceal cancer treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): Overview of 481 cases. Ann. Surg. Oncol. 2015, 22, 1274–1279. [Google Scholar] [CrossRef]
- McCoy, A.N.; Araujo-Perez, F.; Azcarate-Peril, A.; Yeh, J.J.; Sandler, R.S.; Keku, T.O. Fusobacterium is associated with colorectal adenomas. PLoS ONE 2013, 8, e53653. [Google Scholar] [CrossRef]
- Bashir, A.; Miskeen, A.Y.; Bhat, A.; Fazili, K.M.; Ganai, B.A. Fusobacterium nucleatum: An emerging bug in colorectal tumorigenesis. Eur. J. Cancer Prev. 2015, 24, 373–385. [Google Scholar] [CrossRef]
- Mima, K.; Sukawa, Y.; Nishihara, R.; Qian, Z.R.; Yamauchi, M.; Inamura, K.; Kim, S.A.; Masuda, A.; Nowak, J.A.; Nosho, K.; et al. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol. 2015, 1, 653–661. [Google Scholar] [CrossRef]
- Chen, T.; Li, Q.; Wu, J.; Wu, Y.; Peng, W.; Li, H.; Wang, J.; Tang, X.; Peng, Y.; Fu, X. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol. Immunother. 2018, 67, 1635–1646. [Google Scholar] [CrossRef]
- Vitetta, L.; Chen, J.; Clarke, S. The vermiform appendix: An immunological organ sustaining a microbiome inoculum. Clin. Sci. 2019, 133, 1–8. [Google Scholar] [CrossRef]
- Swidsinski, A.; Dorffel, Y.; Loening-Baucke, V.; Tertychnyy, A.; Biche-Ool, S.; Stonogin, S.; Guo, Y.; Sun, N.D. Mucosal invasion by fusobacteria is a common feature of acute appendicitis in Germany, Russia, and China. Saudi J. Gastroenterol. 2012, 18, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, H.; Ugai, T.; Takashima, Y.; Okadome, K.; Shimizu, T.; Mima, K.; Akimoto, N.; Haruki, K.; Arima, K.; Zhao, M.; et al. Appendectomy and Long-term Colorectal Cancer Incidence, Overall and by Tumor Fusobacterium nucleatum Status. Ann. Surg. 2024, 282, 319–327. [Google Scholar] [CrossRef]
- Khamzina, Y.; King, M.C.; Nieroda, C.; Merrell, D.S.; Sardi, A.; Gushchin, V. The Role of Microorganisms in Appendiceal Pseudomyxoma Peritonei: A Review. Curr. Oncol. 2022, 29, 3576–3584. [Google Scholar] [CrossRef]
- Gilbreath, J.J.; Semino-Mora, C.; Friedline, C.J.; Liu, H.; Bodi, K.L.; McAvoy, T.J.; Francis, J.; Nieroda, C.; Sardi, A.; Dubois, A.; et al. A core microbiome associated with the peritoneal tumors of pseudomyxoma peritonei. Orphanet J. Rare Dis. 2013, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Semino-Mora, C.; Testerman, T.L.; Liu, H.; Whitmire, J.M.; Studeman, K.; Jia, Y.; McAvoy, T.J.; Francis, J.; Nieroda, C.; Sardi, A.; et al. Antibiotic treatment decreases microbial burden associated with pseudomyxoma peritonei and affects beta-catenin distribution. Clin. Cancer Res. 2013, 19, 3966–3976. [Google Scholar] [CrossRef]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017, 358, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Serna, G.; Ruiz-Pace, F.; Hernando, J.; Alonso, L.; Fasani, R.; Landolfi, S.; Comas, R.; Jimenez, J.; Elez, E.; Bullman, S.; et al. Fusobacterium nucleatum persistence and risk of recurrence after preoperative treatment in locally advanced rectal cancer. Ann. Oncol. 2020, 31, 1366–1375. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Y.; Yang, H.; Liang, M.; Wang, X.; Wang, M.; Kong, J.; Yuan, X.; Zhou, F. Clinical Significance of Fusobacterium nucleatum Infection and Regulatory T Cell Enrichment in Esophageal Squamous Cell Carcinoma. Pathol. Oncol. Res. 2021, 27, 1609846. [Google Scholar] [CrossRef]
- Borgognone, A.; Serna, G.; Noguera-Julian, M.; Alonso, L.; Parera, M.; Catala-Moll, F.; Sanchez, L.; Fasani, R.; Paredes, R.; Nuciforo, P. Performance of 16S Metagenomic Profiling in Formalin-Fixed Paraffin-Embedded versus Fresh-Frozen Colorectal Cancer Tissues. Cancers 2021, 13, 5421. [Google Scholar] [CrossRef]
- Park, H.; Knotts, C.; Blodgett, R.; Lewis, C.R.; Bartlett, D.L.; Dadgar, N.; Wagner, P.L. Fusobacterium nucleatum in appendiceal cancer: Prevalence and influence on the tumor immune microenvironment. Surg. Oncol. Insight 2025, 2, 100122. [Google Scholar] [CrossRef]
- Wagner, P.L.; Xie, J.; Flaherty, D.C.; Park, H.; Knotts, C.; Debelius, J.; Tilves, C.; Dadgar, N.; Xiao, K.; Zaidi, A.H.; et al. Intra-tumoral lymphocyte scoring in colorectal cancer: Improving prognostic utility and correlation with underlying cancer biology. Front. Gastroenterol. 2024, 3, 1493949. [Google Scholar] [CrossRef]
- Galeano Nino, J.L.; Wu, H.; LaCourse, K.D.; Kempchinsky, A.G.; Baryiames, A.; Barber, B.; Futran, N.; Houlton, J.; Sather, C.; Sicinska, E.; et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 2022, 611, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Borowsky, J.; Haruki, K.; Lau, M.C.; Dias Costa, A.; Vayrynen, J.P.; Ugai, T.; Arima, K.; da Silva, A.; Felt, K.D.; Zhao, M.; et al. Association of Fusobacterium nucleatum with Specific T-cell Subsets in the Colorectal Carcinoma Microenvironment. Clin. Cancer Res. 2021, 27, 2816–2826. [Google Scholar] [CrossRef]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Gyorffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef]
- Janczewski, L.M.; Browner, A.E.; Cotler, J.H.; Nelson, H.; Kakar, S.; Carr, N.J.; Hanna, N.N.; Holowatyj, A.N.; Goldberg, R.M.; Washington, M.K.; et al. Survival outcomes used to validate version 9 of the American Joint Committee on Cancer staging system for appendiceal cancer. CA Cancer J. Clin. 2023, 73, 590–596. [Google Scholar] [CrossRef]
- Cavallucci, V.; Palucci, I.; Fidaleo, M.; Mercuri, A.; Masi, L.; Emoli, V.; Bianchetti, G.; Fiori, M.E.; Bachrach, G.; Scaldaferri, F.; et al. Proinflammatory and Cancer-Promoting Pathobiont Fusobacterium nucleatum Directly Targets Colorectal Cancer Stem Cells. Biomolecules 2022, 12, 1256. [Google Scholar] [CrossRef]
- Liu, H.; Yu, Y.; Dong, A.; Elsabahy, M.; Yang, Y.W.; Gao, H. Emerging strategies for combating Fusobacterium nucleatum in colorectal cancer treatment: Systematic review, improvements and future challenges. Exploration 2024, 4, 20230092. [Google Scholar] [CrossRef]
- Swidsinski, A.; Dorffel, Y.; Loening-Baucke, V.; Theissig, F.; Ruckert, J.C.; Ismail, M.; Rau, W.A.; Gaschler, D.; Weizenegger, M.; Kuhn, S.; et al. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut 2011, 60, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S. Bacterial pattern of appendix in acute and chronic appendicitis with its clinical correlation. J. Evol. Med. Dent. Sci. 2016, 5, 418–422. [Google Scholar] [CrossRef]
- Park, H.Y.; Knotts, C.; Blodgett, R.J.; Omstead, A.; Xiao, K.; Bartlett, D.L.; Zaidi, A.; Wagner, P.L. Fusobacterium nucleatum Is Prevalent in Appendiceal Cancer and Negatively Associated with Intra-Tumoral Lymphocytes. Surg. Oncol. J. Am. Coll. Surg. 2024, 239, S437–S472. [Google Scholar]
- Barot, S.V.; Sangwan, N.; Nair, K.G.; Schmit, S.L.; Xiang, S.; Kamath, S.; Liska, D.; Khorana, A.A. Distinct intratumoral microbiome of young-onset and average-onset colorectal cancer. EBioMedicine 2024, 100, 104980. [Google Scholar] [CrossRef] [PubMed]
- Kharofa, J.; Apewokin, S.; Alenghat, T.; Ollberding, N.J. Metagenomic analysis of the fecal microbiome in colorectal cancer patients compared to healthy controls as a function of age. Cancer Med. 2023, 12, 2945–2957. [Google Scholar] [CrossRef]
- Narii, N.; Zha, L.; Sobue, T.; Kitamura, T.; Shiba, S.; Mizutani, S.; Yamada, T.; Yachida, S. Association Between Diet and Fusobacterium nucleatum in the Feces of Healthy Adults: A Hospital-based Cross-sectional Study. Cancer Prev. Res. 2023, 16, 119–126. [Google Scholar] [CrossRef]
- Mehta, R.S.; Nishihara, R.; Cao, Y.; Song, M.; Mima, K.; Qian, Z.R.; Nowak, J.A.; Kosumi, K.; Hamada, T.; Masugi, Y.; et al. Association of Dietary Patterns With Risk of Colorectal Cancer Subtypes Classified by Fusobacterium nucleatum in Tumor Tissue. JAMA Oncol. 2017, 3, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Merrell, D.S.; McAvoy, T.J.; King, M.C.; Sittig, M.; Millar, E.V.; Nieroda, C.; Metcalf, J.L.; Blum, F.C.; Testerman, T.L.; Sardi, A. Pre- and post-operative antibiotics in conjunction with cytoreductive surgery and heated intraperitoneal chemotherapy (HIPEC) should be considered for pseudomyxoma peritonei (PMP) treatment. Eur. J. Surg. Oncol. 2019, 45, 1723–1726. [Google Scholar] [CrossRef] [PubMed]
- Guinane, C.M.; Tadrous, A.; Fouhy, F.; Ryan, C.A.; Dempsey, E.M.; Murphy, B.; Andrews, E.; Cotter, P.D.; Stanton, C.; Ross, R.P. Microbial composition of human appendices from patients following appendectomy. mBio 2013, 4, e00366-12. [Google Scholar] [CrossRef]
- Randal Bollinger, R.; Barbas, A.S.; Bush, E.L.; Lin, S.S.; Parker, W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J. Theor. Biol. 2007, 249, 826–831. [Google Scholar] [CrossRef]
- Gebbers, J.O.; Laissue, J.A. Bacterial translocation in the normal human appendix parallels the development of the local immune system. Ann. N. Y Acad. Sci. 2004, 1029, 337–343. [Google Scholar] [CrossRef]
- Arlt, A.; Bharti, R.; Ilves, I.; Hasler, R.; Miettinen, P.; Paajanen, H.; Brunke, G.; Ellrichmann, M.; Rehman, A.; Hauser, C.; et al. Characteristic changes in microbial community composition and expression of innate immune genes in acute appendicitis. Innate Immun. 2015, 21, 30–41. [Google Scholar] [CrossRef]
- Zhong, D.; Brower-Sinning, R.; Firek, B.; Morowitz, M.J. Acute appendicitis in children is associated with an abundance of bacteria from the phylum Fusobacteria. J. Pediatr. Surg. 2014, 49, 441–446. [Google Scholar] [CrossRef]
- Shi, F.; Liu, G.; Lin, Y.; Guo, C.L.; Han, J.; Chu, E.S.H.; Shi, C.; Li, Y.; Zhang, H.; Hu, C.; et al. Altered gut microbiome composition by appendectomy contributes to colorectal cancer. Oncogene 2023, 42, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ma, Q.; Guo, Y.; You, F. The Role of Fusobacterium nucleatum in Colorectal Cancer Cell Proliferation and Migration. Cancers 2022, 14, 5350. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, C.W.; Ma, X.; Paranjpe, A.; Jewett, A.; Lux, R.; Kinder-Haake, S.; Shi, W. Fusobacterium nucleatum outer membrane proteins Fap2 and RadD induce cell death in human lymphocytes. Infect. Immun. 2010, 78, 4773–4778. [Google Scholar] [CrossRef]
- Wagner, P.L.; Knotts, C.M.; Donneberg, V.S.; Dadgar, N.; Cruz Pico, C.X.; Xiao, K.; Zaidi, A.; Schiffman, S.C.; Allen, C.J.; Donnenberg, A.D.; et al. Characterizing the Immune Environment in Peritoneal Carcinomatosis: Insights for Novel Immunotherapy Strategies. Ann. Surg. Oncol. 2024, 31, 2069–2077. [Google Scholar] [CrossRef]
- Knotts, C.M.; Blodgett, R.; Lewis, C.; Park, H.; Omstead, A.; Dadgar, N.; Zaidi, A.; Bartlett, D.L.; Wagner, P.L. Lymphocyte Density Analysis in Appendiceal Cancer Reveals CD8: CD3 Ratio as a Grade-Independent Prognastic Biomarker. Ann. Surg. Oncol. 2024, 31, S55. [Google Scholar]
- Christopher Sherry, N.D.; Park, H.; Knotts, C.; Greyhack, E.; Blodgett, R.; Zaidi, A.; Xiao, K.; Omstead, A.; Bartlett, D.L.; Wagner, P.L. Tumor-associated macrophage (TAM) subsets in Appendiceal cancer. 2025; pending publication. [Google Scholar]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liu, Y.; Kong, X.; Wu, R.; Peng, Q.; Zhang, Y.; Zhou, L.; Duan, L. Fusobacterium nucleatum Facilitates M2 Macrophage Polarization and Colorectal Carcinoma Progression by Activating TLR4/NF-kappaB/S100A9 Cascade. Front. Immunol. 2021, 12, 658681. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.J.; Ohta, S.; Sheu, K.M.; Spreafico, R.; Adelaja, A.; Taylor, B.; Hoffmann, A. NF-kappaB dynamics determine the stimulus specificity of epigenomic reprogramming in macrophages. Science 2021, 372, 1349–1353. [Google Scholar] [CrossRef]
- Zhuang, L.; Zong, X.; Yang, Q.; Fan, Q.; Tao, R. Interleukin-34-NF-kappaB signaling aggravates myocardial ischemic/reperfusion injury by facilitating macrophage recruitment and polarization. EBioMedicine 2023, 95, 104744. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, Y.; Sun, H.; Bao, S.; Ge, S.; Quan, C. The role of Fusobacterium nucleatum in macrophage M2 polarization and NF-kappaB pathway activation in colorectal cancer. Front. Immunol. 2025, 16, 1549564. [Google Scholar] [CrossRef]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Kaplan, C.W.; He, X.; McHardy, I.; Shi, W.; Lux, R. Characterization of aid1, a novel gene involved in Fusobacterium nucleatum interspecies interactions. Microb. Ecol. 2014, 68, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, C.W.; Lux, R.; Haake, S.K.; Shi, W. The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm. Mol. Microbiol. 2009, 71, 35–47. [Google Scholar] [CrossRef]
- Han, Y.W.; Ikegami, A.; Rajanna, C.; Kawsar, H.I.; Zhou, Y.; Li, M.; Sojar, H.T.; Genco, R.J.; Kuramitsu, H.K.; Deng, C.X. Identification and characterization of a novel adhesin unique to oral fusobacteria. J. Bacteriol. 2005, 187, 5330–5340. [Google Scholar] [CrossRef]
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef]
- Xu, M.; Yamada, M.; Li, M.; Liu, H.; Chen, S.G.; Han, Y.W. FadA from Fusobacterium nucleatum utilizes both secreted and nonsecreted forms for functional oligomerization for attachment and invasion of host cells. J. Biol. Chem. 2007, 282, 25000–25009. [Google Scholar] [CrossRef]




| Characteristic | Total Patients n = 54 | |
|---|---|---|
| age | Median (IQR) | 59 years old (51–68) |
| sex | Male | 46.3% (n = 25) |
| Female | 53.7% (n = 29) | |
| histology | LAMN | 18.5% (n = 10) |
| mAC | 31.5% (n = 17) | |
| gcAC | 20.3% (n = 11) | |
| srcAC | 7.4% (n = 4) | |
| AC NOS | 18.5% (n = 10) | |
| Villous adenoma | 1.9% (n = 1) | |
| Sessile serrated lesion | 1.9% (n = 1) | |
| tumor site | Primary appendiceal | 74.1% (n = 40) |
| Metastatic lesion | 25.9% (n = 14) | |
| grade | n/a | 3.7% (n = 2) |
| 1 | 37.0% (n = 20) | |
| 2 | 29.6% (n = 16) | |
| 3 | 29.6% (n = 16) | |
| stage (AJCC) | Non-invasive | 13.0% (n = 7) |
| I | 1.9% (n = 1) | |
| II | 20.4% (n = 11) | |
| III | 5.6% (n = 3) | |
| IV | 59.3% (n = 32) | |
| nodal status * | Positive | 51.4% (n = 18) |
| Negative | 48.6% (n = 17) | |
| lymphovascular invasion * | Positive | 23.5% (n = 8) |
| Negative | 76.5% (n = 26) | |
| perineural invasion * | Positive | 39.3% (n = 11) |
| Negative | 60.7% (n = 17) | |
| recent chemotherapy | Within 3 months | 21.6% (n = 11) |
| prior cytoreductive surgery | Yes | 15.7% (n = 8) |
| best response to therapy | NED | 40.7% (n = 22) |
| Partial response | 24.1% (n = 13) | |
| Stable disease | 1.9% (n = 1) | |
| Progression | 33.3% (n = 18) | |
| PCI | Median (IQR) | 26 (18–31) |
| CEA | Median (IQR) | 3.0 (1.3–11.1) |
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |||
| Progression | ||||||||
| Fn density | ||||||||
| <40th percentile | Ref. | Ref. | ||||||
| >40th percentile | 0.65 | 0.32, 1.32 | 0.24 | 0.46 | 0.2, 1.1 | 0.07 | ||
| Grade | ||||||||
| I | Ref. | |||||||
| II | 2.4 | 0.9, 6.4 | 0.07 | |||||
| III | 1.5 | 0.6, 3.6 | 0.4 | |||||
| Survival | ||||||||
| Fn density | ||||||||
| <40th percentile | Ref. | |||||||
| >40th percentile | 0.19 | 0.05, 0.73 | 0.02 | 0.11 | 0.02, 0.52 | 0.005 | ||
| Grade | ||||||||
| I | Ref. | |||||||
| II | 10.6 | 1.05, 107.7 | 0.05 | |||||
| III | 5.3 | 0.58, 48.6 | 0.14 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherry, C.; Dadgar, N.; Park, H.; Knotts, C.; Grayhack, E.; Blodgett, R.; Xiao, K.; Omstead, A.N.; Donnenberg, A.D.; Bartlett, D.L.; et al. Fusobacterium nucleatum Is Associated with Tumor Characteristics, Immune Microenvironment, and Survival in Appendiceal Cancer. Microorganisms 2025, 13, 1644. https://doi.org/10.3390/microorganisms13071644
Sherry C, Dadgar N, Park H, Knotts C, Grayhack E, Blodgett R, Xiao K, Omstead AN, Donnenberg AD, Bartlett DL, et al. Fusobacterium nucleatum Is Associated with Tumor Characteristics, Immune Microenvironment, and Survival in Appendiceal Cancer. Microorganisms. 2025; 13(7):1644. https://doi.org/10.3390/microorganisms13071644
Chicago/Turabian StyleSherry, Christopher, Neda Dadgar, Hyun Park, Chelsea Knotts, Erin Grayhack, Rose Blodgett, Kunhong Xiao, Ashten N. Omstead, Albert D. Donnenberg, David L. Bartlett, and et al. 2025. "Fusobacterium nucleatum Is Associated with Tumor Characteristics, Immune Microenvironment, and Survival in Appendiceal Cancer" Microorganisms 13, no. 7: 1644. https://doi.org/10.3390/microorganisms13071644
APA StyleSherry, C., Dadgar, N., Park, H., Knotts, C., Grayhack, E., Blodgett, R., Xiao, K., Omstead, A. N., Donnenberg, A. D., Bartlett, D. L., Donnenberg, V., Goel, A., Zaidi, A. H., & Wagner, P. L. (2025). Fusobacterium nucleatum Is Associated with Tumor Characteristics, Immune Microenvironment, and Survival in Appendiceal Cancer. Microorganisms, 13(7), 1644. https://doi.org/10.3390/microorganisms13071644









